Abstract
In eukaryotes, mRNA is actively transported from nucleus to cytoplasm by a family of nuclear RNA export factors (NXF). While yeast harbors only one such factor (Mex67p), higher eukaryotes encode multiple NXFs. In mouse, four Nxf genes have been identified: Nxf1, Nxf2, Nxf3, and Nxf7. To date, the function of mouse Nxf genes has not been studied by targeted gene deletion in vivo. Here we report the generation of Nxf2 null mutant mice by homologous recombination in embryonic stem cells. Nxf2-deficient male mice exhibit fertility defects that differ between mouse strains. One third of Nxf2-deficient males on a mixed (C57BL/6 × 129) genetic background exhibit meiotic arrest and thus are sterile, whereas the remaining males are fertile. Disruption of Nxf2 in inbred (C57BL/6J) males impairs spermatogenesis, resulting in male subfertility, but causes no meiotic arrest. Testis weight and sperm output in C57BL/6J Nxf2-/Y mice are sharply reduced. Mutant epididymal sperm exhibit diminished motility. Importantly, proliferation of spermatogonia in Nxf2-/Y mice is significantly decreased. As a result, inactivation of Nxf2 causes depletion of germ cells in a substantial fraction of seminiferous tubules in aged mice. These studies demonstrate that Nxf2 plays a dual function in spermatogenesis: regulation of meiosis and maintenance of spermatogonial stem cells.
Keywords: NXF2, spermatogenesis, spermatogonia, stem cell, meiosis, mouse
Introduction
In eukaryotes, transport of mRNA from the nucleus to the cytoplasm is mediated by a family of nuclear RNA export factors (NXF) including NXF1. NXF1 (previously known as TAP) was identified as a factor that binds to the constitutive transport element of the retrovirus Mason-Pfizer monkey virus (MPMV) and that is required for nuclear export of incompletely spliced MPMV transcripts (Gruter et al., 1998). NXF1 can be UV cross-linked to poly(A)+ RNA and participates in nuclear export of bulk cellular mRNA (Kang and Cullen, 1999; Katahira et al., 1999). Inactivation of Mex67p, the only NXF sequence homologue in yeast, blocks export of poly(A)+ RNA and causes lethality (Segref et al., 1997). Interestingly, co-expression of human NXF1 and a cofactor termed p15 rescues the lethal phenotype of Mex67 yeast mutant, showing functional conservation of the nuclear RNA export pathway during eukaryote evolution (Segref et al., 1997).
Metazoans encode several Nxf members. In the mouse genome, four Nxf genes have been identified: Nxf1, Nxf2, Nxf3, and Nxf7 (Sasaki et al., 2005; Tan et al., 2005). These Nxf genes exhibit distinct tissue expression patterns: Nxf1 is widely expressed; Nxf2 is expressed in testis and brain; Nxf3 is only expressed in testis; and Nxf7 is only expressed in embryonic tissues. While Nxf1 is autosomal, Nxf2, Nxf3, and Nxf7 are all X-linked.
In addition to a centrally located RNA-binding domain, NXF1 contains an important C-terminal domain that mediates nuclear export through binding to components of the nuclear pore complex (Bachi et al., 2000; Kang and Cullen, 1999; Katahira et al., 1999). Functionally, NXF2 exhibits the same domain structure as NXF1 and possesses nuclear RNA export activities (Herold et al., 2000; Sasaki et al., 2005; Tretyakova et al., 2005). Notably, NXF3 lacks the C-terminal nuclear pore complex-binding domain that is found in both NXF1 and NXF2, but has evolved a Crm1-binding domain (Yang et al., 2001). NXF7 apparently lacks the nuclear RNA export activity (Sasaki et al., 2005; Tretyakova et al., 2005). These studies suggest that while NXF1, as a housekeeping gene, is responsible for nuclear export of bulk poly(A)+ RNA, the non-ubiquitously expressed NXF factors (NXF2, NXF3, and NXF7) might be involved in nuclear export of a subset of RNA or in translational control.
