Abstract
One host susceptibility factor for ozone identified in epidemiologic studies is NAD(P)H quinone oxidoreductase 1 (NQO1). We hypothesized that after ozone exposure, NQO1 is required to increase 8-isoprostane (also known as F2-isoprostane) production, a recognized marker of ozone-induced oxidative stress, and to enhance airway inflammation and hyperresponsiveness. In this report, we demonstrate that in contrast to wild-type mice, NQO1-null mice are resistant to ozone and have blunted responses, including decreased production of F2-isoprostane and keratinocyte chemokine, decreased airway inflammation, and diminished airway hyperreponsiveness. Importantly, these results in mice correlate with in vitro findings in humans. In primary human airway epithelial cells, inhibition of NQO1 by dicumarol blocks ozone-induced F2-isoprostane production and IL-8 gene expression. Together, these results demonstrate that NQO1 modulates cellular redox status and influences the biologic and physiologic effects of ozone.
Keywords: ozone, NAD(P)H quinone oxidoreductase 1, F2-isoprostane
CLINICAL RELEVANCE
This study demonstrates in vitro and in vivo that NAD(P)H quinone oxidoreductase 1 is a host susceptibility factor that confers oxidative stress, inflammation, and airway obstruction after ozone exposure in mice. To our knowledge, this is the first demonstration relating a cellular redox pathway with a candidate host susceptibility gene that has been previously identified in epidemiologic investigations of asthma risk.
Individuals living in urban centers of the United States frequently are exposed to ambient concentrations of ozone that exceed clean air standards promulgated by the EPA (0.12 ppm for 1 h or 0.08 ppm for 8 h) (1, 2). In epidemiologic studies, ozone levels have been found to directly correlate with emergency department visits for asthma, school absences, and hospitalization rates (1, 3, 4). Ozone levels below the current national standard cause asthma exacerbations in children on controller medication for moderate to severe asthma (5). In addition, ozone exposure remodels airway structure in developing animals (6).
Ozone is extremely reactive with unsaturated fatty acids at the airway surface liquid–epithelial cell membrane interface and does not penetrate the cell membrane. Ozone reacts with the phospholipids that comprise the plasma membrane to generate lipid ozonation products (LOP), including aldehydes, hydroxyhydroperoxides, and hydrogen peroxide (7). LOPs stimulate the activation of phospholipases (8, 9), and the release of eicosanoids (10) and platelet-activating factor (8). Ozone activates transcription factors, including NF-κB (11, 12), NF–IL-6, and AP-1 (11). IL-8, a cytokine that recruits neutrophils into the airway, is up-regulated after ozone exposure (11). In humans, ozone exposure induces neutrophil-dominant airway inflammation (13) and airway hyperresponsiveness (14–16).
However, in laboratory investigations and small cohort, prospective environmental studies, vulnerability to ozone-induced pulmonary injury varies among healthy individuals. For example, inflammatory cells, eosinophilic cationic protein, IL-8 (17), and bronchial epithelial expression of neutrophil chemotactic factors including ENA-78, IL-8, and GRO-α (17, 18) are increased in bronchoalveolar lavage (BAL) fluids or induced sputum samples from sensitive individuals. These studies suggest that susceptible subjects have enhanced proinflammatory responses to ozone, and support the hypothesis that candidate ozone-susceptibility genes influence oxidant signaling initiated by ozone.
Several genotypes are associated with increased risk of asthma in children living in communities with high-oxidant air pollution. TNF-α–308 (19), Glutathione-S-transferase M1 (GSTM1) (20, 21), and NADPH quinone oxidoreductase 1 (NQO1) polymorphisms (22, 23) have been reported to correspond to increased susceptibility to asthma exacerbation by ozone. The combination of wild-type NQO1 and GSTM1-null confers increased risk of ozone-induced oxidant stress and decline in pulmonary function (23) in healthy adults. Importantly, subjects homozygous for the null-NQO1 genotype, NQO1-C609T (187Ser/Ser), have no detectable NQO1 activity; this polymorphism has a protective effect against asthma in children with GSTM1-null genotype and high lifetime ozone exposure (24). These epidemiologic studies indicate that wild-type NQO1 is required for ozone-induced oxidant signaling, particularly in the presence of impaired antioxidant capacity (GSTM1-null).
We have previously reported that susceptibility to pulmonary inflammation and airway hyperresponsiveness in response to ozone (25) occurs differentially in genetically diverse inbred strains of mice. In the present study, we demonstrate for the first time that the presence of NQO1 is required for ozone-induced F2-isoprostane production and neutrophil chemokine gene regulation in an animal model in vivo and human cells in vitro. We propose that NQO1 is an essential enzyme for translation of ozone-induced oxidative stress to a proinflammatory response in the airways.
MATERIALS AND METHODS
Mice
C57BL/6J mice were purchased from the Jackson Laboratories (Bar Harbor, ME). A breeding colony was established at Duke University from breeding pairs of NQO1-null mice (on a C57BL/6 background), obtained from Dr. Frank Gonzalez at the National Cancer Institute (Bethesda, MD). Male C57BL/6J or NQO1-null mice were used at 6 to 8 weeks of age. Experimental protocols were approved by the Institutional Animal Care and Use Committee at Duke University Medical Center and were performed in accordance with the standards established by the U.S. Animal Welfare Act.
Cell Culture
Primary normal human bronchial epithelial (NHBE) cells were harvested from human tracheobronchial tissues from donors obtained from the Lung Transplant Program and the Department of Pathology, Duke University Medical Center, or were purchased commercially (Clonetics/Lonza, Walkersville, MD). The protocol was approved by the Institutional Review Board for Clinical Investigations, Duke University Medical Center. Cells were plated as previously described on 6- or 12-well Transwell Clear chambers (Corning, Corning, NY), in a serum-free growth factor–supplemented media with all-trans-retinoic acid (RA) added fresh at each medium change (26, 27) and cultured in air–liquid interface (ALI) culture conditions. Experiments were performed at Days 10 to 14 after the change of the culture condition from immersed to ALI. NHBE cells were changed to media supplemented with only two factors, bovine serum albumin and RA, for 24 hours before ozone exposure. Cells were preincubated with dicumarol (10 μM) or the equivalent volume of control vehicle (0.1 M sodium hydroxide) and then coincubated with ozone or filtered air (see Ozone Exposure below).
