Abstract
A recent study by Hermosilla et al. [T. Hermosilla, D. Munoz, R. Herrera-Molina, A. Valdivia, N. Munoz, S.U. Nham, P. Schneider, K. Burridge, A.F. Quest, L. Leyton, Direct Thy-1/alphaVbeta3 integrin interaction mediates neuron to astrocyte communication, Biochim Biophys Acta 1783 (2008) 1111–1120] demonstrates that Thy-1 on neurons binds to αvβ3 integrin on astrocytes via a conserved RLD motif, triggering the formation of focal adhesions and stress fibers via tyrosine phosphorylation and RhoA activation. This study adds to growing evidence regarding the signaling mechanisms and biological roles of Thy-1, an important regulator of context-dependent signaling. As knowledge of Thy-1 approaches its fiftieth year, it is critical to begin to synthesize insights from different fields to determine how this heretofore enigmatic molecule modulates cell behavior in both cis and trans.
Keywords: Thy-1, integrin, neuron, astrocyte, context-dependent signaling
Thy-1 (CD90) is a developmentally regulated, evolutionaril conserved cell surface glycoprotein, the biological role of which remains somewhat enigmatic despite hundreds of intriguing publications over the past forty-five years. Part of the reason that Thy-1 is still a bit mysterious is that, though it is by no means ubiquitous, it is expressed in a heterogeneous range of cell types, including thymocytes, lymphocytes, fibroblasts, neuronal cells, hematopoietic and mesenchymal stem cells, ovarian follicular cells, and some cancer cells. It therefore has implications in a number of fields, including neurobiology, immunology, oncology, stem cell biology and wound healing. In each of these areas, investigators have tended to home in on one aspect of the function of Thy-1, in a particular cell type. Thus the field suffers a bit from the well-known “blind men and the elephant” metaphor, in which no one has the big picture. Furthermore, the Thy-1 null mouse is viable and has subtle and diverse phenotypic features. Its characterization has been somewhat piecemeal, in keeping with the above observations. In the minds of many, Thy-1 has been relegated to the role of a mere marker; useful for identifying some fibroblasts, stem cells and neurons (in a colorful, even whimsical way in the case of the Thy-1-Brainbow transgenic mouse [2]), but failing to enter the pantheon of “vitally important” molecules.
Part of Thy-1’s scientific facelessness results from its lack of a defined ligand; its failure to associate with molecules of interest. The article by Hermosilla et al [1] addresses this deficiency in part by clearly demonstrating, using a systematic, combinatorial approach, heterotypic binding of neuronal Thy-1 to αvβ3 integrin on astrocytes, inducing focal adhesion formation and cytoskeletal alterations via RhoA GTPase activation. The findings have implications for neurodevelopment and neural repair, and begin to paint a clearer picture of Thy-1’s role with regard to signaling. The immunologic and non-immunologic functions of Thy-1 and what is known about its signaling mechanisms have been reviewed previously [3–5]; this mini-review will focus on recent findings in two broad paradigms in Thy-1 signaling, attempt to put these findings in a broader biological context, and suggest areas for future exploration.
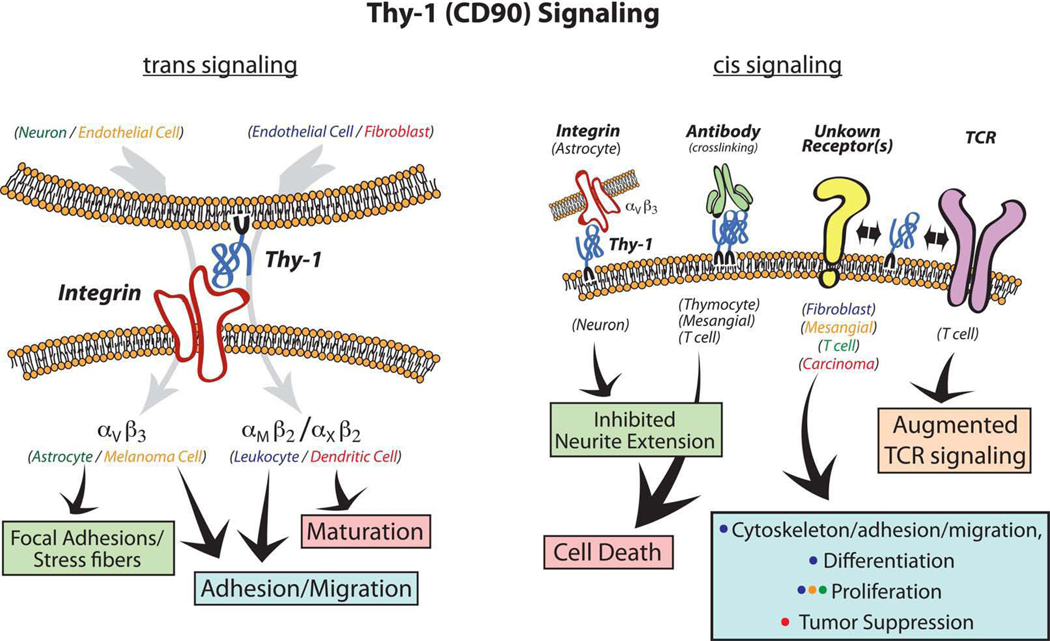
