Abstract
Background
A wide range of possible malaria vaccines is being considered and there is a need to identify which vaccines should be prioritized for clinical development. An important element of the information needed for this prioritization is a prediction of the cost-effectiveness of potential vaccines in the transmission settings in which they are likely to be deployed. This analysis needs to consider a range of delivery modalities to ensure that clinical development plans can be aligned with the most appropriate deployment strategies.
Methods
The simulations are based on a previously published individual-based stochastic model for the natural history and epidemiology of Plasmodium falciparum malaria. Three different vaccine types: pre-erythrocytic vaccines (PEV), blood stage vaccines (BSV), mosquito-stage transmission-blocking vaccines (MSTBV), and combinations of these, are considered each delivered via a range of delivery modalities (Expanded Programme of Immunization – EPI-, EPI with booster, and mass vaccination combined with EPI). The cost-effectiveness ratios presented are calculated for four health outcomes, for assumed vaccine prices of US$ 2 or US$ 10 per dose, projected over a 10-year period.
Results
The simulations suggest that PEV will be more cost-effective in low transmission settings, while BSV at higher transmission settings. Combinations of BSV and PEV are more efficient than PEV, especially in moderate to high transmission settings, while compared to BSV they are more cost-effective in moderate to low transmission settings. Combinations of MSTBV and PEV or PEV and BSV improve the effectiveness and the cost-effectiveness compared to PEV and BSV alone only when applied with EPI and mass vaccinations. Adding booster doses to the EPI is unlikely to be a cost-effective alternative to delivering vaccines via the EPI for any vaccine, while mass vaccination improves effectiveness, especially in low transmission settings, and is often a more efficient alternative to the EPI. However, the costs of increasing the coverage of mass vaccination over 50% often exceed the benefits.
Conclusion
The simulations indicate malaria vaccines might be efficient malaria control interventions, and that both transmission setting and vaccine delivery modality are important to their cost-effectiveness. Alternative vaccine delivery modalities to the EPI may be more efficient than the EPI. Mass vaccination is predicted to provide substantial health benefits at low additional costs, although achieving high coverage rates can lead to substantial incremental costs.
Background
Plasmodium falciparum malaria represents one of the world's major causes of morbidity and mortality[1,2] and there is a pressing need for new effective interventions, which, combined with the existing strategies, could effectively reduce the burden of malaria in endemic areas[3].
Among these potential new interventions are vaccines and, although there is currently no licensed malaria vaccine, a number of candidates are under development. The complexity of the malaria life cycle means that a number of different stages of the parasite can be targeted. The candidate that is most advanced in clinical development[4,5] targets pre-erythrocytic stages of the parasite.
Appraisals of candidate malaria vaccines should not include only efficacy and effectiveness evaluations but also cost effectiveness analyses (CEA) aimed at guiding vaccine developers, funding agencies[6], and policy makers to allocate resources so that social and economic benefits are maximized[7,8]. CEA can help in evaluating alternative health interventions because health decision makers are primarily interested in knowing what health improvements can be bought with a given budget, and not the overall economic impact per se[9,10]. Previously, the likely epidemiological effects[11] and cost-effectiveness[12] of pre-erythrocytic vaccines when delivered in areas of stable endemic malaria via the Expanded Programme on Immunization (EPI) were estimated based on a dynamic model of malaria epidemiology[13]. These simulations showed that at moderate vaccine prices the cost-effectiveness of such vaccines may be similar to that of other preventive and curative interventions against malaria. However, more evidence is needed on the likely cost-effectiveness of different malaria vaccines under development, and on the implications for it of adopting alternative means of deployment. The cost-effectiveness of a malaria vaccine will depend not only on the vaccine profile and the transmission setting, but also on the vaccination coverage that can be achieved, on the vaccine delivery costs, and of the operational feasibility of the delivery modalities adopted to deploy it.
A companion article to the present one[14], reports on the simulations of the likely epidemiological effects of three different malaria vaccine types: pre-erythrocytic vaccines (PEV), blood stage vaccines (BSV), mosquito-stage transmission-blocking vaccines (MSTBV), and combinations of these. A range of different delivery modalities (EPI, EPI with booster, and mass vaccination combined with EPI) were considered. In this article, both the health system and vaccine delivery costs are attached to the events recorded in these simulations to calculate cost-effectiveness ratios for each deployment strategy and each vaccine, and for each of four health outcomes over a 10-year time-horizon.
Methods
Perspective and boundary
The simulations refer to CEA under the perspective of the society as a whole, although only direct costs are included. They thus consider all relevant direct resource inputs and costs to the interventions, and resource consequences, costs, and health impacts resulting from the interventions. The indirect economic impact of malaria such as potential earning forgone of patients and unpaid carers is not included, as its inclusion is controversial in CEA[15,16]. The CEA follows standard cost-effectiveness methodology[8,17-21].
Interventions being compared
The simulations of vaccines are based on a previously described model for the natural history and epidemiology of P. falciparum malaria[13]. This model uses an underlying model based on descriptions of the course of parasite densities in malaria therapy patients[22]. The parameterization of the model for the present simulations is described in the companion article[14] and reviewed in more detail elsewhere[23]. Briefly, each simulated vaccine is characterized by an average initial efficacy (the efficacy reached after completion of a schedule of three doses), and by a half-life of this efficacy, which is assumed to decay exponentially with time. The vaccinated population is assumed to be heterogeneous in the response to vaccination, and to allow for this we assign initial values for efficacy drawn from a beta-distribution[11] (simulated vaccines are delivered at specified ages, and a range of coverage values is considered for vaccination to allow for individuals who do not complete the full schedule).
The effects of the three different vaccine types and four combinations were modeled as follows (see also [14]):
(i) Pre-erythrocytic vaccines (PEV)
Pre-erythrocytic vaccines are assumed to lead to a reduction in the proportion of inoculations from the bites of infected mosquitoes that lead to blood stage infection and the vaccine efficacy is assumed to be equal to the proportion by which this force of infection is reduced.
(ii) Blood stage vaccines (BSV)
A blood stage vaccine is assumed to reduce parasite densities at each time step by a proportion equal to the vaccine efficacy.
(iii) Mosquito stage transmission blocking vaccines (TBV)
Vaccine efficacy is equivalent to the proportional reduction of the probability that a mosquito becomes infected from any one feed on an infectious vaccinated human.
(iv) Combination vaccines
Combination vaccines of PEV with TBV, BSV with TBV, BSV with PEV and also a three-way combination of PEV with both BSV and MSTBV are considered. For each combination, PEV and BSV are assumed to be matched in both the initial efficacy and in their rate of decay. Only combinations of PEV, BSV and of PEV-BSV with high efficacy MSTBV are considered since it is unlikely that a MSTBV with low efficacy would be developed.
Vaccine delivery modalities
The delivery of the three vaccine types and their combinations through the following three strategies are simulated:
(i) EPI
Delivery of the vaccines through the EPI according to the usual diphtheria tetanus pertussis (DTP3) vaccine schedule: age 1, 2 and 3 months.