Identification of NXF2-interacting proteins suggests that NXF2 plays an additional role in the regulation of mRNA stability or trafficking. NXF2 is associated with FMR1 (Fragile X mental retardation syndrome 1), a translational regulator (Lai et al., 2006). Intriguingly, NXF2 and FMR1 appear to destabilize Nxf1 mRNA in cultured neuronal cells since both are present in Nxf1 mRNA-containing ribonucleoprotein particles (Zhang et al., 2007). NXF2 interacts with KIF17, a cytoplasmic motor protein (Takano et al., 2007). NXF2 (and NXF1) also interacts with the microtubule-associated proteins such as MAP1B (Tretyakova et al., 2005). Neuronal mRNA granules move along dendrites in a microtubule-dependent manner. Thus, the presence of NXF2 together with KIF17 and MAP1B in neuronal granules indicates a possible role in the cytoplasmic transport and localization of mRNAs.
Although biochemical and cell biological studies have provided tremendous insight into the function of mammalian NXFs, to date, none of the Nxf genes have been disrupted in mice. We previously identified Nxf2 as a germ cell-specific gene from mouse spermatogonia in a cDNA subtraction screen (Wang et al., 2001). Here we report that disruption of Nxf2 impairs spermatogenesis and provide evidence that Nxf2 plays a role in male meiosis and maintenance of spermatogonial stem cells.
Materials and methods
Antibody production and Western blot analysis
A GST-NXF1 (aa 200-300) fusion protein was expressed in E. coli using the pGEX4T-1 vector. Purified recombinant protein was used to immunize rabbits, resulting in antiserum UP2121. The NXF2 antibody was generated previously (Wang and Pan, 2007). Affinity purified anti-NXF1 (UP2121) and anti-NXF2 (UP1989) antibodies were used for western blotting analysis (1:50). Anti-β-actin was used as a control (1:5,000; Sigma-Aldrich).
Targeted inactivation of the Nxf2 gene
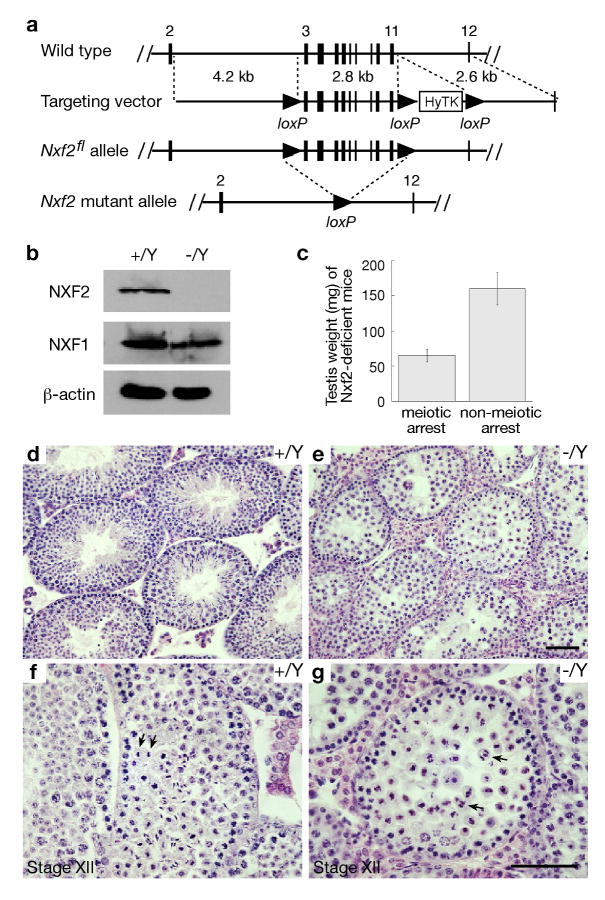
To generate the Nxf2 targeting construct, three DNA fragments (4.2 kb, 2.8 kb, and 2.6 kb) were amplified by high-fidelity PCR using an Nxf2-containing BAC clone (RPCI23-65A22) as template (Fig. 1a). The CMV-HyTK double selection cassette was flanked by loxP sites and enabled hygromycin-positive selection and thymidine kinase-negative selection. Hybrid V6.5 ES cells (C57BL/6 × 129/sv) were electroporated with linearized Nxf2 targeting construct and selected for integration in the presence of hygromycin B (120 μg/ml; Invitrogen). By screening 384 hygromycin-resistant ES cell clones, we identified two Nxf23lox clones that resulted from homologous recombination. These two Nxf23lox ES cell lines were then electroporated with the pOG231 plasmid that transiently expresses Cre recombinase. Two days after electroporation, cells were passaged and then subjected to selection with gancyclovir (2 μM; Sigma) for removal of the HyTK cassette. Ninety-six colonies were picked for each ES line and screened by PCR. Recombination between the immediate HyTK-flanking loxP sites resulted in the Nxf2fl allele (Fig. 1a).