Thy-1 can affect signaling heterotypically (in trans) by “effector cell” Thy-1 binding to a Thy-1 ligand on a “target cell” (Fig. 1A) This is the paradigm illustrated in the Hermosilla paper. Reported ligands for Thy-1 trans-signaling are β2 and β3 integrins. Specifically, human dermal microvascular endothelial cell (HDMEC) Thy-1 binds to αXβ2 (p 150,95, CD11c/CD18) or αMβ2 (Mac-1, CD11b/CD18) on leukocytes, promoting their adhesion and transendothelial migration [6–8]. Melanoma cells appear to bind instead via αvβ3 integrin to Thy-1 on activated endothelium [9, 10], which may facilitate melanoma metastasis. Thy-1 has been known to inhibit neurite outgrowth on astrocytes for many years [11]. Leyton et al initially described how this interaction produces focal adhesion changes in astrocytes as well, and defined a role for β3 integrin in this heterotypic signaling [12]. This group has continued to dissect this interaction, and the current study demonstrates definitively the role of the αvβ3 heterodimer, and more clearly defines the signaling in astrocytes downstream of αvβ3 [1]. A number of novel findings are reported. The b chain of the avb3 heterodimer identified by immunoprecipitation from DI TNC1 rat astrocytes was of smaller-than-expected molecular size, suggesting alternative splicing or post-translational modification. However, “full-length” αvβ3 binds to Thy-1 in vitro by surface plasmon resonance. The αvβ3 heterodimer is not normally expressed on adult astrocytes, but may be upregulated in response to injury. Hermosilla et al also demonstrate a requirement for the RLD tripeptide in Thy-1 in binding to αvβ3 integrin. This sequence is found within a highly conserved region (70% human-rat-mouse homology in the 10 amino acids flanking RLD in both directions) of Thy-1, and is similar to the αMβ2-binding region in fibrinogen [13]. It is suggested that other portions of the Thy-1 molecule, such as the GPI anchor, are not required for trans-signaling through αvβ3, as Thy-1-Fc fusion protein on microspheres appears equally capable of mediating focal adhesion formation on astrocytes. This finding also would seem to negate the need for Thy-1 in “effector” cells (e.g., neurons) to be present within lipid rafts. The signaling pathway stimulated by Thy-1 via αvβ3 involves focal adhesion kinase (FAK) and Rho A GTPase. Interestingly, these are pathways which we have observed in association with homotypic (in cis) Thy-1 signaling in pulmonary fibroblasts [14–16], as discussed further below. The neuron-astrocyte interaction also involves Thy-1 cis-signaling in neurons (neurite outgrowth inhibition); however, the Thy-1-interacting molecules within neurons, and the downstream signaling pathways activated, have yet to be identified. Others have shown that neurite outgrowth inhibition requires lipid raft integrity, as well as the Thy-1-specific GPI anchor [11].
Figure 1.
Thy-1 (CD 90) Signaling. A. Trans Signaling: Simplified scheme of Thy-1 heterotypic signaling. Colored text indicates effector and responder cells for the downstream effects noted. B. Cis Signaling: Colored dots in the light blue box correspond to colored text for the cell types in which the indicated responses have been described. For further details regarding the signaling pathways involved, the reader is referred to the text and to several relevant reviews [3–5].
