Abstract
Y-box proteins belong to the cold shock domain family of proteins that are known to be involved in both transcriptional and translational control. Here, we give a brief overview of the structure, regulation and physiological functions of the Y-box proteins. This is followed by examining the role of Y-box protein 1 (YB-1), the most extensively studied of the Y-box protein in tumorigenesis, and its clinicopathological significance. YB-1 has the potential to be a prognostic marker and predictor of chemoresistance in human cancers.
Keywords: YB-1, structure and regulation, tumorigenesis, clinicopathological significance, prognostication, chemoresistance
INTRODUCTION
Deregulation of proper transcriptional and translational control triggers tumorigenesis. Several multifunctional proteins are involved in both transcriptional and translational control (Wilkinson and Shyu, 2001). Here we focus on a family of such multifunctional proteins, the Y-box protein family, in terms of its significance in cell proliferation and cancer. As different aspects of the Y-box proteins have already been reviewed (Matsumoto and Wolffe, 1998; Swamynathan et al, 1998; Evdokimova and Ovchinnikov, 1999; Kohno et al, 2003), we briefly appraise the structure and functions of the Y-box proteins with the emphasis on recent findings. We then summarize the role of a Y-box protein YB-1 in cancer and its use in the clinical setting.
Y-box proteins (or Y-box binding proteins) are so named because they were originally identified as DNA binding proteins that are capable of associating with the Y-box (inverted CCAAT-box) sequence of the major histocompatibility complex class II gene. Y-box proteins have thus far been known to regulate positively or negatively a number of genes, such as multidrug resistance 1, cyclin A, cyclin B1, matrix metalloproteinase 2 and collagen alpha2(I) (Higashi et al, 2003a; Jurchott et al, 2003; Kohno et al, 2003). However, members of Y-box protein family are also found in the cytoplasm and associated with mRNAs as major components of messenger ribonucleoprotein particles. Y-box proteins regulate translation in a dose-dependent manner; low concentrations of Y-box proteins activate translation and high concentrations repress it (Evdokimova and Ovchinnikov, 1999). Collectively, Y-box proteins have been implicated in the regulation of mRNA metabolism in multiple steps in both the nucleus and the cytoplasm, including transcription, splicing, mRNA stability and translation.
Structure and cellular localization of Y-box proteins
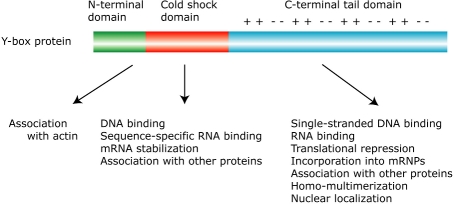
Y-box proteins consist of three domains: the N-terminal domain, the cold shock domain (CSD) and the C-terminal tail domain (Figure 1). The CSD is a highly conserved nucleic acid binding domain that confers RNA- and single-stranded and double-stranded DNA binding activities to the Y-box proteins. Both the short N-terminal and the C-terminal tail domains are less conserved among the Y-box proteins. The charged C-terminal tail domain of vertebrate Y-box proteins, consisting of alternating clusters of acidic/aromatic and basic amino acids, is likely to account for its RNA-binding activity and ability for associating with various proteins. Y-box proteins have been shown to interact with a number of cellular and viral proteins that are involved in various cellular processes (Figure 2).
Figure 1.
Schematic diagram depicting the structure of the Y-box protein. ++ and − − indicate clusters of basic and acidic/aromatic amino acids. Functions attributed to each domain are summarized at the bottom.
Figure 2.
