Abstract
While numerous hypotheses have been proposed to explain the molecular mechanisms underlying the pathogenesis of neurodegenerative diseases, the theory of oxidative stress has received considerable support. Although many correlations have been established and encouraging evidence has been obtained, conclusive proof of causation for the oxidative stress hypothesis is lacking and potential cures have not emerged. Therefore it is likely that other factors, possibly in coordination with oxidative stress, contribute to neuron death. Using Parkinson's disease (PD) as the paradigm, this review explores the hypothesis that oxidative modifications, mitochondrial functional disruption, and impairment of protein degradation constitute three interrelated molecular pathways that execute neuron death. These intertwined events are the consequence of environmental exposure, genetic factors, and endogenous risks and constitute a "Bermuda triangle" that may be considered the underlying cause of neurodegenerative pathogenesis.
Review
A "Bermuda Triangle" of Insults Induces Neurodegeneration
Understanding the molecular basis of neurodegenerative diseases has proven to be a major challenge, yet is vitally important because of the prevalence of these chronic conditions in the aging population. While diverse neurodegenerative disorders, which encompass Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD), and Amyotrophic Lateral Sclerosis (ALS), involve unique proteins and selectively disparate brain regions, they share two key features: formation of insoluble protein aggregates and neuron degeneration [1]. Therefore it is reasonable to speculate that a common causative process underlies the pathogenesis of neurodegenerative disorders. Specifically focusing on PD, this review proposes that neurodegeneration is due to three interrelated molecular mechanisms: oxidative modifications, mitochondrial dysfunction, and impaired protein degradation.
One possible unifying molecular mechanism that can induce both the formation of protein inclusions and neuron degeneration is the oxidative reactions derived from the production of reactive oxygen and nitrogen species. A substantial increase in oxidized proteins, lipids, and DNA has been found in postmortem brain tissue of PD patients as compared to age-matched disease-free subjects [2]. Although these observations do not demonstrate that oxidative processes are the sole cause of neuronal demise, they are consistent with data in animal and cellular model systems (reviewed below) that establish a role for oxidation in neurodegeneration and death.
The four electron reduction of oxygen to water is a fundamental biochemical process largely responsible for the survival of organisms in aerobic environments. Oxidation and reduction reactions are also important in the central nervous system for the formation and metabolic processing of catecholamines, for the production of signaling molecules such as nitric oxide, and for the metabolism of xenobiotics. The coupling of these enzymatic systems ensures that electrons are transferred to the desired substrate, avoiding partial reduction of oxygen to reactive species. However, inappropriate reduction of oxygen does occur occasionally, resulting in the production of superoxide and hydrogen peroxide.
Mitochondria are considered a key source of reactive species. Interruptions or disturbances in the electron transport chain allow electrons to be transferred and reduce molecular oxygen by one electron to form superoxide, or by two electrons to form hydrogen peroxide. In addition to generating ATP, mitochondria also play critical roles in regulating cellular viability. Therefore, functional compromise of this organelle has a large impact on oxidative homeostasis. To protect against reactive species, a network of antioxidant enzymes including Cu, Zn superoxide dismutase (SOD) in the cytosol, Mn SOD in the mitochondria, peroxidases, and catalase secure conversion of these reactive species to water and therefore prevent adverse oxidation of cellular macromolecules.
How then do reactive species induce stress? The answer to this question is not entirely understood but several suggestions have been advanced. A significant increase in the rate of reactive species production, potentially coupled with a decline in the efficiency of the antioxidant networks that remove them may permit secondary reactions with cellular biomolecules (proteins, lipids, DNA/RNA) that result in undesired oxidations. While neuronal homeostasis can be disturbed by these oxidative modifications, protective mechanisms including protein degradation, lipid turnover, and DNA repair sustain cellular homeostasis by repairing or removing the oxidized macromolecules. However, compromise of these defense mechanisms – either by direct oxidative modification or indirectly by the inability to process oxidatively modified substrates – may render the cell incapable of efficiently removing oxidized biomolecules, resulting in their accumulation.
Alteration of protein folding and degradation, due to oxidative stress, mitochondrial dysfunction, or other factors has been strongly associated with neurodegenerative diseases. Protein aggregation is a hallmark of a diverse array of these late-onset neurodegenerative disorders, and thus factors that influence protein folding, processing, and clearance have been a focus of much research. Two major pathways are responsible for the degradation of cellular proteins: the ubiquitin-proteasome system (UPS) [3] and the autophagy-lysosome pathway [4-6].
The UPS is the principal mechanism of degradation for short-lived proteins and proteins that are misfolded in the endoplasmic reticulum [5]. UPS substrates are selectively targeted for degradation by the 20S or 26S proteasome complex after the conjugation of a polyubiquitin tag through a three-step enzymatic cascade [7]. Upon recruitment to the proteasome, the substrates must be unfolded to pass through the narrow barrel of the proteasome where they are degraded [5,7]. The consistent observation that antibodies against ubiquitin label some of the human and rodent protein inclusions suggests that failure of the UPS may contribute to neurodegeneration. However, the effect of UPS inhibition on cell death and protein aggregation in cellular model systems as well as rodent models has yielded conflicting results that have not been entirely resolved [8-13]. These variable results suggest that other factors, including other protein degradation pathways such as autophagy and mitochondrial dysfunction associated with a decline in ATP levels, may contribute to cellular viability. This hypothesis remains to be further explored in cellular and rodent model systems.
The other primary pathway for protein degradation in the cell is through autophagy. While the ultimate result of autophagy is always the delivery of proteins or organelles to the lysosome for degradation, there are three different routes by which this can be accomplished. Macroautophagy is a non-selective method of bulk degradation whose activity is upregulated in response to stress. Microautophagy is also a non-selective process, though it is maintained at a constitutively active state. The final type of autophagy is chaperone-mediated autophagy (CMA). Like macroautophagy, CMA is present at low basal levels in the cell and is upregulated in response to stress. However, CMA is unique from the other two forms of autophagy in that it is a selective process [4,14]. While the UPS, macroautophagy, and CMA have been implicated as potential contributors to neurodegeneration, their precise involvement is controversial and unclear.
Macroautophagy was first implicated in neurodegeneration after it was noted that autophagic structures were present in affected brain regions of patients with neurodegenerative diseases, including PD [15-18]. Initial hypotheses speculated that these autophagic vacuoles were evidence of neurons "eating themselves to death" [15]. This was based on previous observations that autophagic mechanisms can participate in induction of non-apoptotic cell death cascades [19-25]. However, recent evidence has shown that, particularly within the context of neurodegeneration, macroautophagy may instead serve as a protective process by which cells attempt to clear misfolded proteins and damaged organelles[4]. Independently generated data has revealed the neuroprotective role of macroautophagy through manipulation of either Atg7 or Atg5 – two different proteins essential for autophagy. Conditionally knocking out either of these genes in the central nervous system of mice leads to severe neurodegeneration and formation of protein inclusions, accompanied by motor dysfunction and early death [26,27]. In cells, inhibition of macroautophagy at the stage of autophagosome formation by 3-methyladenine (3-MA), at the stage of autophagosome-lysosome fusion by Bafilomycin A1 (BafA1), or at the stage of lysosomal degradation by deficiency of the enzyme cathepsin D, led to enhanced aggregation of polygluatmine, polyalanine, and α-synuclein proteins [28-30]. In contrast, the induction of autophagy leds to increased clearance and reduced toxicity of pathogenic proteins, decreased aggregate formation and neurodegeneration, and improved behavioral phenotype in fly and mouse models [29-35]. Stimulation of autophagy has been accomplished either by rapamycin, which inhibits the negative regulator of autophapgy mammalian target of rapamycin (mTOR), or by several mTOR independent compounds including lithium, trehalose, and small molecules identified in a screen [29-35].
CMA may also be playing a role in cell vulnerability. In CMA deficient cells, baseline levels of survival were unaffected, but stressors such as UV light or multiple types of oxidative stress significantly reduced viability [36]. Additionally, the proteins implicated in neurodegenerative disease, APP, Htt, and α-synuclein, all contain a putative CMA targeting motif, indicating that regulation of this degradation system may have important effects on pathogenic protein homeostasis[14].
The UPS, macroautophagy, and CMA are each involved in the degradation of oxidized proteins. In response to moderate levels of oxidative stress, cells are able to induce a protective upregulation of all three of these protein degradation pathways, supporting an interplay between protein oxidation and protein degradation during normal homeostasis [4,37-43].
However, more severe oxidative stress impairs the degradation of oxidized proteins [39,40,44]. For the UPS system, oxidative modifications that induce crosslinking, misfolding, and aggregation prevent the proper unfolding necessary for substrates to be passed through the barrel of the proteasome for degradation, making these substrates resistant to degradation as well as potentially inhibiting the overall activity of the proteasome [45-48]. Additionally, direct oxidative modification of the proteasome subunits inhibits 20S and 26S catalytic peptidase activity [46,49-54]. In a rat model of ischemia/reperfusion, the lipid peroxidation product 4-hydroxyl-2-noneal (HNE) impaired the peptidase activity of the proteasome by direct oxidative modification of the α-like 20S proteasome subunits iota, C3, and an isoform of XAPC7 [53,54].
Additionally, oxidatively modified proteins may impair the cellular machinery of autophagic degradation [55]. Reactive species can damage the lysosomal membrane and crosslink membrane proteins, resulting in cytosolic leakage of lysosomal hydrolases [56-58]. Some oxidatively modified aggregated species are resistant to degradation by proteases and accumulate within lysosomes. There, the non-degraded proteins become a potential new source of reactive species, further damaging the lysosomal membrane [59].
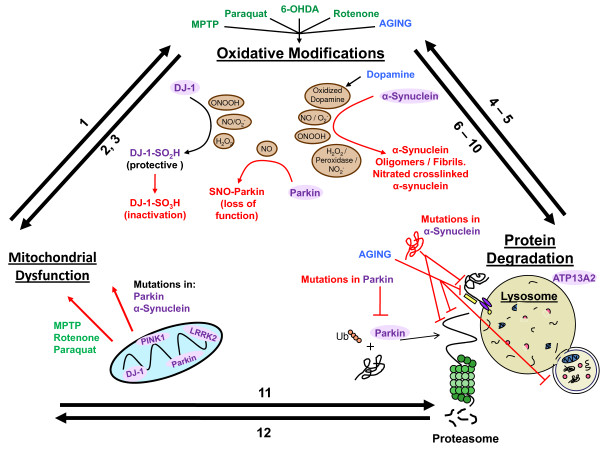
Below we discuss evidence that implicates known environmental, genetic, and endogenous factors as contributors that intitiate oxidative modifications, mitochondrial dysfunction, and protein aggregation in PD (Figure 1). We propose that the combined interactions of these three interrelated molecular pathways – oxidative modifications, mitochondrial dysfunction, and impaired protein degradation – constitute a "Bermuda Triangle" that ultimately induces neuron death.
Figure 1.
A "Bermuda Triangle" of insults leads to neurondeath in PD. Known risk factors for the onset of Parkinson's disease (PD) include environmental (green), genetic (purple), and endogenous (blue) influences. Contributions from these risk factors trigger oxidative modifications, mitochondrial dysfunction, and impaired protein degradation that together form a "Bermuda triangle" of interrelated molecular events that underlie neurodegeneration. The interactions between these pathways are supported by the following (for details and citations, please refer to text): (1) Disturbances in mitochondrial respiration generate reactive oxygen species. (2) Overexpression of SOD is protective against mitochondrial toxins. (3) NOS deficiency or inhibition attenuates MPTP, paraquat, and rotenone toxicity. (4) Inhibition of degradation systems leads to increased sensitivity to oxidative stressors. (5) Impaired degradation leads to an accumulation of substrates, increasing the probability for oxidative modifications. (6) Excessive production of reactive oxygen and nitrogen species modifies proteins, leading to inactivation, crosslinking, and aggregation. (7) α-Synuclein modified by oxidized dopamine impedes CMA. (8) Oxidative modifications modify the lysosomal membrane and crosslink membrane proteins. (9) UPS and CMA are not able to unfold and remove oxidatively proteins. (10) Oxidative modification of proteasome subunits inhibits UPS function. (11) Macroautophagy is the principle mechanism for the degradation of damaged mitochondria. (12) Proteasome inhibition increases mitochondrial reactive species generation and decreases complex I and II activity.
Environmental Toxins
One of the most striking clues into the processes involved in PD came from the observation of rapid-onset motor impairments that replicated most of the features of sporadic PD in individuals accidentally exposed to 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) [60]. Further epidemiological studies have suggested that exposure to other pesticides and environmental toxins are associated with PD development. Through their implied ability to target mitochondria, disrupt dopamine metabolism, and participate in the formation of oxidants, these toxins initiate a cascade of deleterious events that can cause the progressive degeneration observed in disease [61].
In addition to the prototypical example of MPTP, a host of other compounds that generate oxidative and nitrative stress (defined as the formation of nitric oxide-dependent oxidants) have been found to be harmful to neurons. These agents have been used for the creation of intoxication models in rodents and non-human primates that reproduce some of the neuropathological findings and behavioral symptoms of the human disease. These intoxication models described below are valuable in understanding the role of oxidative mechanisms, mitochondrial dysfunction, and protein aggregation in neuron death and the selective vulnerability of the nigrostriatal pathway.
Mechanistically, MPP+, the active metabolite of MPTP, is selectively taken up by dopaminergic neurons where it is suggested to inhibit complex I of the mitochondrial respiratory chain, inhibit the uptake of dopamine, and participate in oxidation-reduction biochemistry [62]. MPTP administration, widely used in non-human primates and mice, has been shown to replicate many features of PD, including motor phenotype, degeneration of nigral dopaminergic neurons, and formation of α-synuclein positive filamentous proteinaceous inclusions resembling Lewy Bodies [63-66].