Ozone Exposure
Mice were exposed to either ozone (OZ) (1 ppm) or filtered air (FA) for 3 hours. The mice were placed individually in stainless steel wire cages and the cages were then placed in a 55-L Hinners-style exposure chamber. Chamber air was kept at 20 to 25°C, and 50 to 65% relative humidity was supplied at a rate of approximately 20 changes/hour. The ozone concentration in the chamber was continuously monitored with an ozone ultraviolet light photometer (Dasibi model 1003AH; Dasibi Environmental Corp., Glendale, CA). Ozone was generated by directing 100% medical grade oxygen through an ultraviolet light ozone generator.
NHBE cells were exposed to either filtered air or 0.4 ppm OZ for 5 hours in in vitro exposure chambers; each gas was provided at 20 L/minute, balanced with 5% CO2, and at 88% relative humidity. Immediately after the exposure, RNA was isolated and cell medium was collected for assessment of IL-8 mRNA expression and 8-isoprostane production, respectively.
Mouse Pulmonary Function Testing
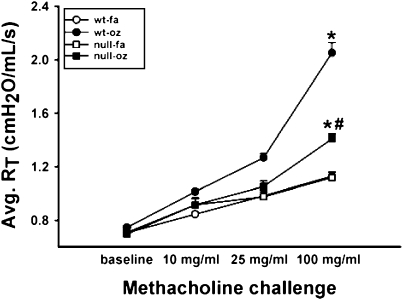
Twenty-four hours after FA or OZ exposure, mice were anesthetized (60 mg/kg Nembutal; Ovation, Deerfield, IL) and surgically prepared with a tracheal cannula, then placed on a computer-controlled ventilator (flexiVent; SCIREQ, Montreal, PQ, Canada) at a constant tidal volume of 6 to 8 ml/kg and a peak expiratory end pressure of 3 cm H2O. The animals were then given a neuromuscular blockade (8 mg/kg Pancuronium Bromide; Sigma-Aldrich, St. Louis, MO) and allowed 5 minutes to adjust to the ventilator. Measurements of airway pressure were made at a side port of the tracheal cannula via a differential pressure transducer, and airway resistance was monitored from pressure and volume data that were generated by applying a 2-second sine wave volume perturbation to the tracheal cannula with an amplitude of 0.2 ml and a frequency of 2.5 Hz. Bronchospasm was induced with methacholine in 0.9% NaCl at increasing concentrations of 10 mg/ml, 25 mg/ml, and 100 mg/ml through an ultrasonic nebulizer (UltraNeb 2000; DeVilbiss, Somerset, PA) placed inline with the ventilator and delivered to the airway cannula for 30 seconds at a rate of 130 breaths/minute. Airway resistance measurements were acquired at baseline and after each methacholine aerosol challenge every 20 seconds for 5 minutes, ensuring that the parameters calculated had peaked. The resistance measurements were then averaged at each dose and graphed linearly (Ave RT cm H2O/mls) along with the initial baseline measurement.
Bronchoalveolar Lavage
Bronchoalveolar lavage (BAL) was performed immediately after exposures or at 6, 12, 24, or 48 hours after air or OZ exposure. The trachea was exposed and intubated with PE-90 tubing (0.86- and 1.27-mm inner and outer diameter, respectively). Sterile saline (3 ml) was instilled 1 ml at a time in the tracheal catheter at a pressure of 25 cm water and retrieved. Return volume was recorded and was consistently greater than 75% of the instilled volume. Cells were isolated by centrifugation (1,500 rpm, 15 min), and the supernatant was stored at −80°C for assessment of cytokine and F2-isoprostane levels. Cells were resuspended in Hanks' balanced salt solution (1 ml) and counted via a hemocytometer. Cell differential was determined from an aliquot of the cell suspension (120 μl) by centrifugation on a slide (Cytospin 3; Shandon, Pittsburgh, PA) and Wright-Giemsa stain (Diff-Quik Stain set; Harleco, Gibbstown, NY). Total and neutrophil cell counts were expressed as number of cells/ ml, means ± SEM for each group of animals.
Total RNA Collection and Real-Time Reverse-Transcriptase Polymerase Chain Reaction
RNA was isolated from NHBE cells or snap frozen mouse lung tissue using Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. For in vivo studies, mouse lungs were snap-frozen and RNA was isolated from pulverized mouse lung tissue using Trizol (1 ml Trizol/100 mg tissue). After quantitation of mouse RNA, a DNase digest step was performed according to the manufacturer's instructions (Invitrogen). RNA was requantitated and one-step real-time RT-PCR was performed for NQO1 on an SDS 7300 machine (Applied Biosystemsm, Foster City, CA) in a 25-μl reaction that included a 6-carboxyfluorescein (FAM) dye-labeled TaqMan minor groove binding (MGB) probe (all real-time RT-PCR reagents and Taqman gene expression assay are from Applied Biosystems) using universal amplification conditions: 50°C for 30 minutes, followed by 95°C for 10 minutes, and then 40 cycles of 95°C, 15 seconds followed by 60°C, 1 minute. Amplification reaction of the β-actin control was similar except that we used a VIC (Applied Biosystems proprietary dye)-labeled probe. For NHBE cells, DNase digest was not required. For NHBE cells, RT-PCR was performed for IL-8 using a Taqman Gene Expression assay from Applied Biosystems according to the manufacturer's instructions and an 18 s rRNA control in a 25-μl reaction as described above for mice. Each sample was amplified in duplicate reactions for both the gene of interest and the control gene. The relative gene expression level was calculated by the ΔΔCt method which represents the fold difference in gene expression corrected for the 18 s rRNA control gene expression, and normalized to the control treated sample (27, 28).
