Abstract
IL-10 is a potent, endogenous anti-inflammatory cytokine known to decrease cytokine and keratinocyte-derived chemokine (KC) expression. Traditionally, in vivo effects of IL-10 were extrapolated from studies employing systemic antibody neutralization. As a result, divergent data regarding the protective and/or harmful roles of IL-10 have been reported. In this study, we used a lung-specific, tetracycline-inducible IL-10 overexpression–transgenic (IL-10 OE) mouse to study the effects of IL-10 overexpression on Pseudomonas aeruginosa–induced lung inflammation and corresponding survival in mice. Overexpression of IL-10 in the lung significantly increased mortality. During the early phase after infection (6-hours after infection), neutrophil recruitment as well as cytokine (TNF-α) and chemokine (KC) expression were significantly decreased in the IL-10 OE mice, which resulted in attenuated bacterial clearance. In contrast, overzealous production of KC and TNF-α intensified neutrophil infiltration and increased vascular leakage in IL-10 OE mice at the later stage of infection (24 hours after infection). Neutrophil depletion showed impaired bacterial clearance in both control and IL-10 OE mice, and further enhanced mouse mortality, whereas exogenous administration of KC reversed this finding. Our data indicate that early neutrophil recruitment is important for combating bacterial infection, and that the inhibition of neutrophil recruitment by IL-10 results in insufficient bacteria clearance in the lung, leading to excessive development of inflammation and increased mortality.
Keywords: IL-10, Pseudomonas, neutrophils, chemokines
CLINICAL RELEVANCE
This work provides insight on how counterregulatory molecules have dual effects on regulation, inflammation, and subsequent pathogen clearance. When they are not properly regulated, the consequences can be equally as detrimental as dysregulated proinflammation.
IL-10 is an important anti-inflammatory cytokine, and plays an important role in down-regulating TNF-α, IL-1, and members of both the CXC and CC keratinocyte-derived chemokine (KC) families (1). We have previously examined the role of IL-10 in regulating acute pulmonary inflammation (2, 3). Exogenous administration of recombinant IL-10 in a rat model of immune complex–mediated alveolitis abrogated the inflammatory response in a manner that was linked to decreased expression of TNF-α expression (4). We and others have also reported on the ability of IL-10 to abrogate chemokine expression in lung inflammation, thereby diminishing neutrophil influx (3); however, the effects of IL-10 regulation could lead to opposite outcomes when different model systems employing live pathogens are used. For example, in models of sepsis the neutralization of IL-10 exaggerated proinflammatory cytokine expression and death (5–7); however, in bacterial pneumonia, the effect of IL-10 on inhibiting this protective innate immune response may be detrimental. For example, neutralization of IL-10 improved survival in a murine model of Klebsiella pneumonia, which was concluded to be a result of increased inflammation and thus improved bacterial killing (8). In a model of sepsis using cecal ligation and puncture, elevated IL-10 was accompanied by increased mortality after a secondary Pseudomonas aeruginosa challenge, whereas blockade of IL-10 improved survival as well as bacterial clearance (9, 10). However, these studies were limited by their inability to examine the function of IL-10 specifically in the lung.
As described previously (11), we developed a transgenic mouse capable of regulatable, lung-specific IL-10 overexpression. Regulation of lung-specific overexpression was achieved by placing the reverse tetracycline transactivator under the control of a rat Clara cell secretory protein promoter so that addition of tetracycline to the chow resulted in rapid (within 24 hours) IL-10 overexpression in the lung (11). Our previous study found that acute lung inflammation was attenuated when the mice were challenged by direct intratracheal injection with LPS in a manner associated with in vivo inhibition of IκK and NF-κB. However, in the setting of a lung challenge with a viable bacterial pathogen, we hypothesized that IL-10 overexpression would alter the immune response and attenuate bacterial clearance. To address this question, we modeled bacterial pneumonia by infecting mice with P. aeruginosa via intratracheal challenge. We investigated the effects of IL-10 on bacterial clearance and mouse survival, and explored the mechanism whereby IL-10 overexpression resulted in enhanced P. aeruginosa–induced lethality.
MATERIALS AND METHODS
Animals
All in vivo studies were performed in accordance with the National Institutes of Health guidelines and University of Michigan's Committee on Use and Care of Animals. The tetracycline-inducible and lung-specific human IL-10 overexpression–transgenic (IL-10 OE) mice were established and characterized previously in the lab (11). To achieve regulatable, lung-specific overexpression of human IL-10, bitransgenic mice possessing reverse tetracycline transactivator under the control of Clara cell secretory protein promoter and a tetracycline-dependent, CMV promoter–driven human IL-10 transgene (tet-O-CMV-IL-10), were bred to homozygosity. In preliminary studies, access to chow containing tetracycline (0.0625%), which was purchased as TestDiet from Purina (Richmond, IN), resulted in rapid (within 24 hours) IL-10 overexpression only in the lungs of mice possessing both transgenes, but in neither single transgenic line alone. Further, bitransgenic mice not provided access to chow containing tetracycline had no detectable expression of human IL-10 (i.e., no transgene “leak” was detected), and no phenotype different from all other control conditions. In light of this observation, and to control for the independent effect of the antibiotic-containing chow on P. aeruginosa clearance, single transgenic mice on the FVB/n genetic background and containing only one of the two active transgenes (tet-O-CMV-IL-10) were provided tetracycline-containing chow, and served as controls. In all cases, mice were provided tetracycline chow ad libidum 5 days before the experiments.
Intratracheal Infection with P. aeruginosa
Bacteria (P. aeruginosa [PA19660 Strain; American Type Culture Collection, Manassas, VA]) were grown in tryptic soy broth (Difco; BD Diagnostics, Franklin Lakes, NJ) for 18 hours at 37°C. The bacteria culture was diluted to the desired concentration. Animals were anesthetized with isoflurane/oxygen mixed using a self-scavenging anesthesia machine (Surgivet, Waukesha, WI). The trachea was exposed by blunt dissection, and the targeted number of bacteria (based on CFUs) in 40 μl PBS was inoculated via a sterile 30-gauge needle during several cycles of inspiration. The skin incision was closed using surgical staples.