Thy-1 also signals homotypically (in cis), through interaction with TCR, via Thy-1 crosslinking, or through unknown mechanisms (Fig. 1B). The latter two must by necessity involve interaction with other molecules, as Thy-1 lacks a transmembrane or cytoplasmic portion. We and others have previously established Thy-1’s interaction with members of the src family kinases (SFKs; e.g., via association of the GPI-anchor with palmitoylated residues on src family members such as fyn and lyn [17]). Immunoprecipitation of Thy-1 from cell lysates co-precipitates SFKs [18]. Evidence for cis signaling of Thy-1 can be derived from pulmonary fibroblast subpopulations sorted based on their surface expression of Thy-1. Thy-1-expressing fibroblasts display altered phenotypic responses to growth factors, cytokines, and adhesion proteins. These responses have been partially explained by regulation of downstream signaling proteins such as phosphoinositide-3 kinase (PI3K) and SFKs. In response to the matricellular protein thrombospondin, Thy-1 expressing fibroblasts will disassemble focal adhesions in a PI3K- and SFK-mediated manner [15]. Lack of cell surface Thy-1 abrogates this effect, which can be rescued by exogenous Thy-1 expression following transfection. Furthermore, the baseline activity of the SFK-p190 Rho GTPase-activating protein (GAP) pathway is dependent on Thy-1 expression. Exogeneous expression of Thy-1 on Thy-1-nonexpressing fibroblasts results in inhibition of SFK activity and subsequent activation of Rho A, leading to focal adhesion assembly and stress fiber formation [14]. Equally important to Thy-1 cis signaling is the integrity of lipid raft micro-and nano-domains. Through its GPI linkage, Thy-1 has been shown to have equal mobility in cholesterol-rich lipid rafts as in the bulk plasma membrane, which facilitates its trafficking into and out of lipid raft domains, whereas transmembrane proteins and other moieties are more spatially restricted [19]. This inherent mobility may play a role in Thy-1’s capacity to facilitate cis signaling as demonstrated by the fact that Thy-1 localization to lipid rafts and lipid raft integrity appear critical to Thy-1’s effect on signaling. Either replacement of the GPI linkage with a membrane-spanning domain or disruption of lipid rafts with methyl-beta-cyclodextrin (MβCD) disrupts Thy-1-mediated signaling [11, 16]. Recently, strong evidence has been presented that implies a role for Thy-1 in the association of lipid rafts with the actin cortex immediately adjacent to the plasma membrane. Clustering of Thy-1 with antibody-coated gold nanoparticles followed by particle tracking demonstrates that Thy-1 sorts into, resides within, and migrates out of distinct lipid nanodomains. The residence time within the nanodomains appears controlled by associations with the cell cytoskeleton [20]. Taken together, these data are suggestive of a role of Thy-1 in the trafficking and partitioning of signaling molecules into and out of specialized lipid rafts for higher order signaling networks associated with the cytoskeleton. In this way Thy-1 may act alternately as an inhibitor, by sequestering, or facilitator, by trafficking, of signaling molecules such as SFKs.
In order to truly understand the effects of Thy-1 on signaling networks in both cis and trans, significant further studies are required. As yet, no comprehensive search for cis binding partners has been reported. While clear demonstration of the trans interaction with αvβ3 via RLD is a significant breakthrough, it is clear that Thy-1 interacts with other cell surface molecules in a planar fashion. Does Thy-1 also associate with integrin in cis? The role of Thy-1 in regulating the Rho GTPases, focal adhesion assembly/disassembly, and stress fiber formation are suggestive that cis interactions with integrin occur, but studies are required to demonstrate this, as well as to systematically determine other Thy-1 binding partners. Furthermore, the mechanisms regulating Thy-1 localization and trafficking to specialized and distinct lipid nano-domains present a significant challenge that is likely to provide further insights into Thy-1’s role in coordinating signaling networks. Substitution of peptide sequences that direct GPI linkages in the endoplasmic reticulum would allow the interrogation of the effects of Thy-1 localization on signaling networks and downstream cell biology. The recent publications describing Thy-1’s relative mobility and residence time in specific plasma membrane domains and the unexpected ability of Thy-1 to connect lipid rafts to underlying cytoskeletal networks uncovers a completely unexplored topic with direct relevance to Thy-1’s documented activity in regulating cell cytoskeletal organization and changes.
The evidence for Thy-1 binding to integrin and regulation of cytoskeletal signaling and organization make it ideally located to affect critical cellular events, such as cell motility, mitotic lift-off, and potentially epithelial to mesenchymal transition. The fact that the molecule is shed from the surface [21, 22] and could thus directly interact as a soluble factor, acting potentially as an inhibitor of cell adhesion, further complicates our understanding of the role of this protein in normal physiology and pathological progression. What remains clear is that the expression of Thy-1 has significant consequences for the phenotype of many cells. Despite our lack of understanding of the nuances of Thy-1 mechanisms, it is clear we can no longer be naïve in the assumption that Thy-1 is simply a marker. It appears instead to be an integral protein in defining cell behavior during normal homeostasis as well as during tissue repair.
