Abstract
Prenatal nicotine exposure impairs normal lung development and leads to diminished pulmonary function after birth. Previous work from our laboratory has demonstrated that nicotine alters lung development by affecting a nonneuronal cholinergic autocrine loop that is expressed in lung. Bronchial epithelial cells (BECs) express choline acetyltransferase, the choline high-affinity transporter and nicotinic acetylcholine (ACh) receptor (nAChR) subunits. We now demonstrate through a combination of morphological and electrophysiological techniques that nicotine affects this autocrine loop by up-regulating and activating cholinergic signaling. RT-PCR showed the expression of α3, α4, α7, α9, α10, β2, and β4 nAChR mRNAs in rhesus monkey lung and cultured BECs. The expression of α7, α4, and β2 nAChR was confirmed by immunofluorescence in the cultured BECs and lung. The electrophysiological characteristics of nAChR in BECs were determined using whole-cell patch–clamp on cultured BECs. Both ACh and nicotine evoked an inward current, with a rapid desensitizing current. Nicotine induced inward currents in a concentration-dependent manner, with an EC50 of 26.7 μM. Nicotine-induced currents were reversibly blocked by the nicotinic antagonists, mecamylamine, dihydro-β-erythroidine, and methyllcaconitine. Incubation of BECs with 1 μM nicotine for 48 hours enhanced nicotine-induced currents by roughly 26%. The protein tyrosine phosphorylation inhibitor, genistein, increased nicotine-induced currents by 58% and enhanced methyllcaconitine-sensitive currents (α7 nAChR activities) 2.3-fold, whereas the protein tyrosine phosphatase inhibitor, pervanadate, decreased the effects of nicotine. These results demonstrate that chronic nicotine exposure up-regulates nAChR activity in developing lung, and that nAChR activity can be further modified by tyrosine phosphorylation.
Keywords: nicotinic acetylcholine receptors, electrophysiology, bronchial epithelial cells, nicotine, lung
CLINICAL RELEVANCE
This research explains the fundamental mechanism by which nicotine affects lung development and function—by activating as opposed to desensitizing nicotinic cholinergic receptors in lung. This will help to develop therapies to block the effects of nicotine on lung.
Nicotinic acetylcholine (ACh) receptors (nAChRs) are the receptors for the neurotransmitter, ACh, as well as the receptor for nicotine. nAChR belong to a family of ligand-gated ion channels (including receptors for γ-aminobutyric acid and glycine) and are composed of five subunits that can be similar or different. Our laboratory has previously reported that developing lung expresses multiple nAChR subtypes in airway epithelial cells, airway fibroblasts, and pulmonary type II cells (1, 2). Nicotine freely passes the placenta, and the interaction of nicotine with nAChR in fetal lung is one mechanism by which smoking during pregnancy impairs normal lung development.
Smoking during pregnancy alters normal lung development, as manifested by decreased pulmonary function in offspring that can be measured immediately after birth and persists at least into adolescence (1). Alterations in pulmonary function can be most sensitively measured by decreases in forced expiratory flows. Clinically, changes in lung development caused by in utero tobacco smoke exposure are reflected by increased incidence of sudden infant death syndrome, increased incidence of childhood asthma, and increased hospitalizations for respiratory illnesses (3). In monkeys, prenatal exposure to nicotine leads to alterations in forced expiratory flows that are very similar to the changes in expiratory flows seen in offspring of human smokers (1). This suggests that nicotine mediates the effects of smoking during pregnancy on offspring pulmonary function.
Our laboratory and others have demonstrated the presence of an intrinsic, nonneuronal cholinergic paracrine signaling system in developing lung (2). Monkey bronchial epithelial cells (BECs) synthesize and secrete ACh, which can then interact with both nicotinic and muscarinic ACh receptors (mAChR) that are expressed on the surface of the BECs. The cholinergic paracrine loop in lung is demonstrated by the expression of choline acetyltransferase, the vesicular ACh transporter, the choline high-affinity transporter, α7, α3, α4, and β2 nAChR subunits, and the nAChR accessory protein, lynx1 (2), in BEC and other lung cell types. Primary culture of BECs confirms the synthesis and secretion of ACh and the activity of cholinesterases (1). Prenatal nicotine exposure dramatically up-regulates nAChR immunostaining in monkey BECs, but the functional significance of this increase is unknown (4).
Chronic exposure to nicotine has complicated effects on nAChR activity. Depending on subunit structure and tissue-specific factors, chronic nicotine exposure can either activate or desensitize nAChR activity, and can increase or decrease nAChR expression. For example, Fenster and colleagues (5) have shown that chronic nicotine exposure causes the lasting functional deactivation of nAChR in Xenopus oocytes. For the heteromeric nAChRs, Fenster and colleagues found that the α subunit makes a significant contribution in determining the apparent nicotine affinity of the active and desensitized states of an nAChR, and that the β subunit makes a significant contribution in determining the overall time course of desensitization (5). Chronic nicotine exposure produces a loss of nicotinic functional activity as a result of rapid and persistent desensitization (6), and chronic nicotine exposure, such as that resulting from smoking, has been reported both to up-regulate and to inactivate several classes of neuronal nAChRs in a long-lasting manner (7). However, it has also been reported that chronic nicotine exposure can alternately up-regulate the function of the α4β2 subtype in the central nervous system (8, 9). In addition, nicotine can act as a chaperone to increase nAChR receptor expression in cell membrane (10). The functional properties of α7 nAChR depend on the tyrosine phosphorylation status of the receptor, and are the result of a balance between tyrosine kinases and phosphatases; dephosphorylated α7 nAChRs cause increased ACh-evoked current, whereas phosphorylated nAChR are less active, showing that tyrosine phosphorylation and Src-family kinases (SFKs) negatively regulate receptor activity (11). Kuryatov and colleagues (10) have also reported that nicotine causes human α4β2 nAChR up-regulation by inducing an active or desensitized conformation, which assembles more efficiently.