Fig. 1.
Inactivation of Nxf2 causes male meiotic arrest in mice of mixed (C57BL/6 × 129) backgrounds. (a) The Nxf2 targeting construct and various Nxf2 alleles. The mouse Nxf2 gene consists of 23 exons and spans a 22-kb genomic region on the X chromosome. In the Nxf2flox allele, one loxP site is inserted in intron 2 and one in intron 11. Exons 3-11 encode amino acids 44-345. (b) Western blot analysis of adult wild type and Nxf2-/Y testes. Equal amounts (30 μg) of testis protein extracts were loaded. Three blots were probed with anti-NXF2, anti-NXF1, and anti-β-actin antibodies respectively. The abundance of NXF1 did not differ between Nxf2-/Y and wild type testes. (c) Testis weight of adult Nxf2-/Y mice. Adult Nxf2-/Y males have either small or large testes. The average testis weight ± standard deviation is shown for each group (22 mice each). (d, f) Histological analysis of testes from adult wild type mice. (e, g) Histological analysis of small testes from 4-month-old Nxf2-/Y mice reveals meiotic arrest. Arrows indicate normal chromosome segregation in wild type anaphase I spermatocytes (f) and chaotic chromosome segregation in Nxf2-/Y anaphase I spermatocytes (g). Scale bars, 50 μm.
Generation and backcross of Nxf2 mutant mice
Two ES clones (A8 and B2) harboring the Nxf2fl allele were injected into B6C3F1 (Taconic) blastocysts that were subsequently transferred to uteri of pseudopregnant ICR females. The Nxf2fl allele was transmitted through the germline in chimera mice derived from both clones. To delete the Nxf2 floxed region, Nxf2fl mice were crossed with TNAP-Cre mice (Lomeli et al., 2000). The TNAP-Cre allele was subsequently excluded from Nxf2 mutant mice by breeding. TNAP-Cre mice were of a mixed (C57BL/6 × 129) genetic background (Kehler et al., 2004). Nxf2+/- mutant mice were backcrossed to the C57BL/6J strain (The Jackson Laboratory) for more than ten generations. Experiments were performed on mice of both mixed (C57BL/6 × 129) and inbred (C57BL/6J) strain background. All offspring were genotyped by PCR. Wild type (243 bp) and floxed (433 bp) alleles were assayed by PCR with the primers CTATCAGTGGTTAATGGTGCC and TGATGGCTGCACACTAGTGCT. The Nxf2 knockout (465 bp) allele was assayed by PCR with the primers TGTTCAGCTCAGTGTGTATTG and CTATCAGTGGTTAATGGTGCC.
Histological, TUNEL, immunofluorescent, and surface spread analyses
For histological analysis, testes were fixed in Bouin's solution, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. For TUNEL and immunofluorescent analyses, testes were fixed in 4% paraformaldehyde, dehydrated in 30% sucrose, frozen, and sectioned. TUNEL assays were performed using the ApopTag Fluorescein In Situ Apoptosis Detection Kit (Chemicon). Immunostaining of testis sections was performed with anti-FMR1 antibody (Cat. No. ab17722, Abcam). Metaphase spread cells were stained with 4% Gurr Giemsa (Invitrogen). Antibodies for immunostaining of surface spread nuclei were described previously (Yang et al., 2008).
Mating test
Each male starting two months of age was housed with two healthy wild type C57BL/6J females. Females were replaced every two months. Cages were observed daily and the litter size was recorded. Six males for each genotype (wild type and Nxf2-/Y) were tested separately for up to 7 months.
Sperm count and sperm motility analysis
For sperm count, cauda epididymides were dissected in phosphate buffered saline solution. Sperm were squeezed out with fine forceps. Epididymides were minced, pipetted repeatedly, and incubated at room temperature for 10 minutes to allow sperm to disperse. Samples were fixed in 4% paraformaldehyde. Sperm were counted using a hematocytometer.