Lung Homogenate Preparation and NQO1 Activity Assay
Lung were harvested from mice; snap frozen; homogenized in buffer containing 50 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM DTT, 1 mM PMSF, 1× protease inhibitor, and 1× phosphatase inhibitor (300 μl/100 mg tissue; all chemicals from Sigma, except EDTA from Invitrogen); sonicated for 3 × 15 seconds; and then incubated with rocking for 1 hour at 4°C. Homogenates were clarified by centrifugation (16,000 × g, 4°C, 30 min). The resulting supernatant corresponded to lung lysate protein, and was quantitated using the DC Protein Assay (Bio-Rad) following the manufacturer's instructions (27, 28).
NQO1 activity was measured in mouse lung lysate protein by a spectrophotometric method (29). Mouse lung glysate protein (400–900 μg) was added to Tris-HCl buffer, pH 7.76 containing 0.2% Tween-20, 2,6-Dichlorophenolindophenol (DCPIP, 80 μM), 0.075% BSA, NADPH (200 μM), in the presence or absence of dicumarol (10 μM), with a total reaction volume of 1 ml (all reagents from Sigma). Enzyme activity was determined by spectrophotometric assay of the dicumarol-inhibitable reduction of DCPIP at 600 nm over time (20 s), and was corrected for protein concentration.
NQO1 Western Analysis
Mouse lung lysate proteins (50 μg) were separated by electrophoresis on a 12% SDS-polyacrylamide gel, transferred to a nitrocellulose membrane, and blocked with 5% nonfat milk in 15 mM Tris, 150 mM NaCl, 0.1% Tween-20 (4°C, overnight or 1 h, room temperature). Membranes were incubated with polyclonal goat anti-NQO1 antibody (1:1,000 [Abcam], room temperature [RT], 1 h), followed by horseradish peroxidase (HRP)-conjugated anti-goat IgG (1:5,000 [Abcam, Cambridge, MA], RT, 1 h). Antigen–antibody complexes were visualized by enhanced chemiluminescence (ECL plus; GE Healthcare Life Sciences, Piscataway, NJ). Membranes were re-probed with a monoclonal antibody to confirm equivalent protein loading. Nonimmune goat IgG or the absence of primary antibody served as negative controls in place of primary NQO1 antibody.
Statistical Analysis
All data were analyzed using the Kruskal-Wallis one-way nonparametric ANOVA and post hoc comparisons by the Wilcoxon rank sum test (30) NQO1 activities were compared using comparison of linear regressions (30). Statistical analysis was performed using Statistix Software (Analytical Software, Tallahasee, FL). Differences were considered significant at P < 0.05.
RESULTS
NQO1 Is Associated with Pulmonary Susceptibility to Ozone In Vivo
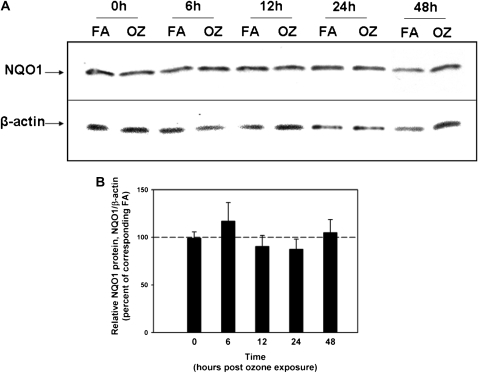
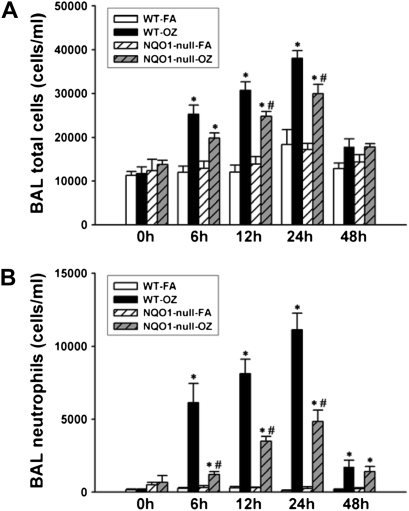
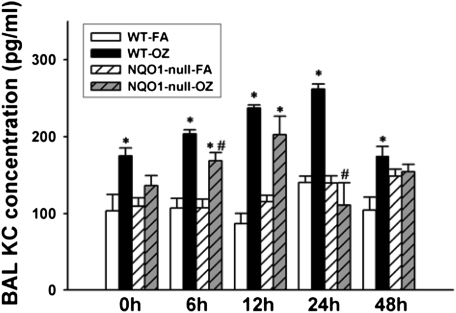
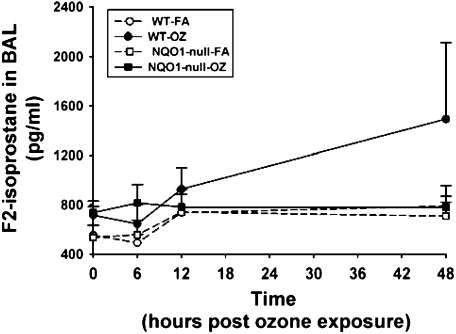
In humans, the combination of the wild-type NQO1 genotype and null-GSTM-1 polymorphism, has been associated with airway obstruction in healthy subjects after ozone exposure (22, 23) and with asthma prevalence in children in Mexico City (24), a city with high levels of ambient ozone. Therefore, we investigated whether NQO1 played an essential and necessary role in susceptibility to ozone-induced oxidant signaling and lung inflammation. We exposed NQO1-null mice and their congenic strain, C57BL6 mice, to FA or OZ. Twenty-four hours after exposures, we evaluated the mice for airway hyperresponsiveness using the flexivent system of impulse oscillometry (Figure 1). We found that absence of NQO1 protected mice from OZ-triggered airway hyperresponsiveness. We next evaluated whether OZ exposure up-regulated NQO1 expression in mouse lungs. Immediately after exposure and up to 48 hours after exposure, lung NQO1 protein levels (Figure 2) and NQO1 activity levels (Table 1) were not significantly altered by OZ in C57BL6 wild-type mice. As expected, NQO1 protein was not detected by Western or by real-time RT-PCR in lung homogenates from the NQO1-null mice (data not shown). Importantly, these results suggest that it is the presence or absence of NQO1 and not increased expression of NQO1 that regulates OZ-induced airway hyperresponsiveness. The suppression of airway hyperresponsiveness in NQO1-null mice correlated temporally with attenuation of OZ-induced airway inflammation in NQO1-null mice (Figure 3). NQO1-null mice had slightly less lung inflammation as measured by total cell counts in the BAL after OZ exposure (Figure 3A); this represented primarily a significant decrease in the number of BAL neutrophils that infiltrated the lung in the NQO1-deficient mice exposed to OZ (Figure 3B). The protection afforded to NQO1-null mice in response to OZ, and attenuation of airway inflammation, also paralleled the decreases in OZ-induced keratinocyte chemokine (KC) in BAL fluids collected from these same mice (Figure 4). Furthermore, OZ induced an increase in oxidant signaling manifest by increased BAL F2-isoprostane content between 12 and 48 hours after ozone exposure that was blunted in NQO1-null mice (Figure 5). These results demonstrate that NQO1 is a necessary factor for amplification of oxidant signaling in lung cells and neutrophil chemokine up-regulation.