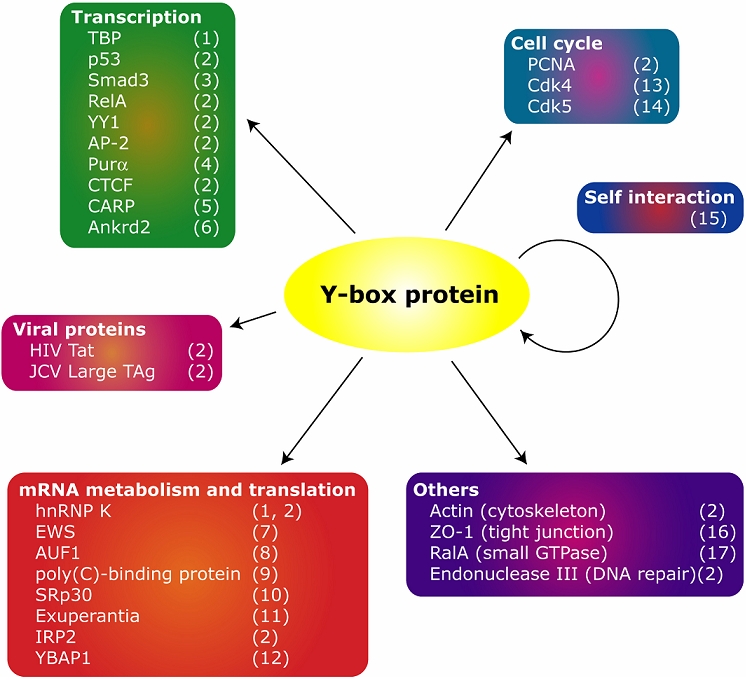
Cellular and viral proteins that interact with the Y-box protein. Numbers in parentheses indicate the references as follows: (1) Shnyreva et al, 2000, (2) Kohno et al, 2003; Swamynathan et al, 1998 and references therein, (3) Higashi et al, 2003b, (4) Safak et al, 1999, (5) Zou et al, 1997, (6) Kojic et al, 2004, (7) Chansky et al, 2001, (8) Moraes et al, 2003, (9) Funke et al, 1996, (10) Raffetseder et al, 2003, (11) Wilhelm et al, 2000, (12) Matsumoto et al, 2005, (13) Balda et al, 2003, (14) Moorthamer et al, 1999, (15) Matsumoto and Wolffe, 1998; Evdokimova and Ovchinnikov, 1999 and references therein, (16) Balda and Matter, 2000, (17) Frankel et al, 2005.
In a variety of cell types, Y-box proteins are predominantly found in the cytoplasm. However, given that Y-box proteins regulate transcription, they are expected to localize to the nucleus. Y-box proteins are translocated into the nucleus by a number of conditions and mechanisms, including UV irradiation, hyperthermia, interferon-gamma treatment, adenovirus infection, interaction with p53 and a splicing factor SRp30c and high levels of ectopic YB-1expression (Higashi et al, 2003a; Kohno 2003; Raffetseder et al, 2003 and references therein; Zhang et al, 2003). Both the CSD and the tail domains are implicated in nuclear localization of YB-1; the tail domain seems to contain a non-canonical nuclear localization signal and the isolated CSD also contributes to nuclear retention (Bader and Vogt, 2005). Y-box proteins are capable of nucleocytoplasmic shuttling, which allows them to contribute to the coupling control of transcription and translation. Y-box proteins become associated with nascent transcripts cotranscriptionally and are presumed to accompany mRNA into the cytoplasm (Soop et al, 2003). Interestingly, a mouse Y-box protein MSY2 preferentially associates with mRNAs that are transcribed from genes containing Y-box sequences in their promoter regions and stores those mRNAs in male germ cells (Yang et al, 2005a).
Regulation of the synthesis of Y-box proteins
Experiments with overexpression or down-regulation of the Y-box proteins in cultured cells or animals have shown that the amount of Y-box proteins must be precisely controlled (see below). Therefore, it is important to understand how the synthesis of Y-box proteins is regulated. Recent data have shown that the synthesis of a Y-box protein, YB-1 (Y-box binding protein-1), is regulated both at transcriptional and post-transcriptional levels. YB-1 mRNA accumulates when cells are treated with cisplatin or UV irradiation (Ohga et al, 1996). Transcription of the YB-1 gene is stimulated by p73 through an enhanced recruitment of the c-Myc-Max complexes to E-box sequences in the YB-1 promoter (Uramoto et al, 2002). Once synthesized, YB-1 mRNA is negatively regulated by its own product. YB-1 protein represses translation of YB-1 mRNA by binding to specific elements in the 5′- and 3′-untranslated regions (Fukuda et al, 2004; Skabkina et al, 2005). This self-regulation may contribute towards maintaining the concentration of YB-1 protein optimal in a cell.
