Abstract
Increased expression of a number of proinflammatory genes, including IL-8, is associated with inflammatory conditions such as asthma. Glucocorticoid receptor (GR)β, one of the GR isoforms, has been suggested to be upregulated in asthma associated with glucocorticoid insensitivity and to work as a dominant negative inhibitor of wild type GRα. However, recent data suggest that GRβ is not a dominant negative inhibitor of GRα in the transrepressive process and has its own functional role. We investigated the functional role of GRβ expression in the suppressive effect of glucocorticoids on tumor necrosis factor (TNF)-α-induced IL-8 release in an airway epithelial cell line. GRβ expression was induced by treatment of epithelial cells with either dexamethasone or TNF-α. GRβ was able to inhibit glucocorticoid-induced transcriptional activation mediated by binding to glucocorticoid response elements (GREs). The suppressive effect of dexamethasone on TNF-α-induced IL-8 transcription was not affected by GRβ overexpression, rather GRβ had its own weak suppressive activity on TNF-α-induced IL-8 expression. Overall histone deacetylase activity and histone acetyltransferase activity were not changed by GRβ overexpression, but TNF-α-induced histone H4 acetylation at the IL-8 promoter was decreased with GRβ overexpression. This study suggests that GRβ overexpression does not affect glucocorticoid-induced suppression of IL-8 expression in airway epithelial cells and GRβ induces its own histone deacetylase activity around IL-8 promoter site.
Keywords: asthma; glucocorticoids; histone acetyltransferases; histone deacetylases; interleukin-8; receptors, glucocorticoid; tumor necrosis factor-α
Introduction
Glucocorticoids are the mainstay of treatment of various inflammatory diseases such as bronchial asthma (Bateman et al., 2008). Glucocorticoids work through the glucocorticoid receptor (GR), and transcriptional activation by GR is often mediated by GR binding to a consensus glucocorticoid response element (GRE). GR forms homodimers upon binding of glucocorticoid, and this homodimer binds to the GRE enabling recruitment of transcriptional co-activators.
Recently, it has been described that multiple isoforms of GR are generated from a single gene by the mechanisms of alternative splicing and alternative translation initiation (Duma et al., 2006). Of these isoforms, two (GRα and GRβ) have been best studied, and they originate by an alternative splicing event within the 9th exon of the gene. GRβ has been suggested to be upregulated in the steroid resistant asthmatics, and to be the most abundant GR isoform in neutrophils. GRβ was more highly expressed in glucocorticoid resistant asthmatics (Leung et al., 1997; Hamid et al., 1999; Sousa et al., 2000), although this is not a universal observation in glucocorticoid dependent (Gagliardo et al., 2000) or stable asthmatics (Torrego et al., 2004) and glucocorticoid resistant nasal polyps (Choi et al., 2006). Neutrophils expressing high levels of GRβ escape glucocorticoid-induced cell death (Strickland et al., 2001). Viral transduction of GRβ into mouse hybridoma cells also resulted in glucocorticoid insensitivity (Hauk et al., 2002). It has been proposed that formation of heterodimers between GRα and GRβ could potentially contribute to dominant negative activity (Oakley et al., 1999; Strickland et al., 2001; Hauk et al., 2002; Yudt et al., 2003). GRβ lacks amino acids that are essential for the coordination of ligand, and since this event precedes the recruitment of coactivator complexes, it has been suggested to lack transcriptional activation function. The DNA binding domain of GRα and GRβ are identical and the DNA binding activity of GRβ is thought to be unperturbed. Therefore, since GRβ lacks activator activity, it has been proposed to be a dominant-negative inhibitor of GRα. However, some recent data show that GRβ is not a dominant negative inhibitor of GRα especially in the transrepression process (Gougat et al., 2002; Kelly et al., 2008).
IL-8 is a member of the family of chemokines involved in the initiation and amplification of acute inflammatory reactions (Harada et al., 1994) and in chronic inflammatory process. Bronchial hyperresponsiveness is induced by repeated administration of IL-8 (Fujimura et al., 1998) and increased levels of IL-8 in sputum precede an exacerbation of asthma (Kurashima et al., 1996). Epithelial expression of IL-8 is observed in allergic rhinitis (Nonaka et al., 1996) and asthma (Marini et al., 1992). In addition epithelial IL-8 was observed in patients with steroid-refractory asthma. IL-8 was more abundant in induced sputum of patients with severe asthma compared to control subjects (Hamilton et al., 2003; Vachier et al., 2005).
Glucocorticoid-induced suppression of inflammatory cytokines is important in the regulation of many inflammatory diseases, and suppression of TNF-α-induced IL-8 expression by glucocorticoids involves changes in IL-8 promoter acetylation (Tsaprouni et al., 2007). Recent in vitro data verified that GRα and GRβ act in a similar manner on IL-5 and IL-13 promoters, serving to repress transcription via recruitment of histone deacetylase (HDAC) and GRβ does not act as a dominant-negative inhibitor of GRα in this transrepression (Kelly et al., 2008). Effects on other cytokines were not tested. In this study, we investigated the functional role of GRβ in the suppressive effect of glucocorticoids on TNF-α-induced IL-8 release in airway epithelial cells by assessing the effect of GRβ overexpression in TNF-α-induced IL-8 expression and IL-8 promotor histone acetylation status.
Results
Expression of GRα and GRβ by transfection of pCMV.hGRβ
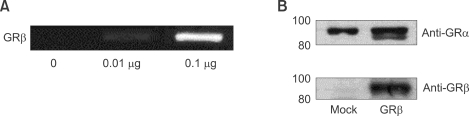
It is well known that GRα is constitutionally expressed, and GRβ is not usually expressed in the airway epithelial cells. We checked baseline expression of GRα and GRβ and the effect of pCMV.hGRβ transfection in A549 cell lines. GRβ expression was easily detected in the airway epithelial cell lines by following transfection of pCMV.hGRβ, as tested by RT-PCR of mRNA and Western blotting (Figure 1). GRβ was expressed in a dose dependent manner (Figure 1A) and was only detected following transfection in contrast to the constitutionally expressed GRα (Figure 1B). This means that in resting cells the functional effect of GRβ can be observed following transfection with pCMV.hGRβ.