Thus, a fundamental question as to the mechanism by which nicotine effects lung development is if the effects reflect an up-regulation of endogenous cholinergic signaling combined with continued activation by chronic nicotine, or do the effects reflect a shutting off of endogenous cholinergic signaling by nAChR deactivation caused by chronic nicotine exposure. In this study, we show that chronic nicotine exposure to BEC increases both nAChR expression and nAChR activity.
MATERIALS AND METHODS
Epithelial Cell Culture
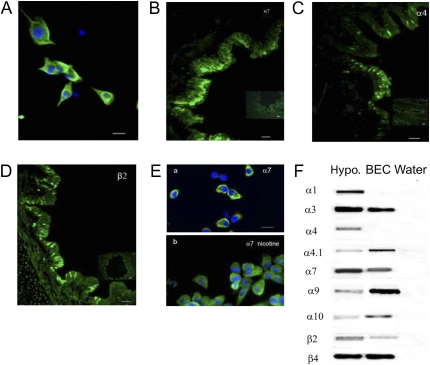
BEC cultures were established as described by Wu and colleagues (12). All animal procedures were approved by the Oregon National Primate Research Center Institutional Animal Care and Utilization Committee. In brief, lungs were obtained from rhesus macaques (gestation, 130 d to 2 yr old) undergoing necropsy for protocols unrelated to lung function or development. Lungs were immersed in ice-cold minimum essential medium (MEM) (MEM + 15 mM Hepes + 50 μg/ml gentamicin + penicillin/streptomycin). The major bronchi were crudely dissected and were washed with media, or opened longitudinally, and then incubated overnight at 4°C in 0.1% protease type 14 (Sigma, St. Louis, MO) in MEM. The cells were then washed from the epithelial cells side surface of the bronchi and collected by centrifugation at 1,000 × g for 10 minutes at 4°C. The cells were resuspended in MEM with 10% FCS, incubated for 5 minutes, washed, and then incubated overnight with bronchial epithelium culture medium (50% Ham's nutrient mixture F12 + 50% Dulbecco's modified Eagle's medium + 1.8 mM calcium chloride, 5.0 μg/ml insulin, 5.0 μg/ml transferrin, 20 ng/ml epidermal growth factor, 0.1 μM dexamethasone, 20 ng/ml cholera toxin, 30 μg/ml bovine hypothalamic extract, and 1.0 μM retinol) containing 2% FCS. The following day, the cells were changed to serum-free bronchial epithelium culture media, then incubated in 5% CO2 at 37°C for 7–10 days. For patch–clamp analysis, the cells were plated on Thermanox 13 mm plastic cover slips (NUNC, Rochester, NY) coated with 1.5 mg/ml collagen type I (Sigma). For immunofluorescence studies, the cells were plated on 13-mm glass coverslips. Identity of cells as BECs was confirmed by staining for cytokeratin and β-tubulin IV. This showed that roughly 90% of cultured cells demonstrated pan-cytokeratin antibody (Ab) staining (Figure 1A).
Figure 1.
Expression of nicotinic acetylcholine (ACh) receptor (nAChR) in monkey cultured bronchial epithelial cells (BECs) and in vivo. BECs were cultured as described in Materials and Methods. (A) Confirming their identity, cultured BECs expressed cytokeratin (4 d in culture). Calibration bar represents 90 μm. (B–D) The α7, α4, and β2 nAChR subunits were observed in lung sections (insets: lack of staining in absence of primary antibody). Calibration bars represent 35 μm. (E, a), α7 nAChR expression in cultured BEC; (E, b) α7 nAChR expression in cultured BECs incubated with 1 μM nicotine for 48 hours. (F) RT-PCR showed the presence of α3, α4.1, α7, α9, α10, β2, and β4 nAChR mRNAs in cultured BECs. Monkey hypothalamus (Hypo) was included as positive control. No bands were seen in negative controls (water), which lacked input RNA.
Immunofluorescence for nAChR Subunits
Whole lungs were obtained from newborn rhesus macaques. Lungs were immersion fixed in 4% paraformaldehyde and processed for cryostat sectioning (5 μM). The sections were washed in PBS, incubated with 0.1% Triton X-100 for 10 minutes, blocked with 10% serum, then incubated overnight at 4°C in the primary antisera. Cultured monkey BECs were fixed in 2% paraformaldehyde for nAChR and in methanol for cytokeratin Ab. nAChR Abs were developed by Jon Lindstrom, as previously described (13). The primary Abs used were: (1) anti-nAChR α7, (mouse, monoclonal 306, 1:200 dilution); (2). anti-nAChR α4 (mouse, monoclonal 299, 1:200 dilution); (3). anti-nAChR β2 (mouse, monoclonal 270, 1:200 dilution); and (4) anti-cytokeratin (mouse, monoclonal, 1:400 dilution, Invitrogen, Carlsbad, CA). The secondary Abs consisted of FITC-conjugated goat anti-mouse IgG (1:400 dilution), and FITC-conjugated rabbit anti-mouse IgG (1:400 dilution; Jackson Immunoresearch Laboratories, West Grove, PA). All the immunofluorescence experiments were repeated at least three times. Samples were viewed under an Axioskop 2 fluorescence microscope (Zeiss, Oberkochen, Germany), and images were acquired with an AxioCam digital camera (Zeiss) and analyzed with Axiovision 4.2 software (Zeiss).
RT-PCR
Total RNA was extracted from monkey hypothalamus and cultured BECs using Trizol (Invitrogen). RT-PCR was performed as previously described (14). Reverse transcription was performed using 0.5 μM of the 5′- and 3′-specific primers for monkey nAChR α1, α3, α4, α7, α9, α10, β2, and β4 (Table 1). Sizes of all amplified bands were consistent with the primers used.
TABLE 1.
