Abstract
Humans learn to speak by a process of vocal imitation that requires the availability of auditory feedback. Similarly, young birds rely on auditory feedback when learning to imitate the songs of adult birds, providing one of the few examples of nonhuman vocal learning. However, whereas humans continue to use auditory feedback to correct vocal errors in adulthood, the mechanisms underlying the stability of adult birdsong are unknown. Here we show that like human speech, adult birdsong is maintained by error correction. We perturbed the pitch (fundamental frequency) of auditory feedback in adult Bengalese finches using custom-designed headphones. Birds compensated for the imposed auditory error by adjusting the pitch of song. When the perturbation was removed, pitch returned to baseline. These results show that adult birds correct vocal errors by comparing auditory feedback to a sensory target and suggest that lifelong error correction is a general principle of learned vocal behavior.
Song learning in many species of birds, like language learning in humans, is based on a process of imitation 1, 2. Learning begins when a young bird is exposed to the song of an adult “tutor”. Subsequently, the young bird refines his initially disordered vocalizations into a mature, “crystallized” song very similar to that of the tutor 3-5. This process parallels speech acquisition in humans, in which language exposure in infancy is thought to establish perceptual categories that then serve as targets for vocal production 2, 6, eventually resulting in a child learning to produce the phonemes of his or her native language. For both adult speech and crystallized song, phonetic structure is highly stereotyped from one rendition to the next and extremely stable throughout the remainder of the individual's life.
Despite the widely-accepted parallels between the acquisition of song and speech7, it is unclear whether the extraordinary stability of adult vocal behavior in birds and humans results from similarly parallel processes. Speech performance in adult humans is thought to rely on an active process in which sensory signals are used to identify and correct vocal motor errors. Recent laboratory studies have shown that when auditory feedback is manipulated, human adults alter their vocal output so as to reduce the experienced auditory error 8, 9, demonstrating the reliance on auditory feedback to correct speech errors.
Here, we test the hypothesis that like humans, birds maintain adult vocal output by comparing auditory feedback to a long-lived auditory target and using the resulting error signals to adaptively modify their vocalizations. This type of error-corrective mechanism requires several things of the adult song system. First, there must be a stable auditory target representing the desired song output. Second, adults must detect small differences between auditory feedback and the target and evaluate which changes in motor output reduce the sensory error. Third, the motor program for song must be modifiable. The first two requirements define error correction, whereas the third merely asserts that the song system is plastic in adulthood.
Manipulations that corrupt or completely eliminate auditory experience (via distortions of auditory feedback or deafening) have been shown to drive degradations of crystallized song 10-12, demonstrating the potential for vocal plasticity in adulthood and showing that the third condition is satisfied. Furthermore, differential reinforcement signals provided by an external evaluator can drive directed changes in adult song 13. However, no prior studies have shown that adult birds naturally perform error correction by monitoring song output to detect and correct deviations from an auditory target. Deafening and feedback distortion paradigms presumably create a mismatch between auditory feedback and the sensory target, but provide no opportunity for the bird to correct these errors, since no alteration of vocal output can restore normal auditory feedback. Reinforcement paradigms circumvent error detection entirely by rewarding or punishing birds based on the experimenter's (rather than the bird's) evaluation of vocal performance. A true test of adult error correction therefore requires an experimental paradigm in which birds both detect song errors and modify their vocal output to reduce them.
We tested the hypothesis of adult error correction by introducing small, correctable perturbations to the pitch (fundamental frequency) of auditory feedback in order to mimic naturally-occurring vocal errors. Pitch is a learned and precisely controlled parameter of song, and the pitch of individual song elements (or “syllables”) is refined during song acquisition and extremely stable in adulthood 5, 14. We predicted that if adult birds maintain their songs by comparing auditory feedback to an auditory target and modifying their songs to correct sensory errors, then shifting the pitch of auditory feedback would cause birds to change the pitch of song in the direction opposite the experimentally imposed feedback shift, thus reducing the experienced auditory error.