Determination of Whole-Lung P. aeruginosa CFUs
At the indicated time points, mice were killed by CO2 overdose and the pulmonary vascular system flushed by perfusing the pulmonary artery with 2 ml PBS. Whole lungs were removed en bloc and the lungs were homogenized in 1 ml PBS with protease inhibitor (Roche Diagnostics, Indianapolis, IN). Homogenates were then serially diluted 1:5 in PBS and plated on blood agar to determine lung CFUs.
Bronchoalveolar Lavage and Polymorphonuclear Leukocyte Counts
At the times indicated after intratracheal challenge, the trachea was exposed and intubated using a 1.7-mm (outside diameter) catheter. Bronchoalveolar lavage (BAL) was performed by instilling 1 ml normal saline, followed by gentle suction with an approximate 0.8-ml volume return. Lavaged cells from each individual mouse were pooled and counted with a hemacytometer. Neutrophil numbers were derived from differential counts determined on the same BAL fluid (BALF) samples subjected to Cytospin centrifugation (700 × g, 5 min) with a slide centrifuge (Shandon, Shandon, PA) that were then stained with Diff-Quick products (Baxter, Miami, FL) for determination of the percent neutrophils.
Whole-Lung Pulmonary Leukocyte Isolation
Animals were killed and lungs perfused via the right heart using PBS until pulmonary vessels were grossly clear. Lungs were harvested and minced to a slurry, and each specimen was suspended in a digest solution containing collagenase (15 mg), DNase I (250 KU units), and complete media (RPMI–10% FCS). The suspension was incubated on a rocker for 30 minutes at 37°C. The cells were dispersed by repetitive suction through a 10 cc syringe and then spun down at 1,100 rpm for 10 minutes. After the supernatant was decanted, each pellet was briefly resuspended with 1-ml sterile distilled, deionized H2O to lyse red blood cells, and then recentrifuged. Cell pellets were resuspended in 5 ml of complete media and passed through a 70-μm cell strainer. Cells were then used for differerential counting.
Lung Myeloperoxidase Assay
Lung myeloperoxidase (MPO) activity (as an assessment of neutrophil influx) was quantitated by a method described previously (12). Briefly, whole lungs were homogenized in 1 ml of a homogenate buffer containing 50 mM potassium phosphate buffer (pH 6.0), with 0.5% hexadecyltrimethylammonium bromide and 5 mM EDTA. The samples were sonicated and centrifuged at 20,000 × g for 15 minutes. The supernatant was mixed 1:15 with assay buffer containing 100 mM potassium phosphate (pH 6.0), with 0.005% H2O2 and 0.5 mM o-dianisidine dihydrochloride, and read at 460 nm in a spectrophotometer (Molecular Devices, Sunnyvale, CA). MPO units were calculated as the change in absorbance over time.
Lung KC, TNF-α, and IL-10 Measurement
Lung KC, TNF-α, mouse IL-10, and human IL-10 levels were measured by the ELISA method (Cytoset; Biosource, Invitrogen, Camarillo, CA) according to the manufacturer's instructions. BALF samples were diluted 1:10 in ELISA diluent buffer to quantify KC and human IL-10 levels, and diluted 1:1 to quantify TNF-α and mouse IL-10 levels. Recombinant mouse KC was purchased from eBiosource.
Determination of Albumin Content in BALF
Mouse albumin levels in BALF were measured using a mouse albumin ELISA kit purchased from Bethyl Labs (Montgomery, TX) (13). The detection limit for this ELISA was 7 ng/ml.
Depletion of Polymorphonuclear Leukocytes
Neutropenia was induced using monoclonal anti-mouse Ly-6G antibody (RB6-8C5; eBioscience, San Diego, CA) in the same manner as previously reported (14). Mice were given 25 μg of RB6 in 200 μl of sterile saline intravenously at 18 hours before the intratracheal injection of P. aeruginosa. Depletion of neutrophils was confirmed by Coulter counting 18 hours after RB6 administration using a Beckman Coulter Counter at the Animal Diagnostic Laboratory at the University of Michigan.
Statistical Analysis
All values are expressed as the mean (±SEM). Significance was assigned at P values less than 0.05. Data sets were analyzed using Student's t test or one-way ANOVA, with individual group means being compared with the Student-Newman-Keuls multiple comparison test.
RESULTS
Mortality from P. aeruginosa Lung Challenge in IL-10–Overexpressing Mice
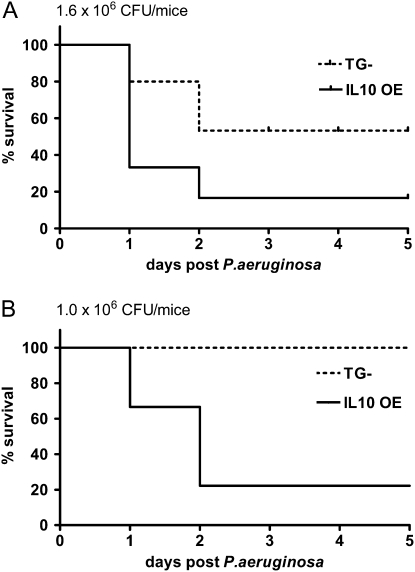
An initial study was performed to assess the effect of the bacterial load on the mortality of the mice. Both control mice (singly transgenic without the ability to overexpress IL-10 [11], designated as transgenic negative [TG−]) and IL-10 OE mice were challenged intratracheally with varying doses of P. aeruginosa. We found that, at a higher dose (1.6 × 106 CFU), the mortality rate was 83% in the IL-10 OE group as compared with 40% in the TG− mice (Figure 1A). When the dose was lowered to 1.0 × 106 CFU, no death was observed in the TG− group; however, IL-10 OE mice still showed a significantly higher mortality rate (80%) (Figure 1B). Thus, IL-10 overexpression in the lung significantly increased the mortality of mice challenged intratracheally with P. aeruginosa bacteria.
Figure 1.
Effects of lung-specific IL-10 overexpression on survival of Pseudomonas aeruginosa–challenged mice. (A) P. aeruginosa at a dose of 1.6 × 106 CFU was administered intratracheally and mice were monitored for 5 d. (B) P. aeruginosa at a dose of 1.0 × 106 CFU was administered intratracheally and mice were monitored for 5 days. Results shown are representative of 3–4 independent experiments, with n = 10/group. IL-10 OE, lung-specific IL-10 overexpression mice; TG−, transgenic negative mice.