(ii) EPI with booster
In addition to the above EPI schedule, this modality includes booster doses at 1, 2, 3 and 4 years after the last EPI schedule. The effect of a booster dose is to restore the protective efficacy to the level achieved after the third dose in the same individual.
(iii) Mass vaccination combined with EPI
Delivery via EPI to infants is supplemented with a mass vaccination campaign at the beginning of the intervention period and additional campaigns after five years.
Vaccine coverage
For vaccines delivered via EPI, the assumed coverage of full vaccination (three doses) corresponds to that reported in Tanzania for three doses of diphtheria tetanus pertussis-hepatitis B virus vaccine in the year 2003, which stood at 89%. The assumed dropout rate from the first to the third dose is 6% since coverage for the first dose of DTP-HBV vaccine was 95%. When booster doses are included, it is assumed that 99% of those that receive the third EPI dose will be given a booster dose 1, 2, 3 and 4 years after the last EPI dose. For mass vaccination the coverage levels of 30, 50 and 70% are simulated.
Case management model
As detailed in the companion article[14], the simulations of the effects of vaccine interventions use a case management model including both formal and informal treatment, similar to a previous study of the authors[24]. An artemisinin-based combination therapy (ACT), artemether-lumefantrine, is used as first line treatment for uncomplicated malaria, as per recent policy changes, and the drug action model was modified accordingly, both in terms of the potential to reduce rates of severe disease, sequelae and death, and the impact on transmission intensity. The model assumes that 90% of patients comply with the ACT treatment schedule and have a cure rate of 85%, while in non-compliers there is no effect.
Measurement of health impact
The effect of vaccines is evaluated by simulating the malaria dynamics in a population of 100,000 people over a 10-year time horizon. For each of the seven vaccine options, and each delivery modality, the simulations start from a reference set of assumptions (Table 1 of the companion article[14]). Each of the 21 vaccine schemes is compared with the reference situation at six different transmission intensities to obtain cost effectiveness results for each of the 126 vaccine scenarios in preventing the following outcomes: uncomplicated episodes, severe episodes, deaths and DALYs.
Table 1.
Vaccine delivery costs – routine EPI – US$ 2006
| Item | Source | Costs (US$) | ||
| Net vacc. purchase cost per dose | Derived | 1.23 | 2.45 | 12.25 |
| Vaccine price per dose | 1 | 2 | 10 | |
| Freight | UNICEF estimates | 0.0417 | 0.0417 | 0.0417 |
| Wastage | WHO estimates | 15% | 15% | 15% |
| Distribution per dose | [29] | 0.09 | 0.09 | 0.09 |
| Storage per dose | [29] | 0.03 | 0.03 | 0.03 |
| Management per dose | [29] | 0.003 | 0.003 | 0.003 |
| Delivery per dose | Derived | 0.13 | 0.13 | 0.13 |
| Syringes | 0.06 | 0.06 | 0.06 | |
| Unit cost per dose | [30] | 0.05 | 0.05 | 0.05 |
| Freight | UNICEF estimates | 0.0417 | 0.0417 | 0.0417 |
| Wastage | WHO estimates | 10% | 10% | 10% |
| Safety boxes | 0.01 | 0.01 | 0.01 | |
| Unit cost per dose | [30] | 0.0122 | 0.0122 | 0.0122 |
| Freight | UNICEF estimates | 0.0417 | 0.0417 | 0.0417 |
| Wastage | WHO estimates | 10% | 10% | 10% |
| Personnel facility level | [29] | 0.06 | 0.06 | 0.06 |
| Waste management | ||||
| Training over 5 years) | [29] | 0.03 | 0.03 | 0.03 |
| Social mobilization (av) | [29] | 0.12 | 0.12 | 0.12 |
| TOTAL COST PER DOSE | Derived | 1.63 | 2.86 | 12.66 |
*adjusted for inflation
Each simulation is repeated three times using different seeds to initialize the random number generator, and each of these simulations is compared with an independent simulation of a reference scenario: a control population with no vaccine, but with the same human demography, baseline transmission, and health system.
To estimate the number of disability adjusted life years (DALYs), years of life lived with disability are calculated on the basis of the duration of disability, and respective disability weights. Weights for different malaria attributable disease conditions have been obtained from the Global Burden of Disease (GBD) study[25]. DALYs are computed with no age weighting to follow standard cost-effectiveness practices[26]. The disability associated with anemia is assigned to the same time period as the malaria infections causing it.
Years of life lost (YLLs) and DALYs are calculated assuming age-specific life expectancies based on the life-table from Butajira, Ethiopia, with an average life expectancy of 46.6 years at birth[27]. This life-table represents that of an East African setting with low malaria transmission and is very similar to that for Hai District, a high altitude and low malaria prevalence site in Tanzania[28]. YLLs for each simulated death are computed under the assumption that this life table would apply in the absence of malaria.
Vaccine delivery costs
The vaccine delivery costs are estimated using the methodology of a previous study by the authors[29], which was based on an ingredient approach requiring information on the quantities of physical inputs needed and their unit costs. The costs of introducing a malaria vaccine into the EPI in Tanzania include those related to an assumed range of vaccine purchase costs, and data collected from Tanzania on costs of distribution, cold chain, management, vaccine delivery by health facilities, training, and social mobilization[30] (Table 1). For booster doses, the per-dose delivery cost is assumed to be the same as that of routine EPI.
The costs of vaccine campaigns are estimated by adding to the purchase costs, those costs associated with distribution, cold chain, management, specific programme activities, personnel, and other capital costs, estimated by a study in Tanzania on a campaign for Vitamin A supplementation[31] (Table 2).
Table 2.
