Abstract
It is well known that many of the actions of 17β-estradiol (E2) in the central nervous system (CNS) are mediated via intracellular receptor/transcription factors that interact with steroid response elements on target genes. However, there is compelling evidence for membrane-associated steroid receptors for E2 in hypothalamic and other brain neurons. Indeed, we are just beginning to understand how E2 signals via membrane receptors, and how these signals impact not only membrane excitability but also gene transcription in neurons. We know that E2 can rapidly alter neuronal activity within seconds, indicating that some cellular effects can occur via membrane delimited events. In addition, E2 can affect second messenger systems including calcium mobilization and a plethora of kinases to alter cell signaling. This review will concentrate on rapid membrane-initiated and intracellular signaling by E2 in the hypothalamus and hippocampus, the nature of receptors involved and how they contribute to CNS functions.
Keywords: ERα, ERβ, GPR30, mER (a plasma membrane E2 receptor that is G protein-coupled)
Evidence for novel estrogen receptors in the CNS
It has been known for a number of years that 17β-estradiol (E2) has acute, membrane-initiated signaling actions in the brain (for review Kelly and Rønnekleiv, 2002, Rønnekleiv and Kelly, 2005, Bryant et al., 2006). Although twenty years ago the nature and significance of these actions were disputed, it is now generally accepted that these rapid actions of E2 cannot be attributed to the classical nuclear-initiated steroid signaling of estrogen receptors α or β (ERα or ERβ). However, there are two viewpoints of how these rapid (non-nuclear) effects are mediated. One view is that both the nuclear and plasma membrane-associated ERs are products of the same genes (Razandi et al., 1999, Boulware et al., 2005, Pedram et al., 2006, Szegõ et al., 2006, Dewing et al., 2007). This hypothesis is derived primarily from findings that many of the rapid effects of E2 can be induced by selective ERα or ERβ ligands, antagonized by the ER antagonist ICI 182,780, and/or absent in animals bearing mutations in the ERα and/or ERβ genes (Couse and Korach, 1999, Singer et al., 1999, Dubal et al., 2001, Wade et al., 2001, Abraham et al., 2003, Boulware et al., 2005, Boulware et al., 2007). Another viewpoint is that E2 activates a novel membrane ER (mER) that is G protein-coupled (Gu et al., 1999, Toran-Allerand, 2004, Toran-Allerand, 2005, Qiu et al., 2003, Qiu et al., 2006b, Matsuda et al., 2008). Evidence is accumulating from numerous brain regions supporting both of these hypotheses. This review, however, will focus primarily on findings in the hypothalamus and hippocampus, since the topic on membrane-initiated actions of E2 in the central nervous system (CNS) is too broad to be covered in a single article. We would direct the readers to some recent reviews on the membrane-initiated actions of E2 in other CNS systems (Bryant et al., 2006, Brann et al., 2007, Fehrenbacher et al., 2009).
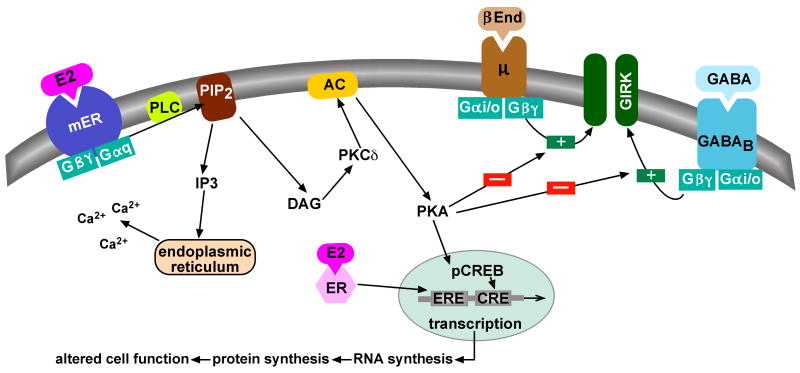
What is the evidence for E2 signaling via G protein-coupled receptors in the CNS? The compelling evidence supporting this hypothesis includes cellular electrophysiological studies in both hippocampal and hypothalamic slices establishing G protein-coupled signaling mechanisms for E2 (Lagrange et al., 1997, Gu and Moss, 1998). Indeed, intracellular and whole cell recording from guinea pig and mouse hypothalamic neurons have characterized a mER that is coupled to Gq (Figure 1) (Lagrange et al., 1997, Qiu et al., 2003, Qiu et al., 2006b). Initially, these studies established that E2 caused a significant reduction in the potency (heterologous desensitization) of μ-opioid and GABAB agonists to activate an inwardly rectifying K+ (Kir3) conductance (Lagrange et al., 1997, Qiu et al., 2003) within a physiologically-relevant concentration (EC50 = 8 nM). Based on the Schild analysis (Schild, 1947) of the antagonism of the response in individual neurons with the antiestrogen ICI 164,384, it was established that the receptor mediating this response has a subnamolar affinity (300 pM) for E2 (Lagrange et al., 1997). This subnanomolar affinity is similar to the binding of the antagonist ICI to ERα in human uterine tissue (Weatherill et al., 1988). Furthermore, 17α-estradiol, even at much higher concentrations (100 fold), did not mimic these actions of E2 indicating that this estrogen receptor discriminates between these two isomers. The rapid effects of E2 were found to be membrane delimited based on the efficacy of E2-BSA, an extracellular constrained moiety, in activating this pathway (Qiu et al., 2003). From a pharmacological perspective, these findings established that this mER is a high-affinity estrogen receptor.
Figure 1. Cellular models of membrane-initiated signaling pathways of E2.
Besides the classical ER-mediated gene activation via an estrogen response element (ERE), E2 can activate a membrane-associated ER (mER) that is Gαq -coupled to activation of phospholipase C that catalyzes the hydrolysis of membrane-bound phosphatidylinositol 4,5-biphosphate (PIP2) to inositol 1,4,5 triphosphate (IP3) and diacylglycerol (DAG). Calcium is released from intracellular stores (endoplasmic reticulum) by IP3, and DAG activates protein kinase Cδ (PKCδ). Through phosphorylation, adenylyl cyclase (AC) activity is upregulated by PKC. The generation of cAMP activates PKA, which can rapidly uncouple GABAB and μ-opioid (μ) receptors from their effector system through phosphorylation of a downstream effector molecule (e.g., G protein-coupled, inwardly rectifying K+ (GIRK) channel). The mER-mediated modulation of kinase pathways reduces the capacity of neuromodulators such as GABA and β-endorphin (β-End) to inhibit POMC neuronal excitability. Finally, mER-mediated activation of PKA can lead to phosphorylation of cAMP-response element binding protein (pCREB), which can then alter gene transcription through its interaction with a CREB response element (CRE).
Furthermore, the E2 mediated desensitization of μ-opioid and GABAB receptors is initiated by a Gαq-coupled membrane ER that is linked to activation of phospholipase C (PLC) -protein kinase C (PKC) -protein kinase A (PKA). Dialyzing cells with a peptide that mimics the C-terminus of Gq or perfusing with a phospholipase C inhibitor abrogates rapid E2 signaling in hypothalamic neurons (Qiu et al., 2003, Qiu et al., 2006b). The effects of E2 are mimicked either by stimulation of adenylyl cyclase with forskolin or by direct PKA activation with Sp-cAMP in a concentration-dependent manner (Lagrange et al., 1997, Qiu et al., 2003). The selective PKA antagonists KT5720 and Rp-cAMP block the effects of E2, suggesting that PKA activation is downstream in the signaling cascade. However, E2 does not compete for the μ-opioid (or GABAB) receptor or alter the affinity for the receptor (Cunningham et al., 1998). This pharmacological profile has defined the mER as G-protein-coupled membrane receptor (Figure 1).
The definitive proof that the mER is not ERα or ERβ is that E2 can activate the Gαq-PLC-PKC-PKA signaling pathway to cause heterologous desensitization of μ-opioid and GABAB receptors in ERα, ERβ and ERα/ERβ knockout mice (Qiu et al., 2006b). Furthermore, a novel selective estrogen receptor modulator (SERM) STX, which does not bind to ERα or ERβ, is twenty times more potent than E2 in desensitizing these receptors (Qiu et al., 2003, Qiu et al., 2006b). Although definitive characterization of this Gq-coupled mER requires cloning of the gene, it is evident that 17β-estradiol can rapidly alter cell function through a novel mER.
In hippocampal CA1 neurons, E2 potentiates kainate-mediated excitation of hippocampal CA1 pyramidal neurons via activation of a cAMP/PKA pathway (Gu and Moss, 1996, Gu and Moss, 1998). In the late 1990's, Gu and Moss hypothesized that E2 activated a Gs-coupled receptor on the extracellular surface of hippocampal neurons, which operated in concert with an internal action of E2 on cAMP-dependent phosphorylation (Gu and Moss, 1998). Importantly, these rapid actions of E2 on kainate-induced currents in hippocampal neurons were still present in animals deficient in ERα, lending further evidence for mER mediating the rapid actions of E2 in the hippocampus (Gu et al., 1999). In parallel studies, Foy and colleagues showed that E2 enhanced N-methyl-D-aspartate (NMDA)-mediated excitatory postsynaptic potentials (EPSPs) in CA1 neurons and long-term potentiation (LTP) following Schaffer (collateral) fiber stimulation (Foy et al., 1999). Furthermore, E2 and E2-BSA, when applied acutely to the hippocampus in ovariectomized animals, produced a sustained reduction of the slow afterhyperpolarization current (IAHP), which is mediated by a Ca2+ - activated K+ conductance, in CA1 pyramidal neurons (Carrer et al., 2003). This provides further evidence for the involvement of a membrane ER.