The concept that oxidative processes are playing a major role in the demise of the catecholaminergic neurons is reinforced by data documenting that mice over-expressing the antioxidant protein cytosolic SOD1 [67] are protected against MPTP toxicity. Additionally, the contribution of reactive nitrogen species to MPTP-induced neuron injury is revealed by studies in nitric oxide synthase (NOS) deficient animals. MPTP toxicity is attenuated in either iNOS or nNOS deficient mice [68,69] or mice that are treated with nNOS inhibitors [70,71] suggesting that nitric oxide-derived oxidants are participants in the oxidative and nitrative processes that lead to MPTP-induced neurodegeneration.
The herbicide paraquat, a biologically redox active molecule, is a toxin that has deleterious effects on neurons. Paraquat is used in mouse models of neurodegeneration and leads to reduced motor activity, cell death selectively within the dopaminergic neurons of the substantia nigra, and degeneration of the striatal fibers in a dose-dependent way [72,73]. Additionally, systemic administration of paraquat results in upregulation of α-synuclein expression and the formation of aggregates [74], similar to changes that have been reported following administration of MPTP [75]. Overexpression of SOD in cells or mice has been shown to protect against paraquat toxicity, supporting the role of oxidative stress in neuron death [76-79]. Delivery of molecules with SOD/catalase and antioxidant scavenging capacity such as MnTBAP or EUK-189 were shown to have a similar effect [80-82], although recent studies have indicated that this protection against paraquat may be due to antioxidant-independent mechanisms of MnTBAP including prevention of mitochondrial Ca2+ accumulation [83,84].
Rotenone is an insecticide that selectively inhibits mitochondrial complex I. It has been used in rat models to produce a Parkinson-like phenotype including selective degeneration of the dopaminergic neurons of the nigrostriatal region, motor impairment, and fibrillar inclusions [85]. Unlike MPTP, rotenone is highly lipophilic and consequently can enter any cell type [86]. Therefore, rotenone could potentially inhibit mitochondrial complex I throughout the brain. However, rats chronically infused with rotenone develop selective nigral degeneration and α-synuclein positive, Lewy body-like inclusions indicating that dopaminergic cells are somehow exquisitely sensitive to mitochondrial impairment [85]. The molecular details underlying this inherent dopaminergic neuron vulnerability still require further investigation, and will be discussed below.
Finally, 6-hydroxydopamine (6-OHDA), a prototypical oxidative stress toxin used in animal models for over 30 years, mimics PD by causing degeneration of the dopaminergic neurons [87,88]. 6-OHDA is structurally similar to dopamine and norepinephrine and thus can accumulate in catecholaminergic cells. In the presence of oxygen and transition metals it oxidizes into para-quinone and hydrogen peroxide, with superoxide (O2.-) and semi-quinone radicals as intermediate species of the reaction [89]. The generation of reactive species and strong electrophiles attacking nucleophilic groups and inactivating macromolecules have been shown to contribute to neurodegeneration [87,88]. Injection of 6-OHDA in the substantia nigra of rats leads to rapid death of dopaminergic neurons, while injection in the stiatum induces a retrograde degeneration of the neurons in the substantia nigra [90-92].
Genetic Links
While the majority of PD cases are sporadic, rare instances of genetic heritability have helped to provide further insight into the mechanisms contributing to disease. Currently, thirteen genetic loci, denoted PARK1–13, have been associated with PD [93]. From these loci, six genes have been established as a causative factor of familial PD: α-synuclein (PARK1/4), parkin (PARK2), PINK1 (PARK6), DJ-1 (PARK7), LRRK2 (PARK8), and ATP13A2 (PARK9) [93-95]. ATP13A2 is a lysosomal P-type ATPase that has been associated with a recessive juvenile form of PD [96]. A recent studies highlighted a genetic interaction between ATP13A2 and α-synuclein and showed that ATP13A2 is able to modulate α-synuclein toxicity [97]. However, while ATP13A2's lysosomal location reinforces the importance of autophagic degradation, this review will focus on the other five PD genes that have been most extensively investigated. Each of these five genes (DJ-1, PINK1, Parkin, LRRK2, and α-synculein) has yielded data supporting critical associations with mitochondrial and oxidative processes and protein degradation.
DJ-1
Mutations and deletions in the gene encoding DJ-1 have been linked to recessive familial PD. DJ-1 is a mitochondrial-associated protein which has been suggested to function as an antioxidant with peroxidredoxin-like activity [98-100]. Mass spectrometry and other methodologies have indentified Cys106 in DJ-1 as the critical amino acid for DJ-1 mediated protection against oxidative stress as well as for the relocation of DJ-1 to the mitochondria during oxidative stress [101]. Irreversible oxidation of this residue renders the protein incapable of protecting cells from oxidant insults [102].
Support for a role of DJ-1 as a protective antioxidant protein is derived from experiments demonstrating that knockout/knockdown of DJ-1 or expression of DJ-1 with a pathogenic mutation in cells leads to an increased sensitivity to oxidative stress [99,103]. Similarly, increased sensitivity to neurotoxins that generate oxidative stress such as MPTP, rotenone, and paraquat has been documented in DJ-1 deficient drosophila and mice [104-108]. Correspondingly, over-expression of DJ-1 protects against oxidative insults. In dopaminergic cell lines, overexpression of wild type, but not mutant, DJ-1 was able to protect cells from hydrogen peroxide and 6-OHDA challenges, leading to reduced levels of reactive species, protein oxidation, and cell death [109,110]. In animal models, overexpression of wild type but not mutant DJ-1 was protective against dopaminergic neural degeneration in mice exposed to MPTP or rats exposed to 6-OHDA [108,110,111].
PINK1
PTEN-induced kinase 1(PINK1) is a mitochondrial associated protein whose loss of function mutations lead to a recessive form of hereditary early onset PD [112]. PINK1 is a putative serine/threonine kinase with an N-terminal mitochondrial targeting sequence [113]. Both endogenous and recombinant PINK1 are localized to the mitochondria in cell culture and a drosophila model [112-114]. Functionally, it is postulated that PINK1 phosphorylates mitochondrial proteins in response to cellular stress and thus protects against mitochondrial dysfunction [112,115]. Additional roles for PINK1 in regulating mitochondrial fusion and fission as well as modulating proteolytic activity through interaction with the serine protease HtrA2 have also been proposed [116-119]. Within the context of disease, lymphoblasts of patients with mutations in PINK1 show increased lipid peroxidation and defects in mitochondrial complex I activity [120,121]. Additionally, abnormal mitochondrial morphology was evident in primary cells derived from patients with two different mutations in PINK1 [120].
PINK1 has been shown to influence cell viability. Knockdown of PINK1 in SH-SY5Y, HeLa, and mouse primary neurons, caused abnormal mitochondrial morphology, compromised mitochondrial function, increased markers of oxidative stress, and ultimately decreased cell viability [120,122]. Additionally, those cells were more vulnerable to challenges by rotenone and the active metabolite of MPTP, MPP+ [120,123,124]. Conversely, overexpression of PINK1 in cell models protected against cell death induced by mitochondrial permeability transition pore opening, oxidative stress, and proteasome inhibitors. Protection of cellular viability was related to the ability of PINK1 to prevent loss of mitochondrial membrane potential, to suppress cytochrome c release from mitochondria, and suppress activation of caspase-3 [112,115,125,126]. Expression of PINK1 with pathogenic mutations, expression of a truncated form of PINK1, or expression of PINK1 lacking the kinase domain eliminated this protective effect [112,115,125,126].
Similar to the cell models, mitochondrial abnormalities and increased sensitivity to stressors have also been documented in PINK1 deficient drosophila [127-130]. This phenotype was able to be rescued by expression of wild type but not mutant PINK1 as well as by expression or administration of SOD-1, further supporting the view that the protective role of PINK1 is related to oxidative processes [128,130].
Interestingly, PINK1 knockout mice do not display generalized mitochondrial defects [131]. However, impaired mitochondrial respiration does occur specifically within the nigrostriatal dopaminergic circuit, and mitochondria isolated from the knockout mice display increased sensitivity to hydrogen peroxide [132]. PINK1 knockout mice also have impaired dopamine release and impaired synaptic plasticity, suggesting a specific role in dopaminergic neuron function [131]. This has important implications for the specificity with which dopaminergic neurons are affected in PD.
Parkin
Identification of loss of function mutations in the gene encoding the ubiquitin ligase parkin in autosomal recessive PD indicates that dysfunction of the ubiquitin proteasome system is a contributing factor in the pathogenesis of PD [133-135]. Additionally, recent evidence implicates parkin in mitochondrial function and oxidative processes.
Parkin is localized to the mitochondria of proliferating cells and influences mitochondrial biogenesis [136]. Attempts to examine the effect of parkin modifications on other proteins have included two dimensional gel electrophoresis combined with proteomic analysis in parkin knockout mice, as well a genetic screen for parkin modifiers and the use of cDNA microarrays to characterize transcriptional alterations in parkin deficient drosophila [137-139]. These studies report that parkin modulates expression of proteins involved in the regulation of energy metabolism, such as subunits of pyruvate dehydrogenase, mitochondrial complexes I and IV, and ATP synthase, as well as in proteins involved protection against oxidative stress, such as peroxiredoxin 1, 2, and 6, Hsp70 related proteins, carbonyl reductase, and thioredoxin reductase [137,138]. Drosophila models deficient in parkin or expressing parkin with a pathogenic mutation exhibit mitochondrial dysfunction and alterations in oxidative response components [139,140]. Additionally, parkin deficient drosophila have increased sensitivity to paraquat [141]. In parkin knockout mice, impaired mitochondrial function and decreased antioxidant capacity is accompanied by nigrostriatal defects, synaptic dysfunction, and dopaminergic behavioral deficits [138,142].
Parkin overexpression in cultured cells helped prevent mitochondrial swelling, cytochrome c release, caspase 3 activation, increased reactive species levels, and cell death [143,144]. In a murine model, viral overexpression of parkin was able to inhibit dopaminergic neural loss in mice exposed to MPTP [111]. As an E3 Ubiquitin ligase, parkin levels are upregulated in response to unfolded protein reponse stress induced by the application of the N-glycosylation inhibitor tunicamycin or the reducing agent 2-mercaptoethanol [145]. Parkin overexpression correspondingly is able to rescue cells from the unfolded protein response (UPR) induced by various stressors [145]. Additionally, parkin overexpression has been shown to protect cells against proteasomal dysfunction and death induced by mutant α-synuclein [146]
Oxidative modifications may also impact parkin itself. S-nitrosylation, a nitric oxide-derived post-translational modification, of parkin occurs in vitro, in a mouse model of PD, and in the brains of PD patients [147]. S-nitrosylation decreases parkin's ubiquitin E3 ligase activity and its protective function in cells expressing α-synuclein and synphilin-1 that were exposed to a proteasome inhibitor [147,148]. Such consequences provide a mechanism by which parkin's function may be disrupted and thus contribute to disease progression in sporadic PD. S-nitrosylation has also been shown to affect the activity of other proteins relevant to neurodegeneration, including protein-disulfide isomerase (PDI), an ER chaperone [149]. S-nitrosylation inhibits PDI's enzymatic activity, preventing it from promoting the proper folding of proteins during times of cellular stress and preventing PDI's protective effect [149].
Recent studies have provided further support for the role of parkin in oxidative processes by establishing that parkin functions downstream of PINK1 within the same pathway. Drosophila mutants that are deficient in either parkin or PINK1 exhibit similar phenotypes. Transgenic expression of parkin is able to rescue the phenotype of PINK1 deficient flies, although the reverse is not true [127-129]. This downstream relationship is supported by the fact that in PINK1 deficient flies, the level of parkin protein is significantly reduced [128]. Additionally, it has been shown that DJ-1 with a pathogenic mutation is able to associate with parkin, and this association is promoted by oxidative stress [150].
Leucine-rich repeat kinase 2
Recently, leucine-rich repeat kinase 2 (LRRK2) has been recognized as a cause of an autosomal dominant late-onset form of familial PD. LRRK2 expression in the brain largely correlates to the nigrostriatal dopaminergic system, although diffuse expression throughout the brain has also been noted, including expression in the cerebral cortex, hippocampus, and cerebellum [151-154]. Within the cell, LRRK2 associates largely with membrane bound structures, including the mitochondria, lysosomes, plasma membrane, synaptic vesicles, golgi apparatus, and endoplasmic reticulum and is likely associated with lipid rafts in these membranes [154-156]. LRRK2 contains putative GTPase, protein kinase, WD40 repeat, and leucine-rich repeat (LRR) domains, but the endogenous function of the protein is still being investigated [157].
In support of the role of mutated LRRK2 in neurodegeneration, expression of LRRK2 with pathogenic mutations in SH-SY5Y cells and primary neurons reduced cell viability [155,158-160]. LRRK2 also affects the ability of the cell to handle oxidative stress. Overexpression of mutant LRRK2 failed to rescue cultured cells from hydrogen peroxide exposure, while expression of wild type LRRK2 successfully attenuated this stress [161]. Additionally, drosophila expressing mutant LRRK2 were significantly more sensitive to paraquat and hydrogen peroxide than flies expressing wild type LRRK2 or deficient in LRRK2 [162]. The magnitude of oxidative damage was lowest in drosophila deficient in LRRK2, while flies expressing the mutant LRRK2 had the highest levels [162]. While these observations support the dominant-negative effect of LRRK2 mutations, it is unclear why wild type LRRK2 is more detrimental than a deficiency of LRRK2. Further studies need to be conducted to fully understand both the normal and pathogenic function of this protein.