Vaccine delivery costs – Campaign – US$ 2006
| Item | Source | Costs (US$) | ||
| Net vacc. purchase cost per dose | Derived | 1.23 | 2.45 | 12.25 |
| Vaccine price per dose | 1 | 2 | 10 | |
| Freight | UNICEF estimates | 0.0417 | 0.0417 | 0.0417 |
| Wastage | WHO estimates | 15% | 15% | 15% |
| Distribution per dose | [29] | 0.09 | 0.09 | 0.09 |
| Storage per dose | [29] | 0.03 | 0.03 | 0.03 |
| Management per dose | [29] | 0.003 | 0.003 | 0.003 |
| Delivery per dose | Derived | 0.07 | 0.07 | 0.07 |
| Syringes | 0.06 | 0.06 | 0.06 | |
| Unit cost per dose | [30] | 0.05 | 0.05 | 0.05 |
| Freight | UNICEF estimates | 0.0417 | 0.0417 | 0.0417 |
| Wastage | WHO estimates | 10% | 10% | 10% |
| Safety boxes | 0.01 | 0.01 | 0.01 | |
| Unit cost per dose | [30] | 0.0122 | 0.0122 | 0.0122 |
| Freight | UNICEF estimates | 0.0417 | 0.0417 | 0.0417 |
| Wastage | WHO estimates | 10% | 10% | 10% |
| Programme-specific costs | 0.07 | 0.07 | 0.07 | |
| Allowances | [31] | 0.1132 | 0.1132 | 0.1132 |
| Fuel & Maintenance | 0.0337 | 0.0337 | 0.0337 | |
| Fax & Telephone | 0.0094 | 0.0094 | 0.0094 | |
| Refreshments | 0.0058 | 0.0058 | 0.0058 | |
| Stationary & Postage | 0.0056 | 0.0056 | 0.0056 | |
| Photocoping | 0.0051 | 0.0051 | 0.0051 | |
| Transport | 0.0050 | 0.0050 | 0.0050 | |
| Social mobilization | 0.0048 | 0.0048 | 0.0048 | |
| Other | 0.0005 | 0.0005 | 0.0005 | |
| Personnel cost | 0.42 | 0.42 | 0.42 | |
| Government | 0.3017 | 0.3017 | 0.3017 | |
| Non Government | 0.1141 | 0.1141 | 0.1141 | |
| Capital cost | 0.07 | 0.07 | 0.07 | |
| Vehicles | 0.0410 | 0.0410 | 0.0410 | |
| Social mobilization | 0.0156 | 0.0156 | 0.0156 | |
| Long term training & studies | 0.0153 | 0.0153 | 0.0153 | |
| Other | 0.0017 | 0.0017 | 0.0017 | |
| TOTAL COST PER DOSE | Derived | 1.98 | 3.20 | 13.01 |
*adjusted for inflation
Potential cost savings of the interventions
The costs of treating those seeking health care for malaria episodes are calculated under the reference and the vaccine scenarios modeled. This allows calculation of expected cost savings associated with the introduction of an efficacious malaria vaccine. The case management cost inputs correspond to those published previously by the authors adjusted for inflation to 2007[24,32-36] (Table 3) and the first line treatment for uncomplicated malaria changed to an ACT (artemether/lumefantrine), for which the public sector price posted by WHO was used (Table 4).
Table 3.
Case management unit costs US$ 2006
| Costs (US$) | Source | |
| Household average out of pocket costs per outpatient visit | ||
| Travel costs | 0.09 | [32] |
| Medical supplies | 0.03 | [32] |
| Non medical supplies | 0.22 | [32] |
| Travel costs | 0.09 | [32] |
| Unit cost of outpatient visit | 0.7176 | derived |
| % of outpatient visits that take place at health centers | 17% | [33] |
| % of outpatient visits that take place at dispensaries | 72% | [33] |
| % of outpatient visits that take place at hospitals | 10% | [33] |
| cost per outpatient visits at health centers | 1.47 | derived |
| cost per outpatient visits at dispensaries | 1.18 | derived |
| cost per outpatient visits at hospitals | 2.54 | derived |
| % of patients using Diagnostic Techniques | 0.1 | [34] |
| unit cost of Diagnostic Technique | 0.3 | [34] |
| % of outpatient visit cost that are recurrent | 69% | [32] |
| % of outpatient visit cost that are fixed | 0.25 | [32] |
| Hospital costs of severe episodes | ||
| Non drug cost per admission when patient fully recorvers | 14.4 | derived |
| Non drug cost per admission when patient recorvers with NS | 32 | derived |
| Non drug cost per admission when patient dies | 6.4 | derived |
| Non drug cost per day of stay | 9.00 | [36] |
| Capital | 2.60 | |
| Recurrent | 6.40 | |
| average length of stay when patient fully recovers | 4.5 | [41] |
| average length of stay when patient recovers with NS | 10 | [41] |
| average length of stay when patient dies | 2 | [41] |
| % of hospital cost that are recurrent | 71.1 | [35] |
| % of hospital recurrent costs that are fixed | 50.0 | [41] |
Table 4.
ACT costs
| Age |
Cost/dose in $ (including 12% dist) |
Cost per course in $ + 25% wastage |
| <3 years – 5 to 14 Kg | 1.008 | 1.260 |
| 3–9 years – 15 to 24 Kg | 1.568 | 1.960 |
| 10–14 years 25 to 34 Kg | 2.128 | 2.660 |
| 15+ years – Above 35 Kg | 2.688 | 3.360 |
Source: http://www.who.int/malaria/cmc_upload/0/000/015/789/CoA_website5.pdf accessed 15 July 2008.
The numbers of uncomplicated and severe malaria episodes averted due to vaccination are multiplied by the case management unit costs, as described above, to estimate the potential cost savings for both the health system and households. The cost savings are subtracted from the vaccine costs to compute the net costs of the interventions.
Calculating cost-effectiveness ratios and interpreting the results
The cost-effectiveness ratios presented are calculated for four health outcomes: uncomplicated and severe malaria episodes averted, DALYs averted, and deaths averted. Future costs and benefits are discounted at 3%. The cost-effectiveness ratios are presented for assumed vaccine prices of US$ 2 or US$ 10 per dose for all the vaccines and vaccine combinations. The results are presented as cost-effectiveness on a 10-year period.
The cost-effectiveness ratios are to be interpreted as incremental cost-effectiveness ratios of implementing the interventions in the simulated scenarios relative to a do-nothing scenario, which corresponds to maintaining only the case management model described above.
Accounting for uncertainty
The cost-effectiveness results are based on an advanced modeling methodology aimed at representing reality as accurately as possible. The large number of scenarios simulated includes some sensitivity analyses of results for key variables, for instance for vaccine efficacy levels. The likely impact on results of other key features of potential malaria vaccines is explored in the companion article[14].
However, many sources of uncertainty cannot be captured by these sensitivity analyses. Probabilistic sensitivity analysis and expected value of information analysis could serve to further assess the impact of uncertainty on the simulation results [37-40], but there are technical problems in implementing and presenting such analyses for a large set of interventions and scenarios. It has been, therefore, planned to run an expected value of information analysis on a sub-set of simulations that will be reported in another article.
Results
Pre-erythrocytic vaccines
At a reference transmission setting with annual entomological inoculation rate (EIR) of 21, the simulations predict that a PEV with 52% initial efficacy could be very cost-effective when delivered via EPI alone. At a vaccine price of US$2 per dose, the cost per uncomplicated malaria episode averted would be around US$ 5, the cost per severe malaria episode averted US$ 269, the cost per DALY averted around US$ 35 and the cost per death averted US$1057 (see table S1 and S2, Additional file 1). The cost-effectiveness ratios are lower for higher effectiveness levels (Figure 1). They increase almost proportionally with vaccine price reaching US$ 160 per DALY averted and US$ 4869 per death averted for a vaccine price of US$ 10 per dose (see table S3 and S4, Additional file 1).