Figure 1.
Expression of GRα and GRβ. (A) A549 cells were transfected with 0.01 µg and 0.1 µg of pCMV.hGRβ and were harvested 18 h after transfection. RT-PCR for GRβ was performed on cDNA derived from these cells. (B) A549 cells were transfected with pCMV.hGRβ and Western blot was carried out with antibodies raised against GRα and GRβ.
Treatment with TNF-α or dexamethasone induces GRβ expression
Previous studies have shown that GRβ expression could be induced with either treatment with dexamethasone or TNF-α in skeletal muscle and some cell lines (Webster et al., 2001; Whorwood et al., 2001). It was unclear whether GRβ expression could be induced in airway epithelial cells after the treatment with certain chemical agents or in some abnormal conditions. Therefore, to determine whether dexamethasone or TNF-α can influence the expression of GRβ in A549 cell lines, cultures were subjected to treatment with dexamethasone or TNF-α. GRβ expression was induced by treatment with either dexamethsone or TNF-α, whereas it was undetectable in resting cells (Figure 2). Relatively low doses of dexamethasone (10-9 M) and TNF-α (1 ng/ml) were able to induce GBβ expression. GRβ was induced 8 days after 10-9 M of dexamethasone and 5 days after 1 ng/ml of TNF-α treatment. These effects are unlikely to be due to subculture of cells, because subcultured cells without drug treatment showed no expression of GRβ.
Figure 2.
Effects of treatment with dexamethasone or TNF-α on expression of GRβ. 10-9 M of dexamethasone or 1 ng/ml of TNF-α was used to treat cells for the times indicated. Cells were harvested, cDNA prepared and RT-PCR were performed.
GRβ can work as an inhibitor of dexamethasone-induced transcriptional activation
GR works through diverse mechanisms and transcriptional activation by GR is mediated by GR binding to a consensus GRE. Anti-inflammatory protein such as lipocortin-1 is generated by this transactivation process (Barnes, 2001).
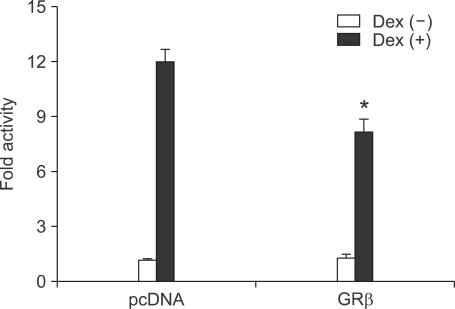
To test the influence of GRβ on genes transcriptionally activated by glucocorticoids, we overexpressed GRβ along with a glucocorticoid sensitive pGRE luc in A549 cells, which were then treated with dexamethasone. GRβ inhibited ligand-induced reporter activity by approximately 30% (P < 0.05, Figure 3), thus GRβ acts as an inhibitor of GRα in the glucocorticoid-induced transactivation, which was similar to the previous reports (Oakley et al., 1996; Kino et al., 2001; Yudt et al., 2003).
Figure 3.
Effects of GRβ expression on dexamethasone-induced transcriptional activation in A549 cell lines. Data are shown as the fold activity over control activity and represent the mean ± SD of three independent experiments. *P < 0.05 versus control activity. Dex, dexamethasone.
GRβ does not act as a dominant negative inhibitor in dexamethasone induced transcriptional repression of IL-8
The anti-inflammatory effects of glucocorticoid are not confined to the synthesis of anti-inflammatory proteins, but also rely on the suppression of expression of activated cytokines in the inflammatory conditions. This suppression of activated cytokines is mediated at least in part by transrepression. Therefore assessment of effects on the transrepressive process is mandatory to see the role of GRβ.
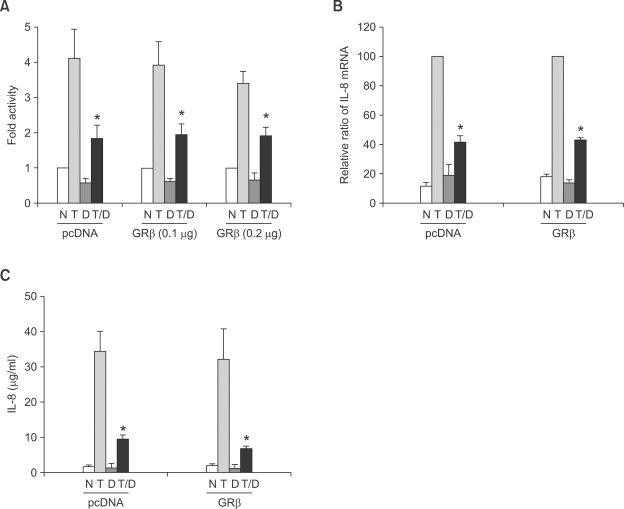
In order to test the influence of GRβ on genes repressed by glucocorticoids, we used two systems, first, using transient transfection of a reporter vector driven by a glucocorticoid repressible promoter, and secondly by investigation of an endogenous gene that is repressed by steroids. In contrast to the result of GRβ effect on the transcriptional activation, GRβ overexpression did not influence transcriptional repression induced by dexamethasone in the A549 IgGκ-NF-κB cell line (Figure 4A). Secondly we measured mRNA expression and secretion of IL-8 with or without GRβ transfection. The suppressive effect of dexamethasone on the production of IL-8 mRNA and secretion of IL-8 were also not significantly affected by GRβ expression (Figure 4B and C). Overall results in the transrepression process suggest that GRβ may not be the dominant inhibitor of GRα in this pathway.
Figure 4.