α-Synuclein
In addition to the discovery that three different autosomal dominant missense mutations in the gene encoding α-synuclein cause early onset, familial PD, wild type α-synuclein has also been identified as one of the primary components of Lewy bodies in sporadic cases [163-167]. α-Synuclein is a soluble, relatively unstructured protein, expressed throughout the central nervous system whose function relates to synaptic vesicular regulation and to chaperone-like activity [168-170]. A hydrophobic region spanning residues 71–82, as well as factors that have not been fully understood, contribute to the orderly assembly of α-synuclein into amyloid fibers that ultimately constitute in part the Lewy bodies and other inclusions [171-173]. α-Synuclein appears to both contribute to mitochondrial dysfunction, oxidative stress, and impaired protein degradation, as well as itself be a target for oxidative modifications that may affect aggregation and neurotoxicity.
In a cell model, overexpression of α-synuclein led to mitochondrial dysfunction and increased levels of reactive species [174]. A similar effect was reported in transgenic mice expressing α-synuclein with the A53T pathogenic mutation. These mice developed mitochondrial degeneration and cell death [175]. α-Synuclein additionally appears to sensitize mice to mitochondrial toxins. Transgenic mice expressing mutant α-synuclein had increased neural degeneration, mitochondrial abnormalities, α-synuclein aggregation, and levels of oxidative and nitrative modifications after exposure to challenges including MPTP, paraquat and maneb [176-179]. Importantly, mice that lack α-synuclein are protected against MPTP toxicity [180-182]. Recent evidence has also shown that α-synuclein accumulates within mitochondria due to an N-terminal targeting sequence, leading to impaired mitochondrial complex I activity and increased production of reactive species [183]. Significantly more α-synuclein was accumulated in mitochondria isolated from the substantia nigra and striatum of patients with sporadic PD than from controls [183].
α-Synuclein may also play a role in disease through its effects on protein degradation. It has been suggested that α-synuclein may initiate UPS inhibition, as it has been shown to disrupt the proteasome in vitro, an effect that is enhanced by the pathogenic α-synuclein mutations [146,184-186]. The mechanisms underlying this inhibition are not fully understood, though possibilities include binding of α-synuclein to a subunit of the proteasome, blockage of the proteasome by aggregated proteins, or potentially an unknown downstream mechanism. Additionally, α-synuclein may play a role in autophagy. In vitro studies have shown α-synuclein is preferentially degraded by CMA [187]. However pathogenic mutations of synuclein or modification by oxidized dopamine cause α-synuclein to bind strongly to the lysosomal CMA receptor. This blocks the uptake and degradation of α-synuclein and other CMA substrates [55,187]. Downstream effects of this disruption may explain how α-synuclein mutations are able to induce cell death – α-synuclein induced impaired CMA degradation of myocyte enhancer factor 2D (MEF2D), a transcription factor required for neuronal survival, resulting in the cytosolic accumulation of MEF2D that bound poorly to DNA, causing an overall decrease in MEF2D function [188].
While α-synuclein can modulate mitochondrial function, oxidative challenges, and protein degradation machinery, oxidation and nitration also appear to modify α-synuclein directly and consequently affect its aggregation. α-Synuclein nitrated on tyrosine residues has been identified in the detergent-insoluble fraction of the brains of PD patients, suggesting that this modification may induce the aggregation of this protein or that aggregated forms of the protein are selectively modified by nitrating oxidants [189]. In cell, mouse, and non-human primate models, treatment with MPTP has been shown to increase oxidative modifications and aggregation of α-synuclein [64,75,190]. Treatment of cells or rats with rotenone and mice with paraquat similarly increased α-synuclein aggregation and inclusion formation and cellular dysfunction [74,85,191].
Collectively, these findings led to biochemical examination of the effect of oxidative or nitrative modification on α-synuclein. Fibrillar α-syncuclein aggregates with a perinuclear localization were formed in cells expressing α-syncuclein upon kinetically controlled exposure to nitric oxide and superoxide [192]. Studies with purified protein revealed that tyrosine nitration effects the ability of α-synuclein to bind to lipid vesicles and slows the rate of degradation by the 20S proteasome and calpain-I [193]. Nitration of α-synuclein monomers and dimers is able to accelerate the rate of fibril formation through the recruitment of non-nitrated α-synuclein, but nitration of oligomers inhibits fibril formation [193-195]. In addition to nitration, exposure of α-synuclein to nitrating oxidants also results in the formation of highly stable o, o'-dityrosine cross linked dimers and oligomers [196]. o, o'-Dityrosine cross linking was found to stabilize pre-formed fibrils, which significantly accelerate the formation of fibrilar aggregates. Site-directed mutation of the four tyrosine residues in α-synuclein discerned that the tyrosine residues are essential for crosslinking and stabilization in response to nitrative insults. [196]. However, oxidative modifications also are able to affect α-synuclein and invoke crosslinking and stable fibril formation independent of tyrosine residues [197]. The C-terminal of α-synuclein has been found to be critical for oligomerization of α-synuclein into detergent insoluble species in response to oxidation by copper and hydrogen peroxide [198].
Due to the regional specificity of pathology in PD patients, the effect of dopamine on α-synuclein has also been investigated. During a chemical compound library screen for molecules that would inhibit formation of α-synuclein fibrils, Lansbury and coworkers discovered that the neurotransmitter dopamine inhibits the formation of α-synuclein fibrils [199]. The interaction of dopamine with α-synuclein appeared to arrest the process of fibril formation at a stage of oligomeric species [199]. We have extended these observations to indicate that dopamine oxidation is essential for this kinetic arrest of α-synuclein oligomers [200]. Since dopamine oxidation generates reactive species and strong electrophiles, mutational analysis of putative amino acid targets in α-synuclein that could be modified by this oxidation was explored [200]. Examination of sites such as the three methionine residues and histidine 50 revealed that covalent modification of these amino acids was not responsible for the effects of oxidized dopamine [200]. The data indicated that the interaction of oxidized dopamine with α-synuclein is directed, not towards a single amino acid, but rather five amino acid residues: tyrosine-glutamate-methionine-proline-serine (YEMPS) in position 125–129 in the C-terminus of the protein [200,201]. Recent studies have confirmed these findings and also indicated that the glutamate 83 residue also participates in stabilizing the interaction of oxidized dopamine with the YEMPS region [202]. The in vitro data has been confirmed in cellular model systems that express A53T α-synuclein or A53T α-synuclein with all 5 amino acids 125–129 mutated, establishing the importance of this C terminal region in the stabilization of α-synuclein oligomers in the presence of oxidized dopamine [201,203]. The decrease in catecholamine levels that has been described as an early even in PD pathogenesis [204] may then allow the formation of insoluble α-synuclein aggregates later in disease [203]. Additionally, α-synuclein modified by oxidized dopamine may have deleterious effects on cellular function, indicating that aggregation may not be a necessary prerequisite for cell death. α-Synuclein modified by oxidized dopamine has been shown to block CMA by binding strongly to the L2A receptor and blocking the uptake of itself and other substrates [55]. Oligomeric α-synuclein was shown to bind to the lysosomal membrane but was unable to be unfolded or taken up into the lysosomes [55]. Furthermore, α-synuclein modified by oxidized dopamine was able to decrease neuronal viability to a degree similar to the effect of L2A RNAi [55]. Therefore α-synuclein may serve as both a modulator and a target of oxidative and nitrative modifications.
Endogenous Factors
In addition to evidence from genetic and environmental risks, the two endogenous factors of aging and dopamine oxidation have implicated oxidative modifications, mitochondrial dysfunction, and impaired protein degradation in PD.
Aging
In PD, the most significant risk factor for developing disease is age. The accumulation of proteins altered by oxidative modifications has been shown to increase with age, which correlates with the late-onset of neurodegenerative pathology [205,206]. Examination of cultured human fibroblasts, human brain tissue, as well as tissues from other organisms have shown that in elderly individuals, approximately one third of proteins have been oxidatively modified [206-208]. This increase is not linear but instead occurs as an initial gradual rise that magnifies several fold in late age [6,206-208]. Oxidative modifications most likely accumulate with age due to a combination of increased production of reactive species, decreased antioxidant function, and impaired ability to repair or remove the modified proteins.
Dysfunctional clearance has been largely supported by findings that the activities of the UPS, macroautophagy and CMA decline with age, consequently diminishing the ability of the cell to clear modified proteins or protect itself from damaging free radicals [47,209-216]. Due to impaired degradation, proteins with oxidative modifications accumulate in the cell, increasing their propensity for aggregation [47,216]. Additionally, once the activity of these degradation pathways is diminished, a feed-forward effect on oxidative damage may result. Sullivan et al. found that proteasomal inhibition increased mitochondrial reactive species generation and decreased mitochondrial complex I and II activity [217]. Therefore, inhibition of the proteasome and autophagy pathways may be further contributing to oxidative damage.
Dopamine Oxidation
The characteristic topology of cell loss that is revealed from neuropathological studies of PD brains, with the relatively selective vulnerability of the ventrolateral and caudal regions of the substantia nigra pars compacta, can provide useful clues on the etiology of the disease. In particular, it has been postulated that the oxidative environment of dopaminergic neurons might be a key component in the pathogenesis of PD. Typically, dopamine is rapidly sequestered within vesicles by the vesicular monoamine transporter, where the acidic pH significantly delays the oxidation of dopamine. However, an oxidative environment can be created if dopamine remains in the cytosol, where it can oxidize at physiological pH to generate reactive ortho-quinones, aminochromes, as well as superoxide and hydrogen peroxide [218,219]. Excessive cytosolic oxidation of catechols has been shown to be neurotoxic in cell culture and rodent models [220-222]. However, it is unclear whether intracellular oxidation of dopamine is able to significantly contribute to the neuron injury.
The gradual accumulation of oxidized dopamine that occurs in normal aging does not appear sufficient to induce neuronal death. However, a consequence of the accrual of oxidized dopamine is the formation of neuromelanin. Neuromelanin, the substance that gives the dopaminergic neurons of the substantia nigra their characteristic dark appearance, is a polymer of oxidized and subsequently heterocyclized dopamine. It has been proposed that the polymer is sequestered within the neurons to form a new cellular organelle of unknown function [223]. In that capacity it has been hypothesized that the neuromelanin polymer might be neuroprotective by further chelating toxins and transition metals such as iron and manganese [223-226]. Since divalent redox capable metals such as iron participate in catalytic reactions with hydrogen peroxide to generate potent oxidizing species, such a role would be crucial to protecting neurons. Efforts to limit the availability of iron have been found to protect neurons from injury and death [227-230].
Alternatively, other studies have revealed a correlation in PD brains between cell loss and the presence of neuromelanin, which suggests that the neuromelanin-pigmented subpopulation of dopaminergic neurons are more vulnerable in disease [231]. Another interesting but unexplored observation is the co-localization of the characteristic protein inclusions (Lewy bodies) in close proximity to neuromelanin in human post mortem PD brains [232,233]. It is possible that the synthesis of neuromelanin, which requires the oxidation of dopamine and the formation of oxidants and electrophiles, promotes the formation of protein aggregates by oxidizing proteins, providing a scaffold for protein filament assembly, or both. In support of its role as a scaffold for aggregation, the melanosome has been shown to be crucial for the assembly of the non-pathogenic natively amyloidogenic protein Pmel17 [234]. Additionally, the melanosome precursor itself assembles into amyloid-like fibrils that may promote the association and assembly of other amyloidogenic proteins [235]. Aggregation may also been promoted by the raft like lipid component of neuromelanin, as hydrophobic interactions would bring macromolecules in close proximity [235,236]. Another interesting observation is that the presence of neuromelanin in dopaminergic neurons is unique to primates, which may explain inconsistencies in the attempts to recapitulate disease in rodent models [237-240].
Conclusion
Examining the "Bermuda triangle" in which dopamine neurons are lost, oxidative modifications, mitochondrial dysfunction, and impaired protein degradation appear to be three interrelated molecular pathways responsible for the pathogenesis of both sporadic and familial PD (Figure 1). Evidence from environmental, genetic, and endogenous factors highlights the interplay of these three mechanisms as the common detrimental denominators inducing neuronal death. Not only do these three processes have clear impacts on cellular viability, but their participation explains other characteristic features of disease, such as the presence of oxidized proteins, inclusions, increased prevalence with late age, and dopaminergic regional selectivity. Together, through their effects on cellular homeostasis and their interactions with one another, oxidative stress, mitochondrial dysfunction, and impaired protein degradation provide the final impetus with which insult to neurons is transformed into neurodegenerative disease.
Currently, treatment for PD is focused merely on alleviating symptoms. As research progresses towards a better understanding of the molecular mechanisms underlying disease, hopefully a more effective therapy can ultimately be designed. Current trials to deliver compounds that can restore mitochondrial function and reduce oxidative burden will be informative and not only improve therapeutic treatment of PD but also provide vital results to guide future studies investigating the molecular mechanisms of neurodegeneration.
Abbreviations
PD: Parkinson's Disease; UPS: Ubiquitin Proteasome System; CMA: Chaperone Mediated Autophagy; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; 6-OHDA: 6-Hydroxy Dopamine; PINK1: PTEN-Induced Kinase 1; LRRK2: Leucine-Rich Repeat Kinase 2; SOD: Superoxide dismutase; NOS: Nitric oxide synthase.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
KAM, ET, and HI wrote the manuscript.
Acknowledgments
Acknowledgements
This work is supported by NIH grants AG13966 and ES013508 NIEHS Center of Excellence in Environmental Toxicology. HI is the Gisela and Dennis Alter Chair in Pediatric Neonatology at the Children's Hospital of Philadelphia.
Contributor Information
Kristen A Malkus, Email: malkus@mail.med.upenn.edu.
Elpida Tsika, Email: tsika@email.chop.edu.
Harry Ischiropoulos, Email: ischirop@mail.med.upenn.edu.