More recent studies in hippocampal CA3-CA1 neuronal cultures have revealed that E2 rapidly stimulates mitogen-activated protein kinase (MAPK)-dependent cAMP-responsive element binding protein (CREB) phosphorylation. In addition, E2 also decreases L-type calcium channel-mediated CREB phosphorylation, and therefore, has both positive and negative influences on CREB activity (Boulware et al., 2005, Boulware et al., 2007). Both effects are mimicked by the membrane-impermeable E2-BSA and inhibited by the antiestrogen ICI 182, 780, which collectively suggests that a membrane ER that has some of the characteristics described by Qiu and coworkers is involved (Qiu et al., 2003). However, the positive modulation of CREB activity appears to involve caveolin-associated ERα interaction with metabotropic glutamate receptors 1(mGluR 1) and the negative modulation of CREB via mGluR 2/3 (Boulware et al., 2005, Boulware et al., 2007), but the exact nature of the protein-protein interaction is currently unknown. The membrane-localized receptors in the hippocampus are hypothesized to be ERα and ERβ based on responses to the ER agonists propylpyrazoletriol (PPT) and diarylpropionitrile (DPN), which are selective for ERα and ERβ, respectively, and transfection studies with mutant ERα (Harrington et al., 2003, Lund et al., 2006, Boulware et al., 2005, Boulware et al., 2007). However, further studies in transgenic mice (ERα and ERβ knockout) mice are needed to more clearly define these hippocampal caveolin-associated (mERs) and whether they are indeed ERα, ERβ and/or splice variants.
Another novel membrane associated ER that is operational in the CNS has been designated ER-X (Toran-Allerand, 2005). ER-X is a plasma membrane-associated, putative ER that is enriched in caveolar-like microdomains of postnatal, but not adult, cortical membranes (Toran-Allerand et al., 1999, Toran-Allerand et al., 2002). In organotypic explants of the developing cerebral cortex, E2 induces tyrosine phosphorylation of both ERK1 (extracellular signal-regulated protein kinase 1) and ERK2, an action very similar to a number of growth factors including nerve growth factor (NGF) (Toran-Allerand, 2005). Interestingly, this E2-induced activation is not antagonized by the antiestrogen, ICI 182,780, and occurs in cortical explants from mice lacking ERα. In addition, this putative mER is particularly responsive to 17α-estradiol as compared to 17β-estadiol (Singh et al., 1999, Singh et al., 2000, Toran-Allerand et al., 2002). These and other findings have led to the hypothesis that ER-X is a novel ER that is ICI-insensitive (for review see Toran-Allerand, 2004, Toran-Allerand, 2005).
An orphan G-protein-coupled receptor, GPR 30, has also received considerable attention because it exhibits binding and signaling characteristics of a membrane ER (Revankar et al., 2005, Thomas et al., 2005, Filardo and Thomas, 2005, Funakoshi et al., 2006, Matsuda et al., 2008) (also Prossnitz et al., in this issue). Although there is some controversy whether this receptor is expressed in the plasma membrane or localized to the endoplasmic reticulum (Revankar et al., 2005, Thomas et al., 2005, Filardo and Thomas, 2005, Funakoshi et al., 2006, Matsuda et al., 2008), there is general agreement on 17β-estradiol-mediated signaling. For example in breast cancer cells that are transfected with GPR30, E2 activates the MAPK kinases ERK1 and ERK2 (Filardo, 2002, Filardo and Thomas, 2005). Accordingly, E2 activates Gβγ-subunits that promote the release and activation of an epidermal growth factor precursor (proHB-EGF). The active HB-EGF binds to the EGF receptor (ErbB) to facilitate receptor dimerization and downstream activation of ERK (Filardo et al., 2000, Filardo et al., 2002, Filardo, 2002). In addition, E2 can also stimulate adenylyl cyclase activity, which leads to PKA-mediated suppression of EGF-induced ERK1/2 activity. Interestingly, the selective estrogen receptor modulator (SERM) tamoxifen and the antiestrogen ICI 182,780 both promote GPR30-dependent transactivation of the EGF receptor and subsequent MAPK activation. Therefore, the pharmacology of GPR30 as defined in cancer cells is different from that of Gq-mER in neurons (Lagrange et al., 1997, Qiu et al., 2003, Qiu et al., 2006b), suggesting that these are different receptors. In fact, the mER-mediated response to estrogen in arcuate neurons is still present in GPR30 KO mice (Qiu et al., 2008). Therefore, although GPR30 has been localized in the brain (Funakoshi et al., 2006, Bologa et al., 2006, Brailoiu et al., 2007, Prossnitz et al., 2007, Matsuda et al., 2008), studies are evolving to establish a definitive role for E2-mediated signaling via GPR30 in the CNS (see below).
17β-estradiol and GnRH neurosecretion
Gonadotropin releasing hormone (GnRH) neurosecretion and the control of the ovulatory cycle in females is dependent on E2 feedback. In spite of recent advances on cellular experimentation, we still do not understand all of the signaling mechanism(s) by which E2 regulates GnRH neurons. It has been obvious for a number of years that GnRH neurons are modulated by E2 in a complex manner. For example, depletion of serum estrogens by ovariectomy disrupts GnRH regulation of pituitary LH secretion and results in elevated levels of plasma LH. This effect is due to the loss of negative feedback actions of E2. However, both negative and positive (induction of the LH surge) feedback regulation of LH (GnRH) secretion can be restored by replacement with E2. The positive feedback is believed to be by an action of E2 in the anteroventral periventricular (AVPV) nucleus (Herbison, 1998, Han et al., 2005, Smith et al., 2006, Wintermantel et al., 2006, Clarkson et al., 2008). Neurons in the AVPV express GABA, opioid peptides and kisspeptin, a neuropeptide encoded by the Kiss 1 gene, all of which are important for regulation of GnRH neurosecretion (Simerly et al., 1988, Wagner et al., 2001a, Jackson and Kuehl, 2002, DeFazio et al., 2002, Smith et al., 2006, Christian and Moenter, 2007, Clarkson et al., 2008). The AVPV area expresses high levels of ERα and also ERβ, and the actions of the gonadal steroids are mediated, in part, via the nuclear-initiated signaling (genomic) mechanism (Shughrue et al., 1997, Wintermantel et al., 2006, Clarkson et al., 2008). However, the AVPV is also sensitive to the rapid actions of gonadal steroids. For example, E2 within 30 min increases the expression of the pCREB in the AVPV (Gu et al., 1996). Interestingly, phosphorylation of CREB by E2 is lost in ERαβ deleted animals (Abraham et al., 2004), an indication that the rapid activation of CREB is dependent on the classical ERs. E2 has other acute actions in AVPV neurons, including modulation of the median afterhyperpolarization current (mIAHP). At the cellular level, in AVPV neurons including GABA neurons, both β-adrenergic and α-adrenergic agonists inhibit the mIAHP, which increases the action potential firing in these neurons (Wagner et al., 2001b). Moreover, the α1-adrenergic, but not β-adrenergic inhibition of the mIAHP is potentiated after acute (15-20 min) exposure to E2, which further increases neuronal excitability (Wagner et al., 2001b). The E2-induced enhancement of the coupling of the α1-adrenergic receptors to calcium-activated K+ (SK) channels (underlying the mIAHP) is initiated within 15 min in vitro and lasts for at least 24 h following systemic steroid administration, suggesting both rapid and sustained effects (Wagner et al., 2001b). Since SK channels are critical for modulating neuronal firing rate and pattern (Stocker et al., 1999, Sah and Davies, 2000), E2-induced modulation of these channels would have significant functional consequences for AVPV neurons and their targets.
Because ERα, the first cloned receptor/transcription factor for E2, has not been localized to native GnRH neurons, the prevailing view has been that estrogen affects GnRH neurons through pre-synaptic mechanisms. However, synaptically-isolated GnRH neurons are rapidly hyperpolarized by E2, an effect that inhibits their firing (Kelly et al., 1984, Condon et al., 1989, Lagrange et al., 1995). It is thought that these hyperpolarizing actions of E2 on GnRH neurons are via a Gαi,o-coupled receptor. Indeed, in GT1-7 cells, an immortalized GnRH neuronal cell line, E2 inhibits adenylyl cyclase activity (cAMP production) via a pertussis toxin-sensitive (Gαi,o coupling) mechanism (Navarro et al., 2003). Because of the rapidity of these E2 effects the involvement of transcription is highly unlikely, but an E2-responsive - Gαi,o-coupled receptor has not been identified. Interestingly, E2 increases the firing in primate nasal placode GnRH neuronal cultures within 10 min (Abe and Terasawa, 2005) and increases Ca+2 oscillations and synchronizations (see below) (Abe et al., 2008). Therefore, E2 may have both inhibitory and excitatory effects on GnRH neuronal activity.
An important milestone for understanding estrogen action in GnRH neurons was the discovery of a second ER, ERβ, in 1996 and the documentation that this receptor was expressed in GnRH neurons (Kuiper et al., 1996, Hrabovszky et al., 2000, Hrabovszky et al., 2001, Kallo et al., 2001, Herbison and Pape, 2001). The latter findings combined with recent technological advances, such as the development of ER mutants and transgenic animals expressing green fluorescent protein (GFP) in GnRH neurons, have greatly facilitated studies to understand the cellular mechanisms by which GnRH neurons are modulated by E2 (Spergel et al., 1999, Suter et al., 2000, Kato et al., 2003, Han et al., 2005, Abraham et al., 2003, Smith et al., 2006, Wintermantel et al., 2006, Zhang et al., 2007). It is, however, important to keep in mind that certain aspects of the ovulatory cycle may be compromised as a result of the genetic manipulations in mice (Suter and O'Farrell, 2008). Therefore, it is important to compare findings in transgenic animals to those of wildtype mice and other mammalian species.