RT-PCR
| mRNA | Sequence (5′–3′) | Length (bp) | Accession No. (GenBank)/Reference |
|---|---|---|---|
| α1 | F: CACCGCTCACCCAGCACCCATGTC | 451 | XM_001091830 |
| R: TTCTGGGGCAGAGCTAGGCTC | |||
| α3 | F: CTGATTGGAGAGTACCTCCTGTTCAC | ||
| R: ATACGATCAATCACCATGGCAACATA | 526 | XM_001107366 | |
| α4 | F: ATCACCTACGCCTTCGTCATCCGGCGGCTG | ||
| R: TCCTTGACCACGGATGGCACTTCATGAGG | 400 | XM_001114265 | |
| α4.1 | F: TTTGGTGCTGCGGGCCTTGAGTGT | ||
| R: ACCCGCCCTCGCCGTCCTGTGT | 449 | XM_001114265 | |
| α7 | F: CGAAAGCGAGGCGGTCTGCAGCGAGTGGAA | ||
| R: AGTTGTGGCGTTTAATGTTGTCCTGGATTA | 259 | NM_001032883 | |
| α9 | F: AAAGGGAGTGAATGGAAGAAGGTG | ||
| R: AAGGGGAACTAAGGGGAAAACGAG | 486 | XM_001094288 | |
| α10 | F: GTGGAGAGCGTGCGGGTTATTAT | ||
| R: GTGGAGAGCGTGCGGGTTATTAT | 392 | BV208429 | |
| β2 | F: GGCTGGCCTGACACAATGGTAG | ||
| R: TCAGGGCAGGGAGGAAGAGTCAG | 547 | XM-001114439 | |
| β4 | F: GGTCTTCCTGCTGCTCATCTCCAA | ||
| R: GACACCTTCTAATGCCTCCTGCAC | 449 | XM-001107489 |
Definition of abbreviations: F, forward primer; R, reverse primer.
Electrophysiology
For electrophysiologic recordings, cells were grown on coverslips, transferred to a recording chamber mounted on the stage of an Axioskop2 microscope (Zeiss). Currents were recorded from confluent cultured monkey BECs (typically after 7–10 d culture) using the standard whole-cell patch–clamp technique (15). The external Krebs solution bathing the BEC had the following composition: 130 mM NaCl, 3 mM KCl, 2.5 mM CaCl2, 1 mM MgCl2, 10 mM NaHCO3, 5 mM Hepes, and 10 mM glucose, pH 7.35–7.40. To isolate inward currents, an internal pipette solution with the following composition was used: 130 mM CsCl, 1 mM CaCl2, 2 mM MgCl2, 10 mM EGTA, 10 mM Hepes, 4 mM Mg-ATP, pH adjusted to 7.2 with CsOH. Pipette resistance was 3 to 5 MΩ, and access resistance was 15 MΩ or less. The chamber, which had a volume of 1 ml, was perfused continuously with Krebs solution at a rate of 6 to 7 ml/minute. All recordings were made from cells at a room temperature.
Drugs were added to the perfusate, and their delivery to the cells was controlled by separate valves. The following drugs used in this study were obtained from Sigma: nicotine, ACh, choline, atropine, mecamylamine, methyllycaconitine (MLA), dihydro-β-erythroidine (DHβE), genistein, and vanadate. Activated pervanadate solution was prepared freshly for each experiment and made by mixing a stock solution of vanadate with H2O2 in an equal molar ratio. Genistein was dissolved in 0.1% DMSO. Stock solution (1–10 mM) of all the drugs were prepared on the day of the experiment in distilled water and diluted with Krebs solution to their final concentration before use.
Currents were recorded with a multiClamp 700B amplifier (Molecular Devices/Axon Instruments, Foster, CA). Data were filtered at 5 KHz. Voltage and current clamp protocols, data acquisition, and analysis were performed using pClamp9 software and DigiData 1322A interface (Molecular Devices/Axon Instruments). All data are given as mean (±SEM), and statistical comparisons were made using the unpaired, two-tailed Student's t test.
RESULTS
Expression of nAChR-α4, -α7, and -β2 in Cultured BECs
Previous studies from our laboratory have demonstrated expression of α7, α4, and β2 nAChR subunits in monkey airway by immunohistochemistry (2). In this study, we first used immunofluorescence to demonstrate that α7, α4, and β2 nAChR subunits are similarly expressed in cultured monkey BECs, establishing them as a valid model (Figure 1). As shown in Figure 1A, cultured cells expressed cytokeratin consistent with the phenotype of BEC. For nAChR characterization, lung sections and cultured cells were stained with the same Abs. In lung sections, expression of α7, α4, and β2 subunits were found in the airway epithelial cell membrane and concentrated in the apical cell membrane (Figures 1B–1D). As shown in Figure 1E, cultured BEC showed similar expression of α7 nAChR, as observed in lung sections. Counting of four randomly selected fields of view per culture showed that the positive percentage of cells staining for α7 was 72 (±2.1) %. The positive percentage of α4 and β2 subunits staining in cultured BECs was 71 (±5.5) % and 77 (±2.9) %, respectively.
The expression of nAChRs transcript in BEC and hypothalamus was examined by RT-PCR (Figure 1F). The α4 subunit has two splice variants (α4 and α4.1). Using specific primers, only the longer α4.1 transcript was present in monkey BECs, whereas both α4 and α4.1 were present in the hypothalamus. RT-PCR was also performed for the α1, α7, α9, and α10 nAChRs, which have been described as potential additional α-bungarotoxin–binding nicotinic receptor subunits in peripheral cells (16). As shown in Figure 1F, α9 and α10 are expressed in BEC. No α1, α5, or α6 transcripts were detected in the BECs.
Effect of ACh and Nicotine on nAChR Expression and Inward Current in Cultured Monkey BECs
Electrophysiology was used to characterize the activity of nAChR expressed by whole-cell patch–clamp analysis. The mean resting membrane potential of BECs was −40.3 (±7) mV (n = 33). Rapid application of nicotine to a voltage-clamped BEC evoked inward currents at negative potential in 21% of cells studies (110 of the 514 BECs); a slightly lower percentage of cells responded similarly to ACh (Figure 2).
