Introduction
The bacteria that contaminate the uterine lumen can be categorised by their pathogenicity as bacteria that are recognised uterine pathogens, potential uterine pathogens or opportunistic contaminant bacteria (41,46). The severity of postpartum endometritis is dependent in part on the pathogenicity of bacteria present; although, establishment and persistence of uterine infection is also influenced by the uterine environment, genetic factors, and the animal’s innate and acquired immunity. The recognised uterine pathogens, Arcanobacterium pyogenes, Escherichia coli, Fusobacterium necrophorum, Prevotella melaninogenicus and Proteus species are associated with greater endometrial inflammation and more severe clinical uterine disease (7,13,29,34). We have recently shown that other potential uterine pathogens or opportunistic contaminant bacteria in the uterine lumen do not have this same relationship (46).
Animals with a greater bacterial growth density in the uterine lumen have smaller ovarian dominant follicles and lower peripheral plasma oestradiol concentrations compared with normal postpartum cows (41). However, it is unclear if suppression of ovarian follicle growth and function is related to the presence of pathogenic, potentially pathogenic or opportunistic contaminant bacteria in the uterus.
The effects of uterine infection on the corpus luteum (CL) are not clear as infections are associated with both premature regression of the corpus luteum and a failure of luteolysis with a resultant extended luteal phase (30). Uterine pathogenic bacteria such as E. coli stimulate prostaglandin E2 secretion by endometrial cell cultures and tissue explants in vitro, which may affect corpus luteum function (14). However, the effect of uterine bacterial infection on formation and function of the first postpartum corpus luteum in the whole animal remains unclear.
The first line of defence against invading bacteria in the uterus is the innate immune system. Part of the innate response is the elaboration of pro-inflammatory cytokines, including as tumour necrosis factor alpha (TNFα), which induce the production of acute phase proteins such as α1-acid glycoprotein (AGP), serum amyloid A (SAA) and haptoglobin (4,12,25,45). Peripheral plasma concentrations of AGP are greater in animals from which recognised uterine pathogens are isolated (46).
The aim of the present study was to examine the relationship between pathogenic bacteria in the postpartum uterine lumen, follicle growth and function and the formation of a competent corpus luteum. In addition, peripheral plasma concentrations of immune mediators were quantified in order to gain further insight into the effects of uterine infection on the immune system.
Materials and Methods
Preliminary Study
To determine if qualitative or quantitative microbiology differs between the previously gravid and non-gravid uterine horns, a preliminary study was conducted using a group of 30 Limousin × Friesian heifers. The heifers were housed in a straw yard and fed concentrate with ad libitum silage throughout. The animals were fed a diet formulated according to standard guidelines (1). Estrus was synchronised by the insertion of an intravaginal progesterone releasing device (Eazi-breed CIDR, Animal Reproductive Technology, Leominster) for 8 days, with an intramuscular injection of 500 μg cloprostenol (Estrumate, Schering-Plough Animal Health, Uxbridge, Middlesex) 24 h before removal of the intravaginal device. Animals were inseminated 48 and 72 h after removal of the device using semen from a single Limousin sire. Those animals that were not pregnant 30 days later, detected by transrectal ultrasonography, were re-synchronised and inseminated again. Twenty three heifers subsequently calved without assistance during a 45 day period, and the uterine lumen was flushed 14 days after calving. A sterile 18 guage silicone foley catheter (AOB Technology, Pullman, Washington, USA) was inserted into the previously gravid uterine horn, guided by palpation per rectum. 50 ml sterile PBS (Sigma, Poole, Dorset) was installed and withdrawn with 3 to 5 gentle back and forth motions using a 50 ml syringe (Becton Dickinson UK Ltd, Cowley, Oxford). More than 80 % of the instilled fluid was recovered, based on the measured volume of fluid in the syringe at the end of the procedure. The catheter was withdrawn from the animal, flushed with sterile water and the procedure repeated for the contralateral uterine horn. A 5 ml aliquot of sample from each horn was submitted for anaerobic and aerobic bacterial culture within 1 h of collection. The remaining intrauterine fluid was transferred on ice to the laboratory within 1 h of collection, and centrifuged at 1000g for 15 min. Approximately 20 ml supernatant was decanted and filtered through a 0.2 μm Polyvinylidene Fluoride Syringe filter (Whatman, Clifton, New Jersey, USA). Concentrations of TNFα and bacterial endotoxin were determined as described below.
Main Study
In the main study a herd of Holstein-Friesian cows was used to determine the effect of uterine pathogen growth density on ovarian function. The herd had an annual average milk yield of 8000 litres and a rolling herd somatic cell count of 183 × 103 cells / ml. The animals were fed a diet formulated according to standard guidelines (1). The diet comprised maize and grass silage, straw, protein pellets, brewers grains, wheat and minerals. The animals were housed in straw-bedded yards from October until April and grazed grass paddocks during the summer. To remove any influence of disease other than bacterial infection of the uterus, cows were excluded from the study if they had a history of a caesarean operation or retained fetal membranes, or the presence of acute mastitis, lameness, abdominal disorders or other intercurrent disease upon clinical examination (16,35,39,42). A total of 71 cows were included in the study, and antimicrobial treatments were not administered to these animals during the study period. All procedures were carried out under Home Office authorisation in compliance with the Animals (Scientific Procedures) Act 1986, and all experimental protocols were approved by the Royal Veterinary College Ethical Review Committee.
Uterine swab collection and bacteriology
For each animal, a transcervical guarded swab was collected from the uterine body on day 21 ± 1 and day 28 ± 1 postpartum, using a previously validated method (27,46). Briefly, the swab comprised a long copper wire bearing a cotton wool tip sheathed in a metal guard tube (8mm external diameter; 58 cm long) and was wrapped and sterilised by autoclaving at 134°C. The distal end of the guard tube was covered by a sterile gelatin half-capsule (Devacaps) to prevent contamination of the swab on insertion. The vulva of each cow was cleaned and the swab inserted through the vagina and cervical canal into the lumen of the uterus, guided by palpation per rectum. Within the uterine body, the swab was extruded from the guard tube, displacing the gelatin capsule and brought into firm contact with the endometrium about 2 cm from the bifurcation of the horns, before being withdrawn into the guard and removed from the uterus. The swab was transferred to a bijou bottle containing 5 mL Stuart Transport Medium (Unipath, Basingstoke, UK) and cultured within one hour of collection. Each swab was cultured at 37°C aerobically for 48 h on sheep blood agar and MacConkey agar (Unipath), and anaerobically for up to seven days on pre-equilibrated sheep blood agar (Unipath). Bacteria were identified on the basis of the characteristics of the colony, Gram stain, morphology, haemolysis, biochemical profile (API systems; bioMerieux, Marcy-‘Etoile, France) and other standard tests (2). Bacterial isolates were categorised (Table 1) according to their expected pathogenic potential in the uterus based on previous reports in the literature (6,10,27-29,34,46). The categories were: bacteria reported to be associated with uterine endometrial lesions (“recognised uterine pathogens”); potential pathogens frequently isolated from the bovine uterine lumen and cases of endometritis, but not commonly associated with uterine lesions; and, opportunist contaminants transiently isolated from the uterine lumen and not associated with endometritis. The bacterial growth density of each bacterial species was scored semi-quantitatively by estimating the number of colonies detected on the plate as follows: 0 = no growth; 1 = < 10 colonies; 2 = 10 to 100 colonies; 3 = 101 to 500 colonies; and 4 = > 500 colonies (28). The day 7 uterine pathogen growth density (UPGD) for each animal was the sum of the individual growth densities of the recognised uterine pathogen species isolated from that animal on day 7 postpartum.