Increased Bacteria Burden in IL-10 OE Mice Challenged with P. aeruginosa
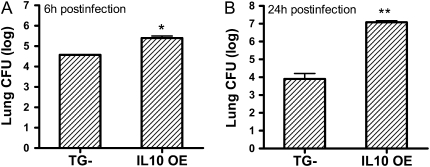
We hypothesized that the substantial increase of lethality observed in IL-10 OE mice challenged with P. aeruginosa was due to impaired bacterial clearance. To test this, we harvested lungs and blood samples of infected mice at 6 and 24 hours after infection. At the 6-hour time point, the bacteria counts in both mouse groups were lower than the initial inoculums (1.0 × 106 CFU; 6.0 CFU log10 scale). The IL-10 OE mice showed a significantly higher count (5.4 CFU log10 scale) than the TG− mice (4.57 CFU log10 scale) (Figure 2A). No bacteria were detected in the blood of either group at this early time point. At the 24-hour time point, TG− mice had a significantly decreased bacteria count (3.9 CFU log10 scale) compared with the initial inoculums (1.1 × 106 CFU; 6.08 log10 scale), whereas the IL-10 OE mice showed a substantially increased bacteria count (7.1 CFU log10 scale) (Figure 2B). Furthermore, unlike the earlier time point of 6 hours, bacteria dissemination into the blood was observed in the IL-10 OE mice at this later time point (3.5 CFU log10 scale and data not shown). No bacteria were detected in the blood of TG− mice at this time point. Our data indicate that lung-specific IL-10 overexpression impaired bacterial clearance, resulting in uncontrolled growth of bacteria in the lungs and modest spillover into the circulation that was associated with the increased mortality of IL-10 OE mice.
Figure 2.
IL-10 OE mice had increased bacteria burden. (A) Mice were given an intratracheal challenge of P. aeruginosa at a dose of 1.0 × 106 CFU and lungs CFU were determined 6 hours later (*P < 0.05). (B) Mice were given 1.1 × 106 CFU of bacteria, and lung CFU were determined at 24 hours after infection (**P < 0.01). In both (A) and (B), Log10 P. aeruginosa CFU are expressed on the vertical axis. Data represent mean (±SEM), with n = 7–8 per experimental group.
IL-10 Inhibited Early Neutrophil Recruitment in Mice Challenged with P. aeruginosa
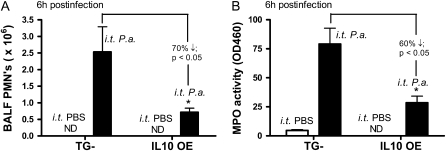
Leukocyte recruitment plays important roles at the early stage of host innate immune response. Therefore, we next examined the effects of IL-10 on early leukocyte recruitment upon viable bacterial challenge by measuring lung leukocyte counts and differentials in BALF. Mice that underwent induction of anesthesia and had intratracheal inoculation with PBS served as negative controls. In both groups of mice (TG− and IL-10 OE), significant neutrophil influx was observed as early as 3 hours after infection as compared with control animals (PBS-challenged) groups and, by 6 hours after challenge, more than 95% of cells recovered by lavage from TG− mice and 30–50% of cells from IL-10 OE mice were neutrophils (data not shown). Between the Pseudomonas-challenged groups, the IL-10 OE mice had a nearly twofold decrease in the numbers of neutrophils at 6 hours after intratracheal administration of 1.0 × 106 CFU as compared with TG− mice similarly challenged (Figure 3A). In addition to quantifying the neutrophil number in BALF, we also measured whole-lung MPO activity as another indicator of neutrophil influx to the lung. Consistent with the BALF neutrophil data, IL-10 OE had a nearly 2.5-fold decrease of MPO activity as compared with TG− mice.
Figure 3.
Defective polymorphonuclear leukocyte (PMN) recruitment and decreased total lung myeloperoxidase (MPO) activity in the IL-10 OE mice 6 hours after bacteria challenge. (A) Mice were given an intratracheal infection with P. aeruginosa at a dose of 1.0 × 106 CFU. Bronchoalveolar lavage (BAL) fluid (BALF) cells were collected at 6 hours, and PMN values were determined from differential counts on cytospin samples (*P < 0.05). (B) Mice were given an intratracheal infection with P. aeruginosa at a dose of 1.0 × 106 CFU. Lungs were collected for MPO activity measurement at 6 hours after administration of bacteria (*P < 0.05). Data are representative of three independent experiments, with n = 7–8 mice per group.
Early Inhibition of Lung Chemokine and TNF-α Expression in IL-10 OE Mice Challenged with P. aeruginosa
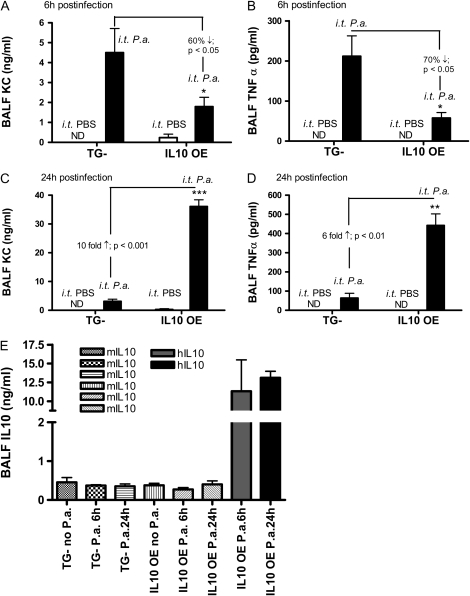
Neutrophils express chemokine receptors, CXCR1 and CXCR2 (15). As a result, neutrophil recruitment to the site of infection is dependent upon chemokine ligands of these receptors, such as KC and macrophage inhibitory protein (MIP)-2, as well as other chemokines, such as MIP-1α (3, 16, 17). To determine the effect of IL-10 overexpression in modulating the expression of chemokines, we measured KC levels in the BALF of infected mice 6 hours after intratracheal administration of bacteria. P. aeruginosa infection resulted in robust KC induction in both TG− mice and IL-10 OE mice (Figure 4A). However, IL-10 OE mice had a more than 60% decrease in KC expression compared with TG− mice (1.79 ± 0.47 ng/ml compared with 4.5 ± 1.21 ng/ml; P < 0.01 [Figure 4A]). Because TNF-α is an important proinflammatory cytokine in lung innate immunity against bacterial pathogens, and has been shown to be modulated by IL-10, we also measured TNF-α expression in the BALF of infected lungs. Lung-specific IL-10 overexpression decreased TNF-α significantly in IL-10 OE mice as compared with control mice (57.5 ± 13.8 pg/ml versus 211.86 ± 38.8 pg/ml, respectively [Figure 4B]). The decrease in KC and TNF-α expression in the IL-10 OE mice was consistent with the defects of neutrophil recruitment to the lung (Figure 3). In addition, neutrophils have been shown to be an important cell that contributes to TNF-α production in the setting of acute lung injury (18); therefore, the decreased expression of TNF-α in IL-10 OE mice could be due to decreased neutrophil recruitment as well, although IL-10 effects on other cell types could not be completely excluded.