Physiological functions of Y-box proteins
In human and mouse, there are three Y-box proteins, two of which are expressed in both somatic cells and germ cells (Table 1). The most extensively studied Y-box protein, YB-1, is ubiquitously expressed in various tissues. Human Contrin and mouse MSY2 are germ cell-specific members of the Y-box protein family. Analyses of the effects of targeting Y-box genes in chicken cells and mice have been widely carried out in the last three years. Chicken B-cell lymphoma DT40 cells are widely used to study functional consequences of disrupting specific genes because of the high frequency of homologous recombination. The YB-1 (or YB-1b) gene in DT40 cells has been disrupted by two independent groups of investigators; one group reported that YB-1+/− cells show slow-growth phenotype and increased DNA content (Swamynathan et al, 2002). The other group of researchers found that heterozygous disruption resulted in no growth defects but homozygous gene disruptants exhibited a slow and cold-sensitive growth phenotype (Matsumoto et al, 2005). One research group tried to disrupt YB-1 gene in mice but encountered difficulties in disrupting both alleles of the YB-1 gene (Shibahara et al, 2004). They found hypersensitivity of the YB-1+/− cells to genotoxic stresses. However, as was the case in chicken cells, another group recently reported homozygous YB-1gene disruption, showing the importance of YB-1 in late stages of embryonic development (Lu et al, 2005). They observed developmental defects of YB-1−/−embryos after embryonic day 13.5 including craniofacial lesions, hemorrhage and respiratory failure, with YB-1−/− MEF cells showing premature senescence and hypersensitivity to different cellular stresses. The reason for the presence or absence of the haplo-insufficient phenotypes is currently unknown. In mice lacking MSY2, both male and female homozygotes are sterile, a consequence of disturbed spermatogenesis due to reduction of postmeiotic germ-cell mRNAs in male and oocyte loss in female (Yang et al, 2005b). Overall, studies designed to reduce or deplete a Y-box protein in cells or whole organisms underscore the significance of Y-box proteins in appropriate cell growth, stress responses and development.
Table 1.
Y-box proteins in human and mouse
| Human | Mouse | Expression | Phenotype in knockout mice |
|---|---|---|---|
| YB-1/DbpB | YB-1/MSY1 | Ubiquitous | Embryonic/perinatal lethality (neurological abnormalities, haemorrhage, respiratory failure and growth retardation)* |
| DbpA | MSY4 | Ubiquitous (abundant in heart, muscle and testis) | Unknown |
| Contrin | MSY2 | Germ cells | Male and female infertility** |
Role of YB-1 in tumorigenesis
The role of YB-1 in cancer progression has attracted attention in recent years.YB-1 has been found to be upregulated during prostate cancer tumor progression (Gimenez-Bonafe et al, 2004). Increased YB-1 expression has been correlated with DNA topoisomerase IIα and proliferating cell nuclear antigen expression in human lung cancer (Gu et al, 2001) and colorectal cancer (Shibao et al, 1999) and linked to markers of cellular proliferation in osteosarcoma (Oda et al, 1998). YB-1 has been identified as a cell cycle stage-specific transcription factor (Jurchott et al, 2003). Nuclear accumulation of YB-1 in HeLa cells was demonstrated to transcriptionally activate cyclin A and B1 genes, which are crucial for cell cycle progression. Increase in cyclin A has been reported to be associated with poor clinical outcome in breast cancer (Michalides et al, 2002).
In addition, YB-1 is believed to promote metastasis by enhancing the transcription of gelatinase A, a matrix metalloproteinase that facilitates cell migration (Cheng et al, 2002). Recently, Berquin et al. (2005) has also shown that YB-1 may induce epidermal growth factor (EGF) independence in mammary epithelial cells via activation of the EGF receptor pathway, thereby contributing to breast tumor aggressiveness. In yet another recent paper, Bergmann and colleagues (2005), using a transgenic mouse model, showed that overexpression of YB-1 may cause breast cancer through the induction of genetic instability.
On the other hand, YB-1 may have anti-oncogenic activity as it is reported to be capable of blocking oncogenic cell transformation (Bader and Vogt, 2005). The phosphoinositide 3-kinase (PI 3-kinase) pathway is known to show gain of function in human cancers (Bader and Vogt 2004). The catalytic subunits of PI 3-Kinase, p110 (of which P3K is a homolog) and Akt are oncoproteins and YB-1 is specifically known to inhibit P3K and Akt-induced transformation involving protein synthesis (Bader et al., 2003). YB-1 may interefere with the synthesis of growth-related proteins including growth factors, receptors, kinases, transcriptional regulators and cell cycle proteins associated with P3K and Akt pathways (Zimmer et al, 2000; Bader and Vogt 2004).
A seminal paper describing YB-1 expression in cancer tissues was first reported by Royer's group in breast cancer (Bargou et al, 1997). The pathological significance of YB-1 in a variety of cancers is shown in Table 2.
Table 2.