A series of recent publications have shown that in mouse hypothalamic neuronal explants and in primate nasal placode cultures, E2 augments synchronous intracellular Ca2+ oscillations in GnRH neurons (Temple et al., 2004, Temple and Wray, 2005, Abe et al., 2008, Noel et al., 2009). This relatively rapid effect is observed within 10-30 min of E2 application. Furthermore, E2 conjugated to BSA at the C-17 position (E2-17 BSA) or to a large non-degradable poly(amido)amine-dendrimer macromolecule (Harrington et al., 2006) mimics the effects of E2 on intracellular calcium oscillations (Temple et al., 2004, Temple and Wray, 2005, Abe et al., 2008). However in the mouse explant experiments (Temple et al., 2004, Temple and Wray, 2005), the E2 -induced calcium oscillations are blocked by ICI 182,780 suggesting that an ER is involved. In contrast, E2-induced calcium oscillations in the primate placode cultures are not blocked by the antagonist ICI 182,780, and ICI 182,780 alone has no effect suggesting that an ER may not be involved (Abe et al., 2008, Noel et al., 2009). The actions of E2-BSA in mouse and monkey GnRH neuronal cultures are abrogated by pertussis toxin treatment and not by inhibition of gene transcription, supporting a role for a G protein-coupled membrane receptor (Temple et al., 2004, Temple and Wray, 2005, Noel et al., 2009). Interestingly, GPR30 is expressed in primate GnRH neurons and appears to play a critical role in the rapid membrane-initiated actions of E2 in these neurons (Noel et al., 2009). However, in vivo studies have provided evidence for ERβ in the E2-induced rapid (15 min) induction of pCREB in mouse GnRH neurons (Abraham et al., 2003). Recently, the Herbison lab (Romanò et al., 2008) has shown that E2 can rapidly (within 15 min) increase calcium transients in GnRH neurons through what appears to be an ERα-dependent release of GABA from presynaptic terminals. Collectively, these findings indicate that there are multiple acute actions of E2 in GnRH neurons, some of which may involve a novel receptor and perhaps GPR30, and some that involve ERα and/or ERβ.
Based on the original experiments, ERβ-deficient mice appeared to exhibit normal sexual behavior and reproduce successfully with the exception that their litter size was reduced, presumably due to a decline in ovarian function (Krege et al., 1998, Dupont et al., 2000). Therefore, the physiological role of ERβ in GnRH neurons has been uncertain. In contrast, ERα, which appears not to be expressed in GnRH neurons, is essential for both sexual behavior and fertility (Ogawa et al., 1998, Dupont et al., 2000). Because the original ERβ mouse mutants displayed alternative splicing transcripts, another ERβ mutant, which is devoid of any transcripts downstream of exon 3, has recently been generated (Antal et al., 2008). Interestingly, both male and female exon 3-deleted βERKO animals are infertile. This underscores a potential critical role for ERβ in GnRH neurons. For example, recent findings have revealed that GnRH neurons express K-ATP channels and the agonist-induced outward (K-ATP channel) current is increased by approximately two-fold in E2-treated animals (Zhang et al., 2007). The increased K-ATP channel activity, which occurs in the presence of TTX and GABA and glutamate blockade, is not the result of increased expression of the channel based on quantitative real-time PCR results. Therefore, it appears that there is an E2-induced change in signaling molecules (kinases) that impinge on the channel, part of which may be nuclear-initiated steroid signaling through ERβ and/or acute phosphorylation events.
Studies are forthcoming on the cross-talk between rapid membrane-initiated and long-term nuclear-initiated steroid actions (Lagrange et al., 1994, Lagrange et al., 1997, Wagner et al., 2001b, Vasudevan et al., 2001, Kow and Pfaff, 2004, Malyala et al., 2005, Qiu et al., 2006b, Qiu et al., 2006a, Roepke et al., 2007, Roepke et al., 2008). For example, it has been found that both acute effects of E2 and the transcriptional changes alter excitability of hypothalamic neurons (Kelly et al., 2003, Malyala et al., 2005, Qiu et al., 2006a). In addition, the estrogen-induced membrane actions in the ventromedial nucleus (VMH) of the hypothalamus can potentiate its genomic effects on lordosis behavior (Kow and Pfaff, 2004). Moreover, this membrane effect in the VMH appears to be mediated by signaling pathways involving PKC and PKA (Kow and Pfaff, 2004). These findings are particularly intriguing in view of other findings that estrogen activates a novel membrane ER, other than ERα or ERβ that is Gαq-coupled to phospholipase C, PKCδ and PKA to alter cell firing (Figure 1) (Qiu et al., 2003, Qiu et al., 2006b). In addition, genetic models have been generated from αERKO animals that possess an ER-knock-in mutation, which allows in vivo distinction between ERE-dependent and ERE-independent mechanisms of E2 action (McDevitt et al., 2008, Glidewell-Kenney et al., 2008). This ERE-independent model was used to study GnRH neurons, using loose cell attached recordings (Christian et al., 2008). It was concluded that an E2-induced decrease in GnRH neuronal firing during the morning (negative feedback model) as well as increased neuronal firing during the afternoon (positive feedback model) are both dependent on the classical ERα and ERE-dependent mechanisms and that these actions of E2 on GnRH neuronal activity during negative and positive feedback are presynaptic. Clearly with the multiple receptors and both pre- and post-synaptic E2 actions, further experiments are needed to fully understand the complex regulation of GnRH neurons by E2.
17β-estradiol and reproductive behavior
Reproductive or sexual behavior is dependent on the ovarian steroid hormones E2 and progesterone, which act sequentially and synergistically in preoptic and basal hypothalamic areas to facilitate the behavior (Etgen and Acosta-Martinez, 2003, Etgen et al., 2006). The role of E2 is to prime the neurons that control reproductive behavior for subsequent action of progesterone and other molecules. In this respect, brain insulin-like growth factor-1 (IGF-1) receptor activity is required for E2 priming of the female reproductive axis (Etgen et al., 2006). Systemic administration of E2 in ovariectomized rats activates insulin-like growth factor-1 (IGF-1) receptors and induces the association between IGF-1 receptors and ERα in the hypothalamus (Quesada and Etgen, 2001, Cardona-Gómez et al., 2002, Mendez et al., 2003). Similar to the effects in cortical neurons, there is an interaction (complex formation) between the p85 subunit of PI3K and ERα within 1-3 h, which leads to activation of Akt (Cardona-Gómez et al., 2002, Mendez et al., 2003). Also, the E2-induced activation of IGF-1 receptors augments α1-adrenergic receptor signaling, which is important for reproductive functions (Quesada and Etgen, 2001). On the other hand, blockade of IGF-1 receptors during E2 priming prevents E2-induced increases in α1-adrenergic receptor binding density as well as IGF-1 enhancement of noradrenergic receptor signaling (Quesada and Etgen, 2002). Collectively, these findings support functional interactions between E2 and IGF-1 signaling. Therefore, these actions of E2 on the IGF-1 receptor signaling pathway may be a key mechanism by which E2 affects synaptic remodeling and neuronal plasticity during the estrous cycle.
Of particular interest are the findings that intracerebroventricular (i.c.v.) infusion of JB-1, a selective competitive antagonist of IGF-1 autophosphorylation, inhibits the E2-induced LH surge and sexual behavior in ovariectomized rats (Quesada and Etgen, 2002). In addition, co-administration (i.c.v.) of blockers of PI3 kinase (wortmannin) and MAPK (PD98059) inhibit the long-term (48 h) effects of E2 to induce the LH surge and facilitate lordosis behavior (Etgen and Acosta-Martinez, 2003). Therefore, facilitation of female sexual behavior by E2 appears to involve activation of both PI3 kinase and MAPK signal transduction pathways. The importance of growth factors for female sexual behavior is further illustrated by observations that epidermal growth factor (EGF) and also IGF-1 can within 1-4 h of i.c.v. administration and in the absence of estrogen and progesterone induce mating behavior in rats and mice, in part, through an ERα-dependent mechanism (Apostolakis et al., 2000). This relatively rapid, ligand-independent ER action is in striking contrast to the well established finding that estrogen priming over a period of at least 24 h is needed for progesterone induction of female reproductive behavior (Etgen et al., 2001). Although it is currently not well understood, the cross-talk between E2 signaling and membrane-initiated growth factor signaling in the hypothalamus is particularly intriguing. The ability of both IGF-1 and E2 to induce female sexual behavior may involve complex interactions between ERα, the IGF-1 receptor and the PI-3 kinase p85 subunit.
The findings that the E2-induced membrane actions in the ventromedial nucleus of the hypothalamus (VMH) can potentiate its genomic effects on female lordosis behavior have generated much interest (Kow and Pfaff, 2004), and studies are forthcoming to understand this membrane-initiated E2 signaling and how it relates to the nuclear-initiated signaling of E2 (Roepke et al., 2008, Micevych and Mermelstein, 2008). An interesting model is that ERα through a protein-protein interaction with group 1 metabotropic glutamate receptors (mGluR1) facilitates reproductive behavior (Micevych and Mermelstein, 2008). According to this model E2 at high levels acts rapidly at caveolin-associated ERs in the arcuate nucleus to excite rostrally projecting POMC neurons to induce μ-opioid receptor internalization in the preoptic area (Mills et al., 2004, Micevych and Mermelstein, 2008). This internalization is the trigger for lordosis behavior, which is also dependent on the genomic actions of E2. Interestingly, μ-opioid receptor internalization and lordosis behavior can be prevented with mGluR1 antagonists during high E2 levels and can also be induced with mGluR1 agonists in the presence of low circulating levels of E2, which by itself is not sufficient for lordosis behavior (Micevych and Mermelstein, 2008). These observations form the basis for the above described hypothesis. Exactly, how ERα and mGluR1 interacts at the membrane is not known. Therefore, further studies are needed to elucidate the specifics of this interaction and whether the mER and mGlur1 signaling are similar or distinct pathways.