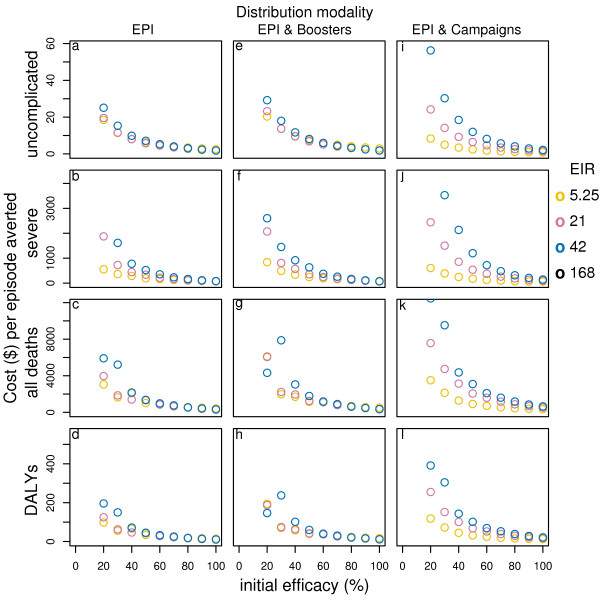
Figure 1.
Effect of initial efficacy on cost-effectiveness of PEV by transmission setting and delivery modality*. Results obtained assuming a vaccine half-life of 10 years, homogeneity value of 10, and vaccine price of US$2. EPI & Campaigns means EPI with 70% mass vaccination. *data for EIR in some cases are not shown in the figure due to a scale problem.
The proportion of events averted by PEV delivered via EPI with booster doses is slightly higher, but the cost per uncomplicated episode averted is 20% higher (see table S1, Additional file 1), and cost per DALY and death averted is around 31% higher (see table S2, Additional file 1).
With EPI and mass vaccination the proportion of events averted is 5% higher for mass vaccination coverage of 50% and 8% higher for coverage of 70%[14], and the cost per uncomplicated episode averted is slightly lower. However, the costs per DALY and death averted are around 60%–66% higher (see table S1 and S2, Additional file 1). For higher efficacy levels the pattern is similar, showing that the incremental benefits of these deployment modalities, in this transmission setting, are modest (Figure 1).
In low transmission settings, while the cost per uncomplicated episode averted under EPI alone is similar to that in the reference transmission setting (see table S1 and S2, Additional file 1), the cost per DALY and death averted are lower at US$ 31 per DALY averted and US$ 925 per death averted at a vaccine price of US$ 2 per dose (see table S2 and S4, Additional file 1). Adding booster doses leads to higher cost-effectiveness ratios for efficacy levels up to around 60%, but at near 100% efficacy the cost-effectiveness ratios become similar (Figure 1). In contrast, when mass vaccination is added to EPI, the cost-effectiveness ratios decrease substantially, by around 70% for the cost per uncomplicated case averted (see table S1 and S3, Additional file 1), and by 24% to 28% for the cost per DALY and death averted (see table S2 and S4, Additional file 1).
In high transmission settings, the effectiveness of PEV is low[14] and the cost-effectiveness ratios are therefore higher than in the other transmission settings irrespective of delivery modality. For some outcomes, vaccination even leads to an increase in the number of clinical events[14], and, therefore, to negative cost-effectiveness ratios and negative case management cost savings (see table S5, Additional file 1).
Across all transmission settings, the incremental benefits of booster doses are small and the cost-effectiveness ratios are higher. Adding mass campaigns has little impact on overall effect when the primary efficacy is low. However, for high vaccine efficacy and high coverage, this strategy is predicted to lead to local elimination of the parasite in low transmission settings and substantially reduce transmission in medium transmission settings[14] at low additional costs. Under these conditions, because of the effects of the vaccine on transmission, delivery via mass campaigns plus EPI becomes a cost-effective alternative to EPI alone.
Blood-stage vaccines
At the reference transmission intensity, BSV of moderate efficacy with a price of US$ 2 per dose applied through EPI achieves a cost per uncomplicated episode averted of about US$ 9 (see table S1, Additional file 1), which is higher than for the corresponding PEV, but the costs per DALY averted (US$ 21) and per death averted (US$ 630) are lower than for PEV (see table S2, Additional file 1). At higher efficacy levels, the cost-effectiveness ratios decrease, following the same patterns as for PEV (Figure 2). Adding booster doses increases the cost-effectiveness ratios somewhat. Mass campaigns also increase the cost-effectiveness ratios except for uncomplicated episodes, where they decrease.
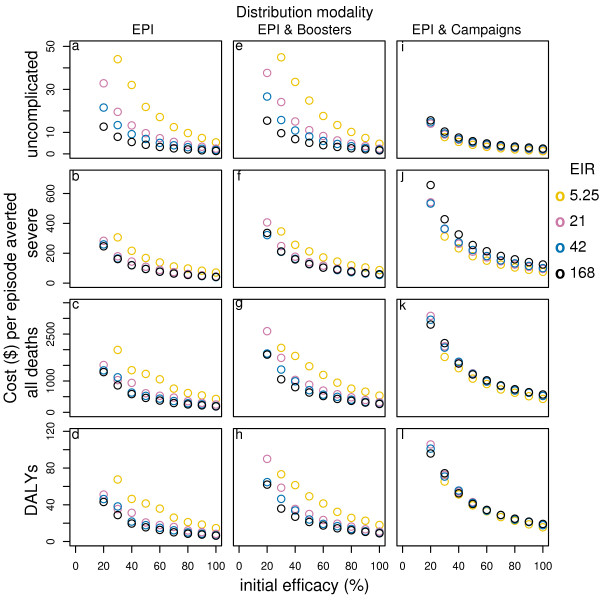
Figure 2.
Effect of initial efficacy on cost-effectiveness of BSV by transmission setting and delivery modality. Results obtained assuming a vaccine half-life of 10 years, homogeneity value of 10, and vaccine price of US$2. EPI & Campaigns means EPI with 70% mass vaccination.
At low transmission intensity BSV averts a lower proportion of uncomplicated and severe cases and deaths than PEV[14] and the cost effectiveness ratios are higher for all outcomes. Adding booster doses leads to slightly higher costs per uncomplicated episode averted (see table S1 and S3, Additional file 1), and much higher costs per DALY and death averted (see table S2 and S3, Additional file 1, and Figure 2). Adding mass campaigns to EPI leads to a dramatic reduction in the cost per uncomplicated episode averted, but the costs per DALY and death averted are only slightly lower (see table S1, S2, S3, Additional file 1, and Figure 3, 4).
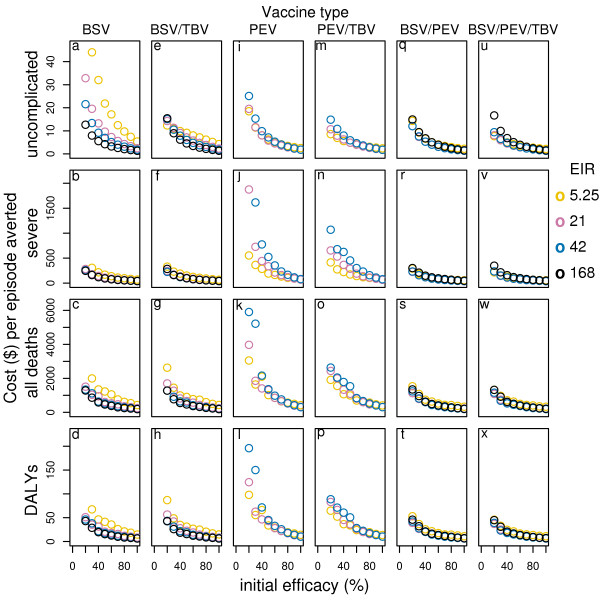
Figure 3.