Effects of GRβ expression on dexamethasone-induced repression of IL-8. A549 IgGκ-NF-κB luciferase cells were transfected with control vector or pCMV.hGRβ. Following 6 h of treatment with TNF-α (5 ng/ml) and/or 10-7 M of dexamethasone, cells were harvested. (A) Luciferase activity. Data are shown as the fold activity over control activity. (B) mRNA of IL-8 expression was measured by RT-PCR. Densitometric data. The results are shown as the relative ratio to TNF-α induced activity. (C) Supernatants were collected and levels of IL-8 were measured by ELISA. Data represent the mean ± SD of three independent experiments. *P < 0.05 versus TNF-α-induced activity. N, no treatment; T, TNF-α treatment; D, dexamethasone treatment.
GRβ does not change the HDAC activities and HAT activities
The mechanisms of action of glucocorticoids in the suppression of cytokine gene expression are not yet clear and have been explained by several ways, of which recruitment of co-repressor like HDAC is a recently introduced attractive mechanism (Jee et al., 2005; Kelly et al., 2008).
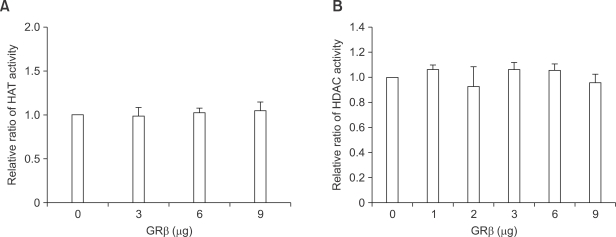
GRβ has some suppressive effects on the transcriptional activation induced by TNF-α although it was not significant (Figure 4A). Increased expression of GRβ caused a reduction in TNF-α-induced IL-8 transcription. We hypothesized that GRβ may have some activity on the recruitment of HDAC.
HAT activites and HDAC activities were measured with MOCK and GRβ transfection. Overexpression of GRβ did not change the HDAC activities nor HAT activities of A549 cell lines (Figure 5).
Figure 5.
Effect of GRβ overexpression on HAT and HDAC activities in A549 cells. GRβ overexpression did not change the (A) HAT and (B) HDAC activities. Results are shown as the relative ratio to HAT or HDAC activity with control vector transfection and the mean ± SD of three independent experiments.
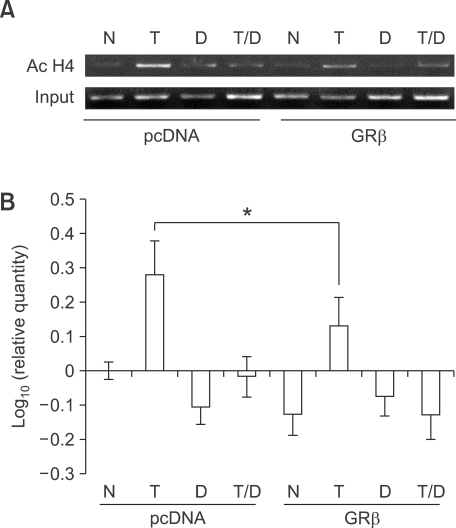
GRβ overexpression inhibit TNF-α-induced histone H4 acetylation at the IL-8 promoter
Because GRβ did not affect the overall HDAC activities and HAT activities, we then applied the semi-quantitative ChiP assay to analyze histone acetylation at the IL-8 promoter. It is known that suppression of TNF-α-induced IL-8 expression by glucocorticoids involves changes in IL-8 promoter acetylation (Tsaprouni et al., 2007). As shown in Figure 6, IPs with antibody against acetylated histone H4 revealed a marked enrichment of the IL-8 promoter DNA after TNF-α treatment. Overexpression of GRβ inhibited this histone H4 acetylation. The levels of H4 acetylation tended to decrease by GRβ overexpression in the untreated cells, although it was not significant.
Figure 6.
GRβ inhibit TNF-α-induced acetylation of histone H4 at IL-8 promoter. (A) Results of a representative chromatin immunoprecipitation (ChIP) experiment showing histone acetylation at IL-8 promoter. A549 cells were transfected with control (pcDNA) or pCMV.hGRβ. Cells were incubated with TNF-α (T; 5 ng/ml) in the presence and absence of dexamethasone (D; 10-7 M). (B) Graphical analysis of the percentage increase in band density of ChIP data. The results are expressed as mean ± SD of three independent experiments. *P < 0.05 versus control vector-induced activity. N, no treatment; T, TNF-α treatment; D, dexamethasone treatment.
Discussion
Our data have shown that treatment with either dexamethasone or TNF-α induced GRβ expression in an epithelial cell line. GRβ overexpression affected neither the TNF-α-induced IL-8 expression nor the suppression of IL-8 expression by dexamethasone, rather it looked like that GRβ had its own weak activity suppressing TNF-α induced IL-8 expression. Furthermore, we demonstrated that GRβ overexpression was able to reduce the degree of histone acetylation detected in the IL-8 promoter region following stimulation by TNF-α. These data confirm that GRβ modulates histone H4 acetylation status at the IL8 promoter area in similar way to GRα and that these changes in histone acetylation by GRβ has its own important role in transcriptional repression to TNF-α-induced IL-8 expression in an airway epithelial cell line.
The study demonstrated that GRβ expression was induced by either persistent treatment with dexamethasone or TNF-α in the airway epithelial cell lines. The previous study has reported the upregulation of GRβ mRNA by glucocorticoids in human skeletal muscle cells (Whorwood et al., 2001). It was also reported that GRβ could be induced with the treatment of TNF-α in HeLa and CEM-C7 cell lines (Webster et al., 2001). Pujols et al. (2002) reported that GRβ mRNA was downregulated in a dose dependent manner after short-term exposure to glucocorticoid (6 h of dexamethasone administration), with GRβ mRNA levels returning to basal expression levels after 24 h, reflecting the differences of GRβ expression with glucocorticoid according to the treatment time and dosage. In this study, the expression of GRβ after treatment of dexamethasone or TNF-α raises the possibility that it may be induced under the conditions, such as longstanding use of glucocorticoid or severe inflammation in airway epithelium.