17β-estradiol and energy homeostasis
In addition to its role in the control of reproduction, E2 is involved in the regulation of appetite, energy expenditure, body weight, adipose tissue deposition and distribution in females (Milewicz et al., 2000, Geary, 2001, Poehlman, 2002). Ovariectomy induces an increase in food intake and decreases ambulatory and wheel running activities in rodents, all of which are reversed with E2 replacement (Ahdieh and Wade, 1982, Colvin and Sawyer, 1969, Shimomura et al., 1990, Asarian and Geary, 2002). In fact, hypo-estrogenic states are associated with decreased activity and an increase in body weight in females (Czaja and Goy, 1975, Butera and Czaja, 1984, Czaja, 1984, McCaffrey and Czaja, 1989, Jones et al., 2000, Asarian and Geary, 2002, Qiu et al., 2006b, Clegg et al., 2006, Clegg et al., 2007). The anorectic effects of estrogen are thought to be mediated through CNS actions based on the findings that direct injections of E2 into the paraventricular nucleus of the hypothalamus (PVH) or arcuate/ventromedial nucleus are effective to reduce food intake, body weight and increase wheel running activity in females (Colvin and Sawyer, 1969, Ahdieh and Wade, 1982, Butera and Czaja, 1984). It is evident that neurons in these hypothalamic nuclei regulate energy homeostasis and are affected by E2. For example, E2 upregulates the expression of the peptide β-endorphin in proopiomelanocortin (POMC) neurons in ovariectomized female guinea pigs (Thornton et al., 1994, Bethea et al., 1995). Furthermore, there is a decrease in hypothalamic β-endorphin levels in the hypothalamus of postmenopausal women who do not take hormone replacement and experience a significant gain in body weight (Leal et al., 1998). In contrast, E2 and the mER agonist STX reverse the ovariectomy-induced increase in arcuate neuropeptide Y (NPY) mRNA expression in rodents (Shimizu et al., 1996, Roepke et al., 2008). Therefore, it appears that the arcuate nucleus and specifically POMC and NPY neurons are a major target for the anorectic actions of estrogen, which underscores their importance in the control of energy homeostasis. Indeed, POMC neurons are critical for the regulation of feeding and are also involved in the rewarding aspects of food intake (Hayward et al., 2002, Appleyard et al., 2003).
Important inhibitory regulators of POMC neuronal activity are the GIRK channels. Both μ-opioid receptor (e.g., by β-endorphin or by selective μ-opioid agonists) or GABAB receptor activation of these GIRK channels directly hyperpolarize and thereby inhibit hypothalamic neurons (Loose et al., 1990, Kelly et al., 1992, Lagrange et al., 1994, Lagrange et al., 1995, Lagrange et al., 1996). As reviewed above, brief (<20 min) application of E2, BSA-E2 or STX in vitro causes a four-fold decrease in the potency of μ-opioid receptor agonists and GABAB receptor agonists to inhibit POMC neurons arguing for the involvement of a mER, which is distinct from from ERα or ERβ (Lagrange et al., 1994, Lagrange et al., 1996, Qiu et al., 2003, Qiu et al., 2006b). Therefore, E2 or STX via a mER-mediated pathway rapidly decreases the potency of μ-opioid and GABAB ligands at their receptors (desensitization), thus increasing POMC neuronal firing and the release of the POMC products, β-endorphin and α-melanocyte stimulating hormone (α-MSH).
Interestingly, inwardly rectifying K+ channels are also a point of convergence for the effects of leptin and insulin in POMC neurons (Plum et al., 2006). Both leptin and insulin activate PI3Kinase (via insulin receptor substrate) that leads to the metabolism of phosphatidylinositol (4,5) biphosphate (PIP2) to phoshatidylinositol (3,4,5)-triphosphate (PIP3) in POMC neurons (Xu et al., 2005). Activation of GIRK channels requires permissive levels of membrane PIP2 and increased channel activity results from Gβγ-mediated stabilization of PIP2-GIRK binding (Huang et al., 1998, Zhang et al., 1999). In fact, the regulation of channel activities by Gβγ, PIP2, or phosphorylation occurs on the time scale of a few seconds which can be resolved by electrophysiological techniques (Suh and Hille, 2002). In recent studies from our lab, we have found that PI3K plays a critical role in facilitating the rapid membrane response to estrogen, as elucidated by the use of PI3K inhibitors (Malyala et al., 2008). Indeed, we found that PI3K inhibitors, which block the phosphorylation of PIP2 and thereby increase the levels of PIP2 in the membrane, augmented GIRK channel activity and attenuated the effects of E2 on the mER signaling pathway.
As proof of principle of the importance of the membrane-initiated estrogen signaling pathway in the control of energy homeostasis, we used STX to selectively target the mER (i.e., in vivo treatment), and found that STX, similar to E2, attenuated the weight gain following ovariectomy (Qiu et al., 2006b, Roepke et al., 2008). The fact that both E2 and STX were fully efficacious in activating this signaling pathway in double-estrogen receptor knock-out mice is further proof for the existence of a novel mER that is involved in critical physiological processes such as the control of energy homeostasis.
Gene expression of neuropeptides, cation channels and channel modulators (signaling molecules) by E2 via membrane-initiated signaling (mER) and the classical receptors ERα and ERβ are all vital for the control of energy homeostasis. In fact, the cross-talk between rapid, membrane-initiated effects of E2 on signaling pathways (and channels) and estrogenic gene regulation of both channels and signaling mechanisms ultimately determines neuronal function and activity. In order to measure gene regulation via the mER using STX, we employed custom gene microarray analysis of arcuate tissue from STX-treated female guinea pigs coupled to quantitative real-time PCR (Roepke et al., 2007, Roepke et al., 2008). The analysis of gene expression with STX was used to evaluate the membrane-initiated versus nuclear-initiated signaling of E2. Because the mER activates signaling molecules that may impinge on channel function (PLC, PI3K, PKC and PKA) (Qiu et al., 2003, Malyala et al., 2008), gene microarray studies focused on the regulation of cation channels, signaling molecules and associated genes (Roepke et al., 2007, Roepke et al., 2008). Indeed, several relevant genes were found to be regulated by long-term STX treatment in the arcuate nucleus. Of particular interest was the significant reduction in NPY mRNA expression in the arcuate nucleus (Roepke et al., 2008) suggesting that STX exerts some of its anorectic effects by suppressing NPY. Although the cellular signalling mechanisms are currently unknown, the anorectic effects of STX may be mediated, in part, by channel modulation in POMC neurons that synapse on NPY neurons to inhibit their activity and gene expression.
Another significant finding from the microarray studies was that E2 induced gene expression of a T-type Ca2+ channel (Cav3.1) subunit in the arcuate nucleus after 24 hr treatment (Qiu et al., 2006a) and after long-term E2 treatment (30 day) (Roepke et al., 2008). The E2-induced increase in Cav3.1 subunit expression had previously been demonstrated to increase the peak T-type Ca2+ current by two-fold in arcuate (POMC) neurons (Qiu et al., 2006a) and the magnitude of Ca+2 (T-type?) currents in the VMH neurons (Lee et al., 2008). Furthermore, the increase in Cav3.1 expression in the arcuate nucleus from E2 treatment was abrogated in αERKO mice (Bosch et al., 2009), indicating that the expression is under the control of ERα. However, STX also significantly up-regulated the Cav3.1 subunit in the arcuate nucleus after long-term treatment (Roepke et al., 2008), suggesting that these two receptor-mediated transcriptional effects converge at the Cav3.1 gene. Since the mER is operable in POMC neurons, one can hypothesize that the up-regulation after STX-treatment is occurring in these neurons. Based on the role of T-type Ca2+ channels in modulating neuronal excitability (Huguenard and McCormick, 1992, Erickson et al., 1993), the augmentation in the T-type Ca2+ current in POMC neurons would facilitate burst firing and increase neurotransmitter (α-MSH) release. Therefore, the regulation of calcium channel expression and activity is a good example of crosstalk between membrane initiated (mER) and ERα/β mediated transcriptional activity.