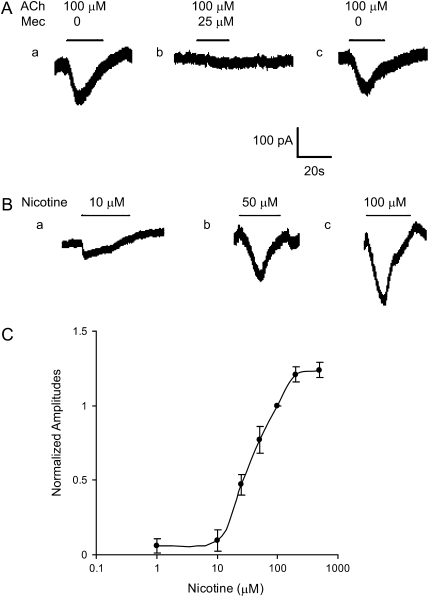
Figure 2.
Detection of ACh- and nicotine-induced currents in cultured BECs. (A, a) ACh (100 μM) induced a rapid inward current in BEC. (A, b) Mecamylamine (Mec; 25 μM) was perfused for 3–5 minutes, then 100 μM ACh plus 25 μM mecamylamine was applied and resulted in a diminished ACh-induced inward current. (A, c) The ACh (100 μM) induced current after washout of mecamylamine. All traces are from the same cell. Holding potential was −60 mV. (B) Inward currents in response to application of nicotine at concentrations shown. (C) Dose–response of BEC response to nicotine. Peak currents evoked at concentrations shown are expressed relative to the peak current evoked by 100 μM of nicotine and plotted against the log of nicotine concentration. Mean response was taken from 5–12 cells. Experimental data were fitted to the Hill equation (EC50 = 26.5 μM; Hill coefficient = 1.0). Data are presented as means (±SEM).
ACh- or nicotine-induced inward current was voltage independent. Application of 100 μM ACh to a BEC voltage clamped at −60 mV elicited a transient inward current (Figure 2A, a). Evoked current ranged from approximately 50 to 98 pA, and mean peak ACh-induced inward current was 68.4 (±5.3) pA (n = 8). The current response induced by ACh was reversibly blocked by the nonselective nAChR antagonist, mecamylamine (Figure 2A, b and c).
The nicotine-induced inward currents were concentration dependent (Figures 2B and 2C). At the holding potential of −60 mV, nicotine (1–500 μM) induced currents (Inic) that displayed a rapid desensitization in the continued presence of the nicotine. The mean peak amplitude of Inic in response to 50 μM nicotine was 86.7 (±6.5) pA (n = 11). To obtain the dose–response relationship, the mean peak current at each nicotine concentration was normalized to that elicited by 100 μM nicotine. The EC50 for activation by nicotine was 26.5 (±2.3) μM (n = 4), and the Hill coefficient was 1.0 (±0.06) (n = 4) (Figure 2C).
Pharmacological Characterization of BEC nAChR
In cultured BECs, the mean current induced by 100 μM nicotine was 175 (±10.7) pA (n = 6) under control conditions and 11.6 (±2.2) pA (n = 5) after 25 μM mecamylamine, corresponding to a reduction of approximately 95% (Figures 3A and 3B). DHβE (50 μM), an antagonist with high affinity for α4 and α3 containing nAChR (17), reduced Inic from 170 (±9.4) pA (n = 5) in control condition to 23.6 (±4) pA (n = 5) after DHβE, a reduction of roughly 86% (Figure 3B).
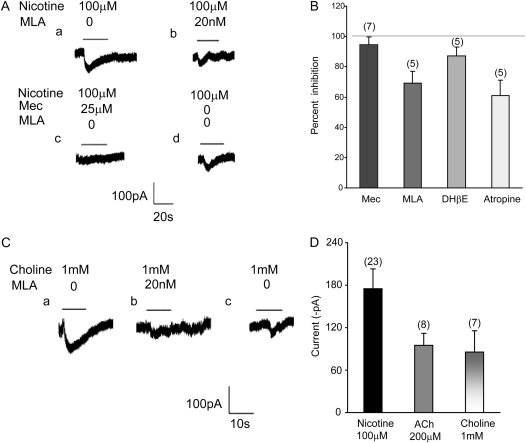
Figure 3.
Effects of nAChR antagonists and agonists on nicotine-induced current in BECs. (A, a and b) The inward current induced by 100 μM nicotine was partially blocked by the α7 nAChR antagonist, methyllycaconitine (MLA) (20 nM). (A, c) The nonselective nAChR antagonist, mecamylamine (25 μM), blocked all current induced by nicotine. (A, d) Nicotine (100 μM) induced current after washout of mecamylamine. (B) Summary of effects of cholinergic antagonists on nicotine-induced current (100 μM). Data are presented as means (±SEM). Gray line shows 100% inhibition of nicotine-induced current. (C, a) Inward current evoked by perfusion of 1 mM choline. (C, b) MLA (20 nM) blocked most of the choline-induced current. (C, c) The choline-induced (1 mM) inward current after washout of MLA. (D) Summary of effects of cholinergic agonists on inward currents in BECs. Data are presented as means (±SEM).
MLA (20 nM), a competitive antagonist specific for α7 nAChR, reversibly inhibited 100 μM nicotine–induced current (Figures 3A and 3B). The mean total nicotine-induced current was 167 (±15.1) pA (n = 7). Using MLA, this current could be divided into MLA-sensitive and -insensitive currents to show the relative role of α7 and non-α7 containing nAChR in mediating Inic. The amplitude of nicotine-induced MLA-sensitive current was 96.8 (±8.1) pA (n = 5), and the amplitude of nicotine-induced, MLA-insensitive current was 55.7 (±7.2) pA (n = 5) (Figures 3A and 3B).
Choline, a key precursor for lipid and surfactant synthesis in lung, is also an α7 nAChR agonist (18, 19). Consistent with this, 17 of 170 BECs sampled (∼10%) responded to 1 mM choline (Figure 3C). The mean amplitude of choline-induced current was 85.5 (±11.7) pA (n = 7, Figure 3D), and the desensitization time constant of choline-induced current was 17.7 (±8) seconds (n = 5). The choline-induced currents were reversibly blocked by 20 nM MLA (Figure 3C). The currents induced by 1 mM choline were equivalent to that induced by 200 μM ACh, and 50% of that induced by 100 μM nicotine.