Overexpression of YB-1 and pathological significance in human cancers
| Organ | Tumors | Pathological Significance | Reference |
|---|---|---|---|
| Breast | Invasive ductal breast cancer | Tumor aggressiveness and axillary lymph node positivity | Huang et al, 2005 |
| Associated with progesterone receptor positivity but no prognostic value | Saji et al, 2003 | ||
| Breast cancer (histologic subtype not specified) | Higher risk for relapse without postoperative chemotherapy | Janz et al, 2002 | |
| Ovary | Surface epithelial neoplasms (serous, mucinous, endometroid & clear cell) | Co-expression with P-glycoprotein associated with poor survival | Huang et al, 2004 |
| Surface epithelial neoplasms (mainly serous) | Higher nuclear expression in recurrent lesions than in primary tumors | Yahata et al, 2002 | |
| Serous adenocarcinoma | Poor prognosis | Kamura et al, 1999 | |
| Lung | Nonsmall cell lung cancer | Nuclear expression correlated with reduced survival | Gessner et al, 2004 |
| Nuclear expression correlated with node metastasis, stage of the disease and poor prognosis | Shibahara et al, 2001 | ||
| Squamous cell carcinoma | Poor prognosis | Shibahara et al, 2001 | |
| Adenocarcinoma | Associated with T3-4 and Stage II-IV tumors | Gu et al, 2001 | |
| Thyroid | Anaplastic (undifferentiated) carcinomas, papillary carcinomas and follicular carcinomas | High expression in anaplastic carcinoma (known to be rapidly progressive) | Ito et al, 2003 |
| Soft tissues | Synovial sarcoma | Poor prognosis | Oda et al, 2003 |
| Large intestine | Colorectal adenocarcinoma | Proliferation associated marker | Shibao et al, 1999 |
YB-1 and chemoresistance in human tumors
Substantial YB-1 expression was demonstrated in multidrug-resistant breast, gastric and pancreatic cell lines (Holm et al, 2004). Altered drug sensitivity to cisplatin, a very potent and widely used anti-cancer agent and mitomycin C has been observed following treatment of cells with antisense YB-1 (Ohga et al, 1996; Torigoe et al, 2005). Expression of YB- 1 protein has been reported to reflect the chemosensitivity of ovarian serous adenocarcinoma (Kamura et al, 1999) and breast cancer (Janz et al, 2002; Huang et al, 2005). Increased nuclear localization of YB-1 has been observed in acquired cisplatin-resistant ovarian cancer (Yahata et al, 2002).
YB-1 expression has also been shown to be associated with P-glycoprotein (Pgp) expression in breast cancer cells resulting in drug resistance (Bargou et al, 1997; Saji et al, 2003; Huang et al, 2005). Pgp, encoded by the MDR1 gene, is a member of the ATP-binding cassette transporter superfamily of proteins involved in the protection of cells from xenobiotics and drugs (Kuwano et al, 2003). Pgp has become an important molecular target for limiting chemoresistance as it plays a major role in the development of multidrug-resistant tumor type and is known to mediate resistance to a wide range of anticancer agents (Kuwano et al, 1999). Bay and co-workers have recently demonstrated a direct interaction between YB-1 and Pgp using the computer-based Resonance Recognition Model (Huang et al, 2005). The same investigators observed the occurrence of raised recurrence rates in breast tumor patients with high YB-1 expression who underwent a chemotherapy regime which contained anthracycline (a Pgp substrate). Besides breast cancer, YB-1 has been correlated with Pgp in ovarian cancer (Huang et al, 2004), prostate cancer (Gimenez-Bonafe et al, 2004) and osteosarcoma (Oda et al, 1998).
YB-1 has been shown to bind p53 (Okamoto et al, 2000) and interaction with p53 could be necessary for the self-defense of cells exposed to DNA-damaging agents (Kuwano et al, 2003). As mentioned earlier, p73, a close relative of the p53 family, has also been observed to stimulate transcription of the YB-1 promoter by enhancing the recruitment of the cMyc-Max complex to its target gene (Uramoto et al, 2002). c-Myc, an oncogene with a dual function in cell proliferation and apoptosis can confer resistance to cisplatin. p73 is known to induce apoptosis (Irwin et al, 2000). and p73 overexpressing clones have been observed to be cisplatin resistant (Gong et al, 1999). Hence, c-Myc and p73 may form a complex necessary in YB-1 mediated drug resistance (Uramoto et al, 2002).
CONCLUSIONS
Expression of the YB-1 protein has a prognostic significance in determining disease progression in human cancers. Perhaps more importantly, YB-1 has the potential to be a biological marker which predicts chemotherapy resistance and aid in the selection of appropriate adjuvant chemotherapy. There has been cumulative evidence in the literature to suggest that YB-1 is involved in pleiotropic resistance to different classes of DNA-targeting drugs (Levenson et al, 2000). As clinical drug resistance hampers effective chemotherapy, a recent focus in cancer therapeutic strategy is to develop molecular cancer therapeutics (Kuwano et al, 2003; Holm et al, 2004). In this regard, YB-1 holds promise as target molecule for the development of novel approaches in overcoming multidrug resistance in cancer chemotherapy (Janz et al, 2002).
ACKNOWLEDGEMENTS
We thank the Singapore National Medical Research Council (grants to BHB) and the Ministry of Education, Culture, Sports, Science, and Technology of Japan (to KM) for funding our work. We are grateful to Ms Song-Lin Bay for technical assistance.