GRβ, as an inhibitor of GRα, may be true only in the glucocorticoid-induced transactivation pathway. This kind of transcriptional activation has been identified in lipocortin-1 (phospholipase A2 inhibitor), β2-adrenoceptor, secretory leukopretease A2 inhibitor, Clara cell protein (CC10, phospholipase A2 inhibitor), IL-1 receptor antagonist, IL-1R2 (decoy receptor), IκB-α (inhibitor of NF-κB), GILZ (glucocorticoid induced leucine zipper), and GITR (glucocorticoid induced TNFR family-related) (Barnes, 2001). The anti-inflammatory effects of glucocorticoid are partially explained by these mechanisms and for this reason, transcriptional activation induced by glucocorticoid is inhibited by GRβ, thus the anti-inflammatory action of glucocorticoid through the production of these anti-inflammatory proteins will be blocked. The result suggests that GRβ is able to interfere with transcriptional activation properties of GRα and this inhibitory action of GRβ on glucocorticoid-induced transcriptional activation could be explained by formation of a heterodimer as GRα-GRβ, which is unable to bind GRE by previous report (Strickland et al., 2001). In this study, we also demonstrated that GRβ acts as an inhibitor of GRα in the glucocorticoid-induced transactivation, which was similar to the previous reports (Oakley et al., 1996; Kino et al., 2001; Yudtet et al., 2003). These data therefore suggest that one of the mechanisms of glucocorticoid resistance is by GRβ acting as an inhibitor of genes activated by glucocorticoids.
The activation and secretion of cytokines is the most important phenomena in the inflammation and the main anti-inflammatory action of glucocorticoid is the suppression of expression of these activated cytokines. There are no consensus GREs in the promoters or enhancers in these inflammatory cytokines and the mechanism of GR in the suppression of cytokines have been explained differently, such as repression by binding to regulatory DNA elements (nGRE) (Drouin et al., 1993), protein-protein interaction (squelching) (Jonat et al., 1990), modification by phosphorylation (Caelles et al., 1997), and recently by recruitment of coactivator or co-repressor complexes to a common subunit (Shi et al., 2001; Jee et al., 2005). Therefore glucocorticoid resistance by GRβ expression must be verified in this glucocorticoid-induced transrepressive process. The effects of GRβ on this glucocorticoid-induced repressive process are still uncertain. Our results show that artificial overexpression of GRβ could not influence the glucocorticoid induced repressive effects in IL-8 production, which means GRβ is not the dominant negative inhibitor in this transrepression process. This suggests that GRβ expression may not be the main mechanism of glucocorticoid resistance in the inflammatory condition induced by inflammatory cytokines. GRβ expression as the reason of steroid resistance has been challenged because GRβ was not usually expressed sufficiently to overcome the function of GR (Hecht et al., 1997; Torrego et al., 2004). Our data suggest that GRβ do not act as the cause of steroid unresponsiveness even when expressed as a significantly higher level in the suppression of IL-8 production.
Chromatin remodeling is critically involved in the rapid shuttling of receptors on and off chromatin templates, and necessarily requires that a large fraction of receptor/genome interactions are coupled to remodeling processes (John et al., 2008). Chromatin remodeling following histone acetylation at the specific gene promoter site is a major transcriptional regulatory mechanism that allows transcription factors to bind to specific gene promoters, initiating gene transcription. Tsaprouni et al. (2007) reported that the ability of TNF-α to induce IL-8 expression in monocyte and T cell lines is regulated by changes in histone acetylation. Our data show that overexpression of GRβ did not show any negative effect on the glucocorticoid-induced transrepression, expression of mRNA, and secretion of IL-8, rather it looked like that GRβ had its own weak transrepressive activity, while it caused inhibition of glucocorticoid-induced transactivation. We suggest that GRβ has a role in glucocorticoid-induced repression of cytokine genes and its own potential recruiting HDAC activity. Activated GR may recruits corepressor proteins, such as HDAC, to the activated inflammatory gene transcriptional complex, resulting in deacetylation of histones and thus, a decrease in inflammatory gene transcription (Barnes et al., 2005). In a similar manner, Kelly and her colleagues have demonstrated a role for altered histone acetylation status in the regulation of IL-5 and IL-13 expression by GRβ (Kelly et al., 2008).
The cell line we used is a cancer cell line and the artificial system provided above-normal level of GRβ expression. These mean that this artificial system might not reflect true physiologic conditions. The chromatin status at critical response elements will diverge in different cell types (John et al., 2008) and chromatin architecture in a given cell type is organized to facilitate receptor template interactions appropriate for that cell. Cell-specific chromatin organization plays a major role in the determination of tissue-selective receptor function. This cell specific response means that our results cannot simply be applicable to other system or condition. But the results are consistent with the findings on promoter activity, mRNA expression, protein production of IL-8, and demonstrate that GRβ suppress TNF-α-induced IL-8 gene transcription in a chromatin-dependent manner through the inhibition of histone H4 acetylation.
GRβ expression may have some meaning other than just bystander or functioning as inhibitor of GRα. For example, GRβ expression can have benefit if it has its own suppressive function in the cytokine secretion to overcome some difficult condition such as severe inflammatory condition, especially in the condition of shortage of glucocorticoid. GRβ do not bind with glucocorticoid and be present abundantly in the nucleus (Oakley et al., 1996; Kino et al., 2001; Yudt et al., 2003). According to some data of previous reports, it appears that GRβ has its own suppressive activity in the NF-κB transactivation (Gougat et al., 2002, Yudt et al., 2003), although Yudt et al. only interpreted the data with the ligand binding. A previous report verified that GRβ form a complex with HDAC cofactor (Kelly et al., 2008), and we confirmed that an enriched histone H4 acetylation of the IL-8 promoter after TNF-α treatment was inhibited by overexpression of GRβ. These increased HDAC activities in IL-8 promoter site by GRβ may influence the transcription of genes such as IL-8 and benefit to suppress the inflammatory condition, especially in the situation of shortage of glucocorticoid. Recently it was confirmed that GRβ bind RU-486, glucocorticoid antagonist, as an ligand and GRβ bound with RU-486 can regulate gene expression, suggesting that GRβ may have some physiological roles other than as an just bystander or inhibitor of GRα (Lewis-Tuffin et al., 2007). The effect of GRβ on IL-8 secretion was not significant in our experiments, which may be due to insufficient ability of GRβ to interact with DNA or be recruited to the area near inflammatory cytokine gene.