References
- Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003;349:583–596. doi: 10.1056/NEJMra023144. [DOI] [PubMed] [Google Scholar]
- Jenner P, Olanow CW. Oxidative stress and the pathogenesis of Parkinson's disease. Neurology. 1996;47:S161–S170. doi: 10.1212/wnl.47.6_suppl_3.161s. [DOI] [PubMed] [Google Scholar]
- Storch A, Jost WH, Vieregge P, Spiegel J, Greulich W, Durner J, Muller T, Kupsch A, Henningsen H, Oertel WH, Fuchs G, Kuhn W, Niklowitz P, Koch R, Herting B, Reichmann H, German Coenzyme, QSG Randomized, double-blind, placebo-controlled trial on symptomatic effects of coenzyme Q(10) in Parkinson disease. Arch Neurol. 2007;64:938–944. doi: 10.1001/archneur.64.7.nct60005. [DOI] [PubMed] [Google Scholar]
- Bandhyopadhyay U, Cuervo AM. Chaperone-mediated autophagy in aging and neurodegeneration: lessons from alpha-synuclein. Exp Geront. 2007;42:120–128. doi: 10.1016/j.exger.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Rubinsztein DC. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 2006;443:780–786. doi: 10.1038/nature05291. [DOI] [PubMed] [Google Scholar]
- Keller JN, Dimayuga E, Chen Q, Thorpe J, Gee J, Ding Q. Autophagy, proteasomes, lipofuscin, and oxidative stress in the aging brain. Int J Biochem Cell Biol. 2004;36:2376–2391. doi: 10.1016/j.biocel.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Ross CA, Pickart CM. The ubiquitin-proteasome pathway in Parkinson's disease and other neurodegenerative diseases. Trends Cell Biol. 2004;14:703–711. doi: 10.1016/j.tcb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- McNaught KSP, Perl DP, Brownell A-L, Olanow CW. Systemic exposure to proteasome inhibitors causes a progressive model of Parkinson's disease. Ann Neurol. 2004;56:149–162. doi: 10.1002/ana.20186. [DOI] [PubMed] [Google Scholar]
- Zeng B-Y, Bukhatwa S, Hikima A, Rose S, Jenner P. Reproducible nigral cell loss after systemic proteasomal inhibitor administration to rats. Ann Neurol. 2006;60:248–252. doi: 10.1002/ana.20932. [DOI] [PubMed] [Google Scholar]
- Schapira AHV, Cleeter MWJ, Muddle JR, Workman JM, Cooper JM, King RHM. Proteasomal inhibition causes loss of nigral tyrosine hydroxylase neurons. Ann Neurol. 2006;60:253–255. doi: 10.1002/ana.20934. [DOI] [PubMed] [Google Scholar]
- Manning-Bog A, Reaney SH, Chou VP, Johnston LC, McCormack AL, Johnston J, Langston JW, Monte DAD. Lack of nigrostriatal pathology in a rat model of proteasome inhibition. Ann Neurol. 2006;60:256–260. doi: 10.1002/ana.20938. [DOI] [PubMed] [Google Scholar]
- Bove J, Zhou C, Jackson-Lewis V, Taylor J, Chu Y, Rideout HJ, Wu D-C, Kordower JH, Petrucelli L, Przedborski S. Proteasome inhibition and Parkinson's disease modeling. Ann Neurol. 2006;60:260–264. doi: 10.1002/ana.20937. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Kanaan NM, Chu Y, Babu RS, III JS, Terpstra BT, Sortwell CE, Steece-Collier K, Collier TJ. Failure of proteasome inhibitor administration to provide a model of Parkinson's disease in rats and monkeys. Ann Neurol. 2006;60:264–268. doi: 10.1002/ana.20935. [DOI] [PubMed] [Google Scholar]
- Massey A, Kiffin R, Cuervo AM. Pathophysiology of chaperone-mediated autophagy. Int J Biochem Cell Biol. 2004;36:2420–2434. doi: 10.1016/j.biocel.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Anglade P, Vyas S, Javoy-Agid F, Herrero MT, Michel PP, Marquez J, Mouatt-Prigent A, Ruberg M, Hirsch EC, Agid Y. Apoptosis and autophagy in nigral neurons of patients with Parkinson's disease. Histol Histopathol . 1997;12:25–31. [PubMed] [Google Scholar]
- Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, Cuervo AM. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol . 2005;64:113–122. doi: 10.1093/jnen/64.2.113. [DOI] [PubMed] [Google Scholar]
- Sapp E, Schwarz C, Chase K, Bhide PG, Young AB, Penney J, Vonsattel JP, Aronin N, DiFiglia M. Huntingtin localization in brains of normal and Huntington's disease patients. Ann Neurol. 1997;42:604–612. doi: 10.1002/ana.410420411. [DOI] [PubMed] [Google Scholar]
- Cataldo AM, Hamilton DJ, Barnett JL, Paskevich PA, Nixon RA. Properties of the endosomal-lysosomal system in the human central nervous system: disturbances mark most neurons in populations at risk to degenerate in Alzheimer's disease. J Neurosci . 1996;16:186–199. doi: 10.1523/JNEUROSCI.16-01-00186.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung J, Koppel H, Clarke P. Endocytosis and autophagy in dying neurons: an ultrastructural study in chick embryos. J Comp Neurol. 1989;283:425–437. doi: 10.1002/cne.902830310. [DOI] [PubMed] [Google Scholar]
- Xue L, Fletcher GC, Tolkovsky AM. Autophagy Is Activated by Apoptotic Signalling in Sympathetic Neurons: An Alternative Mechanism of Death Execution. Molecular and Cellular Neuroscience. 1999;14:180–198. doi: 10.1006/mcne.1999.0780. [DOI] [PubMed] [Google Scholar]
- Yue Z, Horton A, Bravin M, DeJager PL, Selimi F, Heintz N. A Novel Protein Complex Linking the [delta]2 Glutamate Receptor and Autophagy: Implications for Neurodegeneration in Lurcher Mice. Neuron. 2002;35:921–933. doi: 10.1016/S0896-6273(02)00861-9. [DOI] [PubMed] [Google Scholar]
- Borsello T, Croquelois K, Hornung J-P, Clarke PGH. N-methyl-d-aspartate-triggered neuronal death in organotypic hippocampal cultures is endocytic, autophagic and mediated by the c-Jun N-terminal kinase pathway. Eur J Neurosci. 2003;18:473–485. doi: 10.1046/j.1460-9568.2003.02757.x. [DOI] [PubMed] [Google Scholar]
- Yu L-Y, Jokitalo E, Sun Y-F, Mehlen P, Lindholm D, Saarma M, Arumae U. GDNF-deprived sympathetic neurons die via a novel nonmitochondrial pathway. J Cell Biol. 2003;163:987–997. doi: 10.1083/jcb.200305083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, Baehrecke EH, Lenardo MJ. Regulation of an ATG7-beclin 1 Program of Autophagic Cell Death by Caspase-8. Science. 2004;304:1500–1502. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB, Tsujimoto Y. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol. 2004;6:1221–1228. doi: 10.1038/ncb1192. [DOI] [PubMed] [Google Scholar]
- Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Chiba T, Murata S, Iwata J-i, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- Qin Z-H, Wang Y, Kegel KB, Kazantsev A, Apostol BL, Thompson LM, Yoder J, Aronin N, DiFiglia M. Autophagy regulates the processing of amino terminal huntingtin fragments. Hum Mol Genet. 2003;12:3231–3244. doi: 10.1093/hmg/ddg346. [DOI] [PubMed] [Google Scholar]
- Ravikumar B, Duden R, Rubinsztein DC. Aggregate-prone proteins with polyglutamine and polyalanine expansions are degraded by autophagy. Hum Mol Genet. 2002;11:1107–1117. doi: 10.1093/hmg/11.9.1107. [DOI] [PubMed] [Google Scholar]
- Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC. {alpha}-Synuclein Is Degraded by Both Autophagy and the Proteasome. J Biol Chem. 2003;278:25009–25013. doi: 10.1074/jbc.M300227200. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Floto RA, Berger Z, Imarisio S, Cordenier A, Pasco M, Cook LJ, Rubinsztein DC. Lithium induces autophagy by inhibiting inositol monophosphatase. J Cell Biol. 2005;170:1101–1111. doi: 10.1083/jcb.200504035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Davies JE, Huang Z, Tunnacliffe A, Rubinsztein DC. Trehalose, a Novel mTOR-independent Autophagy Enhancer, Accelerates the Clearance of Mutant Huntingtin and {alpha}-Synuclein. J Biol Chem. 2007;282:5641–5652. doi: 10.1074/jbc.M609532200. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Perlstein EO, Imarisio S, Pineau S, Cordenier A, Maglathlin RL, Webster JA, Lewis TA, O'Kane CJ, Schreiber SL, Rubinsztein DC. Small molecules enhance autophagy and reduce toxicity in Huntington's disease models. Nat Chem Biol. 2007;3:331–338. doi: 10.1038/nchembio883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger Z, Ravikumar B, Menzies FM, Oroz LG, Underwood BR, Pangalos MN, Schmitt I, Wullner U, Evert BO, O'Kane CJ, Rubinsztein DC. Rapamycin alleviates toxicity of different aggregate-prone proteins. Hum Mol Genet. 2006;15:433–442. doi: 10.1093/hmg/ddi458. [DOI] [PubMed] [Google Scholar]
- Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scaravilli F, Easton DF, Duden R, O'Kane CJ, Rubinsztein DC. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet. 2004;36:585–595. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]
- Massey AC, Kaushik S, Sovak G, Kiffin R, Cuervo AM. Consequences of the selective blockage of chaperone-mediated autophagy. Proc Natl Acad Sci USA. 2006;103:5805–5810. doi: 10.1073/pnas.0507436103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCray BA, Taylor JP. The role of autophagy in age-related neurodegeneration. Neurosignals. 2008;16:75–84. doi: 10.1159/000109761. [DOI] [PubMed] [Google Scholar]
- Kiffin R, Christian C, Knecht E, Cuervo AM. Activation of chaperone-mediated autophagy during oxidative stress. Mol Biol Cell. 2004;15:4829–4840. doi: 10.1091/mbc.E04-06-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grune T, Reinheckel T, Joshi M, Davies KJ. Proteolysis in cultured liver epithelial cells during oxidative stress. Role of the multicatalytic proteinase complex, proteasome. J Biol Chem. 1995;270:2344–2351. doi: 10.1074/jbc.270.5.2344. [DOI] [PubMed] [Google Scholar]
- Grune T, Blasig IE, Sitte N, Roloff B, Haseloff R, Davies KJ. Peroxynitrite increases the degradation of aconitase and other cellular proteins by proteasome. J Biol Chem. 1998;273:10857–10862. doi: 10.1074/jbc.273.18.10857. [DOI] [PubMed] [Google Scholar]
- Davies KJ. Degradation of oxidized proteins by the 20S proteasome. Biochimie. 2001;83:301–310. doi: 10.1016/S0300-9084(01)01250-0. [DOI] [PubMed] [Google Scholar]
- Grune T, Reinheckel T, Davies KJ. Degradation of oxidized proteins in K562 human hematopoietic cells by proteasome. J Biol Chem. 1996;271:15504–15509. doi: 10.1074/jbc.271.26.15504. [DOI] [PubMed] [Google Scholar]
- Sitte N, Merker K, Grune T. Proteasome-dependent degradation of oxidized proteins in MRC-5 fibroblasts. FEBS lett. 1998;440:399–402. doi: 10.1016/S0014-5793(98)01495-1. [DOI] [PubMed] [Google Scholar]
- Ullrich O, Reinheckel T, Sitte N, Grune T. Degradation of hypochlorite-damaged glucose-6-phosphate dehydrogenase by the 20S proteasome. Free Radic Biol Med. 1999;27:487–492. doi: 10.1016/S0891-5849(99)00060-X. [DOI] [PubMed] [Google Scholar]
- Friguet B, Szweda LI. Inhibition of the multicatalytic proteinase (proteasome) by 4-hydroxy-2-nonenal cross-linked protein. FEBS lett. 1997;405:21–25. doi: 10.1016/S0014-5793(97)00148-8. [DOI] [PubMed] [Google Scholar]
- Friguet B, Stadtman ER, Szweda LI. Modification of glucose-6-phosphate dehydrogenase by 4-hydroxy-2-nonenal. Formation of cross-linked protein that inhibits the multicatalytic protease. J Biol Chem . 1994;269:21639–21643. [PubMed] [Google Scholar]
- Sitte N, Merker K, Von Zglinicki T, Grune T, Davies KJ. Protein oxidation and degradation during cellular senescence of human BJ fibroblasts: part I–effects of proliferative senescence. Faseb J. 2000;14:2495–2502. doi: 10.1096/fj.00-0209com. [DOI] [PubMed] [Google Scholar]
- Sitte N, Huber M, Grune T, Ladhoff A, Doecke W-D, Von Zglinicki T, Davies KJA. Proteasome inhibition by lipofuscin/ceroid during postmitotic aging of fibroblasts. FASEB J. 2000;14:1490–1498. doi: 10.1096/fj.14.11.1490. [DOI] [PubMed] [Google Scholar]
- Keller JN, Huang FF, Dimayuga ER, Maragos WF. Dopamine induces proteasome inhibition in neural PC12 cell line. Free Radic Biol Med. 2000;29:1037–1042. doi: 10.1016/S0891-5849(00)00412-3. [DOI] [PubMed] [Google Scholar]
- Keller JN, Huang FF, Markesbery WR. Decreased levels of proteasome activity and proteasome expression in aging spinal cord. Neuroscience. 2000;98:149–156. doi: 10.1016/S0306-4522(00)00067-1. [DOI] [PubMed] [Google Scholar]
- Keller JN, Huang FF, Zhu H, Yu J, Ho YS, Kindy TS. Oxidative stress-associated impairment of proteasome activity during ischemia-reperfusion injury. J Cereb Blood Flow Metab. 2000;20:1467–1473. doi: 10.1097/00004647-200010000-00008. [DOI] [PubMed] [Google Scholar]