LIST OF ABBREVIATIONS
- CSD
Cold shock domain
- YB-1
Y-Box binding protein-1
- EGF
Epidermal growth factor
- PI-3
Phosphoinositide 3-kinase
- Pgp
P-glycoprotein
STATEMENT OF COMPETING INTERESTS
The authors declared no competing interests.
REFERENCES
- Bader AG, Vogt PK. An essential role for protein synthesis in oncogenic cellular transformation. Oncogene. 2004;23:3145–3150. doi: 10.1038/sj.onc.1207550. [DOI] [PubMed] [Google Scholar]
- Bader AG, Vogt PK. Inhibition of protein synthesis by Y box-binding protein 1 blocks oncogenic cell transformation. Mol Cell Biol. 2005;25:2095–2106. doi: 10.1128/MCB.25.6.2095-2106.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader AG, Felts KA, Jiang N, Chang HW, Vogt PK. Y box-binding protein 1 induces resistance to oncogenic transformation by the phosphatidylinositol 3-kinase pathway. Proc Natl Acad Sci USA. 2003;100:12384–12389. doi: 10.1073/pnas.2135336100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balda MS, Matter K. The tight junction protein ZO-1 and an interacting transcription factor regulate ErbB-2 expression. EMBO J. 2000;19:2024–2033. doi: 10.1093/emboj/19.9.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balda MS, Garrett MD, Matter K. The ZO-1-associated Y-box factor ZONAB regulates epithelial cell proliferation and cell density. J Cell Biol. 2003;160:423–432. doi: 10.1083/jcb.200210020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargou RC, Jurchott K, Wagener C, et al. Nuclear localization and increased levels of transcription factor YB-1 in primary human breast cancers are associated with intrinsic MDR1 gene expression. Nat Med. 1997;3:447–450. doi: 10.1038/nm0497-447. [DOI] [PubMed] [Google Scholar]
- Bergmann S, Royer-Pokora B, Fietze E, et al. YB-1 provokes breast cancer through the induction of chromosomal instability that emerges from mitotic failure and centrosome amplification. Cancer Res. 2005;65:4078–4087. doi: 10.1158/0008-5472.CAN-04-4056. [DOI] [PubMed] [Google Scholar]
- Berquin IM, Pang B, Dziubinski ML, Scott LM, et al. Y-box-binding protein 1 confers EGF independence to human mammary epithelial cells. Oncogene. 2005;24:3177–3186. doi: 10.1038/sj.onc.1208504. [DOI] [PubMed] [Google Scholar]
- Chansky HA, Hu M, Hickstein DD, Yang L. Oncogenic TLS/ERG and EWS/Fli-1 fusion proteins inhibit RNA splicing mediated by YB-1 protein. Cancer Res. 2001;61:3586–3590. [PubMed] [Google Scholar]
- Cheng S, Alfonso-Jaume MA, Mertens PR, Lovett DH. Tumor metastasis suppressor nm23-beta inhibits gelatinase A transcription by interference with transactivator Y-box protein-1. Biochem J. 2002;366:807–816. doi: 10.1042/BJ20020202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evdokimova VM, Ovchinnikov LP. Translational regulation by Y-box transcription factor: involvement of the major mRNA-associated protein, p50. Int J Biochem Cell Biol. 1999;31:139–149. doi: 10.1016/s1357-2725(98)00137-x. [DOI] [PubMed] [Google Scholar]
- Frankel P, Aronheim A, Kavanagh E, et al. RalA interacts with ZONAB in a cell density-dependent manner and regulates its transcriptional activity. EMBO J. 2005;24:54–62. doi: 10.1038/sj.emboj.7600497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T, Ashizuka M, Nakamura T, et al. Characterization of the 5′-untranslated region of YB-1 mRNA and auto-regulation of translation by YB-1 protein. Nucleic Acids Res. 2004;32:611–622. doi: 10.1093/nar/gkh223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funke B, Zuleger B, Benavente R, et al. The mouse poly(C)-binding protein exists in multiple isoforms and interacts with several RNA-binding proteins. Nucleic Acids Res. 1996;24:3821–3828. doi: 10.1093/nar/24.19.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessner C, Woischwill C, Schumacher A, et al. Nuclear YB-1 expression as a negative prognostic marker in nonsmall cell lung cancer. Eur Respir J. 2004;23:14–19. doi: 10.1183/09031936.03.00033203. [DOI] [PubMed] [Google Scholar]