Effect of initial efficacy on cost-effectiveness of all vaccines delivered via EPI by transmission setting*. Results obtained assuming a vaccine half-life of 10 years and homogeneity value of 10, and vaccine price of US$2. *data for EIR in some cases are not shown in the figure due to a scale problem.
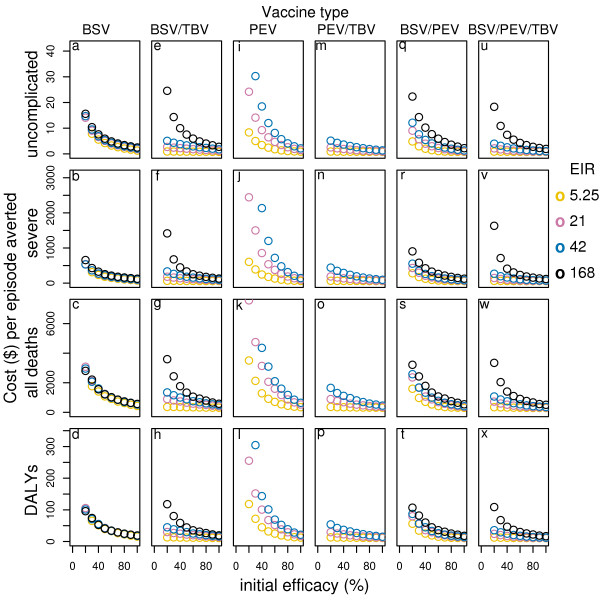
Figure 4.
Effect of initial efficacy on cost-effectiveness of all vaccines delivered via EPI with 70-% mass vaccination by transmission setting*. Results obtained assuming a vaccine half-life of 10 years and homogeneity value of 10, and vaccine price of US$2. *data for EIR in some cases are not shown in the figure due to a scale problem.
In high transmission settings BSV is more effective than PEV especially in averting severe and mortality events[14] and it is also more efficient. Under EPI alone the cost per uncomplicated episode averted, in the highest transmission setting, is US$ 3.8, the cost per DALY averted is US$13.5 and the cost per death averted is US$401, at vaccine price US$ 2 per dose (see table S1 and S2, Additional file 1, and Figure 3). Adding boosters or mass campaigns, leads to higher incremental costs than incremental benefits (see table S1, S2, S3, Additional file 1, and Figure 2).
Across all transmission settings, the incremental costs of adding booster doses to EPI are higher than the incremental benefits and this is particularly true for severe episodes, DALYs, and mortality (see table S1, S2, S3, Additional file 1, and Figure 2). In low transmission settings, campaigns improve cost-effectiveness for uncomplicated episodes averted, but do not change cost-effectiveness estimates for DALYs and deaths averted. However, in moderate to high transmission settings, the incremental costs of campaigns are higher than the incremental benefits (see table S1, S2, S3, Additional file 1, and Figure 2).
Combination vaccines and MSTBV
Combining BSV with PEV (with matched efficacies) in general, improves or matches the cases averted over PEV alone for all transmission settings and vaccine delivery modalities[14]. The cost-effectiveness ratios for this combination are lower than those of PEV in all transmission settings particularly for the cost per DALY and per death averted and in moderate to high transmission settings (see table S1, S2, S3 in Additional file 1, and Figure 3, 4). Compared to BSV alone, the cost-effectiveness ratios of combining BSV with PEV are lower, though the difference is smaller than for PEV and in this case it is higher in moderate to lower transmission settings than in high transmission settings. Adding booster doses to EPI leads to higher cost-effectiveness ratios across all transmission settings for this combination – the costs per uncomplicated episode averted increases by around 19%–23% while those per DALY and death averted show even larger increases (around 30%–40%).
Adding mass campaigns in low to moderate settings lead to incremental uncomplicated episodes averted that are higher than the incremental costs. However, in terms of DALYs and deaths averted the benefits exceed the costs only in the lowest transmission setting, while they are significantly lower in the reference and in high transmission settings. In high transmission settings even the additional uncomplicated episodes averted are lower than the additional costs.
Combinations of MS TBV with PEV or BSV and the triple combination do not improve the effectiveness of the vaccines alone when delivered via EPI or EPI with boosters[14]. However, adding mass campaigns leads to greater effectiveness in all transmission settings (Figure 4). The additional benefits of these combination vaccines are then much higher than the additional costs compared to delivering the vaccines under EPI alone and to all delivery modalities of PEV and BSV alone. In the reference transmission setting, for instance, the cost per uncomplicated episode averted of combining BSV with MSTBV, delivered via EPI and mass campaigns, is (at a vaccine price of US$2) US$1.8 and US$2.3 for 70% and 50% coverage (see table S1, Additional file 1), while the cost per DALY averted is US$20 and US$ 22 for 70% and 50% coverage (see table S2, Additional file 1). The costs per DALY averted vary between US$ 12 and US$40 across transmission settings with the lowest value in the lowest transmission setting where the greatest improvement to effectiveness is observed. The very favourable cost-effectiveness ratios in low transmission settings are related to the case-management cost savings, which may compensate up to more than 50% of the costs of the vaccine intervention (see table S4, Additional file 1).
Effect of delivery modalities
Adding boosters to EPI does not improve effectiveness or cases averted over EPI alone by very much even at the very high coverage level modeled, but it does incur additional costs. This delivery modality does therefore not represent a cost-effective alternative to EPI alone in any scenario (see table S1, S2, S3, Additional file 1).
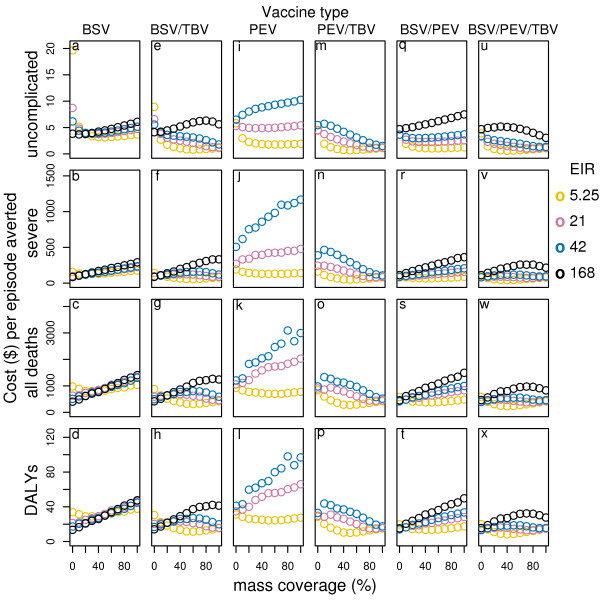
Delivering all vaccines and combinations via population based campaigns improves the effectiveness at mass vaccination coverage of 50%, especially in low transmission settings[14]. Depending on the transmission setting and the vaccine type considered, the incremental costs of delivering vaccines via population based campaigns can be lower than the incremental benefits, leading to a significant reduction in the cost-effectiveness ratios (see table S1, S2, S3, Additional file 1, and Figure 4). Disseminating vaccines via population-based campaign in these cases is predicted to be a more cost-effective way of delivering malaria vaccines than EPI alone. Increasing the coverage of the mass vaccination campaigns increases the effectiveness and cases averted for all vaccine and vaccine combinations under most transmission settings[14]. However, the incremental benefits of increasing coverage are often lower than the incremental costs of achieving it (Figure 5). In some cases, the predictions suggest an optimal cost-effectiveness ratio at intermediate values for the campaign coverage. This is not a consequence of non-proportionality of vaccine delivery costs as a function of coverage (which could be realistic, but not modeled in this study), but of the indirect effects of the vaccines.