17β-estradiol and learning and memory
It is becoming increasingly evident that E2 exerts a positive influence on memory and higher cognitive functions (Smith et al., 2001, Rapp et al., 2003, Sherwin, 2005), which may in part involve its actions in the hippocampus. Morphological and electrophysiological evidence have shown that E2 activates CA1 pyramidal cells both directly via enhancement of glutamate input and indirectly via attenuation of GABAergic input to these neurons (Gu and Moss, 1996, Gu and Moss, 1998, Rudick and Woolley, 2001). Many of the actions of E2 in the hippocampus are by the classical genomic mechanism (Woolley, 2007). However, E2 has been found to produce acute short-term effects, presumed to involve specific membrane actions (Wong and Moss, 1991, Wong and Moss, 1992, Wong and Moss, 1994, Gu and Moss, 1998, Foy et al., 1999, Foy, 2001, Woolley, 2007). Of great interest is that E2 augments long-term potentiation (LTP), which is the quintessential model of activity-dependent enhancement of synaptic activity in the hippocampus (Bliss and Lomo, 1973). Enhanced glutamatergic synaptic transmission underlies hippocampal LTP and may last for hours and is thought to be a cellular model of memory storage (Woolley, 2007). These effects of E2 can be induced within 30 min suggesting a non-classical, membrane-initiated action of E2 (Foy et al., 1999, Foy, 2001). LTP is most often induced by brief, high frequency trains of stimuli to afferents of hippocampal (e.g., CA1) pyramidal neurons. The initiation of LTP at excitatory synapses in CA1 requires the activation of NMDA receptors. The subsequent potentiation of the synaptic strength is through the augmentation of the number and/or function of non-NMDA (AMPA) receptors. The enhancement of LTP by E2 has been shown to be blocked by a tyrosine kinase inhibitor PP2, which also blocks the E2-dependent phosphorylation of NMDA receptors (Bi et al., 2000). E2 also potentiates the non-NMDA (kainate)-mediated excitation of hippocampal CA1 pyramidal neurons via activation of a cAMP/PKA pathway (Gu and Moss, 1996, Gu and Moss, 1998). Importantly, these rapid actions of E2 on kainate-induced currents in hippocampal neurons were still present in mice deficient in ERα, providing evidence for mER mediating the rapid actions of E2 in the hippocampus (Gu et al., 1999). In parallel studies, Foy and colleagues showed that E2 enhanced NMDA-mediated excitatory postsynaptic potentials (EPSPs) in CA1 neurons and LTP following Schaffer (collateral) fiber stimulation (Foy et al., 1999). Furthermore, E2 and E2-BSA, when applied acutely to the hippocampus in ovariectomized animals, produced a sustained reduction of the slow IAHP, which is mediated by a Ca2+ - activated K+ conductance, in CA1 pyramidal neurons (Carrer et al., 2003). Therefore, there are probably multiple mER mediated mechanisms that contribute to the augmentation of LTP in the hippocampus and enhanced learning and memory.
Conclusions
The gonadal steroid E2 participates in numerous physiological functions including growth and development, reproduction, feeding, and learning and memory. It has been known for some time that E2 regulates gene transcription through binding to and activating nuclear receptors (ERα and ERβ) that can stimulate or inhibit gene transcription at specific DNA binding sites. However, it is now evident that E2 can exert its actions through multiple signaling mechanisms including membrane-initiated, cytoplasmic as well as nuclear-initiated signaling pathways (Figure 1). Moreover, there are many examples in the hypothalamus, hippocampus and other brain regions for an E2-induced upregulation of PKC, PKA, PI3K and MAP kinase activity leading to altered neuronal activity as well as transcriptional activity. Although the identity of all of the mERs is still not known, there has been substantial progress towards a better understanding of the full complement of E2 actions in the brain. Also, we are beginning to better understand the cross-talk between rapid signaling events and changes in gene expression. However, many challenges lie ahead to fully identify the nature of membrane steroid receptors, functional properties and integration with nuclear steroid receptors.
Acknowledgments
The authors thank members of their laboratories who contributed to the work described herein, especially Drs. Jian Qiu, Chunguang Zhang, Troy A. Roepke, Anna Malyala and Ms. Martha A. Bosch. Dr. Troy A. Roepke provided helpful comments on earlier versions of the manuscript. The work from the authors' laboratories was supported by PHS grants NS 43330, NS 38809 and DK 68098.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe H, Keen KL, Terasawa E. Rapid action of estrogens on intracellular calcium oscillations in primate LHRH-1neurons. Endocrinology. 2008;149:1155–1162. doi: 10.1210/en.2007-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe H, Terasawa E. Firing pattern and rapid modulation of activity by estrogen in primate luteinizing hormone releasing hormone-1 neurons. Endocrinology. 2005;146:4312–4320. doi: 10.1210/en.2005-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham IM, Han SK, Todman MG, Korach KS, Herbison AE. Estrogen receptor beta mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. J Neurosci. 2003;23:5771–5777. doi: 10.1523/JNEUROSCI.23-13-05771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham IM, Todman MG, Korach KS, Herbison AE. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signaling in mouse brain. Endocrinology. 2004;145:3055–3061. doi: 10.1210/en.2003-1676. [DOI] [PubMed] [Google Scholar]
- Ahdieh HB, Wade GN. Effects of hysterectomy on sexual receptivity, food intake, running wheel activity, and hypothalamic estrogen and progestin receptors in rats. J Comp Physiol Psychol. 1982;96:886–892. [PubMed] [Google Scholar]
- Antal MC, Krust A, Chambon P, Mark M. Sterility and absence of histopathological defects in nonreproductive organs of a mouse ERβ-null mutant. Proc Natl Acad Sci USA. 2008;105:2433–2438. doi: 10.1073/pnas.0712029105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolakis EM, Garai J, Lohmann JE, Clark JH, O'Malley BW. Epidermal growth factor activates reproductive behavior independent of ovarian steroids in female rodents. Mol Endocrinology. 2000;14:1086–1098. doi: 10.1210/mend.14.7.0490. [DOI] [PubMed] [Google Scholar]
- Appleyard SM, Hayward M, Young JI, Butler AA, Cone RD, Rubinstein M, Low MJ. A role for the endogenous opioid beta-endorphin in energy homeostasis. Endocrinology. 2003;144:1753–1760. doi: 10.1210/en.2002-221096. [DOI] [PubMed] [Google Scholar]
- Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav. 2002;42:461–471. doi: 10.1006/hbeh.2002.1835. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Hess DL, Widmann AA, Henningfeld JM. Effects of progesterone on prolactin, hypothalamic beta-endorphin, hypothalamic substance P, and midbrain serotonin in guinea pigs. Neuroendocrinol. 1995;61:695–703. doi: 10.1159/000126897. [DOI] [PubMed] [Google Scholar]
- Bi R, Broutman G, Foy MR, Thompson RF, Baudry M. The tyrosine kinase and mitogen-activated protein kinase pathways mediate multiple effects of estrogen in hippocampus. Proc Natl Acad Sci USA. 2000;97:3602–3607. doi: 10.1073/pnas.060034497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TVP, Lømo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nature Chem Biol. 2006;2:207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- Bosch MA, Hou J, Kelly MJ, Rønnekleiv OK. 17β-estradiol regulation of the mRNA expression of T-type calcium channel subunits: Role of estrogen receptor a and estrogen receptor b. J Comp Neurol. 2009;512:347–358. doi: 10.1002/cne.21901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Kordasiewicz H, Mermelstein PG. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J Neurosci. 2007;27:9941–9950. doi: 10.1523/JNEUROSCI.1647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol. 2007;193:311–321. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM. Neurotrophic and neuroprotective actions of estrogen: Basic mechanisms and clinical implications. Steroids. 2007;72:381–405. doi: 10.1016/j.steroids.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant DN, Sheldahl LC, Marriott LK, Shapiro RA, Dorsa DM. Multiple pathways transmit neuroprotective effects of gonadal steroids. Endocrine. 2006;29:199–207. doi: 10.1385/ENDO:29:2:199. [DOI] [PubMed] [Google Scholar]
- Butera PC, Czaja JA. Intracranial estradiol in ovariectomized guinea pigs: effects on ingestive behaviors and body weight. Brain Res. 1984;322:41–48. doi: 10.1016/0006-8993(84)91178-8. [DOI] [PubMed] [Google Scholar]
- Cardona-Gómez GP, Mendez P, Garcia-Segura LM. Synergistic interaction of estradiol and insulin-like growth factor-I in the activation of PI3K/Akt signaling in the adult rat hypothalamus. Mol Brain Res. 2002;107:80–88. doi: 10.1016/s0169-328x(02)00449-7. [DOI] [PubMed] [Google Scholar]
- Carrer HF, Araque A, Buño W. Estradiol regulates the slow Ca2+- activated K+ current in hippocampal pyramidal neurons. J Neurosci. 2003;23:6338–6344. doi: 10.1523/JNEUROSCI.23-15-06338.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian CA, Glidewell-Kenney C, Jameson JL, Moenter SM. Classical estrogen receptor a signaling mediates negative and postive feedback on gonadotropin-releasing hormone neuron firing. Endocrinology. 2008;149:5328–5334. doi: 10.1210/en.2008-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian CA, Moenter SM. Estradiol induces diurnal shifts in GABA transmission to gonadotropin-releasing hormone neurons to provide a neural signal for ovulation. J Neurosci. 2007;27:1913–1921. doi: 10.1523/JNEUROSCI.4738-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, d'Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci. 2008;28:8691–8697. doi: 10.1523/JNEUROSCI.1775-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55:978–987. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Brown LM, Zigman JM, Kemp CJ, Strader AD, Benoit SC, Woods SC, Mangiaracina M, Geary N. Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes. 2007;56:1051–1058. doi: 10.2337/db06-0015. [DOI] [PubMed] [Google Scholar]