RESULTS
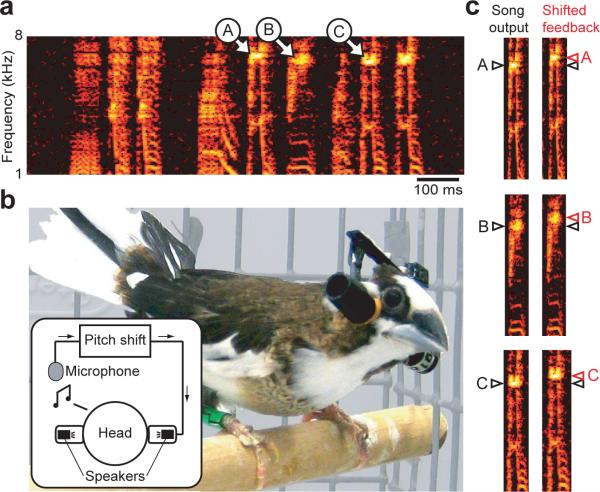
A set of lightweight headphones was custom-fit to each bird in the study in order to generate online shifts in the pitch of auditory feedback. An example of crystallized song from one bird in our study is shown in Figure 1a. A microphone in each bird's cage relayed acoustic signals through sound-processing hardware capable of generating arbitrary shifts in pitch. These pitch-shifted acoustic signals were then played back through speakers in the headphones (Fig. 1b, inset) with an average processing delay of ∼7 msec. Shifts in the pitch of auditory feedback and the resulting changes in the pitch of song are both measured in units of “cents” (see Methods), where 1200 cents corresponds to an octave and 100 cents represents the same pitch interval as a semitone (approximately a 6% change in absolute frequency). Fig. 1c illustrates a 100 cent upward shift in pitch applied to several song syllables.
Figure 1. Technique for manipulating auditory feedback.
a, Crystallized song from an adult Bengalese finch. Spectrographic representation shows the power at each frequency (color scale) as a function of time. Three harmonic features are labeled A, B, and C. b, Each bird was fit with a set of headphones that housed a pair of speakers. A microphone in the cage (see inset) provided input to online sound-processing hardware, which was used to manipulate the pitch of song. Processed acoustic signals were then relayed to the headphone speakers via a flexible cable (not shown in photograph) and played through the speakers. c, An upward (+100 cent) shift in the pitch of auditory feedback introduced by the headphone system. For each of the harmonic features labeled in (a), the left spectrogram shows the bird's acoustic output and the black triangle shows the frequency of the harmonic feature. The right spectrogram shows the pitch-shifted auditory feedback played through the headphones and the red triangle shows the frequency of the harmonic feature in the shifted song. Black triangles are repeated next to the spectrograms on the right for comparison.
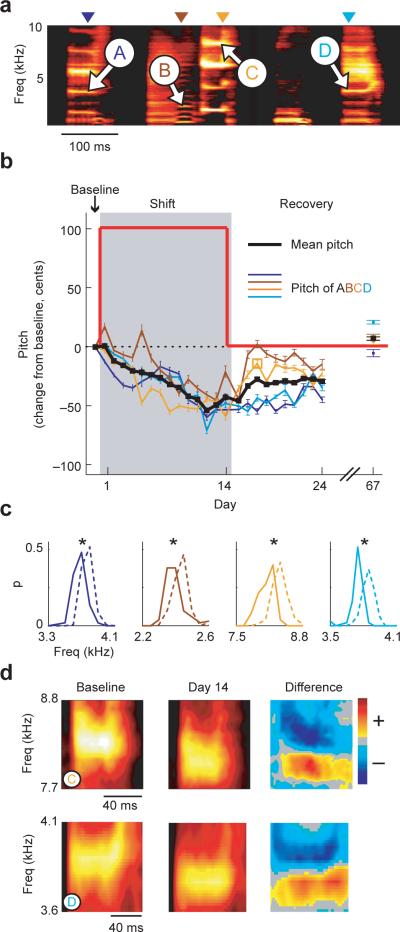
We consistently found that shifting the pitch of auditory feedback led to adaptive changes in song (that is, changes in the direction opposite the imposed pitch shift). We monitored changes in vocal output by repeatedly measuring pitch at particular times (or “spectral frames,” indicated by colored arrowheads in Fig. 2a) during song syllables. Within the spectral frame chosen for each syllable, we quantified changes in pitch by measuring changes in either the fundamental frequency or the frequency of one of the higher harmonics (a “harmonic feature,” see Methods). The four harmonic features measured in one bird's song (labeled A-D) are indicated by white arrows in Figure 2a. Figure 2b shows data from a typical experiment in which a 100 cent upward shift in the pitch of auditory feedback led to a downward change in the pitch of the measured harmonic features over a two-week period. The black line in Figure 2b represents the mean pitch change across all harmonic features, which we use to measure the daily mean change in the pitch of song. This change in the bird's song served to reduce the difference (error) between the pitch of auditory feedback and the baseline pitch. By the end of the shift epoch, the pitch of song had dropped by ∼50 cents. Upon removal of the pitch shift, song returned slowly towards baseline pitch, with a complete return to baseline when song was assessed 67 days after the beginning of the experiment. Figure 2c shows the distribution of frequencies for each harmonic feature in the baseline song (dashed lines) and after 14 days of exposure to the upward pitch shift (solid lines). All four harmonic features had significantly lower frequencies following shift exposure (asterisks indicate p<10−5, 1-tailed t-test). Figure 2d illustrates shift-induced changes in the mean spectrograms of two harmonic features, showing that the gross structure of song is preserved despite significant changes in pitch.