Figure 5.
Cost-effectiveness of vaccines given different levels of mass vaccination coverage by transmission setting*. Results obtained assuming a vaccine half-life of 10 years and homogeneity value of 10, and vaccine price of US$2. *data for EIR in some cases are not shown in the figure due to a scale problem.
Effect of vaccine price
Although the simulations focus on comparative cost-effectiveness of different candidate malaria vaccines and delivery modalities, and not on the sensitivity of cost-effectiveness ratios to vaccine prices, which are hypothetical, it is evident that the cost-effectiveness results are almost directly proportional to the vaccine prices. In fact, at an assumed vaccine price of US$ 10 per dose, most cost-effectiveness ratios are between 4 and 7 times higher than those obtained at US$ 2 per dose (see table S1, S2, S3, Additional file 1). At a vaccine price of US$ 2 per dose, most vaccines and delivery modalities simulated present cost-effectiveness ratios comparable to those of other malaria interventions[9,10,41-43], while at a vaccine price of US$ 10 per dose in many of the simulated scenarios the cost-effectiveness ratios are higher.
Discussion
CEA is a method for evaluating the relative efficiency of alternative interventions and thus can provide important information for assessing the potential implications of the numerous malaria vaccine candidates. This study used stochastic simulations of P. falciparum malaria epidemiology, combined with a case management model, to simulate the cost-effectiveness of potential malaria vaccines under various transmission settings and delivered via different modalities. This is an extension of previous research on pre-erythrocytic vaccines delivered via the EPI[12].
The simulations presented suggest that the cost-effectiveness of candidate malaria vaccines is likely to differ substantially according to the transmission intensity and to the delivery modality adopted. They also suggest that alternative vaccine delivery modalities to the EPI may sometimes, but not always, be more cost-effective than the EPI. In general, at moderate vaccine prices, most vaccines and delivery modalities simulated are likely to present cost-effectiveness ratios, which compare favourably with those of other malaria interventions [41-43], making them potential attractive malaria control strategies, from an economic perspective, in malaria endemic countries.
These simulations have various limitations, as described in the companion article on the epidemiological effects[14]. For the economic analysis, one of the most important limitations is related to the relatively simple case management model used to assess the impact of malaria vaccines on the costs to the health system and to patients. As the case management model used is the same for all scenarios simulated, the relative cost-effectiveness of the vaccines modeled, and, therefore, the comparisons, should only be slightly affected by it. However, further research and modeling of health system characteristics in malaria endemic settings is required. Additionally, the vaccine delivery modalities modeled may not be feasible to implement in all settings as the coverage and the effectiveness of malaria vaccines is likely to depend strongly on the characteristics of the health systems where they will be implemented, including any other malaria intervention being delivered. For instance, the simulations assumed an EPI coverage rate of 89%, which is probably higher than found in some malaria-endemic countries. Lower EPI coverage rates could have an impact on the comparisons between different delivery modalities.
Other limitations of this study include that the comparisons of malaria vaccines – or of combinations of them- with different characteristics, are based on the same assumed vaccine price. In practice, the price might vary according to the characteristics of the vaccines, in particular for combinations of vaccines. This might be important for the result that MSTBV combinations were more efficient than vaccines without MSTBV, especially when delivered via EPI with mass campaigns.
While modeling the costs of different vaccine delivery modalities, the fact that vaccine delivery costs might vary as a function of coverage (as it is the case for other interventions[44,45]) was not taken into account. This aspect was not considered due to the lack of solid evidence on vaccine delivery costs by coverage levels, especially for mass campaigns.
Despite these limitations, the simulations presented provide interesting information for vaccine developers on the potentials of different candidate malaria vaccines. Previous simulation of the cost-effectiveness of PEV[12] suggested that at moderate to low vaccine prices, a vaccine providing partial protection, and delivered via the EPI, may be a cost-effective intervention in countries where malaria is endemic. The simulations presented in this article, also show that these types of vaccines are more effective and cost-effective in low transmission settings, and that the additional costs of delivering a PEV under other modalities than the EPI are likely to be higher than the additional health benefits. The only exception is for the scenario of mass vaccination (added to routine EPI) in low transmission and for high vaccine efficacies and high coverage. In contrast to PEV, BSV are predicted to be more effective and cost-effective at higher transmission settings than low transmission.
Combinations of BSV and PEV are predicted to be more efficient than PEV, in particular in moderate to high transmission settings, but compared to BSV, combinations are more cost-effective in mostly moderate to low transmission settings. The cost-effectiveness ratios of the other delivery modalities simulated are higher than those for EPI alone in almost all scenarios, with the exception of adding mass campaigns to EPI in the lowest transmission setting.
Combinations of MSTBV and PEV or PEV and BSV do not increase the effectiveness or the cost-effectiveness compared to PEV and BSV alone when delivered through the EPI (including with the addition of booster doses). However, when applied with EPI and mass vaccinations, combinations with MSTBV provide substantial incremental health benefits and low incremental costs in all transmission settings. These combination vaccines are therefore predicted to be interesting only for the settings where mass vaccination achieving relatively high coverage rates would be feasible.
According to these simulations, adding booster doses to the EPI is unlikely to be a cost-effective alternative to delivering vaccines via the EPI for any vaccine and transmission setting – i.e. the incremental health benefits are rather low despite the additional costs.
Mass vaccination improves effectiveness, especially in low transmission settings, and in some scenario the cost-effectiveness ratios compare favourably with those of delivering the vaccine via the EPI only – the incremental costs are lower than the incremental health benefits. However, increasing the coverage of mass vaccination over 50%, often leads to incremental costs that exceed the incremental health benefits. In some scenarios, the lowest cost-effectiveness ratios are reached at intermediate coverage rates of campaigns. This result is particularly relevant as it is due to the indirect effect of the vaccines, and not to the increasing vaccine delivery costs of achieving high coverage rates.
In some of the mass vaccination scenarios the simulations predict that local elimination of the parasite would be, in principle, possible. In some of these cases, at moderate vaccine prices, the simulations also predict that the cost-effectiveness ratios of achieving local elimination might be relatively low despite the fact that often the incremental costs of achieving high vaccine coverage are higher than the incremental benefits. However, the cost-effectiveness analyses of these simulations include only part of the economic implications of malaria elimination.