Table 1.
Categorisation of bacteria, isolated by aerobic and anaerobic culture of uterine swabs, according to their expected pathogenic potential in the uterus (6,9,24-26,29,41). Categories are: 1 = recognised uterine pathogens associated with uterine endometrial lesions; 2 = potential pathogens frequently isolated from the bovine uterine lumen and cases of endometritis but not commonly associated with uterine lesions; 3 = opportunist contaminants transiently isolated from the uterine lumen but not usually associated with endometritis.
| Bacterial category | ||
|---|---|---|
| 1 | 2 | 3 |
| Arcanobacterium pyogenes | Bacillus licheniformis | Clostridium perfringens |
| Prevotella melaninogenicus | Enterococcus faecalis | Klebsiella pneumoniae |
| Escherichia coli | Mannhiemia haemolytica | Micrococcus species |
| Fusobacterium necrophorum | Pasteurella multocida | Providencia stuartii |
| Peptostreptococcus species | Proteus species | |
| Staphylococcus aureus | Staphylococcus species, coagulase negative | |
| Non-haemolytic Streptococci | α-haemoltyic Streptococci | |
| Streptococcus acidominimus | ||
| Aspergillus species |
Blood sample collection
Blood samples were collected from the coccygeal vein or artery into evacuated heparinised tubes (BD Vacutainer Systems, Plymouth, UK) and transported on ice to the laboratory. Plasma was separated by centrifugation at 3500g for 10 minutes and stored frozen at - 20°C until required. Serum was separated by centrifugation at 3500g for 10 minutes following 1 h incubation at room temperature and samples were stored frozen at - 20°C until required
Acute phase protein assays
The plasma acute phase proteins α1-acid glycoprotein and haptoglobin were measured by a previously described method using 96-well plates (Life Technologies, Invitrogen, UK) (22,38). Serum amyloid A concentrations were measured in serum using the Tridelta Phase™ range SAA solid phase ELISA kit according to the manufacturers guidelines (Tridelta, Co. Kildare, Ireland). Briefly, 50 μl biotinylated anti-SAA was added to each well followed by 50 μl serum samples in duplicate, which were diluted 1:1000 in diluent buffer supplied. The assay kit standard was reconstituted in dH20 and diluted in diluent buffer as instructed to give concentrations of 300, 150, 75, 37.5, 18.8 and 0 ng/ml, and 50 μl of each standard was added to the plate in duplicate. The plate was then covered and incubated for 1 h at 37°C. Following incubation, the plate was washed 4 times using the wash buffer supplied and 100 μl streptavidin-peroxidase was added to each well. The plate was then incubated in the dark for 30 min at room temperature. A further wash was then followed by the addition of 100 μl TMB substrate to each well and the plate was covered and incubated for 30 min in the dark. Following the final incubation, 50 μl stop solution was added to each well and absorbance read at 450 nm with 630 nm as a reference.
The intra- and interassay coefficients of variation for low, medium and high concentrations within the effective range encountered in the study were all < 12 per cent and < 18 per cent, respectively.
Hormone analysis
Plasma concentrations of oestradiol were analysed using the Oestradiol MAIA radioimmunoassay kit (Biogenesis, Poole, UK) by the method previously described by Prendiville et al (1995) with some modifications. Briefly, a top standard of 50 pg/ml was made by diluting the kit standard of 5000 pg/ml 1 in 100 with assay buffer The top standard was then serially diluted in assay buffer to give the following concentrations: 25, 12.4, 6.25, 3.125, 1.56, 0.78 and 0.39 pg/ml. Aliquots of 200 μl plasma were extracted in duplicate and standards were extracted in triplicate in 16 mm × 100 mm borosilicate glass tubes using 2 ml of diethyl ether (Aristar, BDH). The tubes were then vortexed for 15 min, after which they were transferred to the -80° C freezer for 15 min. The solvent layer was decanted into 12 mm × 75 mm borosilicate glass tubes and evaporated overnight in a fume hood. The standards and samples were reconstituted in 300 μl assay buffer, and 50 μl of the first antibody (kit antibody diluted 1:10 with assay buffer) was added to all tubes except total counts. Tubes were then briefly vortexed and incubated at room temperature for 1 h. Following incubation, 50 μl (approximately 9000 cpm) [125I] estradiol (kit [125I] estradiol diluted 1 in 2 with assay buffer) was added to each tube, which were then briefly vortexed and incubated for 2 h at room temperature. The second antibody (250 μl kit antibody, covalently bound to magnetic particles) was added to all tubes except total counts. Tubes were then briefly vortexed and incubated at room temperature for 20 min. Bound and free fractions were separated using magnetic racks (Biostat). The supernatant was discarded and counts per minute were determined on a gamma counter The intra-assay coefficients of variation were 20.8 and 21.6 %, respectively. Plasma concentrations of progesterone were measured in duplicate using a commercial ELISA kit (Ridgeway Science, Gloucester) following the manufacturers guidelines. The intra- and inter-assay coefficients of variation were 2.7 and 12.2 %, respectively.
Peripheral FSH concentrations were estimated in duplicate using a previously validated radioimmunoassay (9). Internal recovery was 95 % and the intra- and inter-assay coefficients of variation were 13.95 % and 10.47 %, respectively. Plasma concentrations of PGFM were measured in duplicate by direct radioimmunoassay as previously described (20).