It is evident that GR can work through diverse mechanisms rather than just direct binding and activation of transcription. The action of glucocorticoids is highly dynamic and involves chromatin remodeling in addition to the previously proposed mechanism. GR interaction with the genome universally associated with local chromatin reorganization, but multiple remodeling systems are either recruited by the receptor, or operate at a given regulatory site prior to, and coincident with, receptor action (John et al., 2008). Therefore, there might be several mechanisms underlying steroid resistance and these possibilities should be taken into consideration. Recent report showing two different steroid resistant asthma groups showing different ability in terms of histone acetylation also suggest that the cause of steroid resistance is not simple (Matthews et al., 2004).
In summary, the study shows that the increased expression of GRβ does not influence the glucocorticoid-induced suppression of IL-8 in the airway epithelial cell line, rather it has a weak activity suppressing TNF-α induced IL-8 expression, and GRβ induces its own histone deacetylase activity around IL-8 promoter site. Therefore, GRβ may not be the dominant-negative inhibitor of GRα in the glucocorticoid-induced transrepression process of IL-8 and the main cause of glucocorticoid resistance in asthma.
Methods
Cell lines and culture conditions
A549 cells (type II pneumocyte, human lung carcinoma cells, ATCC, MD; CCL-185) were grown in RPMI, supplemented with 10% FCS (Sigma, St. Louis, MO), penicillin (100 IU/ml), and streptomycin (100 µg/ml), at 37℃ and 5% CO2 in humidified air. Where indicated, cells were treated with 5 ng/ml of TNF-α (Sigma, St. Louis, MO) and 10-7 M of dexamethasone (Sigma, St. Louis, MO) for 6 h except in the experiment about GRβ expression with the drug treatment (1 ng/ml of TNF-α and 10-9 M of dexamethasone).
Plasmids and transfections
pGRE luc vector (BD Biosciences Clontech, San Diego, CA), which contains a luciferase reporter under transcriptional control of a GRE, was used to measure the transcriptional activation by glucocorticoid. IgGκ-NF-κB luciferase reporter gene construct, in which a NF-κB binding site of NF-κB dependent IgGκ chain gene was cloned just upstream of minimal IL-8 promoter (promoter -67 to +44), has been described previously (Lee et al., 1999). pCMV.hGRβ were generated as described previously (Kelly et al., 2008). These plasmids were transiently transfected into A549 cells using lipofectamine-plus (Gibco-BRL, Gainthersburg, MD) according to the manufacture's instruction. A549 cell lines had been grown in media containing charcoal-stripped serum before transient transfection. Stripped serum was used to remove steroid from the culture medium that might bind to the endogenous GR of the A549 cell lines and mask the influence of GRβ. Cell viability was measured by MTT assay. Transfection and treatment of cell lines did not induce significant cell death compared to control cell line (data not shown).
Reporter gene assay (Luciferase assay)
Assays were performed according to the manufacturer's protocol (Promega Corp, Medison, WI). Cultured cells were harvested with the addition of lysis buffer (0.1 M HEPES, pH 7.6, 1% Triton-X, 1 mM DTT and 2 mM EDTA) after washing with PBS. Cell pellets were centrifuged at 4℃, 10 min, 14,000 rpm. Protein concentration was measured by Bradford assay (Bio-rad laboratories, Hercules, CA). Luciferase assay mix (25 mM glycylglycine, 15 mM MgSO4, 1 mg/ml BSA, 5 mM ATP and 1 mM D-luciferin) was added to equal amount of protein. Luciferase activity was measured with a Luminometer (Analytical Luminescence Laboratory, San Diego, CA).
RT-PCR
Total cellular RNA was isolated using a Qiagen RNeasy kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions and 1 µg of mRNA was used in first strand synthesis using random hexamers. First strand buffer (0.1 M DTT, 2.5 mM dNTP, RNAase inhibitor, reverse transcriptase) was added and incubated for 1 h at 42℃. cDNA was amplified with 10×PCR buffer (500 mM KCl, 100 mM Tris-HCl pH 8.3, 15 mM MgCl2), 2.5 nM dNTP, Taq DNA polymerase, (TaKaRa Taq, TaKaRa, Shiga, Japan). PCR conditions were an initial 94℃ for 5 min, followed by cycles of 94℃, 55℃ and 74℃ for one minute each. PCR products were analyzed by eletrophoresis through 1.5% agarose gels run in TAE buffer. β-actin was used as an internal control. Used primer sequences were as follows; β-actin sense; 5'-GTGGGGCGCCCCAGGCACCA-3', β-actin antisense; 5'-CTCCTTCCGTCACGCACGATTTC-3', GRα sense; 5'-CCTAAGGACGGTCTGAAGAGC-3', GRα antisense; 5'-GCCAAGTCTTGGCCCTCTAT-3', GRβ sense; 5'-CCTAAGGACGGTCTGAAGAGC-3', GRβ antisense; 5'-CCACGTATCCTAAAAGGGCAC-3'. IL-8 sense; 5'-TTGGCAGCCTTCCTGATT-3', IL-8 antisense; 5'-AACTTCTCCACAACCCTCTG-3'. The size of amplimers: GRα 478 bp, GRβ 366 bp, IL-8 292 bp and β-actin 658 bp.
Western blot analysis
Cells were harvested and whole cell lysates were prepared in lysis buffer (HNET; 50 mM HEPES pH 7.5, 100 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1 mM DTT, 1 mM PMSF). Protein concentration of extracts was measured by Bradford assay (Bio-rad laboratories, Hercules, CA). 20 µg of protein were suspended and eletrophoresed through 12% SDS-polyacrylamide gels. Proteins were transferred to nitrocellulose membranes, blocked in TBS tween/4% milk, and then probed with anti GRα (BD Transduction Laboratories) or anti GRβ (Affinity Bioreagents, Golden, CO). The location of immunoreactive complexes was revealed by ECL.