Chronic Nicotine Exposure Activates nAChR Response in BECs
Depending on both tissue type and subunit composition, chronic nicotine exposure can activate or desensitize nAChR. To determine how chronic nicotine exposure affects nAChR activity in BECs, cultured BECs were incubated with or without 1 μM nicotine for 48 hours, washed, and their responsiveness to varying doses of nicotine tested. Nicotine-induced inward currents were significantly increased by 48-hour incubation with 1 μM nicotine compared with control BECs (Figure 4). The mean current induced by 50 μM nicotine was 80.6 (±4.7) pA (n = 5) for BECs incubated without nicotine, and 119.3 (±15.7) pA for BECs incubated with 1 μM nicotine. Thus, chronic low levels of nicotine up-regulate nAChR activity. Detectable expression of nicotinic receptors in BEC was also increased by chronic nicotine exposure, from 72 (±2.1) % of cells to 90 (±11) % (P < 0.05) of cells (Figure 1 E, b).
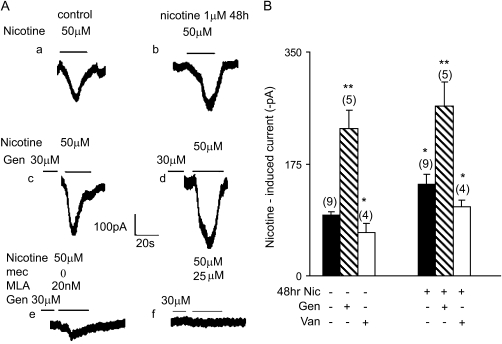
Figure 4.
Effect of chronic nicotine exposure and genistein on nicotine-induced currents in BECs. (A, a) The inward current induced by 50 μM nicotine in a control cell. (A, b) A 48-hour incubation with 1 μM nicotine increased the inward current induced by 50 μM nicotine roughly 26% compared with control. (A, c) The inward current induced by 50 μM nicotine was increased roughly 58% by preincubation with genistein (30 μM) for 2 hours. (A, d) The combination of 48-hour incubation with 1 μM nicotine (50 μM) and 2-hour preincubation with genistein (30 μM) further increased nicotine-induced inward current. (B) Mean effects of inhibition of kinase and phosphatase activity by genistein and vanadate, respectively, on nicotine (50 μM)-induced current. Cells were incubated in control media or media containing 1 μM nicotine for 48 hours. Cells were then incubated for 2 hours with 30 μM genistein or 1 hour with 20 μM vanadate, as shown. Genistein (30 μM) enhanced both acute and chronic nicotine–induced currents, whereas 20 μM vanadate decreased both acute and chronic nicotine-induced currents. Numbers in parentheses indicate the number of cells averaged (mean ± SEM; * P < 0.05; ** P < 0.01).
nAChR Activity Is Regulated by Phosphorylation
In the central nervous system, nAChR activity is up-regulated by tyrosine dephosphorylation in both Xenopus oocytes and rat hippocampal interneurons (20). To determine if similar mechanisms regulate nAChR activity in BECs, we examined the effects of tyrosine kinase and phosphatase inhibitors on nAChR function in BECs.
Cultured BECs were incubated with the tyrosine kinase inhibitor, genistein (30 μM), for 2 hours before nicotine challenge. Genistein (30 μM) significantly enhanced nicotine-induced current from 96.4 (±6.7) pA (n = 4) in control to 229.7 (±29.8) pA in treated (n = 4; P < 0.01), corresponding to an increase of roughly 140% (Figure 4). Genistein also further up-regulated the nicotine-induced currents already up-regulated by chronic exposure to nicotine. Nicotine-induced current after 48-hour exposure to 1 μM nicotine was 229.7 (±32) pA (n = 5), and the combination of genistein and chronic nicotine exposure further increased nicotine-induced current to 265.8 (±74.8) pA, an additional increase of 15% (Figure 4).
Conversely, inhibition of tyrosine phosphatases with pervanadate decreased nicotine-induced current. BECs were incubated with the tyrosine phosphatase inhibitor, pervanadate (20 μM), for 1 hour. Pervanadate decreased nicotine-induced (50 μM) current from 95.6 (±4.6) pA in control to 67.5 (±14.1) pA (n = 4; P < 0.05), corresponding to a reduction by approximately 30% (Figure 4B). Pervanadate also significantly decreased the nicotine-induced currents in BECs exposed to chronic nicotine by roughly 25% from 143.3 (±15.1) pA in controls to 107.5 (±11.9) pA in treated (Figure 4B). These studies indicate that nicotine currents in BECs are regulated by both tyrosine kinases and phosphatases.
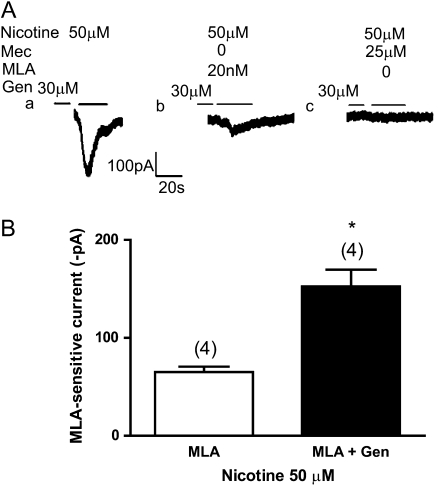
The genistein-enhanced, nicotine-induced current was strongly inhibited by 20 nM MLA, indicating that genistein primarily increased α7 nAChR current. Genistein-enhanced, nicotine-induced, MLA-sensitive current (i.e., α7 nAChR current) was obtained by subtracting genistein-enhanced, MLA-insensitive current from total genistein-enhanced, nicotine-induced current (e.g., Figure 4A, c–e). The mean of genistein-enhanced, nicotine-induced, MLA-sensitive currents was 152.1 (±34) pA (n = 4). Nicotine-induced, MLA-sensitive current was obtained by subtracting nicotine-induced MLA-insensitive current from total nicotine-induced current (e.g., Figure 3A, a–b). The mean of nicotine-induced, MLA-sensitive currents was 65.2 (±6) pA (n = 4). This is quantified in Figure 5, in which it can be seen that the genistein more than doubled the MLA-sensitive nicotine current, from 65.2 pA (n = 4) to 152.1 pA (Figure 5B).