LPS measurement
Concentrations of bacterial lipopolysaccharide (LPS) were measured in samples using the Biowhittaker Kinetic-QCL Limulus Amebocyte Lysate (LAL) Kit following the manufacturers guidelines. Samples were thawed, diluted to 1:10 in endotoxin-free water and heated in a water bath at an optimum temperature for the samples of 75 °C, for the optimum time of 30 min as validated in our laboratory. Samples were then mixed with the LAL substrate reagent and assayed in duplicate in 96-well endotoxin-free microplates (Becton Dickinson, USA) alongside standard curve LPS concentrations of 0.005, 0.01, 0.05, 0.5 and 5 endotoxin units/ml (eu/ml). Internal recovery as determined using positively spiked samples was > 80 % and the intra- and inter assay coefficients of variation were 2.6 and 4.7 %, respectively.
TNFα and NO measurement
Plasma concentrations of bioactive TNFα were measured as previously described (32,44), with some modifications. Rat fibroblast L929 cells were cultured in DMEM (Sigma) supplemented with 12.5 % FBS, 50 IU/ml penicillin and 50 μg/ml streptomycin (Sigma). Cells were plated at a density of 2.5 × 104 cells/well in 96 well plates (Nunc) in 100 μl medium. The standards were made using recombinant human TNFα (Sigma) diluted in Dulbeccos PBS (DPBS; Sigma) to the following concentrations: 10,000, 5,000, 2,500, 1,250, 635, 312, 156, 78, 39, 19.5, 9.8, 4.9, 2.4, 1.2, 0.6 and 0 pg/ml. Cross-reactivity was confirmed using recombinant bovine TNFα (kindly provided by Prof C. Howard, Institute for Animal Health, Compton, UK). Standards and samples were added to the plate in duplicate at a volume of 120 μl. Two μl of 0.5 μg/ml Polymixin B (Sigma) was added to each well to block LPS action and 5 μl of 80 μg/ml Actinomycin D was added to render the cells susceptible to TNFα. The plate was then incubated overnight at 37°C in a 5 % CO2 atmosphere. Cytotoxicity was determined by the colorimetric MTT assay involving the addition of 0.1 μg/ml MTT dye (Sigma) to each well and incubating for 2 to 4 hours at 37 °C in a 5 % CO2 atmosphere. The dye is taken up by the mitochondria in viable cells. The cells were then lysed using 100 μl DMSO (Sigma) per well and colour development read at 560 nm on a Spectra Max 250 (Molecular Devices). Concentrations of TNFα were calculated from the standard curve using Softmax Pro version 1.2.0 software (Molecular Devices). The intra- and inter assay coefficients of variation were 10.1 and 26.1 %, respectively.
Nitric Oxide (NO) was measured using the Greiss Reagent System (Promega) and was carried out according to the manufacturer’s instructions. Standards were made up in stripped plasma to account for the yellow tint of the plasma samples. The intra-assay coefficient of variation was 3.8 %.
Statistical Analysis
The differences in uterine pathogen growth density or inflammatory mediator concentrations between the previously gravid and non-gravid horns were tested using Kaplan-Meier and paired t-tests, respectively. To examine the relationship between uterine pathogenic bacteria and ovarian growth and function, the association of bacteria with follicle diameter were tested using repeated measures ANOVA in a mixed model (41). The fixed variables were the bacterial pathogenicity categories, the time after parturition (7, 14, 21 and 28 days postpartum) and their interactions. The model showed that the significant variable was the recognised uterine pathogen growth density on day 7 post partum. Therefore, for the remainder of the analyses animals were grouped based on the uterine pathogen growth density score on day 7 post partum. Animals with a uterine pathogen bacterial growth density in the upper three quartiles were classed as the ‘high’ uterine pathogen growth density animals (n = 50, high UPGD) and animals with a bacterial growth density score in the lower quartile were ascribed to the ‘low’ uterine pathogen growth density animals (n = 20, low UPGD). Follicle and CL diameters and blood oestradiol, progesterone, FSH, LPS, TNFα, AGP, haptoglobin and SAA concentration were explored using repeated measures ANOVA mixed model in the statistics program SAS 9.1 (19). The fixed explanatory variables were uterine pathogen group, time after parturition and their interaction. The 17 animals that formed an ovarian cyst were removed from the database for analysis of follicle diameter and oestradiol concentrations; and for analysis of corpus luteum diameter and progesterone concentrations, only when the first dominant follicle ovulated were animals included in the dataset (n = 40). Data were analysed for normality and were Log10- transformed to yield variance homogeneity. A compound symmetry model best fitted the data as determined using Akaike’s information criterion. Post hoc comparisons were performed using Bonferroni’s adjustment.
Differences in the location and timing of ovarian events between the groups were compared using the Chi-square test.
Results are quoted as the arithmetic mean ± SEM and significance attributed when P < 0.05.
Results
Preliminary Study
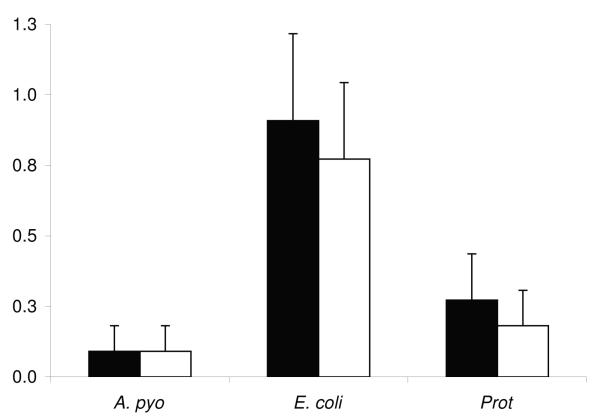
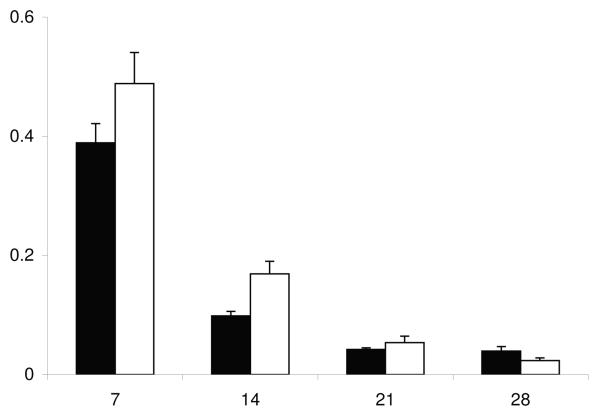
One or more species of uterine bacterial pathogens were isolated from each uterine flush from the group of 30 heifers. However, F. necrophorum and P. melaninogenicus were not isolated from any flush samples. The growth density for the uterine pathogens isolated from uterine fluid on day 14 did not differ between the ipsilateral and contralateral uterine horns (Figure 1).
