Abstract
Protein sumoylation is a post-translational-modification event, in which small ubiquitin-like modifier (SUMO) is covalently attached to protein substrates by a three-step process. Sumoylation has been suggested to regulate multiple cellular processes, including inflammation. Inflammation is initiated in response to pathogenic infections, but uncontrolled inflammatory responses can lead to the development of inflammatory disorders such as rheumatoid arthritis. Recent studies indicate that proinflammatory stimuli, such as tumor necrosis factor α and lipopolysaccharide, can activate PIAS1 [protein inhibitor of activated STAT1 (signal transducer and activator of transcription 1)] SUMO E3 ligase through a SUMO-dependent, inhibitor of κB kinase α(IKKα)-mediated phosphorylation event. Activated PIAS1 is then recruited to inflammatory gene promoters to repress transcription. These findings support a hypothesis that therapies targeting the PIAS1SUMO ligase pathway might be developed for the treatment of inflammatory disorders such as rheumatoid arthritis and atherosclerosis.
Introduction
Post-translational modification by small ubiquitin-like modifier (SUMO) conjugation is achieved through a pathway that involves three steps: activation (E1), conjugation (E2) and ligation (E3) (Box 1). SUMO modification of proteins has been suggested to regulate many physiological processes, such as transcriptional regulation, stress responses and protein localization [1,2]. Recent studies indicate a role for sumoylation in the regulation of inflammation.
Box 1. The small ubiquitin-like modifier (SUMO) conjugation pathway.
The mammalian small ubiquitin-like modifier (SUMO) protein family consists of four members: SUMO1, SUMO2, SUMO3 and SUMO4. The human SUMO2 and SUMO3 are 97% identical to each other, whereas SUMO1 shares only ~50% identity with SUMO2 and SUMO3. SUMO4 shows similarity to SUMO2 and SUMO3, but it is unclear whether SUMO4 can be conjugated to protein substrates. The covalent conjugation of SUMO to substrate proteins is known as protein sumoylation. Sumoylation involves three steps: activation, conjugation and ligation (Figure I). SUMO is activated by the SUMO E1 activating enzyme, which is a heterodimer that consists of SAE (SUMO-activating enzyme) 1 and 2, in an ATP-dependent manner. E1 uses ATP to adenylate the C-terminal glycine residue of SUMO, and a thioester bond is then formed between this residue of SUMO and a cysteine residue in SAE2. Next, SUMO is transferred from E1 to the cysteine residue of the SUMO E2 conjugating enzyme ubiquitin-conjugating enzyme 9 (UBC9). The SUMO E3 ligase then promotes the transfer of SUMO from E2 to the target substrate, forming an isopeptide bond between the C terminus of SUMO and the ε-amino group of a lysine residue in the target protein. Several SUMO E3 ligases, including PIAS proteins, Ras-related nuclear protein (RAN)-binding protein 2 (RANBP2) and polycomb group protein Pc2, have been identified.
Inflammation is initiated in response to tissue damage and infectious agents. Inflammatory responses must be regulated properly, and unrestricted inflammation can lead to inflammatory disorders and cancers. The transcriptional induction of genes involved in inflammatory responses is controlled by various transcription factors, including nuclear factor κB (NF-κB), signal transducer and activator of transcription (STAT) and activator protein-1 (AP-1). Sumoylation can regulate inflammation through the direct modulation of the activity of key transcription factors involved in inflammatory responses. For example, the sumoylation of members of the nuclear-receptor family, such as the peroxisome proliferator-activated receptor γ(PPARγ) and liver X receptors (LXRs), regulates their activity to transrepress inflammatory gene activation [3,4]. Sumoylation has also been suggested to regulate the activity of transcription factor AP-1 and the glucocorticoid receptor, in addition to the type I transforming growth factor-β receptor [5–7].