Figure 2. Vocal error correction driven by an upward shift in the pitch of auditory feedback.
a, Baseline song of Bird 1 (mean spectrogram). Arrowheads above the spectrogram indicate the spectral frames (measurement times within each syllable) at which four harmonic features (white arrows labeled A-D) were measured in order to quantify changes in the pitch of song. b, Changes in pitch in response to a 100 cent upward shift (red line) in the pitch of auditory feedback and subsequent recovery back to baseline. Colored lines show the mean +/− s.e.m. change in pitch (measured in cents, see Methods) of each harmonic feature across time, and the black line shows the mean change in the pitch of song (mean +/− s.e.m. pitch change averaged across harmonic features). After 14 days of shift exposure (gray box), unshifted auditory feedback was restored and the bird was monitored for an additional 10 days. Pitch was also measured on day 67 in order to assess any long-term changes. c, Pre- and post-shift distributions of the frequencies of the harmonic features shown in (a). For each feature, the probability distribution of frequencies during baseline (dashed lines) and day 14 (solid lines) differed significantly (asterisks indicate p<10−5, 1-tailed t-test). Color conventions for each feature as in (b). d, Pitch shift-induced changes in mean spectral structure. Left, mean spectrograms for harmonic features C (top) and D (bottom) during the baseline epoch. Middle, mean spectrograms for features C and D on shift day 14. Right, difference spectrograms obtained by subtracting the baseline spectrograms from the day 14 spectrograms.
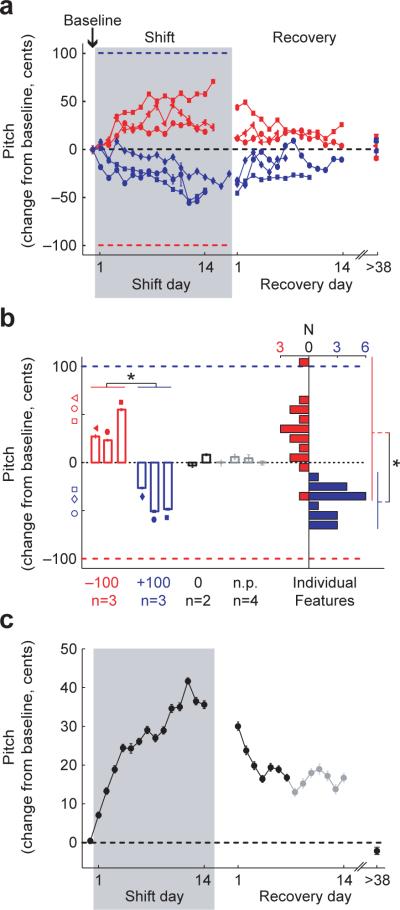
Across experiments, the direction of the behavioral response consistently opposed the direction of the imposed shift, as shown in Figure 3a. Both upward (blue) and downward (red) shifts in the pitch of auditory feedback led to adaptive changes in the pitch of song, and song returned to baseline pitch following shift offset. Figure 3b shows the mean change in song during days 12−14 following downward (empty red bars) or upward (empty blue bars) shifts in the pitch of auditory feedback. Across the six pitch-shift experiments, changes in response to upward versus downward shifts were significantly different (p=0.002, 1-tailed t-test), and no significant difference was found between the magnitudes of adaptive responses to upward and downward shifts (p=0.62, two-tailed t-test). Figure 3b also shows that minimal changes in pitch were observed in two control groups consisting of birds that wore headphones but did not experience a pitch shift (black bars) and birds that were never fitted with headphones (gray bars). Additionally, shifting the pitch of auditory feedback did not significantly affect the amount of song produced, the ordering (syntax) of song syllables, or the relative spectral power found in low versus high harmonics (see Supplementary Figs. 1−3 online).