- Colvin GB, Sawyer CH. Induction of running activity by intracerebral implants of estrogen in overiectomized rats. Neuroendocrinol. 1969;4:309–320. doi: 10.1159/000121762. [DOI] [PubMed] [Google Scholar]
- Condon TP, Rønnekleiv OK, Kelly MJ. Estrogen modulation of the α1-adrenergic response of hypothalamic neurons. Neuroendo. 1989;50:51–58. doi: 10.1159/000125201. [DOI] [PubMed] [Google Scholar]
- Couse JF, Korach KS. Estrogen receptor null mice: What have we learned and where will they lead us? Endocrine Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- Cunningham MJ, Fang Y, Selley DE, Kelly MJ. μ-opioid agonist-stimulated [35S]GTPγS binding in guinea pig hypothalamus: Effects of estrogen. Brain Res. 1998;791:341–346. doi: 10.1016/s0006-8993(98)00201-7. [DOI] [PubMed] [Google Scholar]
- Czaja JA. Sex differences in the activational effects of gonadal hormones on food intake and body weight. Physiol Behav. 1984;33:553–558. doi: 10.1016/0031-9384(84)90370-6. [DOI] [PubMed] [Google Scholar]
- Czaja JA, Goy RW. Ovarian hormones and food intake in female guinea pigs and rhesus monkeys. Horm Behav. 1975;6:329–349. doi: 10.1016/0018-506x(75)90003-3. [DOI] [PubMed] [Google Scholar]
- DeFazio RA, Heger S, Ojeda SR, Moenter SM. Activation of A-type gamma-aminobutyric receptors excites gonadotropin-releasing hormone neurons. Mol Endocrinology. 2002;16:2872–2891. doi: 10.1210/me.2002-0163. [DOI] [PubMed] [Google Scholar]
- Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych P. Membrane estrogen receoptor-α interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J Neurosci. 2007;27:9294–9300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, Kindy MS, Wise PM. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc Natl Acad Sci USA. 2001;98:1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors a (ERα and β (ERβ) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- Erickson KR, Rønnekleiv OK, Kelly MJ. Role of a T-type calcium current in supporting a depolarizing potential, damped oscillations, and phasic firing in vasopressinergic guinea pig supraoptic neurons. Neuroendocrinol. 1993;57:789–800. doi: 10.1159/000126438. [DOI] [PubMed] [Google Scholar]
- Etgen AM, Acosta-Martinez M. Participation of growth factor signal transduction pathways in estradiol facilitation of female reproductive behavior. Endocrinology. 2003;144:3828–3835. doi: 10.1210/en.2003-0157. [DOI] [PubMed] [Google Scholar]
- Etgen AM, Ansonoff MA, Quesada A. Mechanisms of ovarian steroid regulation of norepinephrine receptor-mediated signal transduction in the hypothalamus: Implications for female reproductive physiology. Horm Behav. 2001;40:169–177. doi: 10.1006/hbeh.2001.1676. [DOI] [PubMed] [Google Scholar]
- Etgen AM, González-Flores O, Todd BJ. The role of insulin-like growth factor-I and growth factor-associated signal transduction pathways in estradiol and progesterone facilitation of female reproductive behaviors. Front Neuroendocrinol. 2006;27:363–375. doi: 10.1016/j.yfrne.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Fehrenbacher JC, LoVerme J, Clarke W, Hargreaves KM, Piomelli D, Taylor BK. Rapid pain modulation with nuclear receptor ligands. Brain Res Rev. 2009 doi: 10.1016/j.brainresrev.2008.12.019. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filardo EJ. Epidermal growth factor receptor (EGFR) transactivation by estrogen via the G-protein-coupled receptor, GPR30: a novel signaling pathway with potential significance for breast cancer. J Steroid Biochem Mol Biol. 2002;80:231–238. doi: 10.1016/s0960-0760(01)00190-x. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Bland KI, Frackelton ARJ. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Frackelton AR, Jr, Bland KI. Estrogen action via the G protein-coupled receptor, GPR30: Stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinology. 2002;16:70–84. doi: 10.1210/mend.16.1.0758. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Thomas P. GPR30: a seven-transmembrane-spanning estrogen receptor that triggers EGF release. Trends Endocrinol Metab. 2005;16:362–367. doi: 10.1016/j.tem.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Foy MR. 17β-estradiol: effect on CA1 hippocampal synaptic plasticity. Neurobiol Learn Mem. 2001;76:239–252. doi: 10.1006/nlme.2001.4018. [DOI] [PubMed] [Google Scholar]
- Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW. 17β-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J Neurophysiol. 1999;81:925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- Funakoshi T, Yanai A, Shinoda K, Kawano MM, Mizukami Y. G protein-coupled receptor 30 is an estrogen receptor in the plasma membrane. Biochem Biophys Res Commun. 2006;346:904–910. doi: 10.1016/j.bbrc.2006.05.191. [DOI] [PubMed] [Google Scholar]
- Geary N. Estradiol, CCK and satiation. Peptides. 2001;22:1251–1263. doi: 10.1016/s0196-9781(01)00449-1. [DOI] [PubMed] [Google Scholar]
- Glidewell-Kenney C, Weiss J, Hurley LA, Levine JE, Jameson JL. Estrogen receptor α signaling pathways differentially regulate gonadrotropin subunit gene expression and serum follicle-stimulating hormone in the female mouse. Endocrinology. 2008;149:4168–4176. doi: 10.1210/en.2007-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu G, Rojo AA, Zee MC, Yu J, Simerly RB. Hormonal regulation of CREB phosphorylation in the anteroventral periventricular nucleus. J Neurosci. 1996;16:3035–3044. doi: 10.1523/JNEUROSCI.16-09-03035.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Korach KS, Moss RL. Rapid action of 17β-estradiol on kainate-induced currents in hippocampal neurons lacking intracellular estrogen receptors. Endocrinology. 1999;140:660–666. doi: 10.1210/endo.140.2.6500. [DOI] [PubMed] [Google Scholar]
- Gu Q, Moss RL. 17β-estradiol potentiates kainate-induced currents via activation of the cAMP cascade. J Neurosci. 1996;16:3620–3629. doi: 10.1523/JNEUROSCI.16-11-03620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Moss RL. Novel mechanism for non-genomic action of 17β-estradiol on kainate-induced currents in isolated rat CA1 hippocampal neurones. J Physiol (Lond) 1998;506:745–754. doi: 10.1111/j.1469-7793.1998.745bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington WR, Kim SH, Funk CC, Madak-Erdogan Z, Schiff R, Katzenellenbogen JA, Katzenellenbogen BS. Estrogen dendrimer conjugates that preferentially activate extranuclear, nongenomic versus genomic pathways of estrogen action. Mol Endocrinol. 2006;20:491–502. doi: 10.1210/me.2005-0186. [DOI] [PubMed] [Google Scholar]
- Harrington WR, Sheng S, Barnett DH, Petz LN, Katzenellenbogen JA, Katzenellenbogen BS. Activities of estrogen receptor alpha-and beta-selective ligands at diverse estrogen responsive gene sites mediating transactivation or transrepression. Mol Cell Endocrinol. 2003;206:13–22. doi: 10.1016/s0303-7207(03)00255-7. [DOI] [PubMed] [Google Scholar]
- Hayward MD, Pintar JE, Low MJ. Selective reward deficit in mice lacking β-endorphin and enkephalin. J Neurosci. 2002;22:8251–8258. doi: 10.1523/JNEUROSCI.22-18-08251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocrine Rev. 1998;19:302–330. doi: 10.1210/edrv.19.3.0332. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Pape JR. New evidence for estrogen receptors in gonadotropin-releasing hormone neurons. Front Neuroendocrinol. 2001;22:292–308. doi: 10.1006/frne.2001.0219. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Shughrue PJ, Merchenthaler I, Hajszan T, Carpenter CD, Liposits Z, Petersen SL. Detection of estrogen receptor-β messenger ribonucleic acid and 125I-estrogen binding sites in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2000;141:3506–3509. doi: 10.1210/endo.141.9.7788. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Steinhauser A, Barabas K, Shughrue PJ, Petersen SL, Merchenthaler I, Liposits Z. Estrogen receptor-beta immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2001;142:3261–3264. doi: 10.1210/endo.142.7.8176. [DOI] [PubMed] [Google Scholar]
- Huang CL, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gbg. Nature. 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- Huguenard JR, McCormick DA. Simulation of the currents involved in rhythmic oscillations in thalamic relay neurons. J Neurophysiol. 1992;68:1373–1383. doi: 10.1152/jn.1992.68.4.1373. [DOI] [PubMed] [Google Scholar]
- Jackson GL, Kuehl D. Gamma-aminobutyric acid (GABA) regulation of GnRH secretion in sheep. Reproduction. 2002;59:15–24. [PubMed] [Google Scholar]
- Jones MEE, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J, Oz OK, Leury BJ, Robertson KM, Yao SG, Simpson ER. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci USA. 2000;97:12735–12740. doi: 10.1073/pnas.97.23.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallo I, Butler JA, Barkovics-Kallo M, Goubillon ML, Coen CW. Oestrogen receptor beta-immunoreactivity in gonadotropin releasing hormone-expressing neurones: regulation by oestrogen. J Neuroendocrinol. 2001;13:741–748. doi: 10.1046/j.1365-2826.2001.00708.x. [DOI] [PubMed] [Google Scholar]