If local elimination were feasible, it might be desirable to achieve high vaccine coverage rates even if the incremental costs are high (compared to the incremental health benefits) as elimination would bring future benefits, however to sustain elimination over time, once elimination is achieved there would be a need for strong surveillance and case detection, which would incur substantial additional costs that are not included in our simulations. An assessment of the economic implications of achieving and sustaining local elimination is planned in the next stage of the project.
Conclusion
The simulations presented supports that cost-effectiveness analyses of candidate malaria vaccines may help guide policy makers and vaccine developers, by providing additional evidence that malaria vaccines may be efficient malaria control interventions. The results also indicate that the transmission setting and the vaccine delivery modality adopted are important determinants of the cost-effectiveness of malaria vaccines. While adding booster doses to the EPI is not a cost-effective alternative to the EPI, mass vaccination is predicted to provide substantial health benefits, in particular in low transmission settings, at low additional costs making such a delivery mode, in principle, attractive and feasible, and in some cases lead to local elimination. Nevertheless, achieving high coverage rates can lead to substantial incremental costs compared to the health benefits, while intermediate coverage rates may be a more efficient use of the resources.
While modeling studies such as this one are useful for exploring the potential impact of malaria vaccines at early stages of development, vaccine development and implementation decisions should be also informed by cost-effectiveness studies carefully tailored to the settings where the vaccines are likely to be adopted.
Ultimately, the relative efficiency of malaria vaccines will depend not only on the characteristics of them but also on the other malaria control interventions implemented. As malaria vaccines will eventually be deployed as part of integrated control strategies, the costs and effects of the interactions of vaccine programmes with those of other malaria control interventions should also be evaluated.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
Conceived and designed the study: FT, MP, NM, TS. Analyzed the data: MP, NM, FT. Wrote the paper: FT, TS. Interpretation of the results: FT, MP, NM, TS. Editing of the manuscript: MP. Programming the simulations: AS, MP, NM. Running the simulations: AS, NM.
Supplementary Material
Cost-effectiveness ratios, cost savings, and net costs. the data reported in additional file one are the cost-effectiveness ratios, the cost savings and the net costs, of the different vaccines, by delivery modality and transmission settings
Acknowledgments
Acknowledgements
This project depends on the assistance of many thousands of volunteers who make their computers available to malariacontrol.net, and input to software development from the Africa@home team. Financial support is from the Bill & Melinda Gates Foundation project #39777. We would like to thank Professor Don de Savigny and Professor Marcel Tanner for their help implementing this project and Dr. Allan Shapira for revising the manuscript. The entire responsibility for the study rests of the authors.
Contributor Information
Fabrizio Tediosi, Email: fabrizio.tediosi@unibas.ch.
Nicolas Maire, Email: Nicolas.Maire@unibas.ch.
Melissa Penny, Email: Melissa.Penny@unibas.ch.
Alain Studer, Email: Alain.Studer@unibas.ch.
Thomas A Smith, Email: Thomas-A.Smith@unibas.ch.
References
- Snow R, Guerra C, Noor A, Myint H, Hay S. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood B, Bojang K, Whitty C, Targett G. Malaria. Lancet. 2005:1487–1498. doi: 10.1016/S0140-6736(05)66420-3. [DOI] [PubMed] [Google Scholar]
- Breman JG, Alilio M, Mills A. Conquering the intolerable burden of malaria: what's new, what's needed: a summary. Am J Trop Med Hyg. 2004;71:1–15. [PubMed] [Google Scholar]
- Bojang KMP, Pinder M, Vigneron L, Alloueche A, Kester KE, Ballou WR, Conway D, Reece WHH, Gothard P, Yamuah L, Delchambre M, Voss G, Greenwood BM, Hill A, McAdam KP, Tornieporth N, Cohen JD, Doherty T. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. Lancet. 2001;358:1927–1934. doi: 10.1016/S0140-6736(01)06957-4. [DOI] [PubMed] [Google Scholar]
- Alonso P, Sacarlal J, Aponte J, Leach A, Macete E, Milman J, Mandomando I, Spiessens B, Guinovart C, Espasa M, et al. Efficacy of the RTS, S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet. 2004;364:1411–1420. doi: 10.1016/S0140-6736(04)17223-1. [DOI] [PubMed] [Google Scholar]
- Edejer Tan Torres T. Improving the use of research evidence in guideline development: 11. Incorporating considerations of cost-effectiveness, affordability and resource implications. Health Res Policy Syst. 2006;4:23. doi: 10.1186/1478-4505-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond M, O'Brien B, Stoddart G, Torrance G. Methods for the economic evaluation of health care programmes. Second. New York: Oxford University Press; 1997. [Google Scholar]
- Gold M, Gold S, Weinstein M. Cost-effectiveness in health and medicine. Oxford: Oxford University Press; 1996. [Google Scholar]
- Goodman C, Mills A. The evidence base on the cost-effectiveness of malaria control measures in Africa. Health Policy Plan. 1999;14:301–312. doi: 10.1093/heapol/14.4.301. [DOI] [PubMed] [Google Scholar]
- Goodman CA, Coleman PG, Mills A. Cost-effectiveness of malaria control in sub-Saharan Africa. Lancet. 1999;354:378–385. doi: 10.1016/S0140-6736(99)02141-8. [DOI] [PubMed] [Google Scholar]
- Maire N, Tediosi F, Ross A, Smith T. Predictions of the epidemiologic impact of introducing a pre-erythrocytic vaccine into the expanded program on immunization in sub-Saharan Africa. Am J Trop Med Hyg . 2006;75:111–118. doi: 10.4269/ajtmh.2006.75.111. [DOI] [PubMed] [Google Scholar]
- Tediosi F, Hutton G, Maire N, Smith TA, Ross A, Tanner M. Predicting the cost-effectiveness of introducing a pre-erythrocytic malaria vaccine into the expanded program on immunization in Tanzania. Am J Trop Med Hyg. 2006;75:131–143. doi: 10.4269/ajtmh.2006.75.131. [DOI] [PubMed] [Google Scholar]
- Smith T, Killeen GF, Maire N, Ross A, Molineaux L, Tediosi F, Hutton G, Utzinger J, Dietz K, Tanner M. Mathematical modeling of the impact of malaria vaccines on the clinical epidemiology and natural history of Plasmodium falciparum malaria: Overview. Am J Trop Med Hyg. 2006;75:1–10. doi: 10.4269/ajtmh.2006.75.2_suppl.0750001. [DOI] [PubMed] [Google Scholar]