Figure 5.
Effect of the α7 nAChR antagonist, MLA, on increases in nicotine-induced current caused by genistein. (A, a) Inward current induced by 50 μM nicotine in a control cell (note: same tracing as used in Figure 4A, a). (A, b) MLA (20 nM) inhibited the genistein-enhanced nicotine currents by approximately 66%, with a slower desensitizing current remaining. (A, c) Mec (25 μM) inhibited all the genistein-enhanced nicotine currents. (B) Genistein significantly increased the MLA-sensitive component of nicotine-induced inward current. The MLA-sensitive component of nicotine-induced (50 μM) inward current was 65.2 (±6) pA (n = 4). Genistein (30 μM) increased the MLA-sensitive component of nicotine-induced inward current to 152.1 (±34) pA (n = 4), a 2.3-fold increase. Open bars, MLA; solid bars, MLA + Genistein. Numbers in parentheses indicate the number of cells averaged (mean ± SEM; *P < 0.05).
DISCUSSION
This study shows that fetal and neonatal lung BECs express functional nAChR, and that chronic nicotine exposure significantly up-regulates nAChR activity. The expression of nAChR in monkey BECs was demonstrated by both immunohistochemical and functional studies. Immunofluorescence demonstrated that monkey BECs, both in lung and primary culture, express multiple nAChR subtypes, as determined by well characterized Abs against α4 (monoclonal Ab [mAb] 299), β2 (mAb 270), and α7(mAb 306) nAChR. The specificity of these Abs has recently been demonstrated by Whiteaker and colleagues (21), demonstrating the absence of staining using these Abs in brains of α4 and β2 knockout mice, respectively. In both monkey lung sections and cultured BECs, these Abs showed clear expression of nAChR. Immunostaining was also confirmed by RT-PCR. In addition to the immunostaining shown in Figure 1, RT-PCR also demonstrated expression of α3, α4, α7, α9, α10, and β4 mRNA expression in primary cultures of monkey BECs. Interestingly, BECs only express the 4.1 subtype of the α4.1 nAChR. α4.1 Differs from α4 only in its untranslated region, so this may suggest alterations in mRNA stability and transcription regulation compared with α4 expression in the central nervous system (22).
Whole-cell patch–clamp analysis demonstrated that the nAChR expressed in monkey BECs primary cultures are functional. BEC nAChR are activated by ACh and nicotine, are blocked by mecamylamine, DHβE, and MLA, and have ion-gating properties similar to those of human BECs (23, 24) and neuroepithelial bodies in the lung (25). Only a subset of BECs showed nicotine-induced currents under voltage clamp. This reflected that not all cells stained for nicotinic receptors, and the inability to establish or maintain high-resistance seals when patch clamping the cells.
Interestingly, 50 μM atropine, a nonselective muscarinic antagonist, blocked the nicotine-induced current by approximately 61% (Figure 3B). This suggests that muscarinic receptors may have a modulatory effect on nAChR function in BECs. Similar results have been reported in neuroepithelial cells (25) and petrosal neurons (26, 27). Alternately, atropine has also been reported to act as an α7 antagonist and to also inhibit activity of nAChR containing α9 and α10 subunits, which, as discussed below, are expressed in BECs (Figure 1F) (28, 29). For the human α7 nAChR, atropine has an IC50 of 125 μM (13).
The EC50 for nicotine to activate α7 nAChR is 125 μM, which is consistent with the dose response for nicotine shown in Figure 2 (13). Nicotine has an EC50 of 70 μM for α3β2 nAChR, and an EC50 of 56 μM for α3β4 nAChR (10). By contrast, the EC50 of nicotine for α4β2 nAChR is between 0.1 and 2 μM (10). This suggests that the heteromeric receptors active in BECs contain α3 subunits, and that α4 subunits have a lesser role. α3-Containing nicotinic receptors in BECs are also consistent with the potent inhibition by DHβE seen in Figure 3.
The roles of α9 and α10 are best characterized in auditory pathways. The α9 and α10 nAChR subunits assemble to form the nAChR that mediates synaptic transmission between efferent olivocochlear fibers and hair cells of the cochlea. The α9 and α10 subunits may also form more complex heteromers with α7 in the cochlea, and in sites such as dorsal root ganglion neurons, peripheral blood lymphocytes, developing thymocytes, and skin (30). Homomeric α9 and heteromeric α9α10 nAChR are reversibly inhibited by nanomolar concentrations of α-bungarotoxin and MLA (30–32). In Xenopus oocytes, rat α9 nAChR displays mixed nicotinic–muscarinic features (28), and are inhibited by atropine. Thus, our observations that α9 and α10 mRNAs are expressed in cultured monkey BEC, combined with the inhibitory effects of atropine and MLA, are also consistent with functional α9 and α10 containing nAChR in BECs. The exact role of this new receptor subtype in BECs, as well as subunit stoichiometry, remains to be determined.
In monkey BECs, the time constant of the choline-induced current was 1.8 seconds, which is slower than adult human BEC (400 ms) (23), and nearly equal with neuroepithelial bodies (2.5 s) (25). The possible reason for slower kinetics of the choline-induced current recorded from monkey BECs is that nAChRs in BECs from fetal and neonatal animals could contain α7 assembled with other nAChR subunits (e.g., β2, resulting in slower desensitization compared with homomeric α7 nAChR found in neurons). Given the importance of choline in surfactant synthesis and recycling, signaling by choline could play a role in regulation of surfactant function and synthesis.