Figure 3. Error correction in response to upward and downward shifts in feedback pitch.
a, Mean +/− s.e.m. changes in the pitch of song (across harmonic features) as a function of time for 3 experiments with downward shifts in feedback pitch (−100 cents, red) and 3 experiments with upward shifts (+100 cents, blue). “Recovery day 1” is the first day after unshifted feedback was restored. Symbol shapes identify individual birds. Other conventions as in Figure 2b. b, Mean +/− s.e.m. change in song pitch during shift days 12−14 for downward (empty red bars) and upward (empty blue bars) shifts of auditory feedback. Symbols identify individual birds as in (a). Empty red and blue symbols at left indicate the magnitude of one s.d. of pitch variation in the baseline epoch of each experiment (averaged across harmonic features). Black bars show changes in song pitch across the same interval in birds that wore headphones but did not experience pitch shifts (“0 shift”), and gray bars show the same measure in a group of birds that did not wear headphones at all (“no phones,” or “n.p.”). Longitudinal data from the two 0 shift birds is available as Supplementary Figure 6 online. Filled bars at right show the distribution of pitch changes for individual harmonic features, combined across all downward (red) and upward (blue) shifts of auditory feedback. Asterisks indicate significant differences between the effects of upward and downward shifts (p<0.05, 1-tailed t-tests). Additionally, the changes in song pitch in each +/−100 cent shift group were significantly different from changes in both of the control groups (p<0.05 in all cases, 1-tailed t-tests). c, Combined data from all experiments, reoriented so that changes in the adaptive directions are positive. Days from which data were available from all 6 experiments are plotted in black, recovery days from which data are available from a subset of 4 experiments are plotted in gray.
Analysis of pitch changes in individual harmonic features revealed consistent adaptive learning as well as heterogeneity within the songs of individual birds. Filled bars in Figure 3b show the shift-induced pitch changes in individual harmonic features, demonstrating that in the majority (31/33) of cases the pitches of individual features changed in the adaptive direction (p<10−9, 1-tailed t-test). The magnitude of pitch change (assessed on shift day 14) varied significantly across the harmonic features in each bird's song in all 6 experiments (ANOVA, p<10−9 in all cases). This heterogeneity suggests that the pitch of individual syllables can be adapted separately even if the pitch of auditory feedback is shifted uniformly across song. No correlation was observed between the baseline fundamental frequency of syllables and the magnitude of adaptive learning (see Supplementary Fig. 4 online).
The time course and magnitude of adaptation to pitch shifts suggest that birds compromise between the correction of auditory errors (adaptability) and the maintenance of the established motor program (stability). Figure 3c combines data from all shift experiments (solid black line) to illustrate the average magnitude and time course of learning and suggests that birds balance adaptability and stability by changing their songs relatively slowly (over days) in response to auditory errors. This timescale is comparable to the rate of pitch refinement in juvenile birds first learning their songs 14. Additionally, the acoustic structure of adult Bengalese finch song syllables begins to degrade within one week following deafening 12, 15, possibly as a result of error signals generated in the absence of auditory feedback 16. These results suggest that the intrinsic dynamics of the adult song system only allow for slow modifications of the motor command, perhaps to prevent the destabilization of song during brief periods of aberrant sensory input.