Figure 1.
Airway hyperresponsiveness in C57BL/6 wild-type and NAD(P)H quinone oxidoreductase 1 (NQO1)-null mice after filtered air (FA) or ozone (OZ) exposure. Twenty-four hours after OZ or FA exposure, mice were anesthetized for invasive measures of total pulmonary resistance in response to methacholine by impulse oscillometry with the flexivent ventilator (n = 5 animals/group; mean ± SEM; *significantly different from corresponding air control, P < 0.05; #significantly different from WT-ozone exposed animals, P < 0.05).
Figure 2.
NQO1 Western analyses in C57BL/6 wild-type and NQO1-null mice after FA or OZ exposure. C57BL/6 mice and congenic strain, NQO1-null mice were exposed to OZ (1 ppm, 3 h) or FA. Immediately after exposure (0 h) and 6, 12, 24, and 48 hours after OZ or FA exposure, lungs were harvested for NQO1 protein expression. (A) Western analyses were performed for NQO1. Total lung protein (50 μg) was separated by 12% SDS-PAGE, transferred to a nitrocellulose membrane, and incubated with a polyclonal goat-anti NQO1 antibody (1:1,000), followed by HRP-conjugated anti-goat IgG (1:5,000). Antigen–antibody complexes were visualized by chemiluminescence with ECL plus and autoradiography. Western analyses for β-actin were performed to confirm equivalent protein loading. (B) The graph was made from two separate autoradiographs; the 24-hour time point data, from a separate autoradiograph, was added to the autoradiograph which included the data from the other time points. NQO1 band densities were quantitated and expressed relative to β-actin band densities, and then normalized to the corresponding FA-exposed animals. Dashed line represents 0-time control-FA levels (mean ± SEM, n = 5 animals/group).
TABLE 1.
NQO1 ACTIVITY AT 0, 6, 12, 24, OR 48 HOURS AFTER FILTERED AIR OR OZONE EXPOSURE
| Time: | 0 h | 6 h | 12 h | 24 h | 48 h | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exposure: | FA | OZ | FA | OZ | FA | OZ | FA | OZ | FA | OZ |
| NQO1 activity (×10−4, dA/min/μg protein) | 5.82 ± 0.55 | 4.56 ± 0.35 | 4.16 ± 0.63 | 4.65 ± 0.58 | 3.65 ± 0.47 | 3.70 ± 0.62 | 3.95 ± 0.56 | 3.72 ± 0.25 | 3.70 ± 0.39 | 3.49 ± 0.40 |
Definition of abbreviations: FA, filtered air; NQO1, NAD(P)H quinone oxidoreductase 1; OZ, ozone.
NQO1 activity in mouse lungs at each time point after FA or OZ exposure was determined by spectrophotometric assay measuring the dicumarol-inhibitable reduction of a quinone substrate 2,6-Dichlorophenolindophenol, normalized to total protein (n = 5/group, mean ± SEM) (29). There was no difference in NQO1 activity between FA and OZ exposures by ANOVA or by comparison of linear regression analyses. NQO1 activity was not detectable in NQO1-null mice (data not shown).
Figure 3.
Bronchoalveolar lavage total and neutrophil cell counts in C57BL/6 wild-type and NQO1-null mice after FA or OZ exposure. Immediately after FA or OZ exposure (0 h), and 6, 12, 24, and 48 hours after exposure, bronchoalveolar lavage (BAL) was performed as described in Materials and Methods. (A) Total cell counts and (B) neutrophil counts were determined in wild-type and NQO1-null mice exposed to FA or OZ (n = 5 animals/group). Results are expressed as mean ± SEM. *OZ-exposed significantly different from FA-exposed, P < 0.05; #OZ-exposed NQO1-null mice are significantly different from wild-type OZ-exposed mice, P < 0.05.
Figure 4.
Keratinocyte chemoattractant (KC) levels in BAL by ELISA in C57BL/6 wild-type and NQO1-null mice after OZ exposure. C57BL/6 mice and congenic strain, NQO1-null mice were exposed to ozone (1 ppm, 3 h) or FA. Immediately after OZ exposure (0 h), and 6, 12, 24, and 48 hours after exposure, BAL was performed as described in Materials and Methods. BAL was used for ELISA assays to quantitate the neutrophil chemotactic chemokine, KC (n = 5 animals/group), according to the manufacturer's instructions. Results are expressed as mean ± SEM. *OZ-exposed significantly different from FA-exposed, P < 0.05; #wild-type OZ-exposed mice significantly different from OZ-exposed NQO1-null mice, P < 0.05.
Figure 5.
F2-isoprostane levels in BAL by EIA in C57BL/6 wild-type and NQO1-null mice after OZ exposure. BAL was performed at 0, 6, 12, and 48 hours after ozone (OZ) or filtered air (FA), and then analyzed for F2-isoprostane by EIA according to the manufacturer's instructions. BAL KC quantitation is presented as pg/ml (mean ± SEM, n = 5 animals/group).