Figure 1.
Mean + SEM bacterial growth densities for A. pyogenes (A. pyo), E. coli, and Proteus (Prot) in the ipsilateral (■) and contralateral (□) uterine horns of Limousin-Friesian cattle on day 14 post partum.
Furthermore, the uterine fluid concentrations of LPS were similar in the ipsilateral and contralateral uterine horns (98.0 ± 18.5 vs. 93.9 ± 13.2 μg/ml. The peripheral concentrations of LPS ranged from 0.3 – 6.2 μg/ml. The mean uterine fluid concentrations of TNFα were 0.15 ± 0.03 ng/ml and 0.14 ± 0.03 ng/ml in the ipsilateral and contralateral uterine horns, respectively and ranged from 0.1 – 2.0 ng/ml.
Main Study
General Bacteriology
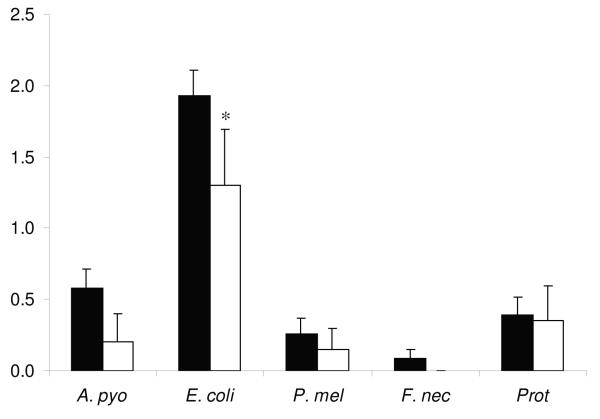
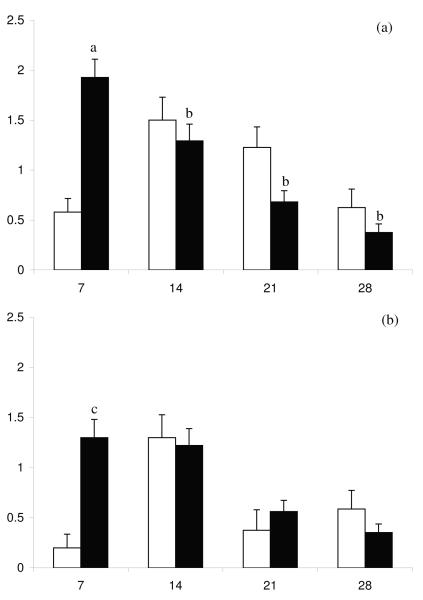
One or more species of uterine bacterial pathogens were isolated at least once from each cow during the postpartum period. The day 7 E. coli, uterine growth density was greater in the high day 7 UPGD cows than in low day 7 UPGD cows (Figure 2), but other pathogen bacterial growth densities did not differ significantly. The relationship between A. pyogenes and E. coli uterine bacterial growth density over the postpartum period is shown in Figure 3 for both high and low day 7 UPGD cows.
Figure 2.
Mean + SEM bacterial growth densities for A. pyogenes (A. pyo), E. coli, F. necrophorum (F. nec), P. melaninogenicus (P. mel) and Proteus (Prot) on day 7 postpartum in Holstein Friesian cows with a high (■) or low (□) day 7 UPGD. Values differ between UPGD groups * P < 0.05.
Figure 3.
Mean + SEM bacterial growth densities for A. pyogenes (□) and E. coli (■) on days 7, 14, 21 and 28 postpartum for cows with a (a) high or (b) low day 7 UPGD. Values differ over time within group abP < 0.001 and between groups acP < 0.05.
In the postpartum cows, peripheral plasma concentrations of LPS ranged from 0.05 – 0.99 μg/ml and were not different between high and low UPGD cows during the study period.
Ovarian folliculogenesis and oestradiol production
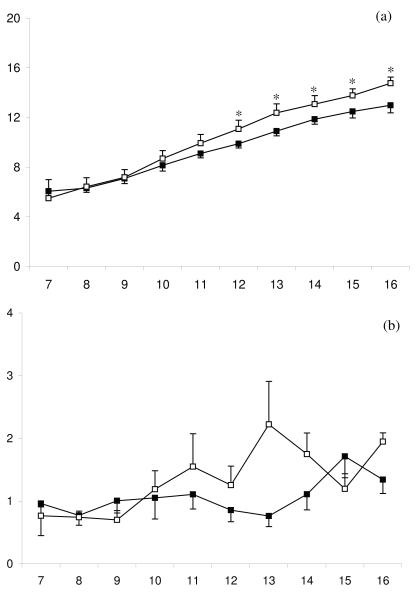
Between days 7 and 16 post partum the internal diameter of the first postpartum dominant follicle increased in all animals (P < 0.001) as did peripheral plasma oestradiol concentrations (P < 0.001). However, in high day 7 UPGD cows, the first postpartum dominant follicle was smaller over days 6 and 11 (Fig 4a, P < 0.05) and peripheral plasma oestradiol concentrations were lower (Fig4b, P < 0.05) than in low day 7 UPGD cows. Mean growth rate of the first post partum dominant follicle tended to be slower in the high than the low day 7 UPGD cows (0.79 ± 0.08 vs. 1.03 ± 0.09 mm/day, respectively, P = 0.05). Peripheral plasma FSH concentrations changed over time (Fig 4c, P < 0.01) but were not different between high and low day 7 UPGD cows.
Figure 4.
Mean ± SEM (a) diameter of the first dominant follicle, (b) peripheral plasma oestradiol concentration between days 7 and 16 postpartum for cows with high (■) or low (□) day 7 UPGD. Values differ between groups within day * P < 0.05.
Location and timing of ovarian events
The number of first wave follicles greater than 4 mm in diameter did not differ between high and low day 7 UPGD cows (Table 2). The interval between calving and the first dominant follicle achieving dominance was 11.8 ± 0.3 days but did not differ between high and low day 7 UPGD cows (Table 2). Fewer high day 7 UPGD cows produced the first dominant follicle in the ipsilateral ovary compared to low day 7 UPGD cows (Table 2). Fewer high UPGD cows ovulated the first postpartum dominant follicle as compared with the low UPGD cows (P < 0.05; Table 2).
Table 2.