The combined data shown in Figure 3c also illustrate that on average birds compensate for only ∼40% of the imposed pitch shift by the end of the shift epoch. This incomplete adaptation does not reflect a physical limitation of the peripheral motor system, since we demonstrate in a separate set of experiments that gradually increasing the size of a shift can drive 100 cent (or larger) changes in the pitch of song17 (see Supplementary Figure 5 online). Incomplete adaptation is also consistently observed in human subjects in response to perturbations of auditory feedback 8, 9, and is the norm in other sensorimotor systems as well 18. Here, the incompleteness of adaptation may reflect a partial reliance on non-auditory sources of information such as proprioceptive signals 19, 20 or the output of an internal (forward) model used to predict the sensory consequences of motor commands21, 22. Furthermore, although the auditory feedback played through the headphone speakers was significantly louder than auditory feedback that reached the bird's ears directly (i.e. leaked through the headphone frame, see Methods), some unshifted feedback signals likely reached the bird via bone conduction 9. Therefore, the observed incomplete adaptation might also have resulted from a conflict between two distinct auditory signals.
The adaptive changes in the pitch of song that we report fall well within the range of both the baseline variability in pitch and the upper limits of pitch plasticity driven using reinforcement techniques 13. Comparison of the mean change in song (empty red and blue bars in Fig. 3b) to the baseline variability (empty symbols at left in Fig. 3b) shows that birds changed the pitch of song by roughly 1 s.d. This comparison reveals that the mean pitch of these syllables could have shifted even further in the adaptive direction without leaving the range of baseline variation, which has a s.d. of 48.0 cents (averaged across birds). Together with data showing that birds can shift the pitch of individual syllables by more than 10 s.d. 13, these results demonstrate that the magnitude of the adaptive pitch changes we report were not limited by physical constraints on the vocal motor periphery (see also Supplementary Figure 5 online).
DISCUSSION
Our results show that adult birds use auditory feedback to correct small errors in the pitch of song, demonstrating that adult song is maintained by a continual process of error correction. Maintaining vocal output through error correction has several important advantages. First, it allows the bird to adapt to changes in vocal output resulting from age-related changes in the strength of vocal muscles and the physical properties of the vocal periphery. Such changes alter the relationship between premotor activity and acoustic output, necessitating changes in the motor command. Second, error correction allows for changes in the neural structures controlling song as synaptic connectivity changes, old neurons die, and new neurons are added in adulthood 23-25. A stable sensory representation of the desired song, therefore, provides for stable behavioral output as both the body and brain change over a bird's lifetime.
As reported here for songbirds, human adults gradually adapt vocal (speech) production to compensate for shifts in the pitch of auditory feedback 9 and furthermore have been shown to compensate for more complex distortions of the structure of individual phonemes 8. Taken together, these results suggest that the use of long-lived perceptual targets to correct auditory errors is a general principle of learned vocal behavior. Interestingly, vocal adaptation in humans is significantly more rapid than that described here, suggesting that the sensorimotor strategy used to maintain speech performance puts relatively greater emphasis on adaptability (and less on stability) than that used to maintain song. Alternately, this difference in timescale might result from experimental differences between our work and prior studies on human speech, which have used bone oscillators or whispered speech to minimize unshifted feedback signals transmitted to the ear via bone conduction 8, 9.
The data presented here are the first direct evidence that adult birds maintain a sensory target representing the desired song output. Such a representation is presumably formed when juvenile birds are exposed to the tutor's song 1, 3, and it is possible that this target persists unchanged into adulthood and drives the adaptive pitch changes we observe. Alternately, auditory feedback might not be compared to a memory of the tutor's song, but rather to a slowly updated memory of the bird's own (relatively stable) vocalizations. Although our experiments do not allow us to distinguish between these possibilities, the adaptive changes in pitch we observe (and, perhaps more importantly, the subsequent return to baseline weeks after pitch shifts are removed) demonstrate that the sensory target is unchanged even after weeks of abnormal sensory experience.
Our results also demonstrate that birds can reduce auditory errors by shifting the pitch of song away from a normally stable baseline (black and gray bars in Fig. 3b). Previous studies have shown that following recovery from partial deafening or the cessation of distortion experiments, song sometimes reverts to its original state 10, 26. However, recent lesion studies suggest that recovery in these settings may be based on the persistence of the baseline motor program to which aberrant neural signals have been added by basal ganglia circuitry27. Furthermore, recovery following deafening or feedback distortion might rely on a somatosensory or motor memory of the baseline song 1, 20 rather than on a process of auditory error correction. A demonstration of adult error correction therefore requires that birds modify their crystallized song in order to reduce experienced auditory errors, as observed here.