Figure 4.
Mean (±SEM) lung keratinocyte–derived chemokine (KC), TNF-α, and IL-10 levels 6 and 24 hours after intratracheal administration of P. aeruginosa. In (A) and (B), mice were infected with P. aeruginosa at a dose of 1.1 × 106 CFU. BALFs were collected at 6 hours after infection and measured for KC and TNF-α expression by ELISA with determinations made in triplicate. In (C) and (D), BALFs were collected at 24 hours after infection and measured for KC and TNF-α expression. (E) Endogenous IL-10 (mouse IL-10) and exogenous IL-10 (human IL-10) levels were measured by ELISA in a similar manner from 6- and 24-hour BALF samples. *P < 0.05 compared with control group; **P < 0.01 compared with control group; ***P < 0.001 compared with control group. P.a., Pseudomonas aeruginosa.
Late Elevation of Lung Chemokine and TNF-α Expression in IL-10 OE Mice Challenged with P. aeruginosa
We show that, at 24 hours after infection, the IL-10 OE mice had a greater bacterial burden, whereas the control mice displayed significant reduction in bacterial CFU by 24 hours (see Figure 2). To examine whether IL-10 inhibited KC and TNF-α expression at this later time point, BALFs were harvested and similarly analyzed. In contrast to the decreased expression observed at 6 hours in the IL-10 OE mice, both KC and TNF-α levels were substantially elevated in IL-10 OE mice at 24 hours (Figures 4C and 4D). We observed a nearly 10-fold increase in KC expression in IL-10 OE mice (36.0 ± 2.09 ng/ml) as compared with control mice (3.15 ± 0.72 ng/ml) (P < 0.001), and a nearly sixfold increase of TNF-α expression (442.16 ± 60.6 pg/ml in IL-10 OE mice versus 63.75 ± 24.79 pg/ml in control mice; P < 0.01). Of note, the absolute levels of KC and TNF-α present in the BALF at the 24-hour time point were much higher than those at the 6-hour time point. We suspect that the enhanced expression of KC and TNF-α could be due to the high amount of bacteria present in the lungs of IL-10 OE mice as compared with the TG− mice, which intensified and prolonged the proinflammatory response, as characterized by these two mediators. We also measured both IL-10 levels in the BALFs after bacteria challenge. Because the overexpressed IL-10 in transgenic mice is human IL-10, we were able to distinguish endogenous IL-10 (mouse IL-10) and overexpressed IL-10 (human IL-10) expression in the BALF. As shown in Figure 4E, no significant differences of mouse IL-10 were observed between groups after bacteria challenge. However, when human IL-10 was measured, the expression level was 10- to 15-fold higher in the IL-10 OE mice as compared with the endogenous (mouse) IL-10 in TG− mice. No human IL-10 was detected in TG− mice (data not shown).
Albumin Permeability and Polymorphonuclear Leukocyte Counts in Lungs 24 Hours after P. aeruginosa Challenge
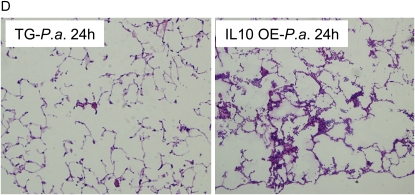
To correlate the degree of lung inflammation with pulmonary vascular leak, we measured leakage of mouse albumin into BALF. At 24 hours after infection, IL-10 OE mice showed a nearly 3.5-fold increase in albumin leakage as compared with the amount found in TG− mice (Figure 5A). This significant increase in lung permeability correlated with the increases in neutrophil accumulation in lungs, as determined by whole-lung polymorphonuclear leukocyte (PMN) counts and MPO content (Figures 5B and 5C). This increased pulmonary vascular leak and MPO activity in the lungs of IL-10 OE mice was further corroborated by observed histologic changes in lungs from similarly challenged mice. As shown in Figure 5D (left panel), modest interstitial inflammatory cell influx was present in control mice 24 hours after bacteria administration. In contrast, in IL-10 OE mice, interstitial accumulation of neutrophils was evident, resulting in substantially denser patchy areas of consolidation (Figure 5D, right panel).
Figure 5.
Lung vascular permeability (albumin levels) and neutrophil recruitment 24 hours after intratracheal administration of P. aeruginosa (1 × 106 CFU). (A) Mouse albumin levels were measured in BALF as an index for vascular leakage, as previously reported. (B) Whole-lung leukocyte isolation is described in Materials and Methods. Neutrophil numbers were determined by differential counting. (C ) Total lung MPO activity was measured at 24 hours after administration of bacteria. In (A)–(C), results are reported as mean (±SEM) from n ≥ 5 mice. Data are representative of three independent experiments. *P < 0.05 compared with control mice. (D) Hematoxylin and eosin staining of lung tissues obtained from mice 24 hours after infection. Magnification: 20×.