A member of the protein inhibitor of activated STAT (PIAS) family, PIAS1, which possesses SUMO E3 ligase activity [8], is a transcriptional repressor of NF-κB and STAT1. PIAS1 functions by blocking the DNA-binding activity of NF-κB and STAT1 on gene promoters (Box 2). Recent studies indicate that PIAS1 is activated by phosphorylation in response to proinflammatory stimuli, such as tumor necrosis factor (TNF) α and lipopolysaccharide (LPS), a process that requires the SUMO ligase activity of PIAS1. Activated PIAS1 is then recruited to inflammatory gene promoters to repress NF-κB and STAT1-mediated transcription [9] (Figure 1). In addition, PIAS1 has also been suggested to promote the sumoylation of PPARγ, resulting in the PPARγ-mediated transrepression of inflammatory gene activation [3] (Figure 1). These findings support a hypothesis that targeting the PIAS1 sumoylation pathway might represent a novel therapeutic strategy for the treatment of inflammatory disorders such as rheumatoid arthritis and atherosclerosis.
Box 2. PIAS-mediated transcriptional repression of STAT and NF-κB pathways.
The mammalian PIAS family consists of four members: PIAS1, PIAS3, PIASx and PIASy. PIAS proteins have several conserved domains (Figure Ia): the SAP domain [scaffold-attachment factor (SAF) A and B, apoptotic chromatin-condensation inducer in the nucleus (ACINUS) and PIAS domain], which contains a conserved Leu-Xaa-Xaa-Leu-Leu (LxxLL) amino-acid motif (‘x’ denotes any amino acid); a conserved Cys3-His-Cys4-type RLD (RING-finger-like zinc-binding domain); the AD (highly acidic domain), which contains a SIM (SUMO1-interaction motif) that is found in all PIAS proteins except PIASy; and the Ser/Thr-rich (ST) region, which is also not present in PIASy. The RLD is necessary for their SUMO E3 ligase activity.
PIAS1 selectively inhibits a subset of STAT1- or nuclear factor κB (NF-κB)-dependent genes in response to treatment with interferon (IFN) and tumor necrosis factor (TNF) or lipopolysaccharide (LPS), respectively, by blocking their DNA-binding activities to gene promoters (Figure Ib).
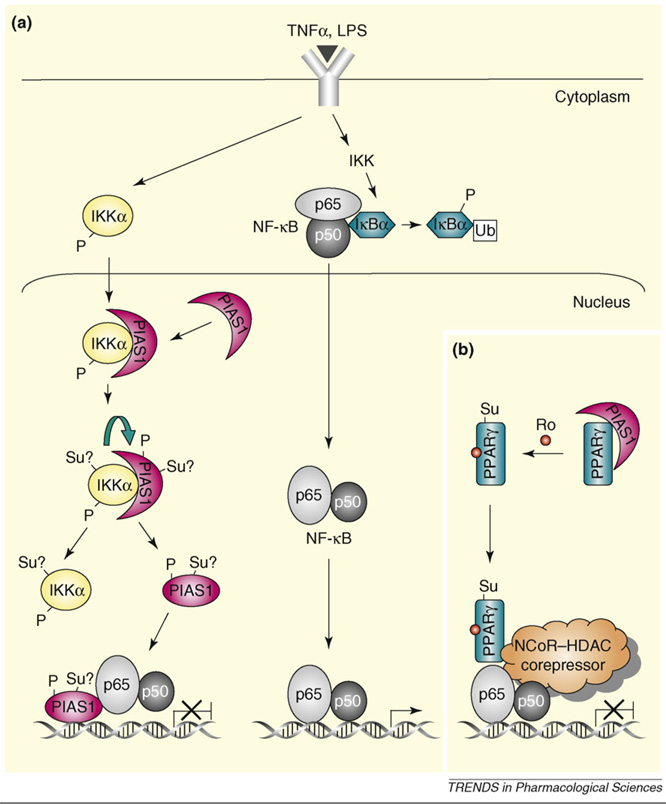
Figure 1.