The location and timing of postpartum ovarian events for animals with high or low day 7 UPGD. Asterisks denote differences between the day 7 UPGD groups
| Event | Low UPGD (n = 20) |
High UPGD (n = 70) |
|---|---|---|
| Number of first wave follicles ≥ 4mm in diameter | 2.1 ± 0.1 | 1.9 ± 0.1 |
| Calving to dominance interval: first dominant follicle (days) |
12.1 ± 0.4 | 11.1 ± 0.4 |
| Number of animals with first dominant follicles on the ipsilateral ovary |
6 (30 %) | 8 (16.3 %) |
| Number of animals with first dominant follicles on the contralateral ovary |
14 (70 %) | 42 (83.7 %)*** |
| Number of first dominant follicles ovulated | 13 (65 %) | 27 (54 %) * |
| Number of first dominant follicles regressed | 2 (10%) | 11 (22 %) * |
| Number of first dominant follicles persisted | 5 (25 %) | 12 (24 %) |
P < 0.05
P < 0.001.
More first postpartum dominant follicles regressed in high day 7 UPGD cows than in low day 7 UPGD cows (P < 0.05, Table 2). Furthermore, more dominant follicles tended to persist in the high day 7 UPGD cows (P = 0.09, Table 2). For all animals in which the first dominant follicle ovulated, the interval from calving to ovulation was 17.1 ± 0.5 days. Plasma progesterone concentrations increased to > 1 ng/ml by day 20.4 ± 0.6. The interval from parturition to dominance of the second dominant follicle was 21.0 ± 0.6 days.
Corpus luteum formation and progesterone production
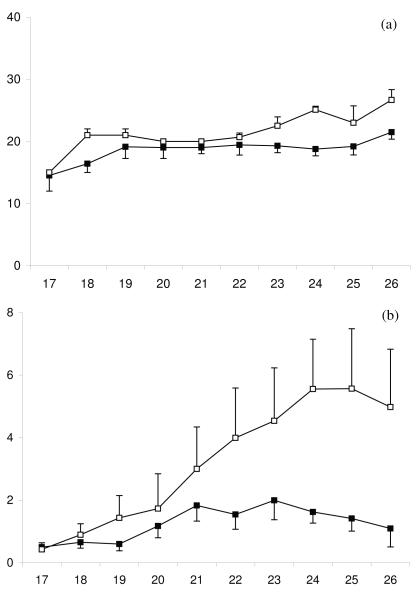
Between days 17 and 26 post partum the internal diameter of the first postpartum CL increased in all ovulating animals (P < 0.001, Fig 5a) as did peripheral plasma progesterone concentrations, (P < 0.001, Fig 5b). However, in animals with a high day 7 UPGD, the first postpartum CL was smaller (P < 0.05) than in low day 7 UPGD animals. The growth rate of the corpus luteum did not differ between high and low UPGD cows (0.8 ± 0.4 vs. 1.3 ± 0.7 mm/day, respectively). Peripheral plasma progesterone concentrations tended to be lower in high day 7 UPGD versus low day 7 UPGD animals over the study period (P = 0.09), and a significant interaction between group and time was observed (P < 0.05). Therefore, comparisons were made between high and low day 7 UPGD groups when a CL was present between 21 and 26 days postpartum. Peripheral progesterone concentrations were lower in animals with a high versus low day 7 UPGD score over this time (P < 0.05).
Figure 5.
Mean ± SEM (a) diameter of the corpus luteum and (b) peripheral plasma progesterone concentrations between days 17 and 26 post partum for cows with high (■) and low (□) day 7 UPGD.
PGFM concentrations
Plasma PGFM concentrations decreased in all animals between days 7 and 28 postpartum (P < 0.001) but concentrations did not differ between the day 7 UPGD groups (Fig 6).
Figure 6.
Mean + SEM peripheral plasma concentrations of PGFM on days 7, 14, 21 and 28 post partum for cows with high (■) or low (□) day 7 UPGD.
Plasma immune mediator concentrations
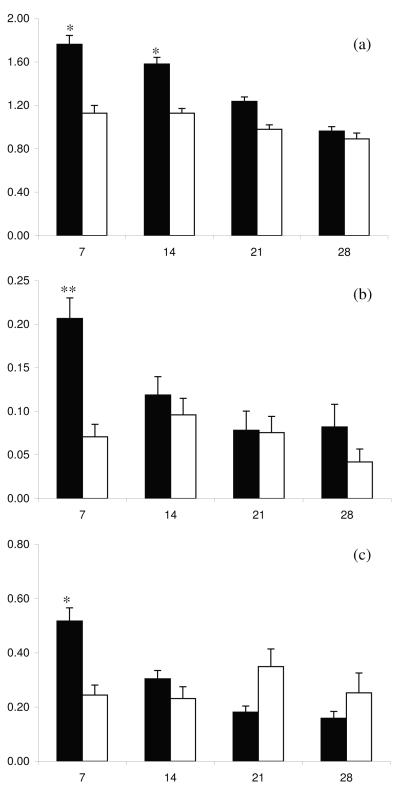
Plasma concentrations of AGP, SAA and Haptoglobin decreased between day 7 and day 28 postpartum (P < 0.01). High day 7 UPGD animals had higher peripheral concentrations of AGP (P < 0.05), SAA (P < 0.01) and Haptoglobin (P < 0.05) during the postpartum period compared to low day 7 UPGD cows, especially on days 7 and 14 postpartum (Figure 7). Peripheral concentrations of TNFα ranged from 0.001 – 47.5 ng/ml and were not different between high and low day 7 UPGD cows during the study period.
Figure 7.
Mean + SEM peripheral plasma concentrations of (a) AGP, (b) SAA and (c) Haptoglobin on days 7, 14, 21 and 28 postpartum for cows with high (■) or low (□) day 7 UPGD. Values differ between UPGD groups within day *P < 0.05, **P < 0.01.
Discussion
Uterine bacterial infections during the postpartum period are associated with lower conception rates, increased intervals from calving to first service or conception and more animals culled for failure to conceive (8,21). The present study provided the evidence that bacterial contamination of the uterine lumen on day 7 post partum with uterine pathogens perturbs ovarian folliculogenesis, resulting in slower growth of the dominant follicle and lower peripheral plasma oestradiol concentrations. Furthermore, after ovulation of the dominant follicle, the first postpartum corpus luteum is smaller and peripheral plasma progesterone concentrations are lower in animals with a high day 7 UPGD. Thus, uterine infection disrupts ovarian function, which is likely to contribute to infertility associated with uterine disease.