As shown in Figure 4, chronic nicotine exposure increased nicotine-induced currents in voltage-clamped BECs. This clearly demonstrates that chronic nicotine does not inactivate nAChR, and suggests that nicotine increases nAChR activity. This likely reflects both increased nicotinic receptor expression induced by nicotine, as shown in Figure 1E, and increased trafficking of nicotinic receptors to the cell membrane (10). Whether chronic nicotine exposure also increases conductance of individual nAChR in BECs cannot be determined without single-unit recording from BEC.
Protein phosphorylation and dephosphorylation are key mechanisms for regulating activity of ligand-gated ion channels, such as the nAChR (20). In SH-SY5Y neuroblastoma cells transfected with α7, inhibition of tyrosine kinase activity increased ACh-evoked current, whereas increased phosphorylation made nAChR less active. In our study, the tyrosine kinase inhibitor, genistein, increased acute nicotine–induced current by 58%, and further increased nicotine-induced current by 16% in the presence of chronic nicotine exposure. As shown in Figure 5, the increase in nicotine currents by genistein primarily reflected increased α7 currents. Cho and colleagues (20) have suggested that genistein increases nAChR signaling by increasing trafficking of nAChR to the cell surface. In that nicotine has also been reported to act as a chaperone to increase nAChR expression on the cell membrane (10), it is not surprising that there is an additive effect between nicotine and genistein on nAChR activity.
Our laboratory has previously reported extensive expression of nAChR in fetal lung, and that prenatal nicotine exposure leads to abnormalities in offspring lung structure (1) and function (33). Our findings here show that chronic nicotine exposure to BEC derived from fetal or neonatal primate lung does not cause nAChR down-regulation, but rather increases nAChR activity. Thus, prenatal nicotine exposure affects lung development by activating nAChR in lung, leading to effects on lung development from both the exogenous nicotine and heightened sensitivity to endogenous ACh. In addition, nAChR activity in developing lung will also be strongly regulated by tyrosine phosphorylation. With the knowledge that effects of smoking on fetal development are caused in part by nAChR up-regulation, one can begin to design therapeutic strategies to block effects of nAChR activation, as well as targeting effects of nAChR regulation by phosphorylation.
This work was supported by National Institutes of Health grants RR00163 and HL087710 (E.R.S.), and NS11323 (J.L.).
Originally Published in Press as DOI: 10.1165/rcmb.2008-0352OC on December 18, 2008
Conflict of Interest Statement: J.L. has given seminars at pharmaceutical companies (Trans Tech Pharma, 2008, $1,000; AstraZeneca, 2007, $1,500; Athenagen, 2006, $2,000; Wyeth, 2005, $1,000), but has not talked about nicotine up-regulation of acetylcholine receptors in bronchial epithelial calls. None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Sekhon HS, Jia Y, Raab R, Kuryatov A, Pankow JF, Whitsett JA, Lindstrom J, Spindel ER. Prenatal nicotine increases pulmonary alpha7 nicotinic receptor expression and alters fetal lung development in monkeys. J Clin Invest 1999;103:637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Proskocil BJ, Sekhon HS, Jia Y, Savchenko V, Blakely RD, Lindstrom J, Spindel ER. Acetylcholine is an autocrine or paracrine hormone synthesized and secreted by airway bronchial epithelial cells. Endocrinology 2004;145:2498–2506. [DOI] [PubMed] [Google Scholar]
- 3.Cutz E, Perrin DG, Hackman R, Czegledy-Nagy EN. Maternal smoking and pulmonary neuroendocrine cells in sudden infant death syndrome. Pediatrics 1996;98:668–672. [PubMed] [Google Scholar]
- 4.Sekhon HS, Keller JA, Proskocil BJ, Martin EL, Spindel ER. Maternal nicotine exposure upregulates collagen gene expression in fetal monkey lung: association with alpha7 nicotinic acetylcholine receptors. Am J Respir Cell Mol Biol 2002;26:31–41. [DOI] [PubMed] [Google Scholar]
- 5.Fenster CP, Whitworth TL, Sheffield EB, Quick MW, Lester RA. Upregulation of surface alpha4beta2 nicotinic receptors is initiated by receptor desensitization after chronic exposure to nicotine. J Neurosci 1999;19:4804–4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ke L, Eisenhour CM, Bencherif M, Lukas RJ. Effects of chronic nicotine treatment on expression of diverse nicotinic acetylcholine receptor subtypes. I. Dose- and time-dependent effects of nicotine treatment. J Pharmacol Exp Ther 1998;286:825–840. [PubMed] [Google Scholar]
- 7.Kawai H, Zago W, Berg DK. Nicotinic alpha 7 receptor clusters on hippocampal GABAergic neurons: regulation by synaptic activity and neurotrophins. J Neurosci 2002;22:7903–7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez-Hernandez GY, Sanchez-Padilla J, Ortiz-Acevedo A, Lizardi-Ortiz J, Salas-Vincenty J, Rojas LV, Lasalde-Dominicci JA. Nicotine-induced up-regulation and desensitization of alpha4beta2 neuronal nicotinic receptors depend on subunit ratio. J Biol Chem 2004;279:38007–38015. [DOI] [PubMed] [Google Scholar]
- 9.Buisson B, Bertrand D. Chronic exposure to nicotine upregulates the human (alpha)4((beta)2 nicotinic acetylcholine receptor function. J Neurosci 2001;21:1819–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuryatov A, Luo J, Cooper J, Lindstrom J. Nicotine acts as a pharmacological chaperone to up-regulate human alpha4beta2 acetylcholine receptors. Mol Pharmacol 2005;68:1839–1851. [DOI] [PubMed] [Google Scholar]