- Shamoto-Nagai M, Maruyama W, Kato Y, Isobe K, Tanaka M, Naoi M, Osawa T. An inhibitor of mitochondrial complex I, rotenone inactivates proteasome by oxidative modification and induces aggregation of oxidized proteins in SH-SY5Y cells. J Neurosci Res. 2003;74:589–597. doi: 10.1002/jnr.10777. [DOI] [PubMed] [Google Scholar]
- Okada K, Wangpoengtrakul C, Osawa T, Toyokuni S, Tanaka K, Uchida K. 4-Hydroxy-2-nonenal-mediated impairment of intracellular proteolysis during oxidative stress. Identification of proteasomes as target molecules. J Biol Chem. 1999;274:23787–23793. doi: 10.1074/jbc.274.34.23787. [DOI] [PubMed] [Google Scholar]
- Bulteau AL, Lundberg KC, Humphries KM, Sadek HA, Szweda PA, Friguet B, Szweda LI. Oxidative modification and inactivation of the proteasome during coronary occlusion/reperfusion. J Biol Chem. 2001;276:30057–30063. doi: 10.1074/jbc.M100142200. [DOI] [PubMed] [Google Scholar]
- Martinez-Vicente M, Talloczy Z, Kaushik S, Massey AC, Mazzulli J, Mosharov EV, Hodara R, Fredenburg R, Wu DC, Follenzi A, Dauer W, Przedborski S, Ischiropoulos H, Lansbury PT, Sulzer D, Cuervo AM. Dopamine-modified alpha-synuclein blocks chaperone-mediated autophagy. J Clin Invest . 2008;118:777–788. doi: 10.1172/JCI32806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan FY, Wang YN, Zhang GJ. The influence of oxidation of membrane thiol groups on lysosomal proton permeability. Biochem J. 2001;360:355–362. doi: 10.1042/0264-6021:3600355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunk UT, Dalen H, Roberg K, Hellquist HB. Photo-oxidative disruption of lysosomal membranes causes apoptosis of cultured human fibroblasts. Free Radic Biol Med. 1997;23:616–626. doi: 10.1016/S0891-5849(97)00007-5. [DOI] [PubMed] [Google Scholar]
- Brunk UT, Terman A. Lipofuscin: mechanisms of age-related accumulation and influence on cell function. Free Radic Biol Med. 2002;33:611–619. doi: 10.1016/S0891-5849(02)00959-0. [DOI] [PubMed] [Google Scholar]
- Ditaranto K, Tekirian TL, Yang AJ. Lysosomal membrane damage in soluble Abeta-mediated cell death in Alzheimer's disease. Neurobiol Dis. 2001;8:19–31. doi: 10.1006/nbdi.2000.0364. [DOI] [PubMed] [Google Scholar]
- Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in Humans due to a Product of Meperidine-Analog Synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson's disease. Annu Rev Neurosci. 1999;22:123–144. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- Choi WS, Kruse SE, Palmiter RD, Xia Z. Mitochondrial complex I inhibition is not required for dopaminergic neuron death induced by rotenone, MPP+, or paraquat. Proc Natl Acad Sci USA. 2008;105:15136–15141. doi: 10.1073/pnas.0807581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forno LS, Langston JW, DeLanney LE, Irwin I. An electron microscopic study of MPTP-induced inclusion bodies in an old monkey. Brain Res. 1988;448:150–157. doi: 10.1016/0006-8993(88)91111-0. [DOI] [PubMed] [Google Scholar]
- Kowall NW, Hantraye P, Brouillet E, Beal MF, McKee AC, Ferrante RJ. MPTP induces alpha-synuclein aggregation in the substantia nigra of baboons. Neuroreport. 2000;11:211–213. doi: 10.1097/00001756-200001170-00041. [DOI] [PubMed] [Google Scholar]
- Forno L, Langston J, DeLanney L, Irwin I, Ricaurte G. Locus ceruleus lesions and eosinophilic inclusions in MPTP-treated monkeys. Ann Neurol. 1986;20:449–455. doi: 10.1002/ana.410200403. [DOI] [PubMed] [Google Scholar]
- Moratalla R, Quinn B, DeLanney LE, Irwin I, Langston JW, Graybiel AM. Differential vulnerability of primate caudate-putamen and striosome-matrix dopamine systems to the neurotoxic effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc Natl Acad Sci USA. 1992;89:3859–3863. doi: 10.1073/pnas.89.9.3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przedborski S, Chen Q, Vila M, Giasson BI, Djaldatti R, Vukosavic S, Souza JM, Jackson-Lewis V, Lee VM, Ischiropoulos H. Oxidative post-translational modifications of alpha-synuclein in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson's disease. J Neurochem. 2001;76:637–640. doi: 10.1046/j.1471-4159.2001.00174.x. [DOI] [PubMed] [Google Scholar]
- Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WG, Dawson VL, Dawson TM, Przedborski S. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med. 1999;5:1403. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- Matthews RT, Beal MF, Fallon J, Fedorchak K, Huang PL, Fishman MC, Hyman BT. MPP+Induced Substantia Nigra Degeneration Is Attenuated in nNOS Knockout Mice. Neurobiol Dis. 1997;4:114–121. doi: 10.1006/nbdi.1997.0141. [DOI] [PubMed] [Google Scholar]
- Schulz BJ, Henshaw DR, Matthews RT, Beal MF. Coenzyme Q10 and nicotinamide and a free radical spin trap protect against MPTP neurotoxicity. Exp Neurol. 1995;132:279–283. doi: 10.1016/0014-4886(95)90033-0. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Jackson-Lewis V, Yokoyama R, Shibata T, Dawson VL, Dawson TM. Role of neuronal nitric oxide in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic neurotoxicity. Proc Natl Acad Sci USA. 1996;93:4565–4571. doi: 10.1073/pnas.93.10.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks AI, Chadwick CA, Gelbard HA, Cory-Slechta DA, Federoff HJ. Paraquat elicited neurobehavioral syndrome caused by dopaminergic neuron loss. Brain Res. 1999;823:1–10. doi: 10.1016/S0006-8993(98)01192-5. [DOI] [PubMed] [Google Scholar]
- McCormack AL, Thiruchelvam M, Manning-Bog AB, Thiffault C, Langston JW, Cory-Slechta DA, Di Monte DA. Environmental Risk Factors and Parkinson's Disease: Selective Degeneration of Nigral Dopaminergic Neurons Caused by the Herbicide Paraquat. Neurobiol Dis. 2002;10:119–127. doi: 10.1006/nbdi.2002.0507. [DOI] [PubMed] [Google Scholar]
- Manning-Bog AB, McCormack AL, Li J, Uversky VN, Fink AL, Di Monte DA. The herbicide paraquat causes up-regulation and aggregation of alpha-synuclein in mice: paraquat and alpha-synuclein. J Biol Chem. 2002;277:1641–1644. doi: 10.1074/jbc.C100560200. [DOI] [PubMed] [Google Scholar]
- Vila M, Vukosavic S, Jackson-Lewis V, Neystat M, Jakowec M, Przedborski S. Alpha-Synuclein Up-Regulation in Substantia Nigra Dopaminergic Neurons Following Administration of the Parkinsonian Toxin MPTP. J Neurochem. 2000;74:721–729. doi: 10.1046/j.1471-4159.2000.740721.x. [DOI] [PubMed] [Google Scholar]
- Elroy-Stein O, Bernstein Y, Groner Y. Overproduction of human Cu/Zn-superoxide dismutase in transfected cells: extenuation of paraquat-mediated cytotoxicity and enhancement of lipid peroxidation. EMBO J . 1986;5:615–622. doi: 10.1002/j.1460-2075.1986.tb04255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Clair DK, Oberley TD, Ho Y-S. Overproduction of human Mn-superoxide dismutase modulates paraquat-mediated toxicity in mammalian cells. FEBS lett. 1991;293:199–203. doi: 10.1016/0014-5793(91)81186-C. [DOI] [PubMed] [Google Scholar]
- Thiruchelvam M, Prokopenko O, Cory-Slechta DA, Richfield EK, Buckley B, Mirochnitchenko O. Overexpression of Superoxide Dismutase or Glutathione Peroxidase Protects against the Paraquat + Maneb-induced Parkinson Disease Phenotype. J Biol Chem. 2005;280:22530–22539. doi: 10.1074/jbc.M500417200. [DOI] [PubMed] [Google Scholar]
- Dong A, Shen J, Krause M, Akiyama H, Hackett SF, Lai H, Campochiaro PA. Superoxide dismutase 1 protects retinal cells from oxidative damage. J Cell Physiol. 2006;208:516–526. doi: 10.1002/jcp.20683. [DOI] [PubMed] [Google Scholar]
- Patel M, Day BJ, Crapo JD, Fridovich I, McNamara JO. Requirement for Superoxide in Excitotoxic Cell Death. Neuron. 1996;16:345–355. doi: 10.1016/S0896-6273(00)80052-5. [DOI] [PubMed] [Google Scholar]
- Day BJ, Shawen S, Liochev SI, Crapo JD. A metalloporphyrin superoxide dismutase mimetic protects against paraquat-induced endothelial cell injury in vitro. J Pharmacol Exp Ther . 1995;275:1227–1232. [PubMed] [Google Scholar]
- Peng J, Stevenson FF, Doctrow SR, Andersen JK. Superoxide Dismutase/Catalase Mimetics Are Neuroprotective against Selective Paraquat-mediated Dopaminergic Neuron Death in the Substantial Nigra: Implications For Parkinson Disease. J Biol Chem. 2005;280:29194–29198. doi: 10.1074/jbc.M500984200. [DOI] [PubMed] [Google Scholar]
- Tauskela JS, Brunette E, O'Reilly N, Mealing G, Comas T, Gendron TF, Monette R, Morley P. An alternative Ca2+-dependent mechanism of neuroprotection by the metalloporphyrin class of superoxide dismutase mimetics. FASEB J. 2005;19:1734–1736. doi: 10.1096/fj.05-3795fje. [DOI] [PubMed] [Google Scholar]
- Rebouças J, Spasojević I, Batinić-Haberle I. Pure manganese(III) 5,10,15,20-tetrakis(4-benzoic acid)porphyrin (MnTBAP) is not a superoxide dismutase mimic in aqueous systems: a case of structure-activity relationship as a watchdog mechanism in experimental therapeutics and biology. J Biol Inorg Chem. 2008;13:289–302. doi: 10.1007/s00775-007-0324-9. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/S0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Dunnett SB, Bjorklund A. Prospects for new restorative and neuroprotective treatments in Parkinson's disease. (cover story) Nature. 1999;399:A32. doi: 10.1038/19899. [DOI] [PubMed] [Google Scholar]
- Jenner P, Olanow CW. Understanding cell death in Parkinson's disease. Ann Neurol . 1998;44:S72–S84. doi: 10.1002/ana.410440712. [DOI] [PubMed] [Google Scholar]
- Cohen G, Heikkila RE, MacNamee D. The Generation of Hydrogen Peroxide Superoxide Radical and Hydroxyl Radical by 6-Hydroxydopamine Dialuric Acid and Related Cytotoxic Agents. J Biol Chem. 1974;249:2447–2452. [PubMed] [Google Scholar]
- Jeon BS, Jackson-Lewis V, Burke RE. 6-Hydroxydopamine lesion of the rat substantia nigra: time course and morphology of cell death. Neurodegeneration. 1995;4:131–137. doi: 10.1006/neur.1995.0016. [DOI] [PubMed] [Google Scholar]
- Sauer H, Oertel WH. Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: A combined retrograde tracing and immunocytochemical study in the rat. Neuroscience. 1994;59:401–415. doi: 10.1016/0306-4522(94)90605-X. [DOI] [PubMed] [Google Scholar]
- Przedbroski S, Leviver M, Jiang H, Ferreira M, Jackson-Lewis V, Donaldson D, Togasaki DM. Dose-dependent lesions of the dopaminergic nigrostriatal pathway induced by instrastriatal injection of 6-hydroxydopamine. Neuroscience. 1995;67:631–647. doi: 10.1016/0306-4522(95)00066-R. [DOI] [PubMed] [Google Scholar]
- Thomas B, Beal MF. Parkinson's disease. Hum Mol Genet. 2007;16:R183–194. doi: 10.1093/hmg/ddm159. [DOI] [PubMed] [Google Scholar]
- Lesage S, Brice A. Parkinson's disease: from monogenic forms to genetic susceptibility factors. Hum Mol Genet. 2009;18:R48–59. doi: 10.1093/hmg/ddp012. [DOI] [PubMed] [Google Scholar]
- Gasser T. Mendelian forms of Parkinson's disease. Biochim Biophys Acta. 2009 doi: 10.1016/j.bbadis.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Ramirez A, Heimbach A, Grundemann J, Stiller B, Hampshire D, Cid LP, Goebel I, Mubaidin AF, Wriekat A-L, Roeper J, Al-Din A, Hillmer AM, Karsak M, Liss B, Woods CG, Behrens MI, Kubisch C. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat Genet. 2006;38:1184–1191. doi: 10.1038/ng1884. [DOI] [PubMed] [Google Scholar]
- Gitler A, Chesi A, Geddie M, Strathearn K, Hamamichi S, Hill K, Caldwell K, Caldwell G, Cooper A, Rochet J, Lindquist S. Alpha-synuclein is part of a diverse and highly conserved interaction network that includes PARK9 and manganese toxicity. Nat Genet. 2009;41:308–315. doi: 10.1038/ng.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres-Mateos E, Perier C, Zhang L, Blanchard-Fillion B, Greco TM, Thomas B, Ko HS, Sasaki M, Ischiropoulos H, Przedborski S, Dawson TM, Dawson VL. DJ-1 gene deletion reveals that DJ-1 is an atypical peroxiredoxin-like peroxidase. Proc Natl Acad Sci USA. 2007;104:14807–14812. doi: 10.1073/pnas.0703219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira T, Saito Y, Niki T, Iguchi-Ariga SM, Takahashi K, Ariga H. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 2004;5:213–218. doi: 10.1038/sj.embor.7400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsumoto A, Nakagawa Y, Takeuchi A, Okawa K, Iwamatsu A, Takanezawa Y. Oxidized forms of peroxiredoxins and DJ-1 on two-dimensional gels increased in response to sublethal levels of paraquat. Free Radic Res. 2001;35:301–310. doi: 10.1080/10715760100300831. [DOI] [PubMed] [Google Scholar]