Regulation of PIAS1-mediated transcriptional repression by phosphorylation and sumoylation. (a) PIAS1 directly binds to chromatin and represses NF-κB-mediated transcription in response to inflammatory stimuli, which is regulated by ligand-induced Ser90 phosphorylation of PIAS1. The IKKα kinase is activated by inflammatory stimuli such as TNF and LPS, which then translocates into the nucleus where it interacts with PIAS1 and phosphorylates PIAS1 at Ser90. The IKKα-mediated PIAS1 phosphorylation is dependent on the SUMO E3 ligase activity of PIAS1, indicating that the possible sumoylation (represented as ‘Su?’) of PIAS1 and/or IKKα might be involved in this process. Phosphorylated PIAS1 disassociates from IKKα and then binds to the promoters of PIAS1-regulated genes for transcriptional repression. (b) PIAS1 also participates in PPARγ-mediated transrepression by acting as SUMO E3 ligase for PPARγ. In response to a PPARγ agonist, such as rosiglitazone (Ro), PPARγ is activated via PIAS1-mediated sumoylation and recruited to the NCoR–HDAC3 corepressor complex, preventing the degradation of the NCoR–HDAC3 complex by the ubiquitin–proteosome pathway and resulting in transrepression. Abbreviation: Ub, ubiquitylation.
PIAS1 regulates NF-κB and STAT1
NF-κB and STATs are two important families of transcription factors that are activated by a variety of inflammatory stimuli. Through distinct signaling mechanisms, NF-κB and STATs are activated rapidly in the cytoplasm and then translocate into the nucleus to activate transcription [10–12]. The SUMO conjugation system participates in the regulation of NF-κB and STAT signaling pathways [8,13].
Biochemical and genetic studies have demonstrated that PIAS1 is a physiologically important negative regulator of NF-κB and STAT1 [14,15]. PIAS1 can bind directly to NF-κB-p65 and STAT1, which leads to the inhibition of the transcriptional activity of NF-κB and STAT1, respectively (Box 2).
A recent study provides evidence to support the role of PIAS1 SUMO ligase activity in the regulation of NF-κB-and STAT1-mediated inflammatory gene activation [9] (Figure 1). Upon stimulation by proinflammatory stimuli, such as TNFα or LPS, PIAS1 becomes rapidly phosphorylated on Ser90, an event that triggers the recruitment of PIAS1 to the promoters of NF-κB or STAT1 target genes, resulting in the inhibition of the promoter-binding activity of NF-κB or STAT1. Inhibitor of κB kinase α (IKKα) has been identified as the kinase responsible for PIAS1 Ser90 phosphorylation that is induced by proinflammatory stimuli. Consistently, an elevated inflammatory gene induction was observed in IKKα-null macrophages [16]. Interestingly, the ability of IKKα to phosphorylate PIAS1 in vivo is dependent on the SUMO ligase activity of PIAS1. The precise role of PIAS1 SUMO ligase in IKKα-mediated PIAS1 Ser90 phosphorylation remains to be understood. It is possible that the sumoylation of IKKα or PIAS1 itself might be necessary for the efficient phosphorylation of PIAS1 by IKKα in vivo.
It should be noted that, although sumoylation has been suggested to regulate inhibitor of κBα (IκBα), which is an inhibitor of NF-κB [17], PIAS1 does not seem to have a role in the process. The SUMO ligase involved in the sumoylation of IκBα remains to be determined. In addition, there is no evidence that NF-κB itself is regulated by sumoylation. Interestingly, it has been reported that STAT1 can be sumoylated by PIAS1 on Lys703 [18–20]. However, the effect of STAT1 sumoylation on STAT1-mediated gene activation is controversial. Although the results from some groups do not support a role of STAT1 sumoylation in gene regulation [18,20], other studies indicate that the sumoylation of STAT1 affects the transcriptional activity of STAT1, probably in a gene-dependent manner [19,21].
One important discovery from Pias1 gene-targeting studies is the finding that PIAS1 displays specificity in the regulation of NF-κB- and STAT1-mediated gene activation. Quantitative polymerase chain reaction (Q-PCR) analysis and systematic gene-profiling studies indicate that PIAS1 selectively regulates the induction of a subset of NF-κB- and STAT1-target genes (PIAS1-sensitive genes), with a preference for inflammatory cytokines and chemokines [14,15].