The songbird system provides a unique opportunity to study the brain mechanisms underlying the learning and control of vocal behavior. After exposure to the tutor's song, juvenile birds can practice and refine their songs in complete social isolation and without any external source of reinforcement 14, 28. Based on this and other findings (such as the inability of young birds to acquire normal song if they are deafened after tutor song exposure 1), it is widely assumed that vocal refinement in young birds reflects a process of error correction similar to the one demonstrated here in the adult 29, 30. Numerous studies have documented anatomical and molecular changes that accompany song acquisition and therefore distinguish the brains of juvenile and adult birds 31, 32. Our results demonstrate that the capacity for adaptive vocal plasticity persists far into adulthood and suggest that the error-correcting processes thought to underlie vocal plasticity during song acquisition are active in the adult. If juvenile song acquisition and adult song maintenance are indeed based on the same process of error correction, a common neural mechanism for sensory-guided plasticity may persist throughout the dramatic neural changes that occur at the close of the critical period for song acquisition.
METHODS
Subjects
Adult (>190 days old) male Bengalese finches (Lonchura striata var. domestica) were used. All procedures were approved by the University of California, San Francisco Institutional Animal Care and Use Committee (IACUC). Birds were individually housed in sound isolation chambers throughout the experiments and were maintained on a 14h:10h light/dark cycle, with lights on from 7am-9pm.
Manipulating auditory feedback
Headphones constructed from lightweight carbon fiber (Hobby Lobby, Inc.) were custom-fit to each bird and held a miniature speaker (Knowles Inc., EH-7157−000) within 3 mm of the entrance to each ear canal. Additionally, a miniature microphone (Knowles Inc., EM-3046) was placed between ear and speaker on one side to calibrate and monitor the performance of the pitch-shifting hardware. The amplitude of the acoustic signal played through the speakers was ∼2 log units greater than direct auditory feedback leaking through the carbon fiber frame. A condenser microphone inside each isolation chamber relayed all cage sounds (including but not limited to song) through sound-processing hardware (Ultraharmonizer DSP7000, Eventide Inc.) that introduced upward, downward, or null shifts in pitch. Acoustic signals were then played back through the headphone speakers with an average loop delay of ∼7 msec. All recordings are from undirected song (i.e. no female was present).
Quantifying behavioral changes
Bengalese finch song consists of distinct syllables that can be identified across multiple renditions of song. For each syllable that had a well-defined pitch (3−8 in each bird), we identified an 8- or 16 msec segment of time (or “spectral frame”) to use for our pitch measurements. In rare cases where a complex syllable was composed of two spectrally distinct “notes,” we measured spectral frames for each distinct note (e.g. Fig. 2a, frames “B” and “C”). The beginning of each spectral frame was defined relative to the onset of the syllable. In each spectral frame, we measured pitch by quantifying the frequency of a harmonic feature (either the fundamental frequency or one of the higher harmonics). The frequency of this harmonic feature was then repeatedly measured over the course of each experiment and changes in its frequency were analyzed as described below.
After being fitted with headphones, birds sang for 3−7 days with zero shift. Each day we analyzed all songs produced in a 2-hour window between 10:00 am and 12:00 noon. (We obtained similar results when song produced in the evening was used for analysis, see Supplementary Fig. 7 online.) The baseline pitch of each harmonic feature was defined as the mean pitch in the last two analysis days preceding shift onset. After the last baseline window, a +/− 100 cent pitch shift was introduced in a single step and maintained for 14−17 days, after which the pitch shift was reset to zero for 7−14 days. Headphones were subsequently removed and birds were maintained in social isolation for 25−123 days. Headphones were then reattached (with zero pitch shift) in order to assess long-term effects on pitch. The pitch of each iteration of a harmonic feature was quantified in units of cents:
where cx is the pitch (in cents) of the feature, hx is the pitch (in Hz) of the feature, and b is the baseline pitch (in Hz) of that feature. Note that because the frequency of harmonics are related to each other by integer multiples, when the pitch of a syllable changes, the frequency of every harmonic changes by the same number of cents. Therefore, the computed change in cents describes the change in the fundamental frequency. Note also that this method is equivalent to measuring the frequency of a given harmonic, dividing that frequency by the appropriate integer to compute the fundamental frequency, and computing the change in cents as described above.