IL-8 ELISA
ELISA (BD Biosciences, SD) was done according to the manufacturer's instruction. Briefly, anti-human IL-8 monoclonal antibody was used to coat 96 well polystyrene plates over 16 h. 200 µl of 10% FBS was added after three washes with 0.05% Tween-20 and incubated for 1 h at room temperature. 100 µl of samples was added and the sealed plate was incubated for 2 h at room temperature. Diluted biotinylated anti-human IL-8 monoclonal antibody and avidin-HPR conjugate (100 µl, each) were added and incubated for 1 h at room temperature. Absorbance was measured at 450 nm by ELISA reader using TMB substrate reagent set.
Extraction of nuclear protein
Cultured cells were trypsinized and washed once in PBS. They were resuspended in buffer A (10 mM Hepes. pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM DTT) and sonicated on ice (sonifier UP200S, dr.hielscher, 40 s, amplitude 50%, cycle 0.5). This step was repeated until no more intact cells were observed. The resulting homogenate was centrifuged at 2,000 rpm for 10 min, washed twice in 1 ml buffer A and centrifuged again. The resulting pellet contains purified nuclei. Nuclei were resuspended in buffer B (20 mM Hepes, pH 7.9, 1.5 mM MgCl2, 0.42 M NaCl, 0.2 mM EDTA, 25%v/v glycerine, 0.5 mM DTT, 0.5 mM PMSF) and the solution was incubated while shaking for further 30 min on ice.
Histone deacetylase activity
Assays were performed using the fluorometric HDAC activity assay from Upstate (Upstate Biotechnology, CA) according to the manufacturer's instructions. Briefly, 30 µg of HeLa nuclear extracts (positive control) or tested samples were diluted in 15 µl of ddH2O; then, 10 µl of HDAC assay buffer (25 mM Tris pH 8.0, 137 mM NaCl, 2.7 mM KCl, 1 mM MgCl2) were added, followed by addition of 15 µl of 267 µM assay substrate (acetyl-histone H4); samples were incubated at 37℃ for 1 h. Subsequently, the reaction was stopped by adding 20 µl of diluted activator solution and left for additional 15 min at room temperature. Released fluorophore from the deacetylated substrate were then read in a fluorescence spectrophotometer (Varian, Austria; excitation = 350-380 nm and emission = 440-460 nm) within 60 min. HDAC activity was expressed as relative OD values comparing the activity between control and GRβ overexpression.
Histone acetyltransferase (HAT) activity
HAT activity of nuclear lysates was determined by an indirect ELISA for the detection of acetyl residues, according to the manufacturer's recommendations (Upstate Biotechnology, CA). An aliquot of the reaction was immobilized onto streptavidin-coated plates. Lysates were washed twice in TBS and incubated in 50 µl of HAT assay buffer (250 mM Tris, pH 8.0, 50% glycerol, 0.5 mM EDTA and 5 mM DTT) containing acetyl-CoA (500 µM) and biotinylated histone H3 peptide or H4 peptide (0.5 µg) for 5 min at 30℃. Acetylated histone H3 and H4 were detected using rabbit polyclonal anti-acetyl-Lysine antibodies. The reaction was stopped by adding 50 µl of 1 M sulfuric acid. Samples were then read in an ELISA plate reader (Bio-Rad, CA) at a wavelength of 450 nm and 570 nm.
Chromatin immunoprecipitation assay (ChIP)
Histone H4 acetylation at the IL-8 gene promoter site was analyzed using ChIP assay kit (Upstate Biotechnology, CA) according to the manufacturer's instructions. Briefly, formaldehyde was added directly to culture medium to a final concentration of 1% and incubated for 10 min to fix protein-DNA complexes. Chromatin pellets from these cells sheared by sonication were pre-cleared with protein A agarose/salmon sperm DNA (50% slurry). One portion of the soluble chromatin was used as DNA input control, and the remains were sub-aliquoted and then precipitated using specific antibodies against acetylated histone H4. DNA from the immunoprecipitated Ab-protein-DNA complex was purified and amplified by PCR using primers specific for the IL-8 promoter. IL-8 promoter sense; 5'-TTCACCAAATTGTGGAGCTT-3', IL-8 promoter antisense; 5'-GAAGCTTGTGTGCTCTGCTG-3'.
Statistical analysis
Error bars represent SD in all cases. Statistics were performed with the Mann-Whitney U test using SPSS 11.0. A P-value of 0.05 or less was considered significant.
Acknowledgements
This study was supported by a grant of the Korea Health 21 R&D Project, Ministry for Health, Welfare and Family Affairs, R.O.K. (A030001).