- Canet-Aviles RM, Wilson MA, Miller DW, Ahmad R, McLendon C, Bandyopadhyay S, Baptista MJ, Ringe D, Petsko GA, Cookson MR. The Parkinson's disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc Natl Acad Sci USA. 2004;101:9103–9108. doi: 10.1073/pnas.0402959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulener MC, Xu K, Thomson L, Ischiropoulos H, Bonini NM. Mutational analysis of DJ-1 in Drosophila implicates functional inactivation by oxidative damage and aging. Proc Natl Acad Sci USA. 2006;103:12517–12522. doi: 10.1073/pnas.0601891103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinat C, Shendelman S, Jonason A, Leete T, Beal MF, Yang L, Floss T, Abeliovich A. Sensitivity to oxidative stress in DJ-1-deficient dopamine neurons: an ES- derived cell model of primary Parkinsonism. PLoS biology. 2004;2:e327. doi: 10.1371/journal.pbio.0020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Gehrke S, Haque ME, Imai Y, Kosek J, Yang L, Beal MF, Nishimura I, Wakamatsu K, Ito S, Takahashi R, Lu B. Inactivation of Drosophila DJ-1 leads to impairments of oxidative stress response and phosphatidylinositol 3-kinase/Akt signaling. Proc Natl Acad Sci USA. 2005;102:13670–13675. doi: 10.1073/pnas.0504610102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulener M, Whitworth AJ, Armstrong-Gold CE, Rizzu P, Heutink P, Wes PD, Pallanck LJ, Bonini NM. Drosophila DJ-1 mutants are selectively sensitive to environmental toxins associated with Parkinson's disease. Curr Biol. 2005;15:1572–1577. doi: 10.1016/j.cub.2005.07.064. [DOI] [PubMed] [Google Scholar]
- Park J, Kim SY, Cha GH, Lee SB, Kim S, Chung J. Drosophila DJ-1 mutants show oxidative stress-sensitive locomotive dysfunction. Gene. 2005;361:133–139. doi: 10.1016/j.gene.2005.06.040. [DOI] [PubMed] [Google Scholar]
- Menzies FM, Yenisetti SC, Min KT. Roles of Drosophila DJ-1 in survival of dopaminergic neurons and oxidative stress. Curr Biol. 2005;15:1578–1582. doi: 10.1016/j.cub.2005.07.036. [DOI] [PubMed] [Google Scholar]
- Kim RH, Smith PD, Aleyasin H, Hayley S, Mount MP, Pownall S, Wakeham A, You-Ten AJ, Kalia SK, Horne P, Westaway D, Lozano AM, Anisman H, Park DS, Mak TW. Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc Natl Acad Sci USA. 2005;102:5215–5220. doi: 10.1073/pnas.0501282102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Freed CR. DJ-1 up-regulates glutathione synthesis during oxidative stress and inhibits A53T alpha-synuclein toxicity. J Biol Chem. 2005;280:43150–43158. doi: 10.1074/jbc.M507124200. [DOI] [PubMed] [Google Scholar]
- Inden M, Taira T, Kitamura Y, Yanagida T, Tsuchiya D, Takata K, Yanagisawa D, Nishimura K, Taniguchi T, Kiso Y, Yoshimoto K, Agatsuma T, Koide-Yoshida S, Iguchi-Ariga SM, Shimohama S, Ariga H. PARK7 DJ-1 protects against degeneration of nigral dopaminergic neurons in Parkinson's disease rat model. Neurobiol Dis. 2006;24:144–158. doi: 10.1016/j.nbd.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Paterna JC, Leng A, Weber E, Feldon J, Bueler H. DJ-1 and Parkin modulate dopamine-dependent behavior and inhibit MPTP-induced nigral dopamine neuron loss in mice. Mol Ther. 2007;15:698–704. doi: 10.1038/sj.mt.6300154. [DOI] [PubMed] [Google Scholar]
- Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, Gonzalez-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, Wood NW. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- Sim CH, Lio DS, Mok SS, Masters CL, Hill AF, Culvenor JG, Cheng HC. C-terminal truncation and Parkinson's disease-associated mutations down-regulate the protein serine/threonine kinase activity of PTEN-induced kinase-1. Hum Mol Genet. 2006;15:3251–3262. doi: 10.1093/hmg/ddl398. [DOI] [PubMed] [Google Scholar]
- Silvestri L, Caputo V, Bellacchio E, Atorino L, Dallapiccola B, Valente EM, Casari G. Mitochondrial import and enzymatic activity of PINK1 mutants associated to recessive parkinsonism. Hum Mol Genet. 2005;14:3477–3492. doi: 10.1093/hmg/ddi377. [DOI] [PubMed] [Google Scholar]
- Petit A, Kawarai T, Paitel E, Sanjo N, Maj M, Scheid M, Chen F, Gu Y, Hasegawa H, Salehi-Rad S, Wang L, Rogaeva E, Fraser P, Robinson B, St George-Hyslop P, Tandon A. Wild-type PINK1 prevents basal and induced neuronal apoptosis, a protective effect abrogated by Parkinson disease-related mutations. J Biol Chem. 2005;280:34025–34032. doi: 10.1074/jbc.M505143200. [DOI] [PubMed] [Google Scholar]
- Deng H, Dodson MW, Huang H, Guo M. The Parkinson's disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc Natl Acad Sci USA. 2008;105:14503–14508. doi: 10.1073/pnas.0803998105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole AC, Thomas RE, Andrews LA, McBride HM, Whitworth AJ, Pallanck LJ. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci USA. 2008;105:1638–1643. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Ouyang Y, Yang L, Beal MF, McQuibban A, Vogel H, Lu B. Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc Natl Acad Sci USA. 2008;105:7070–7075. doi: 10.1073/pnas.0711845105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plun-Favreau H, Klupsch K, Moisoi N, Gandhi S, Kjaer S, Frith D, Harvey K, Deas E, Harvey RJ, McDonald N, Wood NW, Martins LM, Downward J. The mitochondrial protease HtrA2 is regulated by Parkinson's disease-associated kinase PINK1. Nat Cell Biol. 2007;9:1243–1252. doi: 10.1038/ncb1644. [DOI] [PubMed] [Google Scholar]
- Exner N, Treske B, Paquet D, Holmstrom K, Schiesling C, Gispert S, Carballo-Carbajal I, Berg D, Hoepken HH, Gasser T, Kruger R, Winklhofer KF, Vogel F, Reichert AS, Auburger G, Kahle PJ, Schmid B, Haass C. Loss-of-function of human PINK1 results in mitochondrial pathology and can be rescued by parkin. J Neurosci. 2007;27:12413–12418. doi: 10.1523/JNEUROSCI.0719-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoepken HH, Gispert S, Morales B, Wingerter O, Del Turco D, Mulsch A, Nussbaum RL, Muller K, Drose S, Brandt U, Deller T, Wirth B, Kudin AP, Kunz WS, Auburger G. Mitochondrial dysfunction, peroxidation damage and changes in glutathione metabolism in PARK6. Neurobiol Dis. 2007;25:401–411. doi: 10.1016/j.nbd.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Wood-Kaczmar A, Gandhi S, Yao Z, Abramov AS, Miljan EA, Keen G, Stanyer L, Hargreaves I, Klupsch K, Deas E, Downward J, Mansfield L, Jat P, Taylor J, Heales S, Duchen MR, Latchman D, Tabrizi SJ, Wood NW. PINK1 is necessary for long term survival and mitochondrial function in human dopaminergic neurons. PLoS ONE. 2008;3:e2455. doi: 10.1371/journal.pone.0002455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H, Jankovic J, Guo Y, Xie W, Le W. Small interfering RNA targeting the PINK1 induces apoptosis in dopaminergic cells SH-SY5Y. Biochem Biophys Res Commun. 2005;337:1133–1138. doi: 10.1016/j.bbrc.2005.09.178. [DOI] [PubMed] [Google Scholar]
- Tang B, Xiong H, Sun P, Zhang Y, Wang D, Hu Z, Zhu Z, Ma H, Pan Q, Xia JH, Xia K, Zhang Z. Association of PINK1 and DJ-1 confers digenic inheritance of early-onset Parkinson's disease. Hum Mol Genet. 2006;15:1816–1825. doi: 10.1093/hmg/ddl104. [DOI] [PubMed] [Google Scholar]
- Pridgeon JW, Olzmann JA, Chin LS, Li L. PINK1 Protects against Oxidative Stress by Phosphorylating Mitochondrial Chaperone TRAP1. PLoS biology. 2007;5:e172. doi: 10.1371/journal.pbio.0050172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Chou AH, Yeh TH, Li AH, Chen YL, Kuo YL, Tsai SR, Yu ST. PINK1 mutants associated with recessive Parkinson's disease are defective in inhibiting mitochondrial release of cytochrome c. Neurobiol Dis. 2007;28:216–226. doi: 10.1016/j.nbd.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Park J, Lee SB, Lee S, Kim Y, Song S, Kim S, Bae E, Kim J, Shong M, Kim JM, Chung J. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441:1157–1161. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- Yang Y, Gehrke S, Imai Y, Huang Z, Ouyang Y, Wang JW, Yang L, Beal MF, Vogel H, Lu B. Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proc Natl Acad Sci USA. 2006;103:10793–10798. doi: 10.1073/pnas.0602493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, Yoo SJ, Hay BA, Guo M. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–1166. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- Wang D, Qian L, Xiong H, Liu J, Neckameyer WS, Oldham S, Xia K, Wang J, Bodmer R, Zhang Z. Antioxidants protect PINK1-dependent dopaminergic neurons in Drosophila. Proc Natl Acad Sci USA. 2006;103:13520–13525. doi: 10.1073/pnas.0604661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada T, Pisani A, Porter DR, Yamaguchi H, Tscherter A, Martella G, Bonsi P, Zhang C, Pothos EN, Shen J. Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc Natl Acad Sci USA. 2007;104:11441–11446. doi: 10.1073/pnas.0702717104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier CA, Kitada T, Shen J. Loss of PINK1 causes mitochondrial functional defects and increased sensitivity to oxidative stress. Proc Natl Acad Sci USA. 2008;105:11364–11369. doi: 10.1073/pnas.0802076105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- Shimura H, Hattori N, Kubo S-i, Mizuno Y, Asakawa S, Minoshima S, Shimizu N, Iwai K, Chiba T, Tanaka K, Suzuki T. Familial Parkinson disease gene product parkin is a ubiquitin-protein ligase. Nat Genet. 2000;25:302–305. doi: 10.1038/77060. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gao J, Chung KKK, Huang H, Dawson VL, Dawson TM. Parkin functions as an E2-dependent ubiquitin- protein ligase and promotes the degradation of the synaptic vesicle-associated protein CDCrel-1. Proc Natl Acad Sci USA. 2000;97:13354–13359. doi: 10.1073/pnas.240347797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda Y, Mitsui T, Kunishige M, Shono M, Akaike M, Azuma H, Matsumoto T. Parkin enhances mitochondrial biogenesis in proliferating cells. Hum Mol Genet. 2006;15:883–895. doi: 10.1093/hmg/ddl006. [DOI] [PubMed] [Google Scholar]
- Periquet M, Corti O, Jacquier S, Brice A. Proteomic analysis of parkin knockout mice: alterations in energy metabolism, protein handling and synaptic function. J Neurochem. 2005;95:1259–1276. doi: 10.1111/j.1471-4159.2005.03442.x. [DOI] [PubMed] [Google Scholar]
- Palacino JJ, Sagi D, Goldberg MS, Krauss S, Motz C, Wacker M, Klose J, Shen J. Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J Biol Chem. 2004;279:18614–18622. doi: 10.1074/jbc.M401135200. [DOI] [PubMed] [Google Scholar]
- Greene JC, Whitworth AJ, Andrews LA, Parker TJ, Pallanck LJ. Genetic and genomic studies of Drosophila parkin mutants implicate oxidative stress and innate immune responses in pathogenesis. Hum Mol Genet. 2005;14:799–811. doi: 10.1093/hmg/ddi074. [DOI] [PubMed] [Google Scholar]
- Greene JC, Whitworth AJ, Kuo I, Andrews LA, Feany MB, Pallanck LJ. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc Natl Acad Sci USA. 2003;100:4078–4083. doi: 10.1073/pnas.0737556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesah Y, Pham T, Burgess H, Middlebrooks B, Verstreken P, Zhou Y, Harding M, Bellen H, Mardon G. Drosophila parkin mutants have decreased mass and cell size and increased sensitivity to oxygen radical stress. Development. 2004;131:2183–2194. doi: 10.1242/dev.01095. [DOI] [PubMed] [Google Scholar]
- Goldberg MS, Fleming SM, Palacino JJ, Cepeda C, Lam HA, Bhatnagar A, Meloni EG, Wu N, Ackerson LC, Klapstein GJ, Gajendiran M, Roth BL, Chesselet MF, Maidment NT, Levine MS, Shen J. Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. J Biol Chem. 2003;278:43628–43635. doi: 10.1074/jbc.M308947200. [DOI] [PubMed] [Google Scholar]
- Darios F, Corti O, Lucking CB, Hampe C, Muriel MP, Abbas N, Gu WJ, Hirsch EC, Rooney T, Ruberg M, Brice A. Parkin prevents mitochondrial swelling and cytochrome c release in mitochondria-dependent cell death. Hum Mol Genet. 2003;12:517–526. doi: 10.1093/hmg/ddg044. [DOI] [PubMed] [Google Scholar]