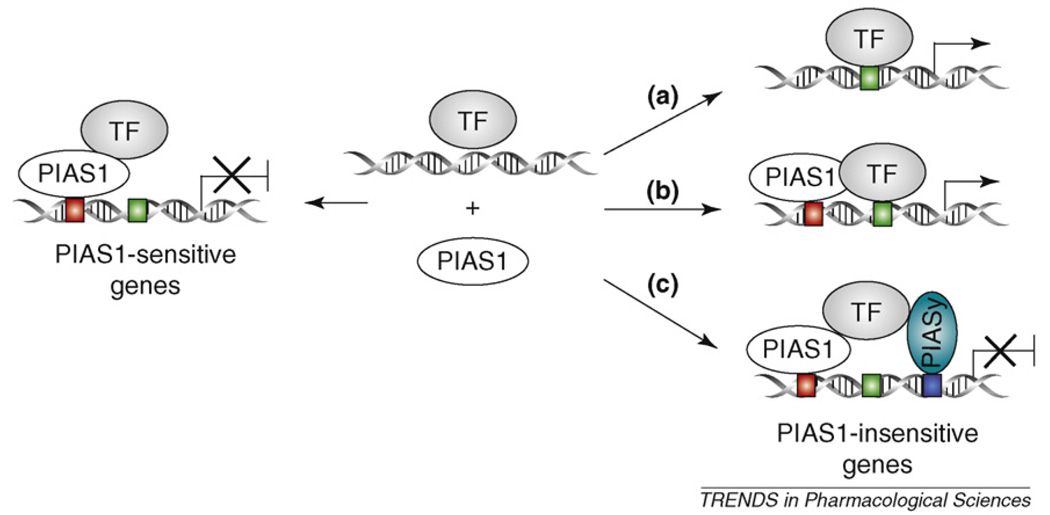
Three possible mechanisms might contribute to the observed specificity of PIAS1 in the regulation of NF-κB and STAT1 (Figure 2). First, PIAS1 needs to be recruited to gene promoters to repress transcription [9]. It is possible that PIAS1 might be selectively recruited to the promoters of its target genes (PIAS1-sensitive genes) that contain a PIAS1-binding sequence, which is absent in PIAS1-insensitive genes. This model can be tested by future studies on the molecular basis of PIAS1-promoter recruitment. Second, PIAS1 target genes containing low-affinity NF-κB or STAT1 DNA-binding sequences in their promoters might be affected preferentially by Pias1 disruption. This is reasonable given the finding that PIAS1 regulates the DNA-binding activity of NF-κB and STAT1. At least in the case of STAT1, experimental data do support this hypothesis [14]. Finally, the redundancy among PIAS family members might also contribute to the specificity of PIAS1-mediated gene regulation. The lack of PIAS1 effect on the induction of certain PIAS1-insensitive genes might be because of a redundant role of another PIAS family member. Indeed, recent studies have shown that PIASy is also involved in the negative regulation of NF-κB and STAT1 signaling [22]. Through genetic-crossing studies between Pias1- and Piasy-null mice, it has been shown that PIASy and PIAS1 display specific and redundant roles in the induction of NF-κB and STAT1 target genes and that the cooperative action of PIAS1 and PIASy controls the specificity and magnitude of NF-κB- and STAT1-mediated gene activation.
Figure 2.
The proposed mechanisms of PIAS1 specificity in gene regulation. In response to signals, PIAS1 selectively represses the induction of a subset of genes (PIAS1- sensitive genes) by blocking the DNA-binding activity of transcription factors (TFs), such as STAT1 and NF-κB. For genes that are not regulated by PIAS1 (PIAS1-insensitive genes), three possible mechanisms might contribute. (a) The promoters of these genes do not contain sequence-specific binding site(s) for PIAS1 (red box). (b) The TF has a high affinity for the gene promoters that cannot be inhibited by PIAS1. (c) Genes might be regulated by PIAS family members, such as PIAS1 and PIASy. In the absence of PIAS1, PIASy can still inhibit gene transcription.