Abbreviations
- GR
glucocorticoid receptor
- GREs
glucocorticoid response elements
- HAT
histone acetyltransferase
- HDAC
histone deacetylase
References
- 1.Barnes PJ, Adcock IM, Ito K. Histone acetylation and deacetylation: importance in inflammatory lung diseases. Eur Respir J. 2005;25:552–563. doi: 10.1183/09031936.05.00117504. [DOI] [PubMed] [Google Scholar]
- 2.Barnes PJ. Molecular mechanisms of corticosteroids in allergic diseases. Allergy. 2001;56:928–936. doi: 10.1034/j.1398-9995.2001.00001.x. [DOI] [PubMed] [Google Scholar]
- 3.Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, Gibson P, Ohta K, O'Byrne P, Pedersen SE, Pizzichini E, Sullivan SD, Wenzel SE, Zar HJ. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31:143–178. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 4.Caelles C, Gonzalez-Sancho JM, Munoz A. Nuclear hormone receptor antagonism with AP-1 by inhibition of the JNK pathway. Genes Dev. 1997;11:3351–3364. doi: 10.1101/gad.11.24.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi BR, Kwon JH, Gong SJ, Kwon MS, Cho JH, Kim JH, Oh S, Roh HJ, Kim DE. Expression of glucocorticoid receptor mRNAs in glucocorticoid-resistant nasal polyps. Exp Mol Med. 2006;38:466–473. doi: 10.1038/emm.2006.55. [DOI] [PubMed] [Google Scholar]
- 6.Drouin J, Sun YL, Chamberland M, Gauthier Y, De Lean A, Nemer M, Schmidt TJ. Novel glucocorticoid receptor complex with DNA element of the hormone-repressed POMC gene. EMBO J. 1993;12:145–156. doi: 10.1002/j.1460-2075.1993.tb05640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duma D, Jewell CM, Cidlowski JA. Multiple glucocorticoid receptor isoforms, mechanisms of post-translational modification. J Steroid Biochem Mol Biol. 2006;102:11–21. doi: 10.1016/j.jsbmb.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Fujimura M, Xiu Q, Tsujiura M, Tachibana H, Myou S, Matsuda T, Matsushima K. Role of leukotriene B4 in bronchial hyperresponsiveness induced by interleukin-8. Eur Respir J. 1998;11:306–311. doi: 10.1183/09031936.98.11020306. [DOI] [PubMed] [Google Scholar]
- 9.Gagliardo R, Chanez P, Vignola AM, Bousquet J, Vachier I, Godard P, Bonsignore G, Demoly P, Mathieu M. Glucocorticoid receptor alpha and beta in glucocorticoid dependent asthma. Am J Respir Crit Care Med. 2000;162:7–13. doi: 10.1164/ajrccm.162.1.9911032. [DOI] [PubMed] [Google Scholar]
- 10.Gougat C, Jaffuel D, Gagliardo R, Henriquet C, Bousquet J, Demoly P, Mathieu M. Overexpression of the human glucocorticoid receptor alpha and beta isoforms inhibits AP-1 and NF-kappaB activities hormone independently. J Mol Med. 2002;80:309–318. doi: 10.1007/s00109-001-0302-6. [DOI] [PubMed] [Google Scholar]
- 11.Hamid QA, Wenzel SE, Hauk PJ, Tsicopoulos A, Wallaert B, Lafitte JJ, Chrousos GP, Szefler SJ, Leung DY. Increased glucocorticoid receptor beta in airway cells of glucocorticoid-insensitive asthma. Am J Respir Crit Care Med. 1999;159:1600–1604. doi: 10.1164/ajrccm.159.5.9804131. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton LM, Torres-Lozano C, Puddicombe SM, Richter A, Kimber I, Dearman RJ, Vrugt B, Aalbers R, Holgate ST, Djukanovic R, Wilson SJ, Davies DE. The role of the epidermal growth factor receptor in sustaining neutrophil inflammation in severe asthma. Clin Exp Allergy. 2003;33:233–240. doi: 10.1046/j.1365-2222.2003.01593.x. [DOI] [PubMed] [Google Scholar]
- 13.Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol. 1994;56:559–564. [PubMed] [Google Scholar]
- 14.Hauk PJ, Goleva E, Strickland I, Vottero A, Chrousos GP, Kisich KO, Leung DY. Increased glucocorticoid receptor Betaexpression converts mouse hybridoma cells to a corticosteroid-insensitive phenotype. Am J Respir Cell Mol Biol. 2002;27:361–367. doi: 10.1165/rcmb.4861. [DOI] [PubMed] [Google Scholar]
- 15.Hecht K, Carlstedt-Duke J, Stierna P, Gustafsson J, Bronnegard M, Wikstrom AC. Evidence that the beta-isoform of the human glucocorticoid receptor does not act as a physiologically significant repressor. J Biol Chem. 1997;272:26659–26664. doi: 10.1074/jbc.272.42.26659. [DOI] [PubMed] [Google Scholar]
- 16.Jee YK, Gilmour J, Kelly A, Bowen H, Richards D, Soh C, Smith P, Hawrylowicz C, Cousins D, Lee T, Lavender P. Repression of interleukin-5 transcription by the glucocorticoid receptor targets GATA3 signaling and involves histone deacetylase recruitment. J Biol Chem. 2005;280:23243–23250. doi: 10.1074/jbc.M503659200. [DOI] [PubMed] [Google Scholar]
- 17.John S, Sabo PJ, Johnson TA, Sung MH, Biddie SC, Lightman SL, Voss TC, Davis SR, Meltzer PS, Stamatoyannopoulos JA, Hager GL. Interaction of the glucocorticoid receptor with the chromatin landscape. Mol Cell. 2008;29:611–624. doi: 10.1016/j.molcel.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 18.Jonat C, Rahmsdorf HJ, Park KK, Cato AC, Gebel S, Ponta H, Herrlich P. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell. 1990;62:1189–1204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- 19.Kelly A, Bowen H, Jee YK, Mahfiche N, Soh C, Lee T, Hawrylowicz C, Lavender P. The glucocorticoid receptor beta isoform can mediate transcriptional repression by recruiting histone deacetylases. J Allergy Clin Immunol. 2008;121:203.e1–208.e1. doi: 10.1016/j.jaci.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Kino T, Stauber RH, Resau JH, Pavlakis GN, Chrousos GP. Pathologic human GR mutant has a transdominant negative effect on the wild-type GR by inhibiting its translocation into the nucleus: importance of the ligand-binding domain for intracellular GR trafficking. J Clin Endocrinol Metab. 2001;86:5600–5608. doi: 10.1210/jcem.86.11.8017. [DOI] [PubMed] [Google Scholar]