- Kato M, Ui-Tei K, Watanabe M, Sakuma Y. Characterization of voltage-gated calcium currents in gonadotropin-releasing hormone neurons tagged with green fluorescent protein in rats. Endocrinology. 2003;144:5118–5125. doi: 10.1210/en.2003-0213. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Loose MD, Rønnekleiv OK. Estrogen suppresses μ-opioid and GABAB-mediated hyperpolarization of hypothalamic arcuate neurons. J Neurosci. 1992;12:2745–2750. doi: 10.1523/JNEUROSCI.12-07-02745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MJ, Qiu J, Wagner EJ, Rønnekleiv OK. Rapid effects of estrogen on G protein-coupled receptor activation of potassium channels in the central nervous system (CNS) J Steroid Biochem Mol Biol. 2003;83:187–193. doi: 10.1016/s0960-0760(02)00249-2. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Rønnekleiv OK. Rapid membrane effects of estrogen in the central nervous system. In: Pfaff DW, editor. Hormones Brain and Behavior. Academic Press; San Diego: 2002. pp. 361–380. [Google Scholar]
- Kelly MJ, Rønnekleiv OK, Eskay RL. Identification of estrogen-responsive LHRH neurons in the guinea pig hypothalamus. Brain Res Bull. 1984;12:399–407. doi: 10.1016/0361-9230(84)90112-6. [DOI] [PubMed] [Google Scholar]
- Kow LM, Pfaff DW. The membrane actions of estrogens can potentiate their lordosis behavior-facilitating genomic actions. Proc Natl Acad Sci USA. 2004;101:12354–12357. doi: 10.1073/pnas.0404889101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JÅ, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci USA. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JÅ. Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrange AH, Rønnekleiv OK, Kelly MJ. Modulation of G protein-coupled receptors by an estrogen receptor that activates protein kinase A. Mol Pharmacol. 1997;51:605–612. doi: 10.1124/mol.51.4.605. [DOI] [PubMed] [Google Scholar]
- Lagrange AH, Rønnekleiv OK, Kelly MJ. Estradiol-17β and μ-opioid peptides rapidly hyperpolarize GnRH neurons: A cellular mechanism of negative feedback? Endocrinology. 1995;136:2341–2344. doi: 10.1210/endo.136.5.7720682. [DOI] [PubMed] [Google Scholar]
- Lagrange AH, Rønnekleiv OK, Kelly MJ. The potency of μ-opioid hyperpolarization of hypothalamic arcuate neurons is rapidly attenuated by 17β-estradiol. J Neurosci. 1994;14:6196–6204. doi: 10.1523/JNEUROSCI.14-10-06196.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrange AH, Wagner EJ, Rønnekleiv OK, Kelly MJ. Estrogen rapidly attenuates a GABAB response in hypothalamic neurons. Neuroendocrinol. 1996;64:114–123. doi: 10.1159/000127106. [DOI] [PubMed] [Google Scholar]
- Leal S, Andrade JP, Paula-Barbosa MM, Madeira MD. Arcuate nucleus of the hypothalamus: Effects of age and sex. J Comp Neurol. 1998;401:65–88. doi: 10.1002/(sici)1096-9861(19981109)401:1<65::aid-cne5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Lee AW, Kyrozis A, Chevaleyre V, Kow LM, Zhou J, Devidze N, Zhang Q, Etgen AM, Pfaff DW. Voltage-dependent calcium channels in ventromedial hypothalamic neurones of postnatal rats: modulation by oestradiol and phenylephrine. J Neuroendocrinol. 2008;20:188–198. doi: 10.1111/j.1365-2826.2007.01637.x. [DOI] [PubMed] [Google Scholar]
- Loose MD, Rønnekleiv OK, Kelly MJ. Membrane properties and response to opioids of identified dopamine neurons in the guinea pig hypothalamus. J Neurosci. 1990;10:3627–3634. doi: 10.1523/JNEUROSCI.10-11-03627.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund TD, Hinds LR, Handa RJ. The androgen 5α-dihydrotestosterone and its metabolite 5α-androstan-3β, 17 β-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor β-expressing neurons in the hypothalamus. J Neurosci. 2006;26:1448–1456. doi: 10.1523/JNEUROSCI.3777-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malyala A, Kelly MJ, Rønnekleiv OK. Estrogen modulation of hypothalamic neurons: Activation of multiple signaling pathways and gene expression changes. Steroids. 2005;70:397–406. doi: 10.1016/j.steroids.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Malyala A, Zhang C, Bryant D, Kelly MJ, Rønnekleiv OK. PI3K signaling effects in hypothalamic neurons mediated by estrogen. J Comp Neurol. 2008;506:895–911. doi: 10.1002/cne.21584. [DOI] [PubMed] [Google Scholar]
- Matsuda K, Sakamoto H, Mori H, Hosokawa K, Kawamura A, Itose M, Nishi M, Prossnitz ER, Kawata M. Expression and intracellular distribution of the G protein-coupled receptor 30 in rat hippocampal formation. Neurosci Lett. 2008;441:94–99. doi: 10.1016/j.neulet.2008.05.108. [DOI] [PubMed] [Google Scholar]
- McCaffrey TA, Czaja JA. Diverse effects of estradiol-17 beta: Concurrent suppression of appetite, blood pressure and vascular reactivity in conscious, unrestrained animals. Physiol Behav. 1989;45:649–657. doi: 10.1016/0031-9384(89)90086-3. [DOI] [PubMed] [Google Scholar]
- McDevitt MA, Glidewell-Kenney C, Jimenez MA, Ahearn PC, Weiss J, Jameson JL, Levine JE. New insights into the classical and non-classical actions of estrogen: evidence from estrogen receptor knock-out and knock-in mice. Mol Cell Endocrinol. 2008;290:24–30. doi: 10.1016/j.mce.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez P, Azcoitia I, Garcia-Segura LM. Estrogen receptor alpha forms estrogen-dependent multimolecular complexes with insulin-like growth factor receptor and phospatidylinositol 3-kinase in the adult rat brain. Mol Brain Res. 2003;112:170–176. doi: 10.1016/s0169-328x(03)00088-3. [DOI] [PubMed] [Google Scholar]
- Micevych PE, Mermelstein PG. Membrane estrogen receptors acting through metabotropic glutamate receptors: an emerging mechanism of estrogen action in brain. Mol Neurobiol. 2008;38:66–77. doi: 10.1007/s12035-008-8034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milewicz A, Bidzinska B, Mikulski E, Demissie M, Tworowska U. Influence of obesity and menopausal status on serum leptin, cholecystokinin, galanin and neuropeptide Y levels. Gynecol Endocrinol. 2000;14:196–203. doi: 10.3109/09513590009167682. [DOI] [PubMed] [Google Scholar]
- Mills RH, Sohn RK, Micevych PE. Estrogen-induced mu-opioid receptor internalization in the medial preoptic nucleus is mediated via neuropeptide Y-Y1 receptor activation in the arcuate nucleus of female rats. J Neurosci. 2004;24:947–955. doi: 10.1523/JNEUROSCI.1366-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro CE, Saeed SA, Murdock C, Martinez-Fuentes AJ, Arora KK, Krsmanovic LZ, Catt KJ. Regulation of cyclic adenosine 3′,5′- monophosphate signaling and pulsatile neurosecretion by Gi-coupled plasma membrane estrogen receptors in immortalized gonadotrophin-releasing hormone neurons. Mol Endocrinol. 2003;17:1792–1804. doi: 10.1210/me.2003-0040. [DOI] [PubMed] [Google Scholar]
- Noel SD, Keen KL, Baumann DI, Filardo EJ, Terasawa E. Involvement of G-protein couple receptor 30 (GPR30) in rapid action of estrogen in primate LHRH neurons. Mol Endocrinol. 2009;23:349–359. doi: 10.1210/me.2008-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor-alpha gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998;139:5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinology. 2006;20:1996–2009. doi: 10.1210/me.2005-0525. [DOI] [PubMed] [Google Scholar]
- Plum L, et al. Enhanced PIP3 signaling in POMC neurons causes Katp channel activation and leads to diet-sensitive obesity. Journal of Clinical Investigation. 2006;116:1886–1901. doi: 10.1172/JCI27123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poehlman ET. Menopause, energy expenditure, and body composition. Acta Obstet Gynecol Scand. 2002;81:603–611. doi: 10.1034/j.1600-0412.2002.810705.x. [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Arterburn JB, Sklar LA. GPR30: A G protein-coupled receptor for estrogen. Mol Cell Endocrinol. 2007;265-266:138–142. doi: 10.1016/j.mce.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Jamali K, Xue C, Kelly MJ, Rønnekleiv OK. Estrogen upregulates T-type calcium channels in the hypothalamus and pituitary. J Neurosci. 2006a;26:11072–11082. doi: 10.1523/JNEUROSCI.3229-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Rønnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Krust A, Graham S, Murphy S, Korach KS, Chambon P, Scanlan TS, Rønnekleiv OK, Kelly MJ. A G protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J Neurosci. 2006b;26:5649–5655. doi: 10.1523/JNEUROSCI.0327-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Rønnekleiv OK, Kelly MJ. Modulation of hypothalamic neuronal activity through a novel G-protein coupled estrogen membrane receptor. Steroids. 2008;73:985–991. doi: 10.1016/j.steroids.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada A, Etgen AM. Insulin-like growth factor-1 regulation of α1-adrenergic receptor signaling is estradiol dependent in the preoptic area and hypothalamus of female rats. Endocrinology. 2001;142:599–607. doi: 10.1210/endo.142.2.7946. [DOI] [PubMed] [Google Scholar]
- Quesada A, Etgen AM. Functional interactions between estrogen and insulin-like growth factor-I in the regulation of α1B-adrenoceptors and female reproductive function. J Neurosci. 2002;22:2401–2408. doi: 10.1523/JNEUROSCI.22-06-02401.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003;23:5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: Studies of ERα and ERβ expressed in Chinese hamster ovary cells. Mol Endocrinology. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- Roepke TA, Malyala A, Bosch MA, Kelly MJ, Rønnekleiv OK. Estrogen regulation of genes important for K+ channel signaling in the arcuate nucleus. Endocrinology. 2007;148:4937–4951. doi: 10.1210/en.2007-0605. [DOI] [PubMed] [Google Scholar]