PIAS1 regulates nuclear receptors
Members of the nuclear-receptor family of ligand-dependent transcription factors, such as PPARγ and LXRs, can inhibit inflammatory gene expression directly by antagonizing the activities of other transcription factors, such as NF-κB and AP-1; this is a molecular process termed trans-repression [23]. Sumoylation of PPARγ and LXRs has been shown to play an important part in the regulation of transrepression of inflammatory genes by PPARγ and LXRs [3,4].
In macrophages, PPARγ mediates transrepression of a group of inflammatory genes, such as inducible nitric oxide synthase (iNOS), by blocking the signal-dependent clearance of nuclear-receptor corepressor (NCoR)–histone deacetylase (HDAC)-3 complexes on inflammatory promoters [3]. Upon LPS stimulation, the NCoR–HDAC3 corepressor complexes are normally removed from inflammatory gene promoters via the recruitment of the ubiquitinylation–19S proteosome machinery, resulting in the rapid induction of inflammatory genes. Interestingly, PPARγ agonists induce ligand-dependent conjugation of SUMO1 to PPARγ, which targets PPARγ to NCoR–HDAC3 corepressor complexes on inflammatory gene promoters and, consequently, prevents the clearance of NCoR–HDAC3 complexes by the ubiquitin–proteosome pathway. The ligand-induced sumoylation of PPARγ is mediated through PIAS1 E3 ligase. These results reveal a role for protein sumoylation in the PPARγ-mediated anti-inflammatory responses [3]. As described, PIAS1 can participate directly in the inhibition of LPS-induced NF-κB-mediated inflammatory gene activation. Thus, PIAS1 seems to have a dual functional role in PPARγ-mediated transrepression of inflammatory gene induction (Figure 1).
Recently, a similar sumoylation-dependent regulatory mechanism has also been shown to be involved in the regulation of LXR-mediated transrepression of inflammatory genes [4]. However, SUMO2 and/or SUMO3, but not SUMO1, is conjugated to LXRs. In addition, HDAC4, but not PIAS1, is suggested to function as the SUMO E3 ligase to promote the ligand-dependent sumoylation of LXRs. These studies indicate that the sumoylation-dependent regulatory mechanism might be a general molecular strategy for transrepression.
Interestingly, a high degree of gene specificity is observed in the sumoylation-dependent transrepression by PPARγ and LXRs. In fact, SUMO1–PPARγ and SUMO2–LXRs and/or SUMO3–LXRs seem to inhibit distinct inflammatory response genes through a promoter-and signal-specific manner [4]. Together with the finding that PIAS proteins display specificity in the regulation of NF-κB- and STAT1-mediated inflammatory gene activation, these studies argue for an interesting role of protein sumoylation in the control of specificity in gene expression.
Design of sumoylation-based therapies to control inflammation
The studies discussed here have revealed the involvement of the sumoylation pathway in the regulation of inflammatory responses, and they raise an interesting possibility that therapeutic strategies targeting the PIAS1 SUMO ligase pathway might be developed for the treatment of inflammatory diseases such as rheumatoid arthritis. However, drugs that target the global sumoylation system might not be effective to restrict inflammation. This is because sumoylation is involved in the regulation of multiple biological processes and is necessary for normal cellular functions [24,25]. In addition, there is no clear correlation between the level of global protein sumoylation and inflammation. Rather, it seems likely that the biological effect of sumoylation on inflammation might be largely dependent on individual proteins that are modified by sumoylation. Thus, targeting specific sumoylation events rather than the global SUMO pathway might prove to be a more rational and effective strategy. How can we achieve this goal? Among various components of the SUMO conjugation system, SUMO ligases are most important for the control of substrate specificity. Recent studies indicate that the PIAS SUMO ligase family has an important role in the regulation of immune responses. Of particular interest is the finding that PIAS1 selectively affects the induction of a subset of NF-κB and STAT1 target genes, with a preference for inflammatory cytokines and chemokines [14,15,22]. Similarly, PIAS1 selectively affects the ability of PPARγ to inhibit inflammatory gene expression [3,4]. These findings argue for PIAS1 as a potential therapeutic target for the treatment of inflammatory disorders.