- 21.Kurashima K, Mukaida N, Fujimura M, Schroder JM, Matsuda T, Matsushima K. Increase of chemokine levels in sputum precedes exacerbation of acute asthma attacks. J Leukoc Biol. 1996;59:313–316. doi: 10.1002/jlb.59.3.313. [DOI] [PubMed] [Google Scholar]
- 22.Lee KY, Chang W, Qiu D, Kao PN, Rosen GD. PG490 (triptolide) cooperates with tumor necrosis factor-alpha to induce apoptosis in tumor cells. J Biol Chem. 1999;274:13451–13455. doi: 10.1074/jbc.274.19.13451. [DOI] [PubMed] [Google Scholar]
- 23.Leung DY, Hamid Q, Vottero A, Szefler SJ, Surs W, Minshall E, Chrousos GP, Klemm DJ. Association of glucocorticoid insensitivity with increased expression of glucocorticoid receptor beta. J Exp Med. 1997;186:1567–1574. doi: 10.1084/jem.186.9.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis-Tuffin LJ, Jewell CM, Bienstock RJ, Collins JB, Cidlowski JA. Human glucocorticoid receptor beta binds RU-486 and is transcriptionally active. Mol Cell Biol. 2007;27:2266–2282. doi: 10.1128/MCB.01439-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marini M, Vittori E, Hollemborg J, Mattoli S. Expression of thepotent inflammatory cytokines, granulocyte- macrophage-colony-stimulating factor and interleukin-6 and interleukin-8, in bronchial epithelial cells of patients with asthma. J Allergy Clin Immunol. 1992;89:1001–1009. doi: 10.1016/0091-6749(92)90223-o. [DOI] [PubMed] [Google Scholar]
- 26.Matthews JG, Ito K, Barnes PJ, Adcock IM. Defective glucocorticoid receptor nuclear translocation and altered histone acetylation patterns in glucocorticoid-resistant patients. J Allergy Clin Immunol. 2004;113:1100–1108. doi: 10.1016/j.jaci.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 27.Nonaka M, Nonaka R, Jordana M, Dolovich J. GM-CSF, IL-8, IL-1R, TNF-alpha R, and HLA-DR in nasal epithelial cells in allergic rhinitis. Am J Respir Crit Care Med. 1996;153:1675–1681. doi: 10.1164/ajrccm.153.5.8630619. [DOI] [PubMed] [Google Scholar]
- 28.Oakley RH, Sar M, Cidlowski JA. The human glucocorticoid receptor beta isoform. Expression, biochemical properties, and putative function. J Biol Chem. 1996;271:9550–9559. doi: 10.1074/jbc.271.16.9550. [DOI] [PubMed] [Google Scholar]
- 29.Oakley RH, Jewell CM, Yudt MR, Bofetiado DM, Cidlowski JA. The dominant negative activity of the human glucocorticoid receptor beta isoform. Specificity and mechanisms of action. J Biol Chem. 1999;274:27857–27866. doi: 10.1074/jbc.274.39.27857. [DOI] [PubMed] [Google Scholar]
- 30.Pujols L, Mullol J, Roca-Ferrer J, Torrego A, Xaubet A, Cidlowski JA, Picado C. Expression of glucocorticoid receptor alpha- and beta-isoforms in human cells and tissues. Am J Physiol Cell Physiol. 2002;283:C1324–C1331. doi: 10.1152/ajpcell.00363.2001. [DOI] [PubMed] [Google Scholar]
- 31.Shi Y, Downes M, Xie W, Kao HY, Ordentlich P, Tsai CC, Hon M, Evans RM. Sharp, an inducible cofactor that integrates nuclear receptor repression and activation. Genes Dev. 2001;15:1140–1151. doi: 10.1101/gad.871201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sousa AR, Lane SJ, Cidlowski JA, Staynov DZ, Lee TH. Glucocorticoid resistance in asthma is associated with elevated in vivo expression of the glucocorticoid receptor beta-isoform. J Allergy Clin Immunol. 2000;105:943–950. doi: 10.1067/mai.2000.106486. [DOI] [PubMed] [Google Scholar]
- 33.Strickland I, Kisich K, Hauk PJ, Vottero A, Chrousos GP, Klemm DJ, Leung DY. High constitutive glucocorticoid receptor beta in human neutrophils enables them to reduce their spontaneous rate of cell death in response to corticosteroids. J Exp Med. 2001;193:585–593. doi: 10.1084/jem.193.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torrego A, Pujols L, Roca-Ferrer J, Mullol J, Xaubet A, Picado C. Glucocorticoid receptor isoforms alpha and beta in in vitro cytokine-induced glucocorticoid insensitivity. Am J Respir Crit Care Med. 2004;170:420–425. doi: 10.1164/rccm.200308-1143OC. [DOI] [PubMed] [Google Scholar]
- 35.Tsaprouni LG, Ito K, Adcock IM, Punchard N. Suppression of lipopolysaccharide- and tumour necrosis factor-alpha-induced interleukin (IL)-8 expression by glucocorticoids involves changes in IL-8 promoter acetylation. Clin Exp Immunol. 2007;150:151–157. doi: 10.1111/j.1365-2249.2007.03484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vachier I, Bonnans C, Chavis C, Farce M, Godard P, Bousquet J, Chanez P. Severe asthma is associated with a loss of LX4, an endogenous anti-inflammatory compound. J Allergy Clin Immunol. 2005;115:55–60. doi: 10.1016/j.jaci.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 37.Webster JC, Oakley RH, Jewell CM, Cidlowski JA. Proinflammatory cytokines regulate human glucocorticoid receptor gene expression and lead to the accumulation of the dominant negative beta isoform: a mechanism for the generation of glucocorticoid resistance. Proc Natl Acad Sci USA. 2001;98:6865–6870. doi: 10.1073/pnas.121455098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whorwood CB, Donovan SJ, Wood PJ, Phillips DI. Regulation of glucocorticoid receptor alpha and beta isoforms and type I 11beta-hydroxysteroid dehydrogenase expression in human skeletal muscle cells: a key role in the pathogenesis of insulin resistance? J Clin Endocrinol Metab. 2001;86:2296–2308. doi: 10.1210/jcem.86.5.7503. [DOI] [PubMed] [Google Scholar]
- 39.Yudt MR, Jewell CM, Bienstock RJ, Cidlowski JA. Molecular origins for the dominant negative function of human glucocorticoid receptor beta. Mol Cell Biol. 2003;23:4319–4330. doi: 10.1128/MCB.23.12.4319-4330.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]