NQO1 Mediates Human Airway Epithelial Responses to Ozone In Vitro
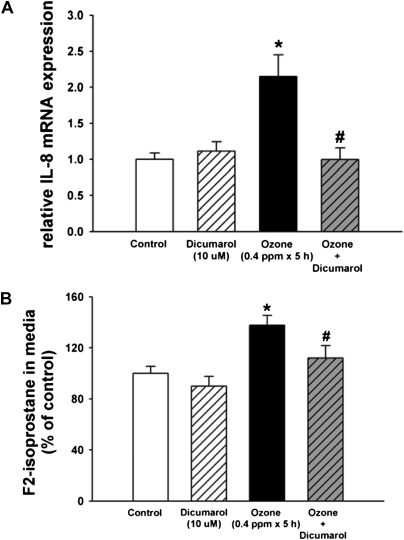
We tested whether NQO1 is necessary for OZ-induced oxidant signaling in human airway epithelial cells, by evaluating IL-8 gene expression in human airway epithelial cells exposed in vitro to ozone. We employed primary cultures of normal human bronchial epithelial cells cultured at air–liquid interface to induce mucociliary differentiation (27). Cells were exposed to FA or OZ (0.4 ppm × 5 h) in the presence or absence of dicumarol (10 μM), a competitive inhibitor of NQO1. RNA was isolated for IL-8 mRNA expression by real-time RT-PCR, and the culture media was collected for EIA analysis of F2-isoprostane. The OZ exposure conditions were based on previous reports demonstrating OZ-induced oxidant stress (31) and up-regulation of IL-8 (11) in airway epithelial cells. Dicumarol (10 μM) blocks NQO1 activity in NHBE cells based on spectrophotometric assays of enzyme activity and inhibition of neutrophil elastase (NE)-generated reactive oxygen species (27). We found that dicumarol blocked the OZ-induced increase in IL-8 mRNA expression (Figure 6A), suggesting that NQO1 is required for IL-8 gene up-regulation after OZ exposure. Dicumarol also inhibited ozone-generated F2-isoprostane production (Figure 6B). Taken together, the in vivo mouse model results and the human in vitro data strongly suggest that NQO1 is required for OZ-induced pulmonary oxidant signaling and KC/IL-8 mRNA expression.
Figure 6.
IL-8 gene expression by real-time RT-PCR and F2-isoprostane quantitation by EIA in cultured primary normal human bronchial epithelial cells after OZ exposure. NHBE cells cultured at air–liquid interface (ALI) were preincubated and coincubated with NQO1 inhibitor, dicumarol (10 nM, 1 h), or control vehicle at the apical and basolateral compartments, and then exposed to OZ (0.4 ppm, 5 h) or FA with media ± dicumarol only in the basolateral compartments. At the end of the exposure period, total RNA was collected using Trizol reagent, and medium was collected for F2-isoprostane measurements by EIA. As per the manufacturer's instructions, BHT was added to the medium to a final concentration of 0.005%. (A) Real-time RT-PCR analysis of IL-8 mRNA expression was performed as described in Materials and Methods (n = 6). (B) F2-isoprostane levels in the cell culture medium were quantitated and expressed as a percentage of the control for each experiment (n = 11–12; two to four separate experiments, mean ± SEM are shown). *OZ-exposed cells significantly greater than control, P < 0.05; #OZ+dicumarol–exposed cells were significantly different from OZ-exposed cells, P < 0.05.
DISCUSSION
Our results suggest that NQO1 is a key factor for cellular regulation of pulmonary tissue and epithelial cell susceptibility to ozone. In addition, we have demonstrated that absence or inhibition of NQO1 blocked OZ-induced F2-isoprostane production, diminished IL-8 mRNA or KC protein expression, decreased neutrophilic inflammation in the lung, and attenuated airway hyperresponsiveness.
NQO1 is well positioned as a control factor for OZ susceptibility due to its localization and cellular functions in the lung. NQO1 is highly expressed in airway epithelial cells (32). Although NQO1 is predominantly a cytosolic enzyme, under oxidant stress conditions, NQO1 may translocate to the plasma membrane (33, 34), the initial site of OZ deposition upon epithelial surfaces. NQO1 uses either NADH or NADPH as a reducing cofactor to catalyze the obligate two-electron reduction of quinones to hydroquinones (35). Depending on the quinone substrate, NQO1 can function as an anti- or pro-oxidant enzyme. Thus, NQO1 may reduce endogenous quinones such as ubiquinone and α-tocopherone; these reduced molecules have antioxidant properties and serve to protect cellular membranes against lipid peroxidation (36). However, NQO1 may also catalyze the reduction of a hydroquinone to a redox-labile product and consequently generate ROS (36–39). Comproportionation reactions between substrates and reduced quinone products contribute to the generation of semiquinones and superoxide (38).
We have recently reported that NE, an important mediator in many inflammatory airway diseases, up-regulates mucin MUC5AC gene expression in vitro in normal human bronchial epithelial cells by a NQO1-dependent mechanism (27). Others have demonstrated that NE levels in BAL collected from OZ-exposed human volunteers are significantly elevated (13). Importantly, NE induces lipid peroxidation, as assayed by lipid carbonyl production, a biomarker of oxidative stress, and this effect is inhibited by dicumarol (27). Thus, this prior report suggests that NQO1 exerts a key role in reactive oxygen species (ROS) signaling that is a requirement for NE-induced MUC5AC gene expression.
Dicumarol has other functions in addition to being an inhibitor of NQO1. It blocks murine glutathione S transferase A1-1, mitochondrial electron transport, microtubule stabilization, and SAPK/JNK activation (27). These effects may augment oxidant stress after dicumarol treatment; in contrast, our results using dicumarol are consistent with decreased production of F2-isoprostane, a marker of oxidative stress, and its role as a competitive inhibitor of NQO1.
NQO1 generates ROS through the bioactivation of chemotherapeutic agents, resulting in ROS generation in several cancers. The antitumor agent β-lapachone selectively kills non–small cell lung cancer cells that overexpress NQO1. This agent stimulates ROS generation, and subsequent DNA damage and poly(ADP-ribose) polymerase-1–mediated cell death, and both responses rely upon NQO1-dependent mechanisms (40). Other anti-cancer chemotherapeutic agents have also been reported to take advantage of the bioactivation/bioreductive activity of NQO1. Phenothiazinium redox cyclers induce cancer cell apoptosis by an NQO1-dependent bioreductive generation of ROS (41). NQO1 has also been reported to bioactivate antitumor quinones such as RH1, resulting in cytotoxicity as measured by growth inhibition and DNA strand breaks (42).