- Gimenez-Bonfe P, Fedoruk MN, Whitmore TG, et al. YB-1 is upregulated during prostate cancer tumor progression and increases P-glycoprotein activity. Prostate. 2004;59:337–349. doi: 10.1002/pros.20023. [DOI] [PubMed] [Google Scholar]
- Gong JG, Costanzo A, Yang HQ, et al. The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature. 1999;399:806–809. doi: 10.1038/21690. [DOI] [PubMed] [Google Scholar]
- Gu C, Oyama T, Osaki T, Kohno K, Yasumoto K. Expression of Y box-binding protein-1 correlates with DNA topoisomerase II alpha and proliferating cell nuclear antigen expression in lung cancer. Anticancer Res. 2001;21:2357–2362. [PubMed] [Google Scholar]
- Higashi K, Inagaki Y, Suzuki N, et al. Y-box-binding protein YB-1 mediates transcriptional repression of human alpha 2(I) collagen gene expression by interferon-gamma. J Biol Chem. 2003a;278:5156–5162. doi: 10.1074/jbc.M208724200. [DOI] [PubMed] [Google Scholar]
- Higashi K, Inagaki Y, Fujimori K, et al. Interferon-gamma interferes with transforming growth factor-beta signaling through direct interaction of YB-1 with Smad3. J Biol Chem. 2003b;278:43470–43479. doi: 10.1074/jbc.M302339200. [DOI] [PubMed] [Google Scholar]
- Holm PS, Lage H, Bergmann S, et al. Multidrug-resistant cancer cells facilitate E1-independent adenoviral replication: impact for cancer gene therapy. Cancer Res. 2004;64:322–328. doi: 10.1158/0008-5472.can-0482-2. [DOI] [PubMed] [Google Scholar]
- Huang X, Ushijima K, Komai K, et al. Co-expression of Y box-binding protein-1 and P-glycoprotein as a prognostic marker for survival in epithelial ovarian cancer. Gynecol Oncol. 2004;93:287–291. doi: 10.1016/j.ygyno.2004.01.040. [DOI] [PubMed] [Google Scholar]
- Huang J, Tan PH, Li KB, Matsumoto K, Tsujimoto M, Bay BH. Y-box binding protein, YB-1, as a marker of tumor aggressiveness and response to adjuvant chemotherapy in breast cancer. Int J Oncol. 2005;26:607–613. [PubMed] [Google Scholar]
- Irwin M, Marin MC, Phillips AC. Role for the p53 homologue p73 in E2F-1-induced apoptosis. Nature. 2000;407:645–648. doi: 10.1038/35036614. [DOI] [PubMed] [Google Scholar]
- Ito Y, Yoshida H, Shibahara K, et al. Y-box binding protein expression in thyroid neoplasms: its linkage with anaplastic transformation. Pathol Int. 2003;53:429–433. doi: 10.1046/j.1440-1827.2003.01494.x. [DOI] [PubMed] [Google Scholar]
- Janz M, Harbeck N, Dettmar P, et al. Y-box factor YB-1 predicts drug resistance and patient outcome in breast cancer independent of clinically relevant tumor biologic factors HER2, uPA and PAI-1. Int J Cancer. 2002;97:278–282. doi: 10.1002/ijc.1610. [DOI] [PubMed] [Google Scholar]
- Jurchott K, Bergmann S, Stein U, et al. YB-1 as a cell cycle-regulated transcription factor facilitating cyclin A and cyclin B1 gene expression. J Biol Chem. 2003;278:27988–27996. doi: 10.1074/jbc.M212966200. [DOI] [PubMed] [Google Scholar]
- Kamura T, Yahata H, Amada S, et al. Is nuclear expression of Y box-binding protein-1 a new prognostic factor in ovarian serous adenocarcinoma? Cancer. 1999;85:2450–2454. doi: 10.1002/(sici)1097-0142(19990601)85:11<2450::aid-cncr21>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Kohno K, Izumi H, Uchiumi T, Ashizuka M, Kuwano M. The pleiotropic functions of the Y-box-binding protein, YB-1. Bioessays. 2003;25:691–698. doi: 10.1002/bies.10300. [DOI] [PubMed] [Google Scholar]
- Kojic S, Medeot E, Guccione E, et al. The Ankrd2 protein, a link between the sarcomere and the nucleus in skeletal muscle. J Mol Biol. 2004;339:313–325. doi: 10.1016/j.jmb.2004.03.071. [DOI] [PubMed] [Google Scholar]
- Kuwano M, Toh S, Uchiumi T, Takano H, Kohno K, Wada M. Multidrug resistance-associated protein subfamily transporters and drug resistance. Anticancer Drug Res. 1999;14:123–131. [PubMed] [Google Scholar]