In the present study, there were more colonies of E. coli isolated from the high than isolated from the low UPGD cows but the numbers of A. pyogenes, P. melaninogenicus, F. necrophorum and Proteus were not different between the groups. Thus the effects of uterine pathogen infection may be particularly mediated by the actions of E. coli. In cases of clinical endometritis, E. coli are usually the most common bacteria present in lochial secretions (47). Although it is widely accepted that A. pyogenes is the most severe pathogen of the genital tract, it is rarely isolated alone in the postpartum period (5,21,22,37,47). In the first few days after calving, E. coli dominates the uterus and A. pyogenes is found later in animals with severe clinical endometritis (17,18). In the present study, E.coli numbers decreased with time over the postpartum period whilst A. pyogenes increased to a peak on day 14 postpartum before declining. In addition, E. coli numbers were significantly higher on day 7 in the high UPGD animals versus the low UPGD animals. Therefore, it appears that E. coli preceeds the rise in the number of A. pyogenes and increases the susceptibility of the endometrium to infection with A. pyogenes.
Contamination of the uterine lumen with uterine pathogens on day 7 post partum in the present study, suppressed growth and function of the first postpartum dominant follicle. The data is in agreement with previous studies where uterine bacterial infection or bacterial products perturbed ovarian follicular growth and function (3,31,41). However, in the present study we have identified that it is pathogenic bacteria that must likely mediate this effect. The mechanism could involve localised or systemic pathways but was not investigated in the present study. Bacterial products such as LPS infused into the uterus or peripheral circulation can disrupt LH secretion from the pituitary (3,31). In the present study LH concentrations were not measured, therefore, it cannot be determined whether the suppression of follicular growth and function and the fewer ovulations in the high uterine pathogen growth density cows is related to an inhibition in pulsatile LH secretion.
Plasma FSH concentrations did not differ between the day 7 pathogen growth density groups in the present study or between cattle with standard or high uterine bacterial score categories in a previous study (41). These results and those of the present study may suggest that bacterial infection is associated with a reduction in the capacity of the ovaries to respond to FSH, rather than with the secretion of FSH itself.
The present study shows that in animals with a high day 7 uterine pathogen growth density score, the first postpartum corpus luteum was smaller and secreted less progesterone than in low group cows. This phenomenon is similar to that reported in a previous study where Bovine Viral Diarrhoea Virus infection resulted in a suppression of progesterone concentrations (11). Bacterial products such as LPS may act on luteal cells, indicating a potential mechanism through which uterine pathogenic infection may compromise pregnancy in cows.
Uterine concentrations of E. coli LPS were approximately 64 times higher than peripheral concentrations in the present study. The mechanism by which LPS escapes from the uterus into the peripheral circulation is unknown, however it has been suggested that following leakage through the oviduct into the peritoneal cavity, LPS may then access the bloodstream via transmural movement in the peritoneal cavity (31). Alternatively, bacterial products such as LPS may signal indirectly from the uterus to the ovary via prostaglandins (14). In the present study we found no difference in LPS concentrations in peripheral plasma between high and low day 7 UPGD animals. Recognition of LPS in the uterus results in the production of pro-inflammatory cytokines including TNFα, which act on liver hepatocyte cells to induce the production of acute phase proteins. These proteins act at the site of tissue damage to limit further injury and promote repair (4). In the present study, TNFα concentrations were not different between the bacterial groups during the postpartum period. However, production of TNFα is up regulated rapidly and returns to basal concentrations within a few hours after pathogenic insult (15). Therefore, the sampling time in the present study may explain the absence of any differences between the groups. Additionally, concentrations of TNFα in local tissues rather than peripheral concentrations have been suggested to be a more effective measure of biological activity and the extent of a bacterial challenge (25). Day 7 uterine pathogen growth density was correlated to the production of the acute phase proteins AGP, SAA and haptoglobin. We previously reported that the severity of uterine bacterial contamination, as determined by total numbers of bacteria, was correlated with the peripheral circulating concentrations of AGP (38). Furthermore, AGP concentrations were particularly increased in those animals from which uterine pathogens were isolated, particularly E. coli (38,46). Circulating concentrations of the acute phase proteins are related to the severity of disease and the extent of the tissue damage in the affected area (4). Thus, due to the restrictions in measuring TNFα and other cytokines in the periphery, analysis of acute phase protein concentrations may be a better indicator of uterine pathogenic infection during the postpartum period (40).
In postpartum cattle, PGFM is an indicator of uterine PGF2α secretion (20). Cows with severe endometritis have been shown to have higher plasma concentrations of PGFM than cows with mild endometritis and measurement of plasma PGFM concentrations has been suggested as an indicator of postpartum uterine infection (24,26,36,43). In the present study, concentrations of PGFM decreased to basal concentrations within 21 days, which is in agreement with previous reports (23). However, there was no difference in PGFM concentrations over the postpartum period between the uterine pathogen growth density groups. These results conflict with earlier reports perhaps because, in the present study, animals have been grouped based on the number of recognised uterine pathogens isolated on day 7 postpartum whereas previous studies have focused on animals with clinical endometritis, which is usually not diagnosed before 21 days postpartum. In one study, uterine fluid profiles of PGFM were similar in animals with mild or heavy endometritis whereas plasma PGFM concentrations were significantly higher. This observation suggests that plasma concentrations, although a good measure of peripheral PGF2α concentrations, may not be a reliable measure of uterine PGF2α synthesis (43).
In conclusion, the present study provides evidence that uterine infection in dairy cows with the recognised uterine pathogens A. pyogenes, E. coli, F. necrophorum, P. melaninogenicus and Proteus, is related to a disruption in the normal ovarian events during the postpartum period. Animals with high numbers of these pathogens in utero on day 7 post partum have perturbed ovarian follicle growth and function, and, in animals that ovulated, the corpus luteum was smaller and produced less progesterone. Furthermore, uterine pathogen infection on day 7 post partum is correlated with an increase in the circulating concentrations of acute phase proteins during the postpartum period. The mechanisms by which bacteria, bacterial products or immune mediators affect ovarian function remain to be determined. However, the results of the present study show that uterine infection perturbs ovarian function during the postpartum period.
Acknowledgements
This study was supported by The Wellcome Trust, Project Grant No. 064155 and a Royal Veterinary College Internal Grant. The authors thank Professor Mac Johnston for access to the dairy herd and Charlotte Verity and Mark Whalley for assistance with animal handling. We thank Sylvia Reedy, Danielle Aw, Maggie Bushnell, Hilary Purcell and Jean Routley for technical assistance. Many thanks to Professor Joe Brownlie, Dr Shan Herath and Dr Brendan Jackson for advice.