- Penny M, Maire N, Studer A, Smith T. What should Vaccine Developers Ask? Simulation of the Effectiveness of Malaria Vaccines. PLoS ONE. 2008;3:e3193. doi: 10.1371/journal.pone.0003193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan-Torres Edejer T, Baltussen R, Adam T, Hutubessy R, Acharya A, Evans DB, Murray CJL. Making choices in health: WHO guide to cost-effectiveness analysis. World Health Organization, Geneva; 2003. [Google Scholar]
- Olsen JA, Richardson J. Production gains from health care: what should be included in cost-effectiveness analyses? Soc Sci Med. 1999;49:17–26. doi: 10.1016/S0277-9536(99)00116-1. [DOI] [PubMed] [Google Scholar]
- Drummond M, O'Brien B, Stoddart GL, Torrance G. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 1997. [Google Scholar]
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic sumissions to the BMJ. BMJ. 1996;313:275–283. doi: 10.1136/bmj.313.7052.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luce BR, Elixhauser A. Estimating costs in the economic evaluation of medical technologies. International Journal of Technology Assessment in Health Care. 1990;6:57–75. doi: 10.1017/S026646230000893X. [DOI] [PubMed] [Google Scholar]
- Weinstein M, Siegel J, Gold M, Kamlet M, Russell L. Recommendations of the panel of cost-effectiveness in medicine. JAMA. 1996;276:1253–1341. doi: 10.1001/jama.276.15.1253. [DOI] [PubMed] [Google Scholar]
- Weinstein MC, O'Brien B, Hornberger J, Jackson J, Johannesson M, McCabe C, Luce BR. Principles of good practice of decision analytic modeling in health care evaluation: Report of the ISPOR Task Force on Good Research Practices-Modeling Studies. Value Health. 2003;6:9–17. doi: 10.1046/j.1524-4733.2003.00234.x. [DOI] [PubMed] [Google Scholar]
- Maire N, Smith T, Ross A, Owusu-Agyei S, Dietz K, Molineaux L. A model for natural immunity to asexual blood stages of Plasmodium falciparum malaria in endemic areas. Am J Trop Med Hyg. 2006;75:19–31. doi: 10.4269/ajtmh.2006.75.19. [DOI] [PubMed] [Google Scholar]
- Smith T, Maire N, Ross A, Penny M, Chitnis N, Schapira A, Studer A, Genton B, Lengeler C, Tediosi F, et al. Towards a comprehensive simulation model of malaria epidemiology and control. Parasitology. 2008;135:1507–1516. doi: 10.1017/S0031182008000371. [DOI] [PubMed] [Google Scholar]
- Tediosi F, Maire N, Smith T, Hutton G, Utzinger J, Ross A, Tanner M. An approach to model the costs and effects of case management of Plasmodium falciparum malaria in sub-saharan Africa. Am J Trop Med Hyg. 2006;75:90–103. doi: 10.4269/ajtmh.2006.75.90. [DOI] [PubMed] [Google Scholar]
- Murray CJL, Lopez AD. The global burden of disease: a comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020. Harvard: Harvard University Press; 1996. [Google Scholar]
- Fox-Rushby J, Hanson K. Calculating and presenting disability adjusted life years (DALYs) in cost-effectiveness analysis. Health Policy Plan. 2001;16:326–331. doi: 10.1093/heapol/16.3.326. [DOI] [PubMed] [Google Scholar]
- Indepth Network . Model Life Tables for Sub-Saharan Africa. Aldershot, England: Ashgate; 2004. [Google Scholar]
- Indepth Network . Population, Health and Survival at INDEPTH Sites. Ottawa: IDRC; 2002. [Google Scholar]
- Hutton G, Tediosi F. The costs of introducing a malaria vaccine through the expanded program on immunization in Tanzania. Am J Trop Med Hyg. 2006;75:119–130. doi: 10.4269/ajtmh.2006.75.119. [DOI] [PubMed] [Google Scholar]
- Msd Medical Store Department price Catalogue 2006. 2006. http://www.msd.or.tz
- Most . Cost analysis of the national twice-yearly vitamin A supplementation program in Tanzania. Arlington VA: USAID; 2005. [Google Scholar]
- Adam T, Kakundwa C, Manzi F, Schellenberg JA, Mgalula L, de Savigny D, Mbuya C, Wilczynska K. Analysis report on the costs of IMCI in Tanzania. Multi-country evaluation of the Integrated Management of Childhood Illness (IMCI)., World Health Organization. Geneva: Department of Child and Adolescent Health and Development, World Health Organisation; 2004. [Google Scholar]
- National Malaria Control Program Global Fund 4th round Proposal. Introducing Artemisin Combination Therapy in Tanzania. 2004.
- National Malaria Control Programme . Monitoring Malaria Situation and Control Activities in Tanzania 2001–2003. Health Facility and Community Survey. Edited by Dar es Salaam TNMCPMaE, Programme. UNMC; 2004. [Google Scholar]
- Health Research for Action . Health care financing in Tanzania: Costing study of health services. Final Report. Laarstraat, Belgium; 1999. [Google Scholar]
- Alonso-Gonzalez M, Menendez C, Font F, Kahigwa E, Kimario J, Mshinda H, Tanner M, Bosch-Capblanch X, Alonso PL. Cost-effectiveness of iron supplementation and malaria chemoprophylaxis in the prevention of anaemia and malaria among Tanzanian infants. Bull World Health Organ. 2000;78:97–107. [PMC free article] [PubMed] [Google Scholar]
- Claxton K. Exploring uncertainty in cost-effectiveness analysis. Pharmacoeconomics. 2008;26:781–798. doi: 10.2165/00019053-200826090-00008. [DOI] [PubMed] [Google Scholar]
- Brennan A, Kharroubi S, O'hagan A, Chilcott J. Calculating partial expected value of perfect information via Monte Carlo sampling algorithms. Med Decis Making. 2007;27:448–470. doi: 10.1177/0272989X07302555. [DOI] [PubMed] [Google Scholar]
- Fenwick E, Claxton K, Sculpher MJ, Briggs AH. Improving the efficiency and relevance of health technology assessment: The role of iterative decision analytic modelling. University of York. 2000.
- Felli JC, Hazen GB. Sensitivity analysis and the expected value of perfect information. Med Decis Making. 1998;18:95–109. doi: 10.1177/0272989X9801800117. [DOI] [PubMed] [Google Scholar]
- Goodman CA, Coleman PG, Mills A. Economic analysis of malaria control in sub-Saharan Africa. Geneva: Global Forum for Health Research; 2000. [Google Scholar]
- Goodman CA, Coleman PG, Mills AJ. The cost-effectiveness of antenatal malaria prevention in sub-Saharan Africa. Am J Trop Med Hyg. 2001;64:45–56. doi: 10.4269/ajtmh.2001.64.45. [DOI] [PubMed] [Google Scholar]
- Morel CM, Lauer JA, Evans DB. Cost effectiveness analysis of strategies to combat malaria in developing countries. BMJ. 2005;331:1299. doi: 10.1136/bmj.38639.702384.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns B, Torres TT. Costs of scaling up health interventions: a systematic review. Health Policy Plan. 2005;20:1–13. doi: 10.1093/heapol/czi001. [DOI] [PubMed] [Google Scholar]
- Johns B, Baltussen R. Accounting for the cost of scaling-up health interventions. Health Econ. 2004;13:1117–1124. doi: 10.1002/hec.880. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cost-effectiveness ratios, cost savings, and net costs. the data reported in additional file one are the cost-effectiveness ratios, the cost savings and the net costs, of the different vaccines, by delivery modality and transmission settings