- Jiang H, Ren Y, Zhao J, Feng J. Parkin protects human dopaminergic neuroblastoma cells against dopamine-induced apoptosis. Hum Mol Genet. 2004;13:1745–1754. doi: 10.1093/hmg/ddh180. [DOI] [PubMed] [Google Scholar]
- Imai Y, Soda M, Takahashi R. Parkin Suppresses Unfolded Protein Stress-induced Cell Death through Its E3 Ubiquitin-protein Ligase Activity. J Biol Chem. 2000;275:35661–35664. doi: 10.1074/jbc.C000447200. [DOI] [PubMed] [Google Scholar]
- Petrucelli L, O'Farrell C, Lockhart PJ, Baptista M, Kehoe K, Vink L, Choi P, Wolozin B, Farrer M, Hardy J, Cookson MR. Parkin Protects against the Toxicity Associated with Mutant [alpha]-Synuclein: Proteasome Dysfunction Selectively Affects Catecholaminergic Neurons. Neuron. 2002;36:1007–1019. doi: 10.1016/S0896-6273(02)01125-X. [DOI] [PubMed] [Google Scholar]
- Chung KK, Thomas B, Li X, Pletnikova O, Troncoso JC, Marsh L, Dawson VL, Dawson TM. S-nitrosylation of parkin regulates ubiquitination and compromises parkin's protective function. Science. 2004;304:1328–1331. doi: 10.1126/science.1093891. [DOI] [PubMed] [Google Scholar]
- Yao D, Gu Z, Nakamura T, Shi Z-Q, Ma Y, Gaston B, Palmer LA, Rockenstein EM, Zhang Z, Masliah E, Uehara T, Lipton SA. Nitrosative stress linked to sporadic Parkinson's disease: S-nitrosylation of parkin regulates its E3 ubiquitin ligase activity. Proc Natl Acad Sci USA. 2004;101:10810–10814. doi: 10.1073/pnas.0404161101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara T, Nakamura T, Yao D, Shi ZQ, Gu Z, Ma Y, Masliah E, Nomura Y, Lipton SA. S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature. 2006;441:513–517. doi: 10.1038/nature04782. [DOI] [PubMed] [Google Scholar]
- Moore DJ, Zhang L, Troncoso J, Lee MK, Hattori N, Mizuno Y, Dawson TM, Dawson VL. Association of DJ-1 and parkin mediated by pathogenic DJ-1 mutations and oxidative stress. Hum Mol Genet. 2005;14:71–84. doi: 10.1093/hmg/ddi007. [DOI] [PubMed] [Google Scholar]
- Galter D, Westerlund M, Carmine A, Lindqvist E, Sydow O, Olson L. LRRK2 expression linked to dopamine-innervated areas. Ann Neurol. 2006;59:714–719. doi: 10.1002/ana.20808. [DOI] [PubMed] [Google Scholar]
- Higashi S, Moore DJ, Colebrooke RE, Biskup S, Dawson VL, Arai H, Dawson TM, Emson PC. Expression and localization of Parkinson's disease-associated leucine-rich repeat kinase 2 in the mouse brain. J Neurochem. 2007;100:368–381. doi: 10.1111/j.1471-4159.2006.04246.x. [DOI] [PubMed] [Google Scholar]
- Higashi S, Biskup S, West AB, Trinkaus D, Dawson VL, Faull RLM, Waldvogel HJ, Arai H, Dawson TM, Moore DJ, Emson PC. Localization of Parkinson's disease-associated LRRK2 in normal and pathological human brain. Brain Res. 2007;1155:208–219. doi: 10.1016/j.brainres.2007.04.034. [DOI] [PubMed] [Google Scholar]
- Biskup S, Moore DJ, Celsi F, Higashi S, West AB, Andrabi SA, Kurkinen K, Yu S-W, Savitt JM, Waldvogel HJ, Faull RLM, Emson PC, Torp R, Ottersen OP, Dawson TM, Dawson VL. Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann Neurol. 2006;60:557–569. doi: 10.1002/ana.21019. [DOI] [PubMed] [Google Scholar]
- West AB, Moore DJ, Biskup S, Bugayenko A, Smith WW, Ross CA, Dawson VL, Dawson TM. Parkinson's disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci USA. 2005;102:16842–16847. doi: 10.1073/pnas.0507360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatano T, Kubo S-i, Imai S, Maeda M, Ishikawa K, Mizuno Y, Hattori N. Leucine-rich repeat kinase 2 associates with lipid rafts. Hum Mol Genet. 2007;16:678–690. doi: 10.1093/hmg/ddm013. [DOI] [PubMed] [Google Scholar]
- MacLeod D, Dowman J, Hammond R, Leete T, Inoue K, Abeliovich A. The familial Parkinsonism gene LRRK2 regulates neurite process morphology. Neuron. 2006;52:587–593. doi: 10.1016/j.neuron.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Greggio E, Jain S, Kingsbury A, Bandopadhyay R, Lewis P, Kaganovich A, Brug MP van der, Beilina A, Blackinton J, Thomas KJ, Ahmad R, Miller DW, Kesavapany S, Singleton A, Lees A, Harvey RJ, Harvey K, Cookson MR. Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol Dis. 2006;23:329–341. doi: 10.1016/j.nbd.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Smith WW, Pei Z, Jiang H, Moore DJ, Liang Y, West AB, Dawson VL, Dawson TM, Ross CA. Leucine-rich repeat kinase 2 (LRRK2) interacts with parkin, and mutant LRRK2 induces neuronal degeneration. Proc Natl Acad Sci USA. 2005;102:18676–18681. doi: 10.1073/pnas.0508052102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan EK, Zhao Y, Skipper L, Tan MG, Di Fonzo A, Sun L, Fook-Chong S, Tang S, Chua E, Yuen Y, Tan L, Pavanni R, Wong MC, Kolatkar P, Lu CS, Bonifati V, Liu JJ. The LRRK2 Gly2385Arg variant is associated with Parkinson's disease: genetic and functional evidence. Hum Genet. 2007;120:857–863. doi: 10.1007/s00439-006-0268-0. [DOI] [PubMed] [Google Scholar]
- Liou AK, Leak RK, Li L, Zigmond MJ. Wild-type LRRK2 but not its mutant attenuates stress-induced cell death via ERK pathway. Neurobiol Dis. 2008;32:116–124. doi: 10.1016/j.nbd.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y, Gehrke S, Wang HQ, Takahashi R, Hasegawa K, Oota E, Lu B. Phosphorylation of 4E-BP by LRRK2 affects the maintenance of dopaminergic neurons in Drosophila. EMBO J. 2008;27:2432–2443. doi: 10.1038/emboj.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez Tortosa E, del Ser T, Munoz DG, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- Chartier-Harlin M-C, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, DestÈe A. [alpha]-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, Rohan de Silva HA, Kittel A, Saitoh T. The precursor protein of non-A[beta] component of Alzheimer's disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14:467–475. doi: 10.1016/0896-6273(95)90302-X. [DOI] [PubMed] [Google Scholar]
- Chandra S, Gallardo G, Fernandez-Chacon R, Schluter OM, Sudhof TC. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123:383–396. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Maroteaux L, Campanelli JT, Scheller RH. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci . 1988;8:2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giasson BI, Murray IV, Trojanowski JQ, Lee VM. A hydrophobic stretch of 12 amino acid residues in the middle of alpha-synuclein is essential for filament assembly. J Biol Chem. 2001;276:2380–2386. doi: 10.1074/jbc.M008919200. [DOI] [PubMed] [Google Scholar]
- Conway KA, Lee S-J, Rochet J-C, Ding TT, Williamson RE, Lansbury PT. Acceleration of oligomerization, not fibrillization, is a shared property of both α-synuclein mutations linked to early-onset Parkinson's disease: Implications for pathogenesis and therapy. Proc Natl Acad Sci USA. 2000;97:571–576. doi: 10.1073/pnas.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg MS, Lansbury PT., Jr Is there a cause-and-effect relationship between [alpha]-synuclein fibrillization and Parkinson/'s disease? Nat Cell Biol. 2000;2:E115–E119. doi: 10.1038/35041081. [DOI] [PubMed] [Google Scholar]
- Hsu LJ, Sagara Y, Arroyo A, Rockenstein E, Sisk A, Mallory M, Wong J, Takenouchi T, Hashimoto M, Masliah E. Alpha-Synuclein Promotes Mitochondrial Deficit and Oxidative Stress. Am J Pathol. 2000;157:401–410. doi: 10.1016/s0002-9440(10)64553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Pan Y, Price AC, Sterling W, Copeland NG, Jenkins NA, Price DL, Lee MK. Parkinson's disease alpha-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J Neurosci. 2006;26:41–50. doi: 10.1523/JNEUROSCI.4308-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song DD, Shults CW, Sisk A, Rockenstein E, Masliah E. Enhanced substantia nigra mitochondrial pathology in human alpha-synuclein transgenic mice after treatment with MPTP. Exp Neurol. 2004;186:158–172. doi: 10.1016/S0014-4886(03)00342-X. [DOI] [PubMed] [Google Scholar]
- Nieto M, Gil-Bea FJ, Dalfo E, Cuadrado M, Cabodevilla F, Sanchez B, Catena S, Sesma T, Ribe E, Ferrer I, RamÌrez MJ, Gomez-Isla T. Increased sensitivity to MPTP in human [alpha]-synuclein A30P transgenic mice. Neurobiol Aging. 2006;27:848–856. doi: 10.1016/j.neurobiolaging.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Yu W, Matsuoka Y, Sziráki I, Hashim A, LaFrancois J, Sershen H, Duff K. Increased Dopaminergic Neuron Sensitivity to 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine (MPTP) in Transgenic Mice Expressing Mutant A53T α-Synuclein. Neurochem Res. 2008;33:902–911. doi: 10.1007/s11064-007-9533-4. [DOI] [PubMed] [Google Scholar]
- Norris EH, Uryu K, Leight S, Giasson BI, Trojanowski JQ, Lee VM. Pesticide exposure exacerbates alpha-synucleinopathy in an A53T transgenic mouse model. Am J Pathol. 2007;170:658–666. doi: 10.2353/ajpath.2007.060359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer W, Kholodilov N, Vila M, Trillat A-C, Goodchild R, Larsen KE, Staal R, Tieu K, Schmitz Y, Yuan CA, Rocha M, Jackson-Lewis V, Hersch S, Sulzer D, Przedborski S, Burke R, Hen R. Resistance of α-synuclein null mice to the parkinsonian neurotoxin MPTP. Proc Natl Acad Sci USA. 2002;99:14524–14529. doi: 10.1073/pnas.172514599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klivenyi P, Siwek D, Gardian G, Yang L, Starkov A, Cleren C, Ferrante RJ, Kowall NW, Abeliovich A, Beal MF. Mice lacking alpha-synuclein are resistant to mitochondrial toxins. Neurobiol Dis. 2006;21:541–548. doi: 10.1016/j.nbd.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Drolet RE, Behrouz B, Lookingland KJ, Goudreau JL. Mice Lacking [alpha]-Synuclein have an Attenuated Loss of Striatal Dopamine Following Prolonged Chronic MPTP Administration. Neurotoxicology . 2004;25:761–769. doi: 10.1016/j.neuro.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem. 2008;283:9089–9100. doi: 10.1074/jbc.M710012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder H, Mensah K, Theisler C, Lee J, Matouschek A, Wolozin B. Aggregated and Monomeric alpha -Synuclein Bind to the S6' Proteasomal Protein and Inhibit Proteasomal Function. J Biol Chem. 2003;278:11753–11759. doi: 10.1074/jbc.M208641200. [DOI] [PubMed] [Google Scholar]
- Stefanis L, Larsen KE, Rideout HJ, Sulzer D, Greene LA. Expression of A53T mutant but not wild-type alpha-synuclein in PC12 cells induces alterations of the ubiquitin-dependent degradation system, loss of dopamine release, and autophagic cell death. J Neurosci. 2001;21:9549–9560. doi: 10.1523/JNEUROSCI.21-24-09549.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Engelender S, Igarashi S, Rao RK, Wanner T, Tanzi RE, Sawa AL, Dawson V, Dawson TM, Ross CA. Inducible expression of mutant {alpha}-synuclein decreases proteasome activity and increases sensitivity to mitochondria-dependent apoptosis. Hum Mol Genet. 2001;10:919–926. doi: 10.1093/hmg/10.9.919. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–1295. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- Yang Q, She H, Gearing M, Colla E, Lee M, Shacka JJ, Mao Z. Regulation of Neuronal Survival Factor MEF2D by Chaperone-Mediated Autophagy. Science. 2009;323:124–127. doi: 10.1126/science.1166088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- Gomez-Santos C, Ferrer I, Reiriz J, Vinals F, Barrachina M, Ambrosio S. MPP+ increases alpha-synuclein expression and ERK/MAP-kinase phosphorylation in human neuroblastoma SH-SY5Y cells. Brain Res. 2002;935:32–39. doi: 10.1016/S0006-8993(02)02422-8. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Stout AK, Lund S, Baptista M, Panov AV, Cookson MR, Greenamyre JT. An in vitro model of Parkinson's disease: linking mitochondrial impairment to altered alpha-synuclein metabolism and oxidative damage. J Neurosci . 2002;22:7006–7015. doi: 10.1523/JNEUROSCI.22-16-07006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinou E, Chen Q, Weisse M, Giasson BI, Norris EH, Rueter SM, Trojanowski JQ, Lee VMY, Ischiropoulos H. Induction of {alpha}-Synuclein Aggregation by Intracellular Nitrative Insult. J Neurosci . 2001;21:8053–8061. doi: 10.1523/JNEUROSCI.21-20-08053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodara R, Norris EH, Giasson BI, Mishizen-Eberz AJ, Lynch DR, Lee VMY, Ischiropoulos H. Functional Consequences of {alpha}-Synuclein Tyrosine Nitration: diminished binding to lipid vesicles and increased fibril formation. J Biol Chem. 2004;279:47746–47753. doi: 10.1074/jbc.M408906200. [DOI] [PubMed] [Google Scholar]