The Effect of IL-10 OE on PMN Recruitment and Bacterial Clearance Is Attenuated by Coadministration of KC
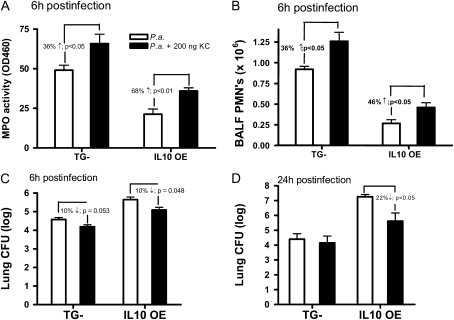
Our observations led us to hypothesize that the reduction of bacterial clearance in the IL-10 OE mice was a result of attenuated neutrophil influx. By coadministering exogenous KC along with bacteria in the IL-10 OE mice, we expected to see a reversed effect (i.e., increase) of neutrophil recruitment. In TG− and, to a greater degree, IL-10 OE mice, intratracheal coadministration of 200 ng KC significantly increased MPO activity in the lung (Figure 6A). These data correlated with similar increases in BALF neutrophil numbers (by 36% in the TG− and 46% in the IL-10 OE, respectively) (Figure 6B). Of note, addition of KC could not fully recover the neutrophil recruitment in the IL-10 OE mice, as, even with coadministration of KC, neutrophil counts and MPO activity in the IL-10 OE mice were still lower than those of control mice without KC treatment (Figures 6A and 6B, compare first and fourth vertical bars). No further improvement was observed when the KC dose was increased up to 1 μg (data not shown). In both TG− and IL-10 OE mice, bacteria CFUs at 6 hours after infection were modestly decreased by the coadministration of KC (Figure 6C). At 24 hours after infection, addition of exogenous KC significantly decreased bacteria CFUs only in the IL-10 OE mice (Figure 6D; P < 0.05). Of note, at both 6 and 24 hours after infection, addition of KC did not lower the bacteria CFUs in the IL-10 OE mice to that of TG− mice without KC treatment (Figures 6C and 6D, compare first and fourth vertical bars). Thus, although these data suggest that KC is an important chemokine, directing neutrophil recruitment in this model, bacterial infection is expected to induce activation of a myriad of chemoattractant molecules, and the additive effects of these likely contribute to the full recruitment of neutrophils and antibacterial defense.
Figure 6.
Coadministration of KC with P. aeruginosa increased PMN recruitment and enhanced bacterial clearance. In (A)–(C), mice were given an intratracheal infection with P. aeruginosa at a dose of 0.8 × 106 CFU with or without the coadministration of 200 ng KC. (A) Lung MPO activity measured 6 hours after administration of bacteria. (B) BALFs were collected at 6 hours, and PMN values determined from total number of leukocytes (Coulter counted) multiplied by the percent neutrophils determined by differential counts on cytospin samples. (C ) Lung CFU determination 6 hours after infection. (D) Mice were given an intratracheal infection of 1.0 × 106 CFU of P. aeruginosa with or without KC. Lung CFU were measured at 24 hours after infection. Data are means (±SEM), and are representative of two independent experiments, with n = 7–8 mice/group.
Neutrophil Depletion Enhanced Bacteria Burden in Both IL-10 OE Mice and Control Mice Challenged with P. aeruginosa
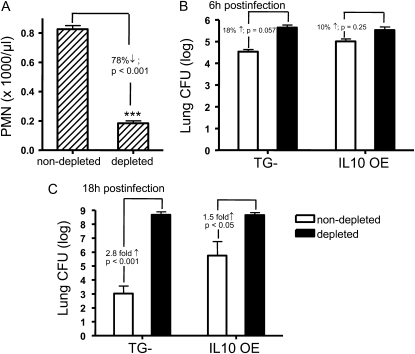
Others have reported that, after intravenous injection of anti-mouse Ly-6G antibody, neutrophils are effectively depleted and mice become neutropenic (14). To further elucidate the role of neutrophils in combating bacterial infection in the lung, we used this approach to assess the effects on bacteria clearance in both TG− and IL-10 OE mice. First, we determined the effective dose of blocking antibody in our mouse strain. As shown in Figure 7A, mice received 25 μg of monoclonal anti-mouse Ly-6G intravenously 18 hours before bacterial challenge, which significantly reduced circulating neutrophil counts. As hypothesized, the induction of neutropenia substantially increased mortality, with no mice surviving in either group at 24 hours after infection with 1.0 × 106 CFU of bacteria (data not shown). However, by modifying the bacteria inoculum and infecting mice with a lower dose of bacteria (0.8 × 106 CFU; 5.9 CFU log10 scale), 80% survival was achieved in both groups at 18 hours after infection. Using this dose, whole lungs were harvested at 6 hours (Figure 7B) and 18 hours (Figure 7C) for bacteria CFU determination. In non–PMN-depleted groups, the results were consistent with data shown in Figure 2, which demonstrate an effective clearance in the TG− mice (4.54 CFU log10 scale at 6 hours and 3.02 CFU log10 scale at 18 hours), but a defect in clearance in the IL-10 OE mice (5.0 CFU log10 scale at 6 hours and 5.52 CFU log10 scale at 18 hours). However, upon neutrophil depletion, both TG− and IL-10 OE mice were unable to clear bacteria. A heavy bacterial burden at both 6 hours (5.5 CFU log10 scale) and 18 hours after infection (8.6 CFU log10 scale) was observed equally in both groups. Taken together, our data demonstrate that neutrophils play a crucial role in the host immune response against bacterial infection, and that IL-10 overexpression inhibits this neutrophil recruitment at the early stage of infection, subsequently leading to uncontrolled bacterial growth, lung injury, and death.
Figure 7.
Neutrophil depletion increased lung bacterial burden in both control mice and IL-10 OE mice 18 hours after intratracheal administration of P. aeruginosa (0.80 × 106 CFU). (A) Significant reductions in circulating PMNs were detected 18 hours after a single injection of 25 μg of monoclonal anti-mouse Ly-6G (clone RB6–8C5). ***P < 0.001 compared with control, non–PMN-depleted group. In (B) and (C ), mice received anti-PMN treatment 18 hours before bacteria infection. Lung CFU were determined at 6 hours (B) and 18 hours (C ) after infection. Data are means (±SEM) representative of two independent experiments each, with n = 7–8 mice/group.