- Roepke TA, Xue C, Bosch MA, Scanlan TS, Kelly MJ, Rønnekleiv OK. Genes associated with membrane-initiated signaling of estrogen and energy homeostasis. Endocrinology. 2008;149:6113–6124. doi: 10.1210/en.2008-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanò N, Lee K, Ábrahám IM, Jasoni CL, Herbison AE. Non-classical estrogen modulation of presynaptic GABA terminals modulates calcium dynamics in gonadotropin-releasing hormone (GnRH) neurons. Endocrinology. 2008;149:5335–5344. doi: 10.1210/en.2008-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rønnekleiv OK, Kelly MJ. Diversity of ovarian steroid signaling in the hypothalamus. Front Neuroendocrinol. 2005;26:65–84. doi: 10.1016/j.yfrne.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Rudick CN, Woolley CS. Estrogen regulates functional inhibition of hippocampal CA1 pyramidal cells in the adult female rat. J Neurosci. 2001;21:6532–6543. doi: 10.1523/JNEUROSCI.21-17-06532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Davies P. Calcium-activated potassium currents in mammalian neurons. Clin Exp Pharmacol Physiol. 2000;27:657–663. doi: 10.1046/j.1440-1681.2000.03317.x. [DOI] [PubMed] [Google Scholar]
- Schild HO. pA, a new scale for the measurement of drug antagonism. Br J Pharmacol. 1947;2:189–206. doi: 10.1111/j.1476-5381.1947.tb00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB. Surgical menopause, estrogen, and cognitive function in women: what do the findings tell us? Ann N Y Acad Sci. 2005;1052:3–10. doi: 10.1196/annals.1347.001. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Ohtani K, Kato Y, Tanaka Y, Mori M. Estrogen increases hypothalamic neuropeptide Y (NPY) mRNA expression in ovariectomized obese rat. Neurosci Lett. 1996;204:81–84. doi: 10.1016/0304-3940(96)12322-3. [DOI] [PubMed] [Google Scholar]
- Shimomura Y, Shimizu H, Takahashi M, Sato N, Uehara Y, Fukatsu A, Negishi M, Kobayashi I, Kobayashi S. The significance of decreased ambulatory activity during the generation by long-term observation of obesity in ovariectomized rats. Physiol Behav. 1990;47:155–159. doi: 10.1016/0031-9384(90)90055-9. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Simerly RB, McCall LD, Watson SJ. Distribution of opioid peptides in the preoptic region: immunohistochemical evidence for a steroid-sensitive enkephalin sexual dimorphism. J Comp Neurol. 1988;276:442–459. doi: 10.1002/cne.902760309. [DOI] [PubMed] [Google Scholar]
- Singer CA, Figueroa-Masot XA, Batchelor RH, Dorsa DM. The mitogen-activated protein kinase pathway mediates estrogen neuroprotection after glutamate toxicity in primary cortical neurons. J Neurosci. 1999;19:2455–2463. doi: 10.1523/JNEUROSCI.19-07-02455.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Setalo GJ, Guan X, Frail DE, Toran-Allerand CD. Estrogen-induced activation of the mitogen-activated protein kinase cascade in the cerebral cortex of estrogen receptor-alpha knock-out mice. J Neurosci. 2000;20:1694–1700. doi: 10.1523/JNEUROSCI.20-05-01694.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Setalo G, Jr, Guan X, Warren M, Toran-Allerand CD. Estrogen-induced activation of mitogen-activated protein kinase in cerebral cortical explants: Convergence of estrogen and neurotrophin signaling pathways. J Neurosci. 1999;19:1179–1188. doi: 10.1523/JNEUROSCI.19-04-01179.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006;26:6687–6694. doi: 10.1523/JNEUROSCI.1618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith YR, Giordani B, Lajiness-O'Neill R, Zubieta JK. Long-term estrogen replacement is associated with improved nonverbal memory and attentional measures in postmenopausal women. Fertil Steril. 2001;76:1101–1107. doi: 10.1016/s0015-0282(01)02902-8. [DOI] [PubMed] [Google Scholar]
- Spergel DJ, Kruth U, Hanley DF, Sprengel R, Seeburg PH. GABA-and glutamate activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. J Neurosci. 1999;19:2037–2050. doi: 10.1523/JNEUROSCI.19-06-02037.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker M, Krause M, Pedarzani P. An apamin-sensitive Ca2+-activated K+ current in hippocampal pyrmadial neurons. Proc Natl Acad Sci USA. 1999;96:4662–4667. doi: 10.1073/pnas.96.8.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh BC, Hille B. Recovery from muscarinic modulation of M current channels requires phosphatidylinositol 4,5-bisphosphate synthesis. Neuron. 2002;35:507–520. doi: 10.1016/s0896-6273(02)00790-0. [DOI] [PubMed] [Google Scholar]
- Suter KJ, O'Farrell L. Impaired episodic LH secretion in female mice with GFP in GnRH neurons. Am J Physiol Endocrinol Metab. 2008;295:E130–E136. doi: 10.1152/ajpendo.90300.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter KJ, Song WJ, Sampson TL, Wuarin JP, Saunders JT, Dudek FE, Moenter SM. Genetic targeting of green fluorscent protein to gonadotropin-releasing hormone neurons: Characterization of whole-cell electrophysiological properties and morphology. Endocrinology. 2000;141:412–419. doi: 10.1210/endo.141.1.7279. [DOI] [PubMed] [Google Scholar]
- Szegõ ÉM, Barabás K, Balog J, Szilágyi N, Korach KS, Juhász G, Abrahám IM. Estrogen induces estrogen receptor a-dependent cAMP response element-binding protein phosphorylation via mitogen activated protein kinase pathway in basal forebrain cholinergic neurons in vivo. J Neurosci. 2006;26:4104–4110. doi: 10.1523/JNEUROSCI.0222-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple JL, Laing E, Sunder A, Wray S. Direct action of estradiol on gonadotropin-releasing hormone-1 neuronal activity via a transcription-dependent mechanism. J Neurosci. 2004;24:6326–6333. doi: 10.1523/JNEUROSCI.1006-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple JL, Wray S. BSA-estrogen compounds differentially alter gonadotropin-releasing hormone-1 neuronal activity. Endocrinology. 2005;146:558–563. doi: 10.1210/en.2004-1117. [DOI] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G-protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- Thornton JE, Loose MD, Kelly MJ, Rønnekleiv OK. Effects of estrogen on the number of neurons expressing β-endorphin in the medial basal hypothalamus of the female guinea pig. J Comp Neurol. 1994;341:68–77. doi: 10.1002/cne.903410107. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD. Estrogen and the brain: beyond ERα, ER-β and 17β-estradiol. Ann NY Acad Sci. 2005;1052:136–144. doi: 10.1196/annals.1347.009. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD. Minireview: A plethora of estrogen receptors in the brain: Where will it end? Endocrinology. 2004;145:1069–1074. doi: 10.1210/en.2003-1462. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ES, Jr, Nethrapalli IS, Tinnikov AA. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toran-Allerand CD, Singh M, Setalo GJ. Novel mechanisms of estrogen action in the brain: New players in an old story. Front Neuroendocrinol. 1999;20:97–121. doi: 10.1006/frne.1999.0177. [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Kow LM, Pfaff DW. Early membrane estrogenic effects required for full expression of slower genomic actions in a nerve cell line. Proc Natl Acad Sci USA. 2001;98:12267–12271. doi: 10.1073/pnas.221449798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade CB, Robinson S, Shapiro RA, Dorsa DM. Estrogen receptor (ER)alpha and ERbeta exhibit unique pharmacologic properties when coupled to activation of the mitogen-activated protein kinase pathway. Endocrinology. 2001;142:2336–2342. doi: 10.1210/endo.142.6.8071. [DOI] [PubMed] [Google Scholar]
- Wagner EJ, Rønnekleiv OK, Bosch MA, Kelly MJ. Estrogen biphasically modifies hypothalamic GABAergic function concomitantly with negative and positive control of luteinizing hormone release. J Neurosci. 2001a;21:2085–2093. doi: 10.1523/JNEUROSCI.21-06-02085.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner EJ, Rønnekleiv OK, Kelly MJ. The noradrenergic inhibition of an apamine-sensitive small conductance Ca2+ -activated K+ channel in hypothalamic γ-aminobutyric acid neurons: Pharmacology, estrogen sensitivity and relevance to the control of the reproductive axis. J Pharmacol Exp Ther. 2001b;299:21–30. [PubMed] [Google Scholar]
- Weatherill PJ, Wilson APM, Nicholson RI, Davies P, Wakeling AE. Interaction of the antioestrogen ICI 164,384 with the oestrogen receptor. J Ster Bioc Mol Biol. 1988;30:263–266. doi: 10.1016/0022-4731(88)90103-3. [DOI] [PubMed] [Google Scholar]
- Wintermantel TM, Campbell RE, Porteous R, Bock D, Gröne HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to ganadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M, Moss RL. Electrophysiological evidence for a rapid membrane action of the gonadal steroid 17β-estradiol, on CA1 pyramidal neurons of the rat hippocampus. Brain Res. 1991;543:148–152. doi: 10.1016/0006-8993(91)91057-8. [DOI] [PubMed] [Google Scholar]
- Wong M, Moss RL. Long-term and short-term electrophysiological effects of estrogen on the synaptic properties of hippocampal CA1 neurons. J Neurosci. 1992;12:3217–3225. doi: 10.1523/JNEUROSCI.12-08-03217.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M, Moss RL. Patch-clamp analysis of direct steroidal modulation of glutamate receptor-channels. J Neuroendocrinology. 1994;6:347–355. doi: 10.1111/j.1365-2826.1994.tb00592.x. [DOI] [PubMed] [Google Scholar]
- Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- Xu AW, Kaelin CB, Takeda K, Akira S, Schwartz MW, Barsh GS. PI3K integrates the action of insulin and leptin on hypothalamic neurons. J Clin Invest. 2005;115:951–958. doi: 10.1172/JCI24301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Bosch MA, Levine JE, Rønnekleiv OK, Kelly MJ. Gonadotropin-releasing hormone neurons express KATP Channels that are regulated by estrogen and responsive to glucose and metabolic inhibition. J Neurosci. 2007;27:10153–10164. doi: 10.1523/JNEUROSCI.1657-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, He C, Yan X, Mirshashi T, Logothetis DE. Activation of inwardly rectifying K+ channels by distinct PtdIns(4,5)P2 interactions. Nature Cell Biol. 1999;1:183–188. doi: 10.1038/11103. [DOI] [PubMed] [Google Scholar]