References
- 1.AFRC . Energy and protein requirements of ruminants. CAB International; 1993. [Google Scholar]
- 2.Barrow GI, Feltham RKA. Cowan and Steel’s Manual for the Identification of Medical Bacteria. Cambridge University Press; Cambridge: 1993. [Google Scholar]
- 3.Battaglia D, Beaver AB, Harris TG, Tanhehco E, Viguie C, Karsch FJ. Endotoxin Disrupts the Estradiol-Induced Luteinizing Hormone Surge: Interface with Estradiol Signal Reading, Not Surge Release. Endocrinology. 1999;140:2471–2479. doi: 10.1210/endo.140.6.6739. [DOI] [PubMed] [Google Scholar]
- 4.Baumann H, Gauldie J. The acute phase response. Immunol Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 5.Bondurant RH. Inflammation in the Bovine Reproductive Tract. J Anim Sci. 1999;77 doi: 10.2527/1999.77suppl_2101x. [DOI] [PubMed] [Google Scholar]
- 6.Bonnet B, Martin SW, Meek AH. Associations of clinical findings, bacteriological and histological results of endometrial biopsy with reproductive performance of postpartum dairy cows. Preventative Veterinary Medicine. 1993;15:205–220. [Google Scholar]
- 7.Bonnett BN, Martin SW, Gannon VP, Miller RB, Etherington WG. Endometrial biopsy in Holstein-Friesian dairy cows. III. Bacteriological analysis and correlations with histological findings. Can J Vet Res. 1991;55:168–173. [PMC free article] [PubMed] [Google Scholar]
- 8.Borsberry S, Dobson H. Periparturient diseases and their effect on reproductive performance in five dairy herds. Vet Rec. 1989;124:217–219. doi: 10.1136/vr.124.9.217. [DOI] [PubMed] [Google Scholar]
- 9.Dobson H, Ribadu AY, Noble KM, Tebble JE, Ward WR. Ultrasonography and hormone profiles of adrenocorticotrophic hormone (ACTH)-induced persistent ovarian follicles (cysts) in cattle. J Reprod Fertil. 2000;120:405–410. [PubMed] [Google Scholar]
- 10.Farin PW, Ball L, Olson JD, Mortimer RG, Jones RL, Admey WS, McChesney AE. Effect of Actinomyces pyogenes and Gram-negative bacteria on the development of bovine pyometra. Theriogenology. 1989;31:979–989. doi: 10.1016/0093-691x(89)90481-0. [DOI] [PubMed] [Google Scholar]
- 11.Fray MD, Mann GE, Bleach EC, Knight PG, Clarke MC, Charleston B. Modulation of sex hormone secretion in cows by acute infection with bovine viral diarrhoea virus. Reproduction. 2002;123:281–289. [PubMed] [Google Scholar]
- 12.Gayle D, Ilyin SE, Plata-Salaman CR. Feeding status and bacterial LPS-induced cytokine and neuropeptide gene expression in hypothalamus. Am J Physiol. 1999;277:R1188–1195. doi: 10.1152/ajpregu.1999.277.4.R1188. [DOI] [PubMed] [Google Scholar]
- 13.Griffin JFT, Hartigan PJ, Nunn WR. Non-specific uterine infection and bovine fertility.I. Infection patterns and endometritis during the first seven weeks post-partum. Theriogenology. 1974;1:91–106. doi: 10.1016/0093-691x(74)90052-1. [DOI] [PubMed] [Google Scholar]
- 14.Herath S, Dobson H, Bryant CE, Sheldon IM. Use of the cow as a large animal model of uterine infection and immunity. Journal of Reproductive Immunology. 2006;69:13–22. doi: 10.1016/j.jri.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Horadagoda NU, Hodgson JC, Moon GM, Wijewardana TG, Eckersall PD. Development of a clinical syndrome resembling haemorrhagic septicaemia in the buffalo following intravenous inoculation of Pastuerella multocida serotype B:2 endotoxin and the role of tumor necrosis factor - alpha. Research in Veterinary Science. 2002;72:194–200. doi: 10.1053/rvsc.2001.0538. [DOI] [PubMed] [Google Scholar]
- 16.Horadagoda NU, Knox KM, Gibbs HA, Reid SW, Horadagoda A, Edwards SE, Eckersall PD. Acute phase proteins in cattle: discrimination between acute and chronic inflammation. Vet Rec. 1999;144:437–441. doi: 10.1136/vr.144.16.437. [DOI] [PubMed] [Google Scholar]
- 17.Hussain AMD, Daniel RCW, O’Boyle D. Postpartum uterine flora following normal and abnormal puerperium in cows. Theriogenology. 1990;34:291–302. doi: 10.1016/0093-691x(90)90522-u. [DOI] [PubMed] [Google Scholar]
- 18.Huszenicza G, Fodor M, Gacs M, Kulcsar M, Dohmen MJW, Vamos M. Uterine bacteriology, resumption of cyclic ovarian activity and fertility in postpartum cows kept in large-scale dairy herds. Reproduction in Domestic Animals. 1999;34:237–245. [Google Scholar]
- 19.SAS Institute Inc. SAS/STAT Software: Changes and enhancements through release. Vol. 6. SAS Institute Inc; Cary, NC: 1997. pp. 571–702. [Google Scholar]
- 20.Kindahl H, Edqvist LE, Granstrom E, Bane A. The release of prostaglandin F2alpha as reflected by 15-keto-13,14-dihydroprostaglandin F2alpha in the peripheral circulation during normal luteolysis in heifers. Prostaglandins. 1976;11:871–878. doi: 10.1016/0090-6980(76)90194-5. [DOI] [PubMed] [Google Scholar]
- 21.Leblanc SJ, Duffield TF, Leslie KE, Bateman KG, Keefe GP, Walton JS, Johnson WH. Defining and Diagnosing Postpartum Clinical Endometritis and its impact on Reproductive Performance in Dairy Cows. Journal of Dairy Science. 2002;85:2223–2236. doi: 10.3168/jds.S0022-0302(02)74302-6. [DOI] [PubMed] [Google Scholar]
- 22.Lewis GS. Uterine Health and Disorders. Journal of Dairy Science. 1997;80:984–994. doi: 10.3168/jds.S0022-0302(97)76024-7. [DOI] [PubMed] [Google Scholar]
- 23.Lewis GS, Thatcher WW, Bliss EL, Drost M, Collier RJ. Effects of heat stress during pregnancy on postpartum reproductive changes in Holstein cows. Journal of Animal Science. 1984;58:174–186. doi: 10.2527/jas1984.581174x. [DOI] [PubMed] [Google Scholar]
- 24.Mateus L, da Costa LL, Bernardo F, Silva JR. Influence of puerperal uterine infection on uterine involution and postpartum ovarian activity in dairy cows. Reprod Domest Anim. 2002;37:31–35. doi: 10.1046/j.1439-0531.2002.00317.x. [DOI] [PubMed] [Google Scholar]