The design of rational strategies to target PIAS1 requires a thorough understanding of the molecular mechanism of the PIAS1-mediated anti-inflammatory effect. As described, several important features of the PIAS1-mediated anti-inflammatory pathway have been revealed recently. It is conceivable that potential drugs can be developed to target various steps of PIAS1 activation (Figure 1). For example, small molecules that can enhance PIAS1 Ser90 phosphorylation through activating IKKα or inhibiting PIAS1 Ser90 phosphatase might prove to be effective to restrict inflammation. In addition, targeting SUMO proteases involved in the regulation of PIAS1 or IKKα might also be useful. Obviously, future studies are needed to identify the PIAS1 Ser90 phosphatase in addition to the SUMO proteases involved in this process. Finally, drugs can also be developed to target the recruitment of PIAS1 to gene promoters, which requires the clear understanding of the molecular basis of this process.
Concluding remarks
A growing amount of evidence indicates that protein sumoylation is involved in the regulation of inflammatory responses. Members of the PIAS SUMO ligase family display specificity by preferentially regulating the expression of inflammatory genes. These findings indicate that PIAS proteins might be novel therapeutic targets for the treatment of inflammatory disorders. Because sumoylation is a highly dynamic and reversible process, it is possible that SUMO proteases might also be involved in the regulation of inflammation. Further studies on the molecular mechanisms of PIAS-mediated gene regulation and the functional characterization of individual PIAS family members in different immune cells, the identification of SUMO proteases that function in inflammatory responses and the exploration of sumoylation in the regulation of other inflammatory processes should provide important information for the design of novel sumoylation-based anti-inflammatory therapies.
Figure 1.
The SUMO conjugation pathway. Sumoylation involves three discrete steps, activation, conjugation and ligation, which are mediated by E1, E2 and E3, respectively. SUMO modification is reversible. In the mammalian system, SUMO-specific proteases (SENPs) proteolytically remove SUMO from protein substrates.
Figure 1.
PIAS1-mediated repression of STAT1 and NF-κB pathways. (a) The domain structure of PIAS proteins. (b) Upon ligand stimulation, STAT1 and NF-κB translocate into the nucleus, via distinct mechanisms, where they bind to gene promoters and activate transcription. PIAS1 blocks the DNA-binding activity of STAT1 and NF-κB, resulting in transcriptional repression. Abbreviations: P, phosphorylation; SH2–PY, interaction between the SH2 domain and phosphorylated Tyr701; Ub, ubiquitylation.
Acknowledgements
K.S. is supported by the National Institute of Allergy and Infectious Diseases (www.niaid.nih.gov; 1R01 AI063286) and Juvenile Diabetes Research Foundation (www.jdrf.org.uk). B.L. is supported by a Research Scientist Development Award from the National Institutes of Health (www.nih.gov; K01 AR52717–01).