Other important aspects of Thy-1 biology not reviewed here include evolution of Thy-1, regulation of Thy-1 expression, post-translational modification of Thy-1, including glycosylation and shedding, and potential interaction with lectins. Even by focusing merely on Thy-1 signaling in cis and trans, however, it is clear that Thy-1 exerts significant effects on signaling modulating cell-cell and cell-matrix interaction, via interesting and possibly unique mechanisms involving changes in both target and effector cells. Thy-1 thus emerges as an important modulator of context-dependent signaling, which alters interaction of cells with each other and with their immediate environment. Some of the most challenging problems in biomedicine today are disorders of neurodevelopment and neural repair after injury, regulation of stem cell pluripotency and differentiation, cancer metastasis, and progressive fibrotic diseases. Given the fact that Thy-1 has a role in all of these processes, the era of seeing Thy-1 as a marker, or of compartmentalized, nearsighted analysis of its function is over. As Thy-1 enters its second half-century, it’s time to start looking at the big picture.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hermosilla T, Munoz D, Herrera-Molina R, Valdivia A, Munoz N, Nham SU, Schneider P, Burridge K, Quest AF, Leyton L. Direct Thy-1/alphaVbeta3 integrin interaction mediates neuron to astrocyte communication. Biochim Biophys Acta. 2008;1783:1111–1120. doi: 10.1016/j.bbamcr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Livet J, Weissman TA, Kang H, Draft RW, Lu J, Bennis RA, Sanes JR, Lichtman JW. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature. 2007;450:56–62. doi: 10.1038/nature06293. [DOI] [PubMed] [Google Scholar]
- 3.Haeryfar SM, Hoskin DW. Thy-1: more than a mouse pan-T cell marker. J Immunol. 2004;173:3581–3588. doi: 10.4049/jimmunol.173.6.3581. [DOI] [PubMed] [Google Scholar]
- 4.Rege TA, Hagood JS. Thy-1, a versatile modulator of signaling affecting cellular adhesion, proliferation, survival, and cytokine/growth factor responses. Biochim Biophys Acta. 2006;1763:991–999. doi: 10.1016/j.bbamcr.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rege TA, Hagood JS. Thy-1 as a regulator of cell-cell and cell-matrix interactions in axon regeneration, apoptosis, adhesion, migration, cancer, and fibrosis. Faseb J. 2006;20:1045–1054. doi: 10.1096/fj.05-5460rev. [DOI] [PubMed] [Google Scholar]
- 6.Choi J, Leyton L, Nham SU. Characterization of alphaX I-domain binding to Thy-1. Biochem Biophys Res Commun. 2005;331:557–561. doi: 10.1016/j.bbrc.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Wetzel A, Chavakis T, Preissner KT, Sticherling M, Haustein UF, Anderegg U, Saalbach A. Human Thy-1 (CD90) on activated endothelial cells is a counterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18) J Immunol. 2004;172:3850–3859. doi: 10.4049/jimmunol.172.6.3850. [DOI] [PubMed] [Google Scholar]
- 8.Saalbach A, Haustein UF, Anderegg U. A ligand of human thy-1 is localized on polymorphonuclear leukocytes and monocytes and mediates the binding to activated thy-1-positive microvascular endothelial cells and fibroblasts. J Invest Dermatol. 2000;115:882–888. doi: 10.1046/j.1523-1747.2000.00104.x. [DOI] [PubMed] [Google Scholar]
- 9.Saalbach A, Hildebrandt G, Haustein UF, Anderegg U. The Thy-1/Thy-1 ligand interaction is involved in binding of melanoma cells to activated Thy-1-positive microvascular endothelial cells. Microvasc Res. 2002;64:86–93. doi: 10.1006/mvre.2002.2401. [DOI] [PubMed] [Google Scholar]
- 10.Saalbach A, Wetzel A, Haustein UF, Sticherling M, Simon JC, Anderegg U. Interaction of human Thy-1 (CD 90) with the integrin alphavbeta3 (CD51/CD61): an important mechanism mediating melanoma cell adhesion to activated endothelium. Oncogene. 2005;24:4710–4720. doi: 10.1038/sj.onc.1208559. [DOI] [PubMed] [Google Scholar]