DISCUSSION
IL-10 remains one of the most important anti-inflammatory cytokines that plays a key role in sepsis and acute lung injury by modulating both the systemic and pulmonary inflammatory response, respectively. In various model systems, IL-10 has been shown to inhibit production of numerous proinflammatory cytokines (e.g., TNF-α, IL-1β, IL-6) and chemokines (e.g., KC, MIP-2, MIP-1α) that are known to contribute to the development of acute inflammatory states. In the setting of acute lung inflammation, IL-10 is a key regulator of the degree of inflammation. This has been demonstrated both by addition of exogenous IL-10 and by neutralization of endogenous IL-10 in the setting of a myriad of intrapulmonary inflammatory stimuli (4, 19–21). With the purpose of studying the biological effects of IL-10 in the pulmonary system more precisely, we had previously established a lung-specific, tetracycline-inducible IL-10 OE mouse. With this mouse strain, we demonstrated that endotoxin-induced lung inflammation was attenuated (11). These findings, together with the clinical observations that decreased IL-10 concentration in BALF is associated with worse outcome in patients with the acute respiratory distress syndrome, appeared to support the protective role of IL-10 during inflammation triggered by a nonviable pathogen (22).
However, the role of IL-10 becomes more complicated when pathogen clearance is involved in the model system. Experiments have shown that IL-10 neutralization or deficiency improved clearance of bacteria, parasites, and fungi (23–25). Similar effects of IL-10 were observed in immunosuppressed mice where preferential expression of anti-inflammatory cytokines, particularly IL-10, were present systemically (10). In a more complex model, where a bacterial challenge to the lung was the second “hit” after a respiratory virus infection, augmented growth of bacteria in these animals was attributed to elevated IL-10 expression induced by the initial virus infection (26). The current data show that, with overexpression of IL-10 in the lung, the mice were defective in clearing bacteria and, as a result, displayed higher mortality. In the study, we aimed to understand the mechanism of IL-10–regulated effects and emphasized the roles of neutrophils during the process of disease development.
Neutrophils are one of the earliest immune cells recruited to the site of infection. Neutrophils play a key role in the early control of acute bacterial infection by killing pathogens through the production of proinflammatory mediators (e.g., TNF-α, IL-1, and IL-6), release of granular enzymes, and, most importantly, through the production of reactive oxygen species (ROS) (27–29). Local production of CXC chemokines, such as MIP-2 and KC, is essential for recruiting neutrophils to the lung during a bacterial infection (15, 30). We found that, at an early stage of infection with P. aeruginosa, KC production was significantly reduced in the IL-10 OE mice, which was associated with decreased numbers of neutrophils recruited to the airway space. Many cell types, including macrophages, pulmonary epithelial and endothelial cells, and neutrophils, serve as sources of chemokine production in response to a variety of stimulants, including LPS, TNF-α, and IFN-γ (15, 30–32). Previously, we have demonstrated that IL-10 inhibited chemokine (MIP-1α and MIP-2) production by macrophages stimulated with a nonviable pathogen molecular pattern, LPS (3). Thus, IL-10 expression likely serves as an initial regulatory signal for slowing down or stopping the process of neutrophil recruitment. Alternatively, the decreased KC production could be reflective of IL-10's inhibition of TNF-α production from alveolar macrophages, which can drive KC production from alveolar epithelial cells (32, 33), though the kinetics of this regulation support a direct IL-10 effect on KC expression. As the viable P. aeruginosa infection progresses (3 hours after infection), neutrophils become the predominant cell type in the airspace and a potential source of KC (and TNF-α) expression. Therefore, the decreased KC (and TNF-α) production in the IL-10 OE mice at this time point might be principally due to the significantly reduced presence of neutrophils in the lung as a source of chemokine/cytokine production; however, the principal role of recruited neutrophils is to contain and clear the injurious pathogen.
The IL-10 OE mice that displayed a significantly reduced lung neutrophil count displayed significantly higher bacterial burden as compared with TG− mice, consistent with the primary role of neutrophils in bacteria killing. This role was further confirmed in the experiments in which exogenous KC was added to the lung along with bacterial infection, and neutrophil recruitment and bacteria clearance, although not completely reversed, were significantly improved. In addition, when neutrophils were depleted in mice using anti-PMN serum, both TG− and IL-10 OE mice lost their ability to clear bacteria effectively, providing further evidence as to the importance of neutrophil recruitment in the early stage of a bacterial infection. Surprisingly, at the later stage of infection, expression of both KC and TNF-α were substantially increased in the IL-10 OE mice, whereas no escalation of chemokine/cytokine expression was observed over time in the TG− mice. Also at this time point, the bacterial burdens differed significantly between the two groups. In the IL-10 OE mice, a 10-fold increase in the bacterial counts recovered from the lungs was observed (7.1 CFU log10 scale as compared with 6.08 CFU log10 scale of the initial inoculum). However, the bacterial counts isolated from the TG− mice (3.9 CFU log10 scale) were decreased to a level nearly 100-fold lower than the inoculum used as the initial challenge (6.08 CFU log10 scale). In addition to this latter increase in bacterial burden, neutrophil infiltration, as indicated by MPO and immunohistologic analysis, was also intensified in the IL-10 OE mice. Together, the data indicate that, although IL-10 was continuously overexpressed in the IL-10 OE mice (as shown in Figure 4E), the early inhibitory effects of IL-10 on KC, TNF-α expression, and neutrophil recruitment were lost over time. We hypothesize that the prolonged presence of IL-10 overexpression reduced the pathogen-directed proinflammation, resulting in incomplete pathogen clearance and allowing for bacterial overgrowth in the lung. This prolonged presence of viable bacteria likely provide an ongoing stimulus for proinflammatory gene expression, as exemplified by the excessive TNF-α and KC expression, as well as the increased neutrophil sequestration in the lungs. Activated neutrophils in the lung could further aggravate inflammation by synthesizing cytokines and chemokines, producing ROS, and releasing proteases. Overproduction of TNF-α, nitric oxide, and ROS, such as H2O2 from neutrophils, have been linked to tissue damage, increased vascular permeability, and organ injury (30, 34). This possibility is supported by studies demonstrating that CXC chemokine neutralization or neutrophil depletion attenuate tissue injury in a variety of experimental models, such as trauma, hemorrhagic shock, and sepsis (31, 35, 36). Together, these data suggest that neutrophils play dual roles in bacteria-induced lung inflammation. They are needed early during the infectious challenge to combat and eradicate bacterial invasion, whereas, at a later stage, their presence contributes to tissue injury via production of cytokines/chemokines, ROS, and proteases. A recent observation in support of this concept was from a study that showed that neutrophil depletion before cecal ligation and puncture (CLP) resulted in attenuated bacterial clearance and bacteremia, whereas neutrophil depletion after CLP improved survival (14).