The balance between NQO1 anti- and pro-oxidant functions influences the redox state of the cell, and therefore influences the balance of downstream ROS signaling molecules such as isoprostanes. Isoprostanes are free radical–generated peroxidation products of arachidonic acid. F2-isoprostane is considered a measure of ROS production or oxidative stress (43). In vivo, F2-isoprostane has biologic activity in the lung (44, 45). In guinea pigs, intratracheal installation of F2-isoprostane increases airway resistance and increases airway plasma exudation into the airways (46). Similarly, in a hyperoxia-exposed rat model, F2-isoprostanes correlate with pulmonary oxygen toxicity and pulmonary plasma exudation (47). F2-isoprostanes also induce vasoconstriction in rabbits, dogs, and humans (48, 49). However, we do not as yet know whether F2-isoprostanes directly regulate ozone-induced inflammation or proinflammatory cytokine expression in airway epithelial cells; or whether F2-isoprostanes indicate the presence of other ROS responsible for these functions (50).
NQO1, in both our in vivo mouse studies and in vitro human cells, support linking OZ (oxidant) exposure with inflammatory (cellular and neutrophilic cytokine) tissue responses. Deficiency in NQO1 activity (supported by results in the NQO1-null mice) decreased OZ-induced inflammation and, by extension, may be the mechanism that protects humans with the NQO1-null genotype (187 Ser/Ser) from developing airflow obstruction acutely after exposure to OZ.
We demonstrate both in vivo and in vitro that the presence of NQO1 is necessary for up-regulation of IL-8/KC mRNA expression, and neutrophilic inflammation after OZ exposure. In addition, we found that inhibition of NQO1 diminished the production of the OZ-activated ROS marker, 8-isoprostane/F2-isoprostane. Others have reported that after OZ exposure of human volunteers, F2-isoprostanes are significantly elevated in BAL fluids (51) and expired breath condensates compared with FA exposures. Together these results show a strong association between increased 8-isoprostane/F2-isoprostane levels and neutrophilic inflammation after OZ exposure. Importantly, to our knowledge, this is the first demonstration relating a cellular redox pathway with a candidate host susceptibility gene that has been previously identified in epidemiologic or clinical investigations of asthma risk. Controlled laboratory exposure studies in humans have suggested that dietary (Vitamin C and α-tocopherol) antioxidant supplementation ameliorates ozone-induced acute changes in airway obstruction (52). We provide proof of concept that the targeted approach of acute inhibition of a host factor that influences cellular redox status can mitigate the deleterious effects of ozone.
Acknowledgments
The authors thank Denise Lopez Domowicz for technical assistance.
This work was supported by National Institutes of Health grants HL082504 (J.A.V.), HL081763 (B.M.F.), ES07943 (A.K.J.), ES011961, and ES012496 (W.M.F.).
Originally Published in Press as DOI: 10.1165/rcmb.2008-0381OC on December 4, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.American Thoracic Society. Health effects of outdoor air pollution. Part 2. Committee of the Environmental and Occupational Health Assembly of the American Thoracic Society. Am J Respir Crit Care Med 1996;153:477–498. [DOI] [PubMed] [Google Scholar]
- 2.Peden DB. The epidemiology and genetics of asthma risk associated with air pollution. J Allergy Clin Immunol 2005;115:213–219. (quiz 220). [DOI] [PubMed] [Google Scholar]
- 3.Foster W. Effects of oxidants. In: Swift D, Foster W, editors. Air pollutants and the respiratory tract. New York: Marcel Dekker; 1999. pp. 147–179.
- 4.Trasande L, Thurston GD. The role of air pollution in asthma and other pediatric morbidities. J Allergy Clin Immunol 2005;115:689–699. [DOI] [PubMed] [Google Scholar]
- 5.Gent JF, Triche EW, Holford TR, Belanger K, Bracken MB, Beckett WS, Leaderer BP. Association of low-level ozone and fine particles with respiratory symptoms in children with asthma. JAMA 2003;290:1859–1867. [DOI] [PubMed] [Google Scholar]
- 6.Fanucchi MV, Plopper CG, Evans MJ, Hyde DM, Van Winkle LS, Gershwin LJ, Schelegle ES. Cyclic exposure to ozone alters distal airway development in infant rhesus monkeys. Am J Physiol Lung Cell Mol Physiol 2006;291:L644–L650. [DOI] [PubMed] [Google Scholar]
- 7.Pryor WA, Squadrito GL, Friedman M. The cascade mechanism to explain ozone toxicity: the role of lipid ozonation products. Free Radic Biol Med 1995;19:935–941. [DOI] [PubMed] [Google Scholar]
- 8.Wright DT, Adler KB, Akley NJ, Dailey LA, Friedman M. Ozone stimulates release of platelet activating factor and activates phospholipases in guinea pig tracheal epithelial cells in primary culture. Toxicol Appl Pharmacol 1994;127:27–36. [DOI] [PubMed] [Google Scholar]
- 9.Kafoury RM, Pryor WA, Squadrito GL, Salgo MG, Zou X, Friedman M. Lipid ozonation products activate phospholipases A2, C, and D. Toxicol Appl Pharmacol 1998;150:338–349. [DOI] [PubMed] [Google Scholar]