References
- 1.Melchior F. SUMO–nonclassical ubiquitin. Annu. Rev. Cell Dev. Biol. 2000;16:591–626. doi: 10.1146/annurev.cellbio.16.1.591. [DOI] [PubMed] [Google Scholar]
- 2.Johnson ES. Protein modification by SUMO. Annu. Rev. Biochem. 2004;73:355–382. doi: 10.1146/annurev.biochem.73.011303.074118. [DOI] [PubMed] [Google Scholar]
- 3.Pascual G, et al. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-γ. Nature. 2005;437:759–763. doi: 10.1038/nature03988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghisletti S, et al. Parallel SUMOylation-dependent pathways mediate gene- and signal-specific transrepression by LXRs and PPARγ. Mol. Cell. 2007;25:57–70. doi: 10.1016/j.molcel.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garaude J, et al. SUMOylation regulates the transcriptional activity of JunB in T lymphocytes. J. Immunol. 2008;180:5983–5990. doi: 10.4049/jimmunol.180.9.5983. [DOI] [PubMed] [Google Scholar]
- 6.Kang JS, et al. The type I TGF-β receptor is covalently modified and regulated by sumoylation. Nat. Cell Biol. 2008;10:654–664. doi: 10.1038/ncb1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Drean Y, et al. Potentiation of glucocorticoid receptor transcriptional activity by sumoylation. Endocrinology. 2002;143:3482–3489. doi: 10.1210/en.2002-220135. [DOI] [PubMed] [Google Scholar]
- 8.Shuai K, Liu B. Regulation of gene-activation pathways by PIAS proteins in the immune system. Nat. Rev. Immunol. 2005;5:593–605. doi: 10.1038/nri1667. [DOI] [PubMed] [Google Scholar]
- 9.Liu B, et al. Proinflammatory stimuli induce IKKα-mediated phosphorylation of PIAS1 to restrict inflammation and immunity. Cell. 2007;129:903–914. doi: 10.1016/j.cell.2007.03.056. [DOI] [PubMed] [Google Scholar]
- 10.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 11.Darnell JE, Jr, et al. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 12.Shuai K, Liu B. Regulation of JAK-STAT signalling in the immune system. Nat. Rev. Immunol. 2003;3:900–911. doi: 10.1038/nri1226. [DOI] [PubMed] [Google Scholar]
- 13.Mabb AM, Miyamoto S. SUMO and NF-κB ties. Cell. Mol. Life Sci. 2007;64:1979–1996. doi: 10.1007/s00018-007-7005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu B, et al. PIAS1 selectively inhibits interferon-inducible genes and is important in innate immunity. Nat. Immunol. 2004;5:891–898. doi: 10.1038/ni1104. [DOI] [PubMed] [Google Scholar]
- 15.Liu B, et al. Negative regulation of NF-κB signaling by PIAS1. Mol. Cell. Biol. 2005;25:1113–1123. doi: 10.1128/MCB.25.3.1113-1123.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawrence T, et al. IKKα limits macrophage NF-κB activation and contributes to the resolution of inflammation. Nature. 2005;434:1138–1143. doi: 10.1038/nature03491. [DOI] [PubMed] [Google Scholar]
- 17.Desterro JM, et al. SUMO-1 modification of IκBα inhibits NF-κB activation. Mol. Cell. 1998;2:233–239. doi: 10.1016/s1097-2765(00)80133-1. [DOI] [PubMed] [Google Scholar]
- 18.Rogers RS, et al. SUMO modification of STAT1 and its role in PIAS-mediated inhibition of gene activation. J. Biol. Chem. 2003;278:30091–30097. doi: 10.1074/jbc.M301344200. [DOI] [PubMed] [Google Scholar]
- 19.Ungureanu D, et al. PIAS proteins promote SUMO-1 conjugation to STAT1. Blood. 2003;102:3311–3313. doi: 10.1182/blood-2002-12-3816. [DOI] [PubMed] [Google Scholar]
- 20.Song L, et al. Stat1 and SUMO modification. Blood. 2006;108:3237–3244. doi: 10.1182/blood-2006-04-020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ungureanu D, et al. SUMO-1 conjugation selectively modulates STAT1-mediated gene responses. Blood. 2005;106:224–226. doi: 10.1182/blood-2004-11-4514. [DOI] [PubMed] [Google Scholar]
- 22.Tahk S, et al. Control of specificity and magnitude of NF-κB and STAT1-mediated gene activation through PIASy and PIAS1 cooperation. Proc. Natl. Acad. Sci. U. S. A. 2007;104:11643–11648. doi: 10.1073/pnas.0701877104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Straus DS, Glass CK. Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol. 2007;28:551–558. doi: 10.1016/j.it.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Hay RT. SUMO: a history of modification. Mol. Cell. 2005;18:1–12. doi: 10.1016/j.molcel.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 25.Gill G. Something about SUMO inhibits transcription. Curr. Opin. Genet. Dev. 2005;15:536–541. doi: 10.1016/j.gde.2005.07.004. [DOI] [PubMed] [Google Scholar]