Given that the protective or harmful role of neutrophils depends on the progression of disease development, it is likely that the “beneficial” or “harmful” IL-10 effects are also time dependent. In a sepsis model, IL-10 neutralization at the time of CLP significantly increased sepsis-induced lethality, but not when IL-10 was neutralized after CLP (7). Instead, in post-CLP mice, IL-10 neutralization with anti–IL-10 protected the mice against a secondary bacterial challenge (10). An explanation for this time-dependent effect of IL-10 congruent with our current findings is that IL-10 is necessary to attenuate inflammation early in sepsis, whereas, at the later stage (e.g., 24 hours after CLP), IL-10 blockade helps to improve secondary bacterial clearance. In this regard, it is conceivable that the therapeutic potentials of IL-10 against inflammation remain valid if the timing of IL-10 administration can be carefully taken into account. Furthermore, it is clear that the balance between pro- and anti-inflammatory signals will determine whether a recovery or deterioration will occur after exposure to pathogenic insults. Under a variety of severe disease conditions in which anti-inflammatory signals, such as that of IL-10, were expected to be down-regulated, the expression of IL-10 was found to be increased together with pro-inflammatory signals (10, 14). Therefore, IL-10 expression levels alone might not serve as an indicator of inflammatory status; rather, it is a compilation of additional key anti- and proinflammatory cytokines, chemokines, and mediators that will influence the ultimate outcome in time.
This work was supported by National Institutes of Health grant GM066893-07 (T.P.S.).
Originally Published in Press as DOI: 10.1165/rcmb.2008-0202OC on December 18, 2008
Conflict of Interest Statement: T.J.S. received $52,428 annually from Centocor (11/16/06–12/31/08). None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Moore KW, O'Garra A, de Waal Malefyt R, Vieira P, Mosmann TR. Interleukin-10. Annu Rev Immunol 1993;11:165–190. [DOI] [PubMed] [Google Scholar]
- 2.Lentsch AB, Shanley TP, Sarma V, Ward PA. In vivo suppression of NF-kappa B and preservation of I kappa B alpha by interleukin-10 and interleukin-13. J Clin Invest 1997;100:2443–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shanley TP, Vasi N, Denenberg A. Regulation of chemokine expression by IL-10 in lung inflammation. Cytokine 2000;12:1054–1064. [DOI] [PubMed] [Google Scholar]
- 4.Mulligan MS, Warner RL, Foreback JL, Shanley TP, Ward PA. Protective effects of IL-4, IL-10, IL-12, and IL-13 in IgG immune complex–induced lung injury: role of endogenous IL-12. J Immunol 1997;159:3483–3489. [PubMed] [Google Scholar]
- 5.Gerard C, Bruyns C, Marchant A, Abramowicz D, Vandenabeele P, Delvaux A, Fiers W, Goldman M, Velu T. Interleukin 10 reduces the release of tumor necrosis factor and prevents lethality in experimental endotoxemia. J Exp Med 1993;177:547–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Standiford TJ, Strieter RM, Lukacs NW, Kunkel SL. Neutralization of IL-10 increases lethality in endotoxemia: cooperative effects of macrophage inflammatory protein-2 and tumor necrosis factor. J Immunol 1995;155:2222–2229. [PubMed] [Google Scholar]
- 7.Walley KR, Lukacs NW, Standiford TJ, Strieter RM, Kunkel SL. Balance of inflammatory cytokines related to severity and mortality of murine sepsis. Infect Immun 1996;64:4733–4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenberger MJ, Strieter RM, Kunkel SL, Danforth JM, Goodman RE, Standiford TJ. Neutralization of IL-10 increases survival in a murine model of Klebsiella pneumonia. J Immunol 1995;155:722–729. [PubMed] [Google Scholar]
- 9.Reddy RC, Chen GH, Newstead MW, Moore T, Zeng X, Tateda K, Standiford TJ. Alveolar macrophage deactivation in murine septic peritonitis: role of interleukin 10. Infect Immun 2001;69:1394–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steinhauser ML, Hogaboam CM, Kunkel SL, Lukacs NW, Strieter RM, Standiford TJ. IL-10 is a major mediator of sepsis-induced impairment in lung antibacterial host defense. J Immunol 1999;162:392–399. [PubMed] [Google Scholar]
- 11.Spight D, Zhao B, Haas M, Wert S, Denenberg A, Shanley TP. Immunoregulatory effects of regulated, lung-targeted expression of IL-10 in vivo. Am J Physiol Lung Cell Mol Physiol 2005;288:L251–L265. [DOI] [PubMed] [Google Scholar]
- 12.Guo RF, Riedemann NC, Laudes IJ, Sarma VJ, Kunkel RG, Dilley KA, Paulauskis JD, Ward PA. Altered neutrophil trafficking during sepsis. J Immunol 2002;169:307–314. [DOI] [PubMed] [Google Scholar]
- 13.Speyer CL, Neff TA, Warner RL, Guo RF, Sarma JV, Riedemann NC, Murphy ME, Murphy HS, Ward PA. Regulatory effects of iNOS on acute lung inflammatory responses in mice. Am J Pathol 2003;163:2319–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoesel LM, Neff TA, Neff SB, Younger JG, Olle EW, Gao H, Pianko MJ, Bernacki KD, Sarma JV, Ward PA. Harmful and protective roles of neutrophils in sepsis. Shock 2005;24:40–47. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi Y. The role of chemokines in neutrophil biology. Front Biosci 2008;13:2400–2407. [DOI] [PubMed] [Google Scholar]