- 10.Leikauf GD, Zhao Q, Zhou S, Santrock J. Ozonolysis products of membrane fatty acids activate eicosanoid metabolism in human airway epithelial cells. Am J Respir Cell Mol Biol 1993;9:594–602. [DOI] [PubMed] [Google Scholar]
- 11.Jaspers I, Flescher E, Chen LC. Ozone-induced IL-8 expression and transcription factor binding in respiratory epithelial cells. Am J Physiol 1997;272:L504–L511. [DOI] [PubMed] [Google Scholar]
- 12.Nichols BG, Woods JS, Luchtel DL, Corral J, Koenig JQ. Effects of ozone exposure on nuclear factor-kappaB activation and tumor necrosis factor-alpha expression in human nasal epithelial cells. Toxicol Sci 2001;60:356–362. [DOI] [PubMed] [Google Scholar]
- 13.Koren HS, Devlin RB, Graham DE, Mann R, McGee MP, Horstman DH, Kozumbo WJ, Becker S, House DE, McDonnell WF, et al. Ozone-induced inflammation in the lower airways of human subjects. Am Rev Respir Dis 1989;139:407–415. [DOI] [PubMed] [Google Scholar]
- 14.Holtzman MJ, Fabbri LM, O'Byrne PM, Gold BD, Aizawa H, Walters EH, Alpert SE, Nadel JA. Importance of airway inflammation for hyperresponsiveness induced by ozone. Am Rev Respir Dis 1983;127:686–690. [DOI] [PubMed] [Google Scholar]
- 15.Seltzer J, Bigby BG, Stulbarg M, Holtzman MJ, Nadel JA, Ueki IF, Leikauf GD, Goetzl EJ, Boushey HA. O3-induced change in bronchial reactivity to methacholine and airway inflammation in humans. J Appl Physiol 1986;60:1321–1326. [DOI] [PubMed] [Google Scholar]
- 16.Foster W, Brown R, Macri K, Mitchell C. Bronchial reactivity of healthy subjects: 18–20 h postexposure to ozone. J Appl Physiol 2000;89:1804–1810. [DOI] [PubMed] [Google Scholar]
- 17.Hiltermann JT, Lapperre TS, van Bree L, Steerenberg PA, Brahim JJ, Sont JK, Sterk PJ, Hiemstra PS, Stolk J. Ozone-induced inflammation assessed in sputum and bronchial lavage fluid from asthmatics: a new noninvasive tool in epidemiologic studies on air pollution and asthma. Free Radic Biol Med 1999;27:1448–1454. [DOI] [PubMed] [Google Scholar]
- 18.Bosson J, Stenfors N, Bucht A, Helleday R, Pourazar J, Holgate ST, Kelly FJ, Sandstrom T, Wilson S, Frew AJ, et al. Ozone-induced bronchial epithelial cytokine expression differs between healthy and asthmatic subjects. Clin Exp Allergy 2003;33:777–782. [DOI] [PubMed] [Google Scholar]
- 19.Li YF, Gauderman WJ, Avol E, Dubeau L, Gilliland FD. Associations of tumor necrosis factor G-308A with childhood asthma and wheezing. Am J Respir Crit Care Med 2006;173:970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romieu I, Sienra-Monge JJ, Ramirez-Aguilar M, Moreno-Macias H, Reyes-Ruiz NI, Estela del Rio-Navarro B, Hernandez-Avila M, London SJ. Genetic polymorphism of GSTM1 and antioxidant supplementation influence lung function in relation to ozone exposure in asthmatic children in Mexico City. Thorax 2004;59:8–10. [PMC free article] [PubMed] [Google Scholar]
- 21.Romieu I, Ramirez-Aguilar M, Sienra-Monge JJ, Moreno-Macias H, del Rio-Navarro BE, David G, Marzec J, Hernandez-Avila M, London S. GSTM1 and GSTP1 and respiratory health in asthmatic children exposed to ozone. Eur Respir J 2006;28:953–959. [DOI] [PubMed] [Google Scholar]
- 22.Corrradi M, Alinovi R, Goldoni M, Vettori MV, Folesani G, Mozzoni P, Cavazzini S, Bergamaschi E, Rossi L, Mutti A. Biomarkers of oxidative stress after controlled human exposure to ozone. Toxicol Lett 2002;134:219–225. [DOI] [PubMed] [Google Scholar]
- 23.Bergamaschi E, De Palma G, Mozzoni P, Vanni S, Vettori MV, Broeckaert F, Bernard A, Mutti A. Polymorphism of quinone-metabolizing enzymes and susceptibility to ozone-induced acute effects. Am J Respir Crit Care Med 2001;163:1426–1431. [DOI] [PubMed] [Google Scholar]
- 24.David G, Romieu I, Sienra-Monge JJ, Collins WJ, Ramirez-Auilar M, del Rio-Navarro BE, Reyes-Ruiz NI, Morris RW, Marzec JM, London SJ. Nicotinamide adenine dinucleotide (Phosphate) reduced:quinone oxidoreductase and glutathione s-transferase M1 polymorphisms and childhood asthma. Am J Respir Crit Care Med 2003;168:1199–1204. [DOI] [PubMed] [Google Scholar]
- 25.Savov JD, Whitehead GS, Wang J, Liao G, Usuka J, Peltz G, Foster WM, Schwartz DA. Ozone-induced acute pulmonary injury in inbred mouse strains. Am J Respir Cell Mol Biol 2004;31:69–77. [DOI] [PubMed] [Google Scholar]
- 26.Krunkosky TM, Martin LD, Fischer BM, Voynow JA, Adler KB. Effects of TNFalpha on expression of ICAM-1 in human airway epithelial cells in vitro: oxidant-mediated pathways and transcription factors. Free Radic Biol Med 2003;35:1158–1167. [DOI] [PubMed] [Google Scholar]
- 27.Zheng S, Byrd AS, Fischer BM, Grover AR, Ghio AJ, Voynow JA. Regulation of MUC5AC expression by NAD(P)H:quinone oxidoreductase 1. Free Radic Biol Med 2007;42:1398–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Voynow JA, Fischer BM, Malarkey DE, Burch LH, Wong T, Longphre M, Ho SB, Foster WM. Neutrophil elastase induces mucus cell metaplasia in mouse lung. Am J Physiol Lung Cell Mol Physiol 2004;287:L1293–L1302. [DOI] [PubMed] [Google Scholar]
- 29.Ernster LDT. Diaphorase. Methods Enzymol 1967;10:309–317. [Google Scholar]
- 30.Snedecor GW, Cochran WG. Statistical methods. Ames: Iowa State University Press; 1980.