Mean Spectrograms
Mean spectrograms were calculated for each syllable by first computing the spectrogram for each iteration of the syllable being analyzed. Spectrograms were then normalized and aligned to a single prototypical example of the syllable. Alignment was accomplished by shifting and linearly stretching each spectrogram until the cross-correlation between the temporal power profile (total spectral power as a function of time) of the exemplar and the prototype was maximized. After all syllables had been aligned, the mean power at each frequency and time point was computed. Note that mean spectrograms are used for display purposes only (Fig. 2) and that mean changes in pitch shift were computed from measurements of individual syllables.
Supplementary Material
Acknowledgements
We thank P. Sabes and A. Doupe for critical discussions, T. Warren, K. Bouchard, and L. Didier-Sober for technical assistance, and J. Wong and R. Mazumder for animal care.
REFERENCES
- 1.Konishi M. The role of auditory feedback in the control of vocalization in the white-crowned sparrow. Zeitschrift fur Tierpsychologie. 1965;22:770–783. [PubMed] [Google Scholar]
- 2.Kuhl PK. Learning and representation in speech and language. Curr. Opin. in Neurobiol. 1995;4:812–822. doi: 10.1016/0959-4388(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 3.Arnold AP. The effects of castration on song development in zebra finches (Poephila guttata). J. Exp. Zool. 1975;191:261–278. doi: 10.1002/jez.1401910212. [DOI] [PubMed] [Google Scholar]
- 4.Olveczky BP, Andalman AS, Fee MS. Vocal experimentation in the juvenile songbird requires a basal ganglia circuit. PLoS Biol. 2005;3:e153. doi: 10.1371/journal.pbio.0030153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kao MH, Doupe AJ, Brainard MS. Contributions of an avian basal gangliaforebrain circuit to real-time modulation of song. Nature. 2005;433:638–43. doi: 10.1038/nature03127. [DOI] [PubMed] [Google Scholar]
- 6.Kuhl PK, et al. Phonetic learning as a pathway to language: new data and native language magnet theory expanded (NLM-e). Philos Trans R Soc Lond B Biol Sci. 2008;363:979–1000. doi: 10.1098/rstb.2007.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doupe AJ, Kuhl PK. Birdsong and human speech: common themes and mechanisms. Annu Rev Neurosci. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- 8.Houde JF, Jordan MI. Sensorimotor adaptation in speech production. Science. 1998;279:1213–6. doi: 10.1126/science.279.5354.1213. [DOI] [PubMed] [Google Scholar]
- 9.Jones JA, Munhall KG. Perceptual calibration of F0 production: evidence from feedback perturbation. J Acoust Soc Am. 2000;108:1246–51. doi: 10.1121/1.1288414. [DOI] [PubMed] [Google Scholar]
- 10.Leonardo A, Konishi M. Decrystallization of adult birdsong by perturbation of auditory feedback. Nature. 1999;399:466–470. doi: 10.1038/20933. [DOI] [PubMed] [Google Scholar]
- 11.Nordeen KW, Nordeen EJ. Auditory feedback is necessary for the maintenance of stereotyped song in adult zebra finches. Behav. Neural. Biol. 1992;57:58–66. doi: 10.1016/0163-1047(92)90757-u. [DOI] [PubMed] [Google Scholar]
- 12.Woolley SM, Rubel EW. Bengalese finches Lonchura Striata domestica depend upon auditory feedback for the maintenance of adult song. J Neurosci. 1997;17:6380–6390. doi: 10.1523/JNEUROSCI.17-16-06380.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tumer EC, Brainard MS. Performance variability enables adaptive plasticity of ’crystallized’ adult birdsong. Nature. 2007;450:1240–1244. doi: 10.1038/nature06390. [DOI] [PubMed] [Google Scholar]
- 14.Tchernichovski O, Mitra PP, Lints T, Nottebohm F. Dynamics of the vocal imitation process: how a zebra finch learns its song. Science. 2001;291:2564–9. doi: 10.1126/science.1058522. [DOI] [PubMed] [Google Scholar]