- 16.Guo RF, Ward PA. Mediators and regulation of neutrophil accumulation in inflammatory responses in lung: insights from the IgG immune complex model. Free Radic Biol Med 2002;33:303–310. [DOI] [PubMed] [Google Scholar]
- 17.Olszewski MA, Falkowski NR, Surana R, Sonstein J, Hartman A, Moore BB, Huffnagle GB, Toews GB. Effect of laparotomy on clearance and cytokine induction in Staphylococcus aureus infected lungs. Am J Respir Crit Care Med 2007;176:921–929. [DOI] [PubMed] [Google Scholar]
- 18.Riedemann NC, Guo RF, Bernacki KD, Reuben JS, Laudes IJ, Neff TA, Gao H, Speyer C, Sarma VJ, Zetoune FS, et al. Regulation by C5a of neutrophil activation during sepsis. Immunity 2003;19:193–202. [DOI] [PubMed] [Google Scholar]
- 19.Gudmundsson G, Bosch A, Davidson BL, Berg DJ, Hunninghake GW. Interleukin-10 modulates the severity of hypersensitivity pneumonitis in mice. Am J Respir Cell Mol Biol 1998;19:812–818. [DOI] [PubMed] [Google Scholar]
- 20.Huaux F, Louahed J, Hudspith B, Meredith C, Delos M, Renauld JC, Lison D. Role of interleukin-10 in the lung response to silica in mice. Am J Respir Cell Mol Biol 1998;18:51–59. [DOI] [PubMed] [Google Scholar]
- 21.Shanley TP, Schmal H, Friedl HP, Jones ML, Ward PA. Regulatory effects of intrinsic IL-10 in IgG immune complex–induced lung injury. J Immunol 1995;154:3454–3460. [PubMed] [Google Scholar]
- 22.Donnelly SC, Strieter RM, Reid PT, Kunkel SL, Burdick MD, Armstrong I, Mackenzie A, Haslett C. The association between mortality rates and decreased concentrations of interleukin-10 and interleukin-1 receptor antagonist in the lung fluids of patients with the adult respiratory distress syndrome. Ann Intern Med 1996;125:191–196. [DOI] [PubMed] [Google Scholar]
- 23.Hugosson E, Montgomery SM, Premji Z, Troye-Blomberg M, Bjorkman A. Higher IL-10 levels are associated with less effective clearance of Plasmodium falciparum parasites. Parasite Immunol 2004;26:111–117. [DOI] [PubMed] [Google Scholar]
- 24.Lazarus JJ, Meadows MJ, Lintner RE, Wooten RM. IL-10 deficiency promotes increased Borrelia burgdorferi clearance predominantly through enhanced innate immune responses. J Immunol 2006;177:7076–7085. [DOI] [PubMed] [Google Scholar]
- 25.Vazquez-Torres A, Jones-Carson J, Wagner RD, Warner T, Balish E. Early resistance of interleukin-10 knockout mice to acute systemic candidiasis. Infect Immun 1999;67:670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Sluijs KF, Nijhuis M, Levels JH, Florquin S, Mellor AL, Jansen HM, van der Poll T, Lutter R. Influenza-induced expression of indoleamine 2,3-dioxygenase enhances interleukin-10 production and bacterial outgrowth during secondary pneumococcal pneumonia. J Infect Dis 2006;193:214–222. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi SD, Voyich JM, Burlak C, DeLeo FR. Neutrophils in the innate immune response. Arch Immunol Ther Exp (Warsz) 2005;53:505–517. [PubMed] [Google Scholar]
- 28.Pina A, Saldiva PH, Restrepo LE, Calich VL. Neutrophil role in pulmonary paracoccidioidomycosis depends on the resistance pattern of hosts. J Leukoc Biol 2006;79:1202–1213. [DOI] [PubMed] [Google Scholar]
- 29.van Faassen H, KuoLee R, Harris G, Zhao X, Conlan JW, and Chen W. Neutrophils play an important role in host resistance to respiratory infection with Acinetobacter baumannii in mice. Infect Immun 2007;75:5597–5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo RF, Ward PA. Role of oxidants in lung injury during sepsis. Antioxid Redox Signal 2007;9:1991–2002. [DOI] [PubMed] [Google Scholar]
- 31.Lomas-Neira J, Chung CS, Perl M, Gregory S, Biffl W, Ayala A. Role of alveolar macrophage and migrating neutrophils in hemorrhage-induced priming for ALI subsequent to septic challenge. Am J Physiol Lung Cell Mol Physiol 2006;290:L51–L58. [DOI] [PubMed] [Google Scholar]
- 32.Sharma AK, Fernandez LG, Awad AS, Kron IL, Laubach VE. Proinflammatory response of alveolar epithelial cells is enhanced by alveolar macrophage–produced TNF-alpha during pulmonary ischemia–reperfusion injury. Am J Physiol Lung Cell Mol Physiol 2007;293:L105–L113. [DOI] [PubMed] [Google Scholar]
- 33.Bogdan C, Vodovotz Y, Nathan C. Macrophage deactivation by interleukin 10. J Exp Med 1991;174:1549–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seitz DH, Perl M, Mangold S, Neddermann A, Braumuller ST, Zhou S, Bachem MG, Huber-Lang MS, Knöferl MW. Pulmonary contusion induces alveolar type 2 epithelial cell apoptosis: role of alveolar macrophages and neutrophils. Shock 2008;30:537–544. [DOI] [PubMed] [Google Scholar]
- 35.Fan J, Marshall JC, Jimenez M, Shek PN, Zagorski J, Rotstein OD. Hemorrhagic shock primes for increased expression of cytokine-induced neutrophil chemoattractant in the lung: role in pulmonary inflammation following lipopolysaccharide. J Immunol 1998;161:440–447. [PubMed] [Google Scholar]
- 36.Frink M, Hsieh YC, Hsieh CH, Pape HC, Choudhry MA, Schwacha MG, Chaudry IH. Keratinocyte-derived chemokine plays a critical role in the induction of systemic inflammation and tissue damage after trauma-hemorrhage. Shock 2007;28:576–581. [DOI] [PubMed] [Google Scholar]