- Krishnan S, Chi EY, Wood SJ, Kendrick BS, Li C, Garzon-Rodriguez W, Wypych J, Randolph TW, Narhi LO, Biere AL, Citron M, Carpenter JF. Oxidative dimer formation is the critical rate-limiting step for Parkinson's disease alpha-synuclein fibrillogenesis. Biochemistry. 2003;42:829–837. doi: 10.1021/bi026528t. [DOI] [PubMed] [Google Scholar]
- Yamin G, Uversky VN, Fink AL. Nitration inhibits fibrillation of human alpha-synuclein in vitro by formation of soluble oligomers. FEBS lett. 2003;542:147–152. doi: 10.1016/S0014-5793(03)00367-3. [DOI] [PubMed] [Google Scholar]
- Souza JM, Giasson BI, Chen Q, Lee VM, Ischiropoulos H. Dityrosine cross-linking promotes formation of stable alpha -synuclein polymers. Implication of nitrative and oxidative stress in the pathogenesis of neurodegenerative synucleinopathies. J Biol Chem. 2000;275:18344–18349. doi: 10.1074/jbc.M000206200. [DOI] [PubMed] [Google Scholar]
- Norris EH, Giasson BI, Ischiropoulos H, Lee VM. Effects of oxidative and nitrative challenges on alpha-synuclein fibrillogenesis involve distinct mechanisms of protein modifications. J Biol Chem. 2003;278:27230–27240. doi: 10.1074/jbc.M212436200. [DOI] [PubMed] [Google Scholar]
- Paik SR, Shin HJ, Lee JH. Metal-catalyzed oxidation of alpha-synuclein in the presence of Copper(II) and hydrogen peroxide. Arch Biochem Biophys. 2000;378:269–277. doi: 10.1006/abbi.2000.1822. [DOI] [PubMed] [Google Scholar]
- Conway KA, Rochet JC, Bieganski RM, Lansbury PT., Jr Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science. 2001;294:1346–1349. doi: 10.1126/science.1063522. [DOI] [PubMed] [Google Scholar]
- Norris EH, Giasson BI, Hodara R, Xu S, Trojanowski JQ, Ischiropoulos H, Lee VM. Reversible inhibition of alpha-synuclein fibrillization by dopaminochrome-mediated conformational alterations. J Biol Chem. 2005;280:21212–21219. doi: 10.1074/jbc.M412621200. [DOI] [PubMed] [Google Scholar]
- Mazzulli JR, Armakola M, Dumoulin M, Parastatidis I, Ischiropoulos H. Cellular Oligomerization of {alpha}-Synuclein Is Determined by the Interaction of Oxidized Catechols with a C-terminal Sequence. J Biol Chem. 2007;282:31621–31630. doi: 10.1074/jbc.M704737200. [DOI] [PubMed] [Google Scholar]
- Herrera FE, Chesi A, Paleologou KE, Schmid A, Munoz A, Vendruscolo M, Gustincich S, Lashuel HA, Carloni P. Inhibition of alpha-Synuclein Fibrillization by Dopamine Is Mediated by Interactions with Five C-Terminal Residues and with E83 in the NAC Region. PLoS ONE. 2008;3:e3394. doi: 10.1371/journal.pone.0003394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzulli JR, Mishizen AJ, Giasson BI, Lynch DR, Thomas SA, Nakashima A, Nagatsu T, Ota A, Ischiropoulos H. Cytosolic catechols inhibit alpha-synuclein aggregation and facilitate the formation of intracellular soluble oligomeric intermediates. J Neurosci. 2006;26:10068–10078. doi: 10.1523/JNEUROSCI.0896-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatsu T. Change of tyrosine hydroxylase in the parkinsonian brain and in the brain of MPTP-treated mice as revealed by homospecific activity. Neurochem Res. 1990;15:425–429. doi: 10.1007/BF00969928. [DOI] [PubMed] [Google Scholar]
- Grune T, Shringarpure R, Sitte N, Davies K. Age-related changes in protein oxidation and proteolysis in mammalian cells. J Gerontol A Biol Sci Med Sci . 2001;56:B459–B467. doi: 10.1093/gerona/56.11.b459. [DOI] [PubMed] [Google Scholar]
- Stadtman ER, Levine RL. Protein oxidation. Ann N Y Acad Sci. 2000;899:191–208. doi: 10.1111/j.1749-6632.2000.tb06187.x. [DOI] [PubMed] [Google Scholar]
- Oliver CN, Ahn BW, Moerman EJ, Goldstein S, Stadtman ER. Age-related changes in oxidized proteins. J Biol Chem. 1987;262:5488–5491. [PubMed] [Google Scholar]
- Smith CD, Carney JM, Starke-Reed PE, Oliver CN, Stadtman ER, Floyd RA, Markesbery WR. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc Natl Acad Sci USA. 1991;88:10540–10543. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, Dimayuga E, Keller JN. Proteasome regulation of oxidative stress in aging and age-related diseases of the CNS. Antioxid Redox Signal. 2006;8:163–172. doi: 10.1089/ars.2006.8.163. [DOI] [PubMed] [Google Scholar]
- Farout L, Friguet B. Proteasome function in aging and oxidative stress: implications in protein maintenance failure. Antioxid Redox Signal. 2006;8:205–216. doi: 10.1089/ars.2006.8.205. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Dice JF. Age-related decline in chaperone-mediated autophagy. J Biol Chem. 2000;275:31505–31513. doi: 10.1074/jbc.M002102200. [DOI] [PubMed] [Google Scholar]
- Donati A, Cavallini G, Paradiso C, Vittorini S, Pollera M, Gori Z, Bergamini E. Age-related changes in the regulation of autophagic proteolysis in rat isolated hepatocytes. J Gerontol A Biol Sci Med Sci. 2001;56:B288–B293. doi: 10.1093/gerona/56.7.b288. [DOI] [PubMed] [Google Scholar]
- Dice JF. Altered degradation of proteins microinjected into senescent human fibroblasts. J Biol Chem . 1982;257:14624–14627. [PubMed] [Google Scholar]
- Martinez-Vicente M, Sovak G, Cuervo AM. Protein degradation and aging. Exp Gerontol. 2005;40:622–633. doi: 10.1016/j.exger.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Terman A. The effect of age on formation and elimination of autophagic vacuoles in mouse hepatocytes. Gerontology. 1995;41:319–326. doi: 10.1159/000213753. [DOI] [PubMed] [Google Scholar]
- Sitte N, Merker K, Von Zglinicki T, Davies KJ, Grune T. Protein oxidation and degradation during cellular senescence of human BJ fibroblasts: part II–aging of nondividing cells. Faseb J. 2000;14:2503–2510. doi: 10.1096/fj.00-0210com. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Dragicevic NB, Deng JH, Bai Y, Dimayuga E, Ding Q, Chen Q, Bruce-Keller AJ, Keller JN. Proteasome inhibition alters neural mitochondrial homeostasis and mitochondria turnover. J Biol Chem. 2004;279:20699–20707. doi: 10.1074/jbc.M313579200. [DOI] [PubMed] [Google Scholar]
- LaVoie MJ, Hastings TG. Dopamine quinone formation and protein modification associated with the striatal neurotoxicity of methamphetamine: evidence against a role for extracellular dopamine. J Neurosci. 1999;19:1484–1491. doi: 10.1523/JNEUROSCI.19-04-01484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVoie MJ, Ostaszewski BL, Weihofen A, Schlossmacher MG, Selkoe DJ. Dopamine covalently modifies and functionally inactivates parkin. Nat Med. 2005;11:1214–1221. doi: 10.1038/nm1314. [DOI] [PubMed] [Google Scholar]
- Hastings TG, Lewis DA, Zigmond MJ. Role of oxidation in the neurotoxic effects of intrastriatal dopamine injections. Proc Natl Acad Sci USA. 1996;93:1956–1961. doi: 10.1073/pnas.93.5.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudle WM, Richardson JR, Wang MZ, Taylor TN, Guillot TS, McCormack AL, Colebrooke RE, Di Monte DA, Emson PC, Miller GW. Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. J Neurosci. 2007;27:8138–8148. doi: 10.1523/JNEUROSCI.0319-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg PA. Catecholamine toxicity in cerebral cortex in dissociated cell culture. J Neurosci . 1988;8:2887–2894. doi: 10.1523/JNEUROSCI.08-08-02887.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca L, Zucca FA, Albertini A, Rizzio E, Fariello RG. A proposed dual role of neuromelanin in the pathogenesis of Parkinson's disease. Neurology . 2006;67:S8–S11. doi: 10.1212/wnl.67.7_suppl_2.s8. [DOI] [PubMed] [Google Scholar]
- D'Amato RJ, Lipman ZP, Snyder SH. Selectivity of the Parkinsonian Neurotoxin MPTP: toxic metabolite MPP+ binds to neuromelanin. Science. 1986;231:987–989. doi: 10.1126/science.3080808. [DOI] [PubMed] [Google Scholar]
- Luigi Zecca Casella L, Albertini A, Bellei C, Zucca FA, Engelen M, Zadlo A, Szewczyk G, Zareba M, Sarna T. Neuromelanin can protect against iron-mediated oxidative damage in system modeling iron overload of brain aging and Parkinson's disease. J Neurochem. 2008;106:1866–1875. doi: 10.1111/j.1471-4159.2008.05541.x. [DOI] [PubMed] [Google Scholar]
- Faucheux BA, Martin ME, Beaumont C, Hauw JJ, Agid Y, Hirsch EC. Neuromelanin associated redox-active iron is increased in the substantia nigra of patients with Parkinson's disease. J Neurochem . 2003;86:1142–1148. doi: 10.1046/j.1471-4159.2003.01923.x. [DOI] [PubMed] [Google Scholar]
- Guelman LR, Pagotto RM, Di Toro CG, Zieher LM. Deferoxamine antioxidant activity on cerebellar granule cells [gamma]-irradiated in vitro. Neurotoxicol Teratol. 2004;26:477–483. doi: 10.1016/j.ntt.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Santiago M, Matarredona ER, Granero L, Cano J. Neuroprotective Effect of the Iron Chelator Desferrioxamine Against MPP+ Toxicity on Striatal Dopaminergic Terminals. J Neurochem . 1997;68:732–738. doi: 10.1046/j.1471-4159.1997.68020732.x. [DOI] [PubMed] [Google Scholar]
- Shachar DB, Kahana N, Kampel V, Warshawsky A, Youdim MBH. Neuroprotection by a novel brain permeable iron chelator, VK-28, against 6-hydroxydopamine lession in rats. Neuropharmacology. 2004;46:254–263. doi: 10.1016/j.neuropharm.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar D, Eshel G, Finberg JP, Youdim MB. The iron chelator desferrioxamine (Desferal) retards 6-hydroxydopamine-induced degeneration of nigrostriatal dopamine neurons. J Neurochem. 1991;56:1441–1444. doi: 10.1111/j.1471-4159.1991.tb11444.x. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Graybiel AM. Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson's disease. Nature. 1988;334:345–348. doi: 10.1038/334345a0. [DOI] [PubMed] [Google Scholar]
- Fasano M, Giraudo S, Coha S, Bergamasco B, Lopiano L. Residual substantia nigra neuromelanin in Parkinson's disease is cross-linked to [alpha]-synuclein. Neurochem Int. 2003;42:603–606. doi: 10.1016/S0197-0186(02)00161-4. [DOI] [PubMed] [Google Scholar]
- Halliday GM, Ophof A, Broe M, Jensen PH, Kettle E, Fedorow H, Cartwright MI, Griffiths FM, Shepherd CE, Double KL. {alpha}-Synuclein redistributes to neuromelanin lipid in the substantia nigra early in Parkinson's disease. Brain. 2005;128:2654–2664. doi: 10.1093/brain/awh584. [DOI] [PubMed] [Google Scholar]
- Fowler DM, Koulov AV, Alory-Jost C, Marks MS, Balch WE, Kelly JW. Functional Amyloid Formation within Mammalian Tissue. PLoS biology. 2006;4:e6. doi: 10.1371/journal.pbio.0040006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurbain I, Geerts WJC, Boudier T, Marco S, Verkleij AJ, Marks MS, Raposo G. Electron tomography of early melanosomes: Implications for melanogenesis and the generation of fibrillar amyloid sheets. Proc Natl Acad Sci USA. 2008;105:19726–19731. doi: 10.1073/pnas.0803488105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorow H, Pickford R, Hook JM, Double KL, Halliday GM, Gerlach M, Riederer P, Garner B. Dolichol is the major lipid component of human substantia nigra neuromelanin. J Neurochem. 2005;92:990–995. doi: 10.1111/j.1471-4159.2004.02975.x. [DOI] [PubMed] [Google Scholar]
- Marsden CD. Pigmentation in the nucleus substantiae nigrae of mammals. J Anat. 1961;95:256–261. [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287:1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- Tofaris GK, Garcia Reitbock P, Humby T, Lambourne SL, O'Connell M, Ghetti B, Gossage H, Emson PC, Wilkinson LS, Goedert M, Spillantini MG. Pathological changes in dopaminergic nerve cells of the substantia nigra and olfactory bulb in mice transgenic for truncated human alpha-synuclein(1–120): implications for Lewy body disorders. J Neurosci. 2006;26:3942–3950. doi: 10.1523/JNEUROSCI.4965-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richfield EK, Thiruchelvam MJ, Cory-Slechta DA, Wuertzer C, Gainetdinov RR, Caron MG, Di Monte DA, Federoff HJ. Behavioral and Neurochemical Effects of Wild-Type and Mutated Human [alpha]-Synuclein in Transgenic Mice. Exp Neurol. 2002;175:35–48. doi: 10.1006/exnr.2002.7882. [DOI] [PubMed] [Google Scholar]