- 31.Ghio AJ, Turi JL, Madden MC, Dailey LA, Richards JD, Stonehuerner JG, Morgan DL, Singleton S, Garrick LM, Garrick MD. Lung injury after ozone exposure is iron dependent. Am J Physiol Lung Cell Mol Physiol 2007;292:L134–L143. [DOI] [PubMed] [Google Scholar]
- 32.Siegel D, Franklin WA, Ross D. Immunohistochemical detection of NADPH:Quinone oxidoreductase in human lung and lung tumors. Clin Cancer Res 1998;4:2065–2070. [PubMed] [Google Scholar]
- 33.Navarro F, Arroyo A, Martin SF, Bello RI, de Cabo R, Burgess JR, Navas P, Villalba JM. Protective role of ubiquinone in vitamin E and selenium-deficient plasma membranes. Biofactors 1999;9:163–170. [DOI] [PubMed] [Google Scholar]
- 34.Navarro F, Navas P, Burgess JR, Bello RI, De Cabo R, Arroyo A, Villalba JM. Vitamin E and selenium deficiency induces expression of the ubiquinone-dependent antioxidant system at the plasma membrane. FASEB J 1998;12:1665–1673. [DOI] [PubMed] [Google Scholar]
- 35.Ross D, Kepa JK, Winski SL, Beall HD, Anwar A, Siegel D. NADPH:quinone oxidoreductase 1 (NQO1): chemoprotection, bioactivation, gene regulation and genetic polymorphisms. Chem Biol Interact 2000;129:77–97. [DOI] [PubMed] [Google Scholar]
- 36.Ross D, Siegel D. NADPH:Quinone Oxidoreductase 1 (NQO1, Dt-Diaphorase), functions and pharmacogenetics. Methods Enzymol 2004;382:115–145. [DOI] [PubMed] [Google Scholar]
- 37.Cadenas E. Antioxidant and prooxidant functions of DT-diaphorase in quinone metabolism. Biochem Pharmacol 1995;49:127–140. [DOI] [PubMed] [Google Scholar]
- 38.Watanabe N, Forman HJ. Autooxidation of extracellular hydroquinones is a causative event for the cytotoxicity of menadione and DMNQ in A549-S cells. Arch Biochem Biophys 2003;411:145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brar S, Kennedy TP, Whorton AR, Sturrock AB, Huecksteadt TP, Ghio AJ, Hoidal JR. Reactive oxygen species from NAD(P)H: quinone oxidoreductase constitutively activate NF-kB in malignant melanoma cells. Am J Physiol Cell Physiol 2001;280:C659–C676. [DOI] [PubMed] [Google Scholar]
- 40.Bey EA, Bentle MS, Reinicke KE, Dong Y, Yang CR, Girard L, Minna JD, Bornmann WG, Gao J, Boothman DA. An NQO1- and PARP-1-mediated cell death pathway induced in non-small-cell lung cancer cells by beta-lapachone. Proc Natl Acad Sci USA 2007;104:11832–11837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wondrak GT. NQO1-activated phenothiazinium redox cyclers for the targeted bioreductive induction of cancer cell apoptosis. Free Radic Biol Med 2007;43:178–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Winski SL, Swann E, Hargreaves RH, Dehn DL, Butler J, Moody CJ, Ross D. Relationship between NAD(P)H:quinone oxidoreductase 1 (NQO1) levels in a series of stably transfected cell lines and susceptibility to antitumor quinones. Biochem Pharmacol 2001;61:1509–1516. [DOI] [PubMed] [Google Scholar]
- 43.Morrow JD, Roberts LJ. The isoprostanes: their role as an index of oxidant stress status in human pulmonary disease. Am J Respir Crit Care Med 2002;166:S25–S30. [DOI] [PubMed] [Google Scholar]
- 44.Janssen LJ, Catalli A, Helli P. The pulmonary biology of isoprostanes. Antioxid Redox Signal 2005;7:244–255. [DOI] [PubMed] [Google Scholar]
- 45.Janssen LJ. Isoprostanes: an overview and putative roles in pulmonary pathophysiology. Am J Physiol Lung Cell Mol Physiol 2001;280:L1067–L1082. [DOI] [PubMed] [Google Scholar]
- 46.Okazawa A, Kawikova I, Cui ZH, Skoogh BE, Lotvall J. 8-Epi-PGF2alpha induces airflow obstruction and airway plasma exudation in vivo. Am J Respir Crit Care Med 1997;155:436–441. [DOI] [PubMed] [Google Scholar]
- 47.Vacchiano CA, Tempel GE. Role of nonenzymatically generated prostanoid, 8-iso-PGF2 alpha, in pulmonary oxygen toxicity. J Appl Physiol 1994;77:2912–2917. [DOI] [PubMed] [Google Scholar]
- 48.Banerjee M, Kang KH, Morrow JD, Roberts LJ, Newman JH. Effects of a novel prostaglandin, 8-epi-PGF2 alpha, in rabbit lung in situ. Am J Physiol 1992;263:H660–H663. [DOI] [PubMed] [Google Scholar]
- 49.Janssen LJ, Premji M, Netherton S, Coruzzi J, Lu-Chao H, Cox PG. Vasoconstrictor actions of isoprostanes via tyrosine kinase and Rho kinase in human and canine pulmonary vascular smooth muscles. Br J Pharmacol 2001;132:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Repine JE, Bast A, Lankhorst I. Oxidative stress in chronic obstructive pulmonary disease: Oxidative Stress Study Group. Am J Respir Crit Care Med 1997;156:341–357. [DOI] [PubMed] [Google Scholar]
- 51.Hazbun ME, Hamilton R, Holian A, Eschenbacher WL. Ozone-induced increases in substance P and 8-epi-prostaglandin F2 alpha in the airways of human subjects. Am J Respir Cell Mol Biol 1993;9:568–572. [DOI] [PubMed] [Google Scholar]
- 52.Samet JM, Hatch GE, Horstman D, Steck-Scott S, Arab L, Bromberg PA, Levine M, McDonnell WF, Devlin RB. Effect of antioxidant supplementation on ozone-induced lung injury in human subjects. Am J Respir Crit Care Med 2001;164:819–825. [DOI] [PubMed] [Google Scholar]