- 11.Tiveron MC, Barboni E, Rivero FBP, Gormley AM, Seeley PJ, Grosveld F, Morris R. Selective inhibition of neurite outgrowth on mature astrocytes by Thy-1 glycoprotein. Nature. 1992;355:745–748. doi: 10.1038/355745a0. [DOI] [PubMed] [Google Scholar]
- 12.Leyton L, Schneider P, Labra CV, Ruegg C, Hetz CA, Quest AF, Bron C. Thy-1 binds to integrin beta(3) on astrocytes and triggers formation of focal contact sites. Curr Biol. 2001;11:1028–1038. doi: 10.1016/s0960-9822(01)00262-7. [DOI] [PubMed] [Google Scholar]
- 13.Altieri DC, Plescia J, Plow EF. The structural motif glycine 190-valine 202 of the fibrinogen g chain interacts with CD11b/CD18 integrin (aMb2Mac-1) and promotes leukocyte adhesion. J. Biol. Chem. 1993;268:1847–1853. [PubMed] [Google Scholar]
- 14.Barker TH, Grenett HE, MacEwen MW, Tilden SG, Fuller GM, Settleman J, Woods A, Murphy-Ullrich J, Hagood JS. Thy-1 regulates fibroblast focal adhesions, cytoskeletal organization and migration through modulation of p190 RhoGAP and Rho GTPase activity. Exp Cell Res. 2004;295:488–496. doi: 10.1016/j.yexcr.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 15.Barker TH, Pallero MA, MacEwen MW, Tilden SG, Woods A, Murphy-Ullrich JE, Hagood JS. Thrombospondin-1-induced focal adhesion disassembly in fibroblasts requires Thy-1 surface expression, lipid raft integrity, and Src activation. J Biol Chem. 2004;279:23510–23516. doi: 10.1074/jbc.M402169200. [DOI] [PubMed] [Google Scholar]
- 16.Rege TA, Pallero MA, Gomez C, Grenett HE, Murphy-Ullrich JE, Hagood JS. Thy-1, via its GPI anchor, modulates Src family kinase and focal adhesion kinase phosphorylation and subcellular localization, and fibroblast migration, in response to thrombospondin-1/hep I. Exp Cell Res. 2006;312:3752–3767. doi: 10.1016/j.yexcr.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 17.Shenoy-Scaria AM, Kwong J, Fujita T, Olszowy MW, Shaw AS, Lublin DM. Signal transduction through decay-accelerating factor. Interaction of glycosyl-phosphatidylinositol anchor and protein tyrosine kinases p56lck and p59fyn 1. J. Immunol. 1992;149:3535–3541. [PubMed] [Google Scholar]
- 18.Henke RC, Seeto GS, Jeffrey PL. Thy-1 and AvGp50 signal transduction complex in the avian nervous system: c-Fyn and G alpha i protein association and activation of signalling pathways. J Neurosci Res. 1997;49:655–670. doi: 10.1002/(SICI)1097-4547(19970915)49:6<655::AID-JNR1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Zhang F, Crise B, Su B, Hou Y, Rose JK, Bothwell A, Jacobson K. Lateral diffusion of membrane-spanning and glycosylphosphatidylinositol-linked proteins: toward establishing rules governing the lateral mobility of membrane proteins. J Cell Biol. 1991;115:75–84. doi: 10.1083/jcb.115.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y, Thelin WR, Yang B, Milgram SL, Jacobson K. Transient anchorage of cross-linked glycosyl-phosphatidylinositol-anchored proteins depends on cholesterol, Src family kinases, caveolin, and phosphoinositides. J Cell Biol. 2006;175:169–178. doi: 10.1083/jcb.200512116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hagood JS, Prabhakaran P, Kumbla P, Salazar L, MacEwen MW, Barker TH, Ortiz LA, Schoeb T, Siegal GP, Alexander CB, Pardo A, Selman M. Loss of fibroblast Thy-1 expression correlates with lung fibrogenesis. Am J Pathol. 2005;167:365–379. doi: 10.1016/S0002-9440(10)62982-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saalbach A, Wetzig T, Haustein UF, Anderegg U. Detection of human soluble Thy-1 in serum by ELISA. Fibroblasts and activated endothelial cells are a possible source of soluble Thy-1 in serum. Cell Tissue Res. 1999;298:307–315. doi: 10.1007/s004419900079. [DOI] [PubMed] [Google Scholar]