- 25.Miller AJ, Luheshi GN, Rothwell NJ, Hopkins SJ. Local cytokine induction by LPS in the rat air pouch and its relationship to the febrile response. Am J Physiol. 1997;272:R857–861. doi: 10.1152/ajpregu.1997.272.3.R857. [DOI] [PubMed] [Google Scholar]
- 26.Nakao T, Gamal A, Osawa T, Nakada K, Moriyoshi M, Kawata K. Postpartum plasma PGF metabolite profile in cows with dystocia and/or retained placenta, and effect of fenprostalene on uterine involution and reproductive performance. J Vet Med Sci. 1997;59:791–794. doi: 10.1292/jvms.59.791. [DOI] [PubMed] [Google Scholar]
- 27.Noakes DE, Till D, Smith GR. Bovine uterine flora post partum: a comparison of swabbing and biopsy. Vet Rec. 1989;124:563–564. doi: 10.1136/vr.124.21.563. [DOI] [PubMed] [Google Scholar]
- 28.Noakes DE, Wallace L, Smith GR. Bacterial flora of the uterus of cows after calving on two hygienically contrasting farms. Vet Rec. 1991;128:440–442. doi: 10.1136/vr.128.19.440. [DOI] [PubMed] [Google Scholar]
- 29.Olson JD, Ball L, Mortimer RG, Farin PW, Adney WS, Huffman EM. Aspects of bacteriology and endocrinology of cows with pyometra and retained fetal membranes. Am J Vet Res. 1984;45:2251–2255. [PubMed] [Google Scholar]
- 30.Opsomer G, Grohn YT, Hertl J, Coryn M, Deluyker H, de Kruif A. Risk factors for post partum ovarian dysfunction in high producing dairy cows in Belgium: a field study. Theriogenology. 2000;53:841–857. doi: 10.1016/S0093-691X(00)00234-X. [DOI] [PubMed] [Google Scholar]
- 31.Peter AT, Bosu WT, DeDecker RJ. Suppression of preovulatory luteinizing hormone surges in heifers after intrauterine infusions of Escherichia coli endotoxin. Am J Vet Res. 1989;50:368–373. [PubMed] [Google Scholar]
- 32.Pfister H, Hennet T, Jungi TW. Lipopolysaccharide synergizes with tumour necrosis factor-alpha in cytotoxicity assays. Immunology. 1992;77:473–476. [PMC free article] [PubMed] [Google Scholar]
- 33.Prendiville DJ, Enright WJ, Crowe MA, Finnerty M, Hynes N, Roche JF. Immunization of heifers against gonadotropin-releasing hormone: antibody titers, ovarian function, body growth, and carcass characteristics. J Anim Sci. 1995;73:2382–2389. doi: 10.2527/1995.7382382x. [DOI] [PubMed] [Google Scholar]
- 34.Ruder CA, Sasser RG, Williams RJ, Ely JK, Bull RC, Butler JE. Uterine infections in the postpartum cow:II. Possible synergistic effect of Fusobacterium necrophorum and Corynebacterium pyogenes. Theriogenology. 1981;15:573–580. [Google Scholar]
- 35.Scott PR, Murray LD, Penny CD. A preliminary study of serum haptoglobin concentration as a prognostic indicator of ovine dystocia cases. Br Vet J. 1992;148:351–355. doi: 10.1016/0007-1935(92)90087-H. [DOI] [PubMed] [Google Scholar]
- 36.Seals RC, Wulster-Radcliffe MC, Lewis GS. Uterine response to infectious bacteria in oestrous cyclic ewes. Am J Reprod Immunol. 2003;49:269–278. doi: 10.1034/j.1600-0897.2003.00039.x. [DOI] [PubMed] [Google Scholar]
- 37.Sheldon IM. Postpartum Uterine Infections and Their Treatment. Cattle Practice. 1999;7:23–24. [Google Scholar]
- 38.Sheldon IM, Noakes DE, Rycroft AN, Dobson H. Acute phase protein responses to uterine bacterial contamination in cattle after calving. The Veterinary Record. 2001:172–175. doi: 10.1136/vr.148.6.172. [DOI] [PubMed] [Google Scholar]
- 39.Sheldon IM, Noakes DE, Ryecroft AN, Dobson H. Effect of postpartum manual examination of the vagina on uterine bacterial contamination in cows. The Veterinary Record. 2002;151:531–534. doi: 10.1136/vr.151.18.531. [DOI] [PubMed] [Google Scholar]
- 40.Sheldon IM, Noakes DE, Rycroft A, Dobson H. Acute phase protein responses to uterine bacterial contamination in cattle after calving. Vet Rec. 2001;148:172–175. doi: 10.1136/vr.148.6.172. [DOI] [PubMed] [Google Scholar]
- 41.Sheldon IM, Noakes DE, Rycroft AN, Pfeiffer DU, Dobson H. Influence of uterine bacterial contamination after parturition on ovarian dominant follicle selection and follicle growth and function in cattle. Reproduction. 2002a;123:837–845. [PubMed] [Google Scholar]
- 42.Skinner JG, Brown RA, Roberts L. Bovine haptoglobin response in clinically defined field conditions. Vet Rec. 1991;128:147–149. doi: 10.1136/vr.128.7.147. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki C, Yoshioka K, Iwamura S, Hirose H. Endotoxin induces delayed ovulation following endocrine aberration during the proestrous phase in Holstein heifers. Domest Anim Endocrinol. 2001;20:267–278. doi: 10.1016/s0739-7240(01)00098-4. [DOI] [PubMed] [Google Scholar]
- 44.Trost LC, Lemasters JJ. A cytotoxicity assay for tumor necrosis factor employing a multiwell fluorescence scanner. Anal Biochem. 1994;220:149–153. doi: 10.1006/abio.1994.1311. [DOI] [PubMed] [Google Scholar]
- 45.Whicher JTD, P. A. Acute phase proteins. Clinics in Immunology and Allergy. 1985;5:425–446. [Google Scholar]
- 46.Williams EJ, Fischer DP, Pfeiffer DU, England GCW, Noakes DE, Dobson H, Sheldon IM. Clinical evaluation of postpartum vaginal mucus reflects uterine bacterial infection and the immune response in cattle. Theriogenology. 2005;63:102–117. doi: 10.1016/j.theriogenology.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 47.Zerbe H, Ossadnik C, Leibold W, Schuberth HJ. Influence of Escherichia coli and Arcanobacterium pyogenes isolated from bovine puerperal uteri on phenotypic and functional properties of neutrophils. Vet Microbiol. 2001;79:351–365. doi: 10.1016/s0378-1135(00)00368-0. [DOI] [PubMed] [Google Scholar]