- Kuwano M, Uchiumi T, Hayakawa H, et al. The basic and clinical implications of ABC transporters, Y-box-binding protein-1 (YB-1) and angiogenesis-related factors in human malignancies. Cancer Sci. 2003;94:9–14. doi: 10.1111/j.1349-7006.2003.tb01344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson VV, Davidovich IA, Roninson IB. Pleotropic resistance to DNA-interactive drugs is associated with increased expression of genes involved in DNA replication, repair and stress response. Cancer Res. 2000;60:5027–5030. [PubMed] [Google Scholar]
- Lu ZH, Books JT, Ley TJ. YB-1 is important for late-stage embryonic development, optimal cellular stress responses, and the prevention of premature senescence. Mol Cell Biol. 2005;25:4625–4637. doi: 10.1128/MCB.25.11.4625-4637.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Wolffe AP. Gene regulation by Y-box proteins: coupling control of transcription and translation. Trends Cell Biol. 1998;8:318–323. doi: 10.1016/s0962-8924(98)01300-2. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Tanaka KJ, Tsujimoto M. An acidic protein, YBAP1, mediates the release of YB-1 from mRNA and relieves the translational repression activity of YB-1. Mol Cell Biol. 2005;25:1779–1792. doi: 10.1128/MCB.25.5.1779-1792.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalides R, van Tinteren H, Balkenende A, et al. Cyclin A is a prognostic indicator in early stage breast cancer with and without tamoxifen treatment. Br J Cancer. 2002;86:402–408. doi: 10.1038/sj.bjc.6600072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorthamer M, Zumstein-Mecker S, Chaudhuri B. DNA binding protein dbpA binds Cdk5 and inhibits its activity. FEBS Lett. 1999;446:343–350. doi: 10.1016/s0014-5793(99)00248-3. [DOI] [PubMed] [Google Scholar]
- Moraes KC, Quaresma AJ, Maehnss K, Kobarg J. Identification and characterization of proteins that selectively interact with isoforms of the mRNA binding protein AUF1 (hnRNP D) Biol Chem. 2003;384:25–37. doi: 10.1515/BC.2003.004. [DOI] [PubMed] [Google Scholar]
- Oda Y, Sakamoto A, Shinohara N, et al. Nuclear expression of YB-1 protein correlates with P-glycoprotein expression in human osteosarcoma. Clin Cancer Res. 1998;4:2273–2277. [PubMed] [Google Scholar]
- Oda Y, Ohishi Y, Saito T, et al. Nuclear expression of Y-box-binding protein-1 correlates with P-glycoprotein and topoisomerase II alpha expression, and with poor prognosis in synovial sarcoma. J Pathol. 2003;199:251–258. doi: 10.1002/path.1282. [DOI] [PubMed] [Google Scholar]
- Ohga T, Koike K, Ono M, et al. Role of the human Y box-binding protein YB-1 in cellular sensitivity to the DNA-damaging agents cisplatin, mitomycin C, and ultraviolet light. Cancer Res. 1996;56:4224–4228. [PubMed] [Google Scholar]
- Okamoto T, Izumi H, Imamura T, et al. Direct interaction of p53 with the Y-box binding protein, YB-1: a mechanism for regulation of human gene expression. Oncogene. 2000;19:2955–2966. doi: 10.1038/sj.onc.1204029. [DOI] [PubMed] [Google Scholar]
- Raffetseder U, Frye B, Rauen T, et al. Splicing factor SRp30c interaction with Y-box protein-1 confers nuclear YB-1 shuttling and alternative splice site selection. J Biol Chem. 2003;278:18241–18248. doi: 10.1074/jbc.M212518200. [DOI] [PubMed] [Google Scholar]
- Safak M, Gallia GL, Khalili K. Reciprocal interaction between two cellular proteins, Puralpha and YB-1, modulates transcriptional activity of JCVCY in glial cells. Mol Cell Biol. 1999;19:2712–2723. doi: 10.1128/mcb.19.4.2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saji H, Toi M, Saji S, Koike M, Kohno K, Kuwano M. Nuclear expression of YB-1 protein correlates with P-glycoprotein expression in human breast carcinoma. Cancer Lett. 2003;190:191–197. doi: 10.1016/s0304-3835(02)00590-6. [DOI] [PubMed] [Google Scholar]
- Shibahara K, Sugio K, Osaki T, et al. Nuclear expression of the Y-box binding protein, YB-1, as a novel marker of disease progression in non-small cell lung cancer. Clin Cancer Res. 2001;7:3151–3155. [PubMed] [Google Scholar]