- 11.Charpantier E, Wiesner A, Huh KH, Ogier R, Hoda JC, Allaman G, Raggenbass M, Feuerbach D, Bertrand D, Fuhrer C. Alpha7 neuronal nicotinic acetylcholine receptors are negatively regulated by tyrosine phosphorylation and Src-family kinases. J Neurosci 2005;25:9836–9849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu R, Martin WR, Robinson CB, St George JA, Plopper CG, Kurland G, Last JA, Cross CE, McDonald RJ, Boucher R. Expression of mucin synthesis and secretion in human tracheobronchial epithelial cells grown in culture. Am J Respir Cell Mol Biol 1990;3:467–478. [DOI] [PubMed] [Google Scholar]
- 13.Peng X, Gerzanich V, Anand R, Whiting PJ, Lindstrom J. Nicotine-induced increase in neuronal nicotinic receptors results from a decrease in the rate of receptor turnover. Mol Pharmacol 1994;46:523–530. [PubMed] [Google Scholar]
- 14.Sekhon HS, Keller JA, Spindel ER. Maternal nicotine exposure up-regulates collagen and elastin gene expression in fetal nonhuman primate lungs: potential role of a7 nicotinic receptors [abstract]. Am J Respir Crit Care Med 2000;161:A562. [Google Scholar]
- 15.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch–clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch 1981;391:85–100. [DOI] [PubMed] [Google Scholar]
- 16.Gahring LC, Rogers SW. Neuronal nicotinic acetylcholine receptor expression and function on nonneuronal cells. AAPS J 2006;7:E885–E894.AA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harvey SC, Maddox FN, Luetje CW. Multiple determinants of dihydro-beta-erythroidine sensitivity on rat neuronal nicotinic receptor alpha subunits. J Neurochem 1996;67:1953–1959. [DOI] [PubMed] [Google Scholar]
- 18.Alkondon M, Pereira EF, Cortes WS, Maelicke A, Albuquerque EX. Choline is a selective agonist of alpha7 nicotinic acetylcholine receptors in the rat brain neurons. Eur J Neurosci 1997;9:2734–2742. [DOI] [PubMed] [Google Scholar]
- 19.Alkondon M, Albuquerque EX. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. I. Pharmacological and functional evidence for distinct structural subtypes. J Pharmacol Exp Ther 1993;265:1455–1473. [PubMed] [Google Scholar]
- 20.Cho CH, Song W, Leitzell K, Teo E, Meleth AD, Quick MW, Lester RA. Rapid upregulation of alpha7 nicotinic acetylcholine receptors by tyrosine dephosphorylation. J Neurosci 2005;25:3712–3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whiteaker P, Cooper JF, Salminen O, Marks MJ, Clure-Begley TD, Brown RW, Collins AC, Lindstrom JM. Immunolabeling demonstrates the interdependence of mouse brain alpha4 and beta2 nicotinic acetylcholine receptor subunit expression. J Comp Neurol 2006;499:1016–1038. [DOI] [PubMed] [Google Scholar]
- 22.Shacka JJ, Robinson SE. Postnatal developmental regulation of neuronal nicotinic receptor subunit alpha 7 and multiple alpha 4 and beta 2 mRNA species in the rat. Brain Res Dev Brain Res 1998;109:67–75. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Pereira EF, Maus AD, Ostlie NS, Navaneetham D, Lei S, Albuquerque EX, Conti-Fine BM. Human bronchial epithelial and endothelial cells express alpha7 nicotinic acetylcholine receptors. Mol Pharmacol 2001;60:1201–1209. [DOI] [PubMed] [Google Scholar]
- 24.Maus AD, Pereira EF, Karachunski PI, Horton RM, Navaneetham D, Macklin K, Cortes WS, Albuquerque EX, Conti-Fine BM. Human and rodent bronchial epithelial cells express functional nicotinic acetylcholine receptors. Mol Pharmacol 1998;54:779–788. [DOI] [PubMed] [Google Scholar]
- 25.Fu XW, Nurse CA, Farragher SM, Cutz E. Expression of functional nicotinic acetylcholine receptors in neuroepithelial bodies of neonatal hamster lung. Am J Physiol Lung Cell Mol Physiol 2003;285:L1203–L1212. [DOI] [PubMed] [Google Scholar]
- 26.Zhong H, Nurse CA. Nicotinic acetylcholine sensitivity of rat petrosal sensory neurons in dissociated cell culture. Brain Res 1997;766:153–161. [DOI] [PubMed] [Google Scholar]
- 27.Nurse CA, Vollmer C. Role of basic FGF and oxygen in control of proliferation, survival, and neuronal differentiation in carotid body chromaffin cells. Dev Biol 1997;184:197–206. [DOI] [PubMed] [Google Scholar]
- 28.Verbitsky M, Rothlin CV, Katz E, Elgoyhen AB. Mixed nicotinic-muscarinic properties of the alpha9 nicotinic cholinergic receptor. Neuropharmacology 2000;39:2515–2524. [DOI] [PubMed] [Google Scholar]
- 29.Gerzanich V, Anand R, Lindstrom J. Homomers of alpha 8 and alpha 7 subunits of nicotinic receptors exhibit similar channel but contrasting binding site properties. Mol Pharmacol 1994;45:212–220. [PubMed] [Google Scholar]
- 30.McIntosh JM, Plazas PV, Watkins M, Gomez-Casati ME, Olivera BM, Elgoyhen AB. A novel alpha-conotoxin, PeIA, cloned from Conus pergrandis, discriminates between rat alpha9alpha10 and alpha7 nicotinic cholinergic receptors. J Biol Chem 2005;280:30107–30112. [DOI] [PubMed] [Google Scholar]
- 31.Elgoyhen AB, Vetter DE, Katz E, Rothlin CV, Heinemann SF, Boulter J. Alpha10: a determinant of nicotinic cholinergic receptor function in mammalian vestibular and cochlear mechanosensory hair cells. Proc Natl Acad Sci USA 2001;98:3501–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baker ER, Zwart R, Sher E, Millar NS. Pharmacological properties of alpha 9 alpha 10 nicotinic acetylcholine receptors revealed by heterologous expression of subunit chimeras. Mol Pharmacol 2004;65:453–460. [DOI] [PubMed] [Google Scholar]
- 33.Sekhon HS, Keller JA, Benowitz NL, Spindel ER. Prenatal nicotine exposure alters pulmonary function in newborn rhesus monkeys. Am J Respir Crit Care Med 2001;164:989–994. [DOI] [PubMed] [Google Scholar]