- 15.Okanoya K, Yamaguchi A. Adult Bengalese finches (Lonchura striata var. domestica) require real-time auditory feedback to produce normal song syntax. Journal of Neurobiology. 1997;33:343–356. [PubMed] [Google Scholar]
- 16.Brainard MS, Doupe AJ. Interruption of a basal ganglia-forebrain circuit prevents plasticity of learned vocalizations. Nature. 2000;404:762–766. doi: 10.1038/35008083. [DOI] [PubMed] [Google Scholar]
- 17.Linkenhoker BA, Knudsen EI. Incremental training increases the plasticity of the auditory space map in adult barn owls. Nature. 2002;419:293–6. doi: 10.1038/nature01002. [DOI] [PubMed] [Google Scholar]
- 18.Choe CS, Welch RB. Variables affecting the intermanual transfer and decay of prism adaptation. J Exp Psychol. 1974;102:1076–84. doi: 10.1037/h0036325. [DOI] [PubMed] [Google Scholar]
- 19.Sober SJ, Sabes PN. Flexible strategies for sensory integration during motor planning. Nat Neurosci. 2005;8:490–7. doi: 10.1038/nn1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suthers RA, Goller F, Wild JM. Somatosensory feedback modulates the respiratory motor program of crystallized birdsong. Proc Natl Acad Sci U S A. 2002;99:5680–5. doi: 10.1073/pnas.042103199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolpert DM, Ghahramani Z, Jordan MI. An internal model for sensorimotor integration. Science. 1995;269:1880–2. doi: 10.1126/science.7569931. [DOI] [PubMed] [Google Scholar]
- 22.Troyer T, Doupe AJ. An associational model of birdsong sensorimotor learning. I. Efference copy and the learning of song syllables. J. Neurophys. 2000a;84:1204–1223. doi: 10.1152/jn.2000.84.3.1204. [DOI] [PubMed] [Google Scholar]
- 23.Nottebohm F. The road we travelled: discovery, choreography, and significance of brain replaceable neurons. Ann N Y Acad Sci. 2004;1016:628–58. doi: 10.1196/annals.1298.027. [DOI] [PubMed] [Google Scholar]
- 24.Wilbrecht L, Kirn JR. Neuron addition and loss in the song system: regulation and function. Ann N Y Acad Sci. 2004;1016:659–83. doi: 10.1196/annals.1298.024. [DOI] [PubMed] [Google Scholar]
- 25.Mooney R. Synaptic mechanisms for auditory-vocal integration and the correction of vocal errors. Ann N Y Acad Sci. 2004;1016:476–94. doi: 10.1196/annals.1298.011. [DOI] [PubMed] [Google Scholar]
- 26.Woolley SM, Rubel EW. Vocal memory and learning in adult Bengalese Finches with regenerated hair cells. J Neurosci. 2002;22:7774–87. doi: 10.1523/JNEUROSCI.22-17-07774.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson JA, Wu W, Bertram R, Johnson F. Auditory-dependent vocal recovery in adult male zebra finches is facilitated by lesion of a forebrain pathway that includes the basal ganglia. J Neurosci. 2007;27:12308–20. doi: 10.1523/JNEUROSCI.2853-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tchernichovski O, Lints T, Mitra PP, Nottebohm F. Vocal imitation in zebra finches is inversely related to model abundance. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:12901–4. doi: 10.1073/pnas.96.22.12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adret P. In search of the song template. Ann N Y Acad Sci. 2004;1016:303–24. doi: 10.1196/annals.1298.005. [DOI] [PubMed] [Google Scholar]
- 30.Deregnaucourt S, et al. Song development: in search of the error-signal. Ann N Y Acad Sci. 2004;1016:364–76. doi: 10.1196/annals.1298.036. [DOI] [PubMed] [Google Scholar]
- 31.Clayton DF. Songbird genomics: methods, mechanisms, opportunities, and pitfalls. Ann N Y Acad Sci. 2004;1016:45–60. doi: 10.1196/annals.1298.028. [DOI] [PubMed] [Google Scholar]
- 32.White SA. Learning to communicate. Curr Opin Neurobiol. 2001;11:510–20. doi: 10.1016/s0959-4388(00)00242-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.