- Shibahara K, Uchiumi T, Fukuda T, et al. Targeted disruption of one allele of the Y-box binding protein-1 (YB-1) gene in mouse embryonic stem cells and increased sensitivity to cisplatin and mitomycin C. Cancer Sci. 2004;95:348–353. doi: 10.1111/j.1349-7006.2004.tb03214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibao K, Takano H, Nakayama Y, et al. Enhanced coexpression of YB-1 and DNA topoisomerase II alpha genes in human colorectal carcinomas. Int J Cancer. 1999;83:732–737. doi: 10.1002/(sici)1097-0215(19991210)83:6<732::aid-ijc6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Shnyreva M, Schullery DS, Suzuki H, Higaki Y, Bomsztyk K. Interaction of two multifunctional proteins. Heterogeneous nuclear ribonucleoprotein K and Y-box-binding protein. J Biol Chem. 2000;275:15498–15503. doi: 10.1074/jbc.275.20.15498. [DOI] [PubMed] [Google Scholar]
- Skabkina OV, Lyabin DN, Skabkin MA, Ovchinnikov LP. YB-1 autoregulates translation of its own mRNA at or prior to the step of 40S ribosomal subunit joining. Mol Cell Biol. 2005;25:3317–3323. doi: 10.1128/MCB.25.8.3317-3323.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soop T, Nashchekin D, Zhao J, et al. A p50-like Y-box protein with a putative translational role becomes associated with pre-mRNA concomitant with transcription. J Cell Sci. 2003;116:1493–1503. doi: 10.1242/jcs.00353. [DOI] [PubMed] [Google Scholar]
- Swamynathan SK, Nambiar A, Guntaka RV. Role of single-stranded DNA regions and Y-box proteins in transcriptional regulation of viral and cellular genes. FASEB J. 1998;12:515–522. doi: 10.1096/fasebj.12.7.515. [DOI] [PubMed] [Google Scholar]
- Swamynathan SK, Varma BR, Weber KT, Guntaka RV. Targeted disruption of one allele of the Y-box protein gene, Chk-YB-1b, in DT40 cells results in major defects in cell cycle. Biochem Biophys Res Commun. 2002;296:451–457. doi: 10.1016/s0006-291x(02)00875-6. [DOI] [PubMed] [Google Scholar]
- Torigoe T, Izumi H, Ishiguchi H, et al. Cisplatin resistance and transcription factors. Curr Med Chem Anti-Canc Agent. 2005;5:15–27. doi: 10.2174/1568011053352587. [DOI] [PubMed] [Google Scholar]
- Uramoto H, Izumi H, Ise T, et al. p73 interacts with c-Myc to regulate Y-box-binding protein-1 expression. J Biol Chem. 2002;277:31694–31702. doi: 10.1074/jbc.M200266200. [DOI] [PubMed] [Google Scholar]
- Wilhelm JE, Mansfield J, Hom-Booher N, et al. Isolation of a ribonucleoprotein complex involved in mRNA localization in Drosophila oocytes. J Cell Biol. 2000;148:427–440. doi: 10.1083/jcb.148.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson MF, Shyu AB. Multifunctional regulatory proteins that control gene expression in both the nucleus and the cytoplasm. Bioessays. 2001;23:775–787. doi: 10.1002/bies.1113. [DOI] [PubMed] [Google Scholar]
- Yahata H, Kobayashi H, Kamura T, et al. Increased nuclear localization of transcription factor YB-1 in acquired cisplatin-resistant ovarian cancer. J Cancer Res Clin Oncol. 2002;128:621–626. doi: 10.1007/s00432-002-0386-6. [DOI] [PubMed] [Google Scholar]
- Yang J, Medvedev S, Reddi PP, Schultz RM, Hecht NB. The DNA/RNA-binding protein MSY2 marks specific transcripts for cytoplasmic storage in mouse male germ cells. Proc Natl Acad Sci U S A. 2005a;102:1513–1518. doi: 10.1073/pnas.0404685102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Medvedev S, Yu J, et al. Absence of the DNA/RNA-binding protein MSY2 results in male and female infertility. Proc Natl Acad Sci U S A. 2005b;102:5755–5760. doi: 10.1073/pnas.0408718102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YF, Homer C, Edwards SJ, et al. Nuclear localization of Y-box factor YB1 requires wild-type p53. Oncogene. 2003;22:2782–2794. doi: 10.1038/sj.onc.1206357. [DOI] [PubMed] [Google Scholar]
- Zimmer SG, DeBenedetti A, Graff JR. Translational control of malignancy: the mRNA cap-binding protein, eIF-4E, as a central regulator of tumor formation, growth, invasion and metastasis. Anticancer Res. 2000;20:1343–1351. [PubMed] [Google Scholar]
- Zou Y, Evans S, Chen J, et al. CARP, a cardiac ankyrin repeat protein, is downstream in the Nkx2-5 homeobox gene pathway. Development. 1997;124:793–804. doi: 10.1242/dev.124.4.793. [DOI] [PubMed] [Google Scholar]