For motility analysis, sperm were collected from 2-month-old wild type and Nxf2-/Y mice by placing minced cauda epididymides in Krebs-Ringer bicarbonate medium without Ca2+, BSA, and NaHCO3 as previously described (Lee and Storey, 1986). The working “complete” medium was prepared by adding CaCl2 (1.7 mM), pyruvate (1 mM), NaHCO3 (25 mM), and BSA (3 mg/ml), followed by gassing with 5% CO2, 95% O2 to pH 7.3. Aliquots of each sperm suspension were loaded into a 100 μm-deep chamber, prewarmed at 37°C (Conception Technologies). Sperm motility and concentration were quantified using a computer-assisted semen analysis system (CASA) running IVOS (version 12.2L, Hamilton Thorne Research). At least 1000 sperm per sample were analyzed. For statistical analysis, frequencies of eight motion parameters: motility (%), VAP, VSL, VCL, ALH, BCF, STR, and LIN were determined. For statistical testing, sperm motility measurements of each parameter were pooled for each genotype and for time of observation. Considering the log-normal distribution, Student's t-test for independent observations was applied to define differences between wild type and mutant in VAP, VSL, VCL, and BCF means (normalized by natural logarithms). For the same purpose, the nonparametric ALH and STR distributions were tested by Friedman's analysis of variance. Statistical analyses were performed using the InStat program (GraphPad software).
BrdU incorporation assay and spermatogonium count
Adult mice (2-3 months-old) were injected intraperitoneally with 5-bromo-2′-deoxyuridine (BrdU) (50 mg/kg, Sigma) two hours prior to euthanasia. Testes were decapsulated. Seminiferous tubules were incubated with 1 mg/ml collagenase II (Calbiochem) at 37°C for 30 minutes. The pellet was collected and digested in 0.5% trypsin-EDTA (Invitrogen) with 20 μg/ml of DNase I (Roche) at 37°C for 15 minutes. DMEM medium with 10% FBS was added to terminate the digestion. Cells were pelleted by centrifugation, resuspended in DMEM, and fixed with 10% formalin at 4°C for 30 minutes. After washing with PBS, cells were added to slides. Cells (slides) were treated in 1 M HCl at 37°C for 1 hour. Cells were double stained with rat anti-BrdU antibody (Abcam, Cat. Ab-6326) and anti-TEX17 antibody. Texas red or FITC-conjugated secondary antibodies and antifade mounting medium with DAPI (Vector laboratories) were used.
To determine the number of spermatogonia, postnatal day 4 testes were fixed in fresh 4% paraformaldehyde at 4°C overnight, dehydrated in 30% sucrose, and embedded with TBS tissue freezing medium. Frozen sections were cut using a cryo-microtome. Every fifth section was stained with DAPI for counting of spermatogonia. All cross-sections of seminiferous tubules were examined and the number of spermatogonia within each round tubule was recorded. Spermatogonia in longitudinal tubules were not counted. Four mice of each genotype were analyzed. For each mouse, 200 round tubules were examined.
Microarray analysis
Total RNA was prepared from postnatal day 21-old testes by using TRIzol reagent (Invitrogen) and subsequently purified using an RNeasy kit (Qiagen). Samples were analyzed in triplicates (3 Nxf2-/Y and 3 wild type littermates). Five micrograms of total RNA from each sample were used for the generation of biotinylated cRNA. The cRNA samples were hybridized to Mouse Genome 430 2.0 GeneChips (Affymetrix) at the University of Pennsylvania Microarray Core Facility according to the manufacturer's expression analysis technical manual (Affymetrix). We imported microarray data files (.cel) into Partek Genomics Suite software v6.0. GCRMA was applied to calculate log2 transformed probe set signal values. We filtered those values to retain probe sets with values ≥ 5 in at least 2 out of the 6 samples. The filtered list (23,250 genes) was subjected to a two-class unpaired analysis using SAM (Statistical analysis of microarrays), where we calculated q values reflecting FDR (False Discovery Rate) and d scores for every probe set on the list. We identified 342 genes with > 2-fold difference at a 1% FDR (Supplementary Table S1). The microarray data have been deposited in the GEO database under the accession number GSE13526.
Results
Disruption of the Nxf2 gene
Mouse Nxf2 is an X-linked gene expressed specifically in germ cells in the testis (Sasaki et al., 2005; Tan et al., 2005; Wang et al., 2001; Wang and Pan, 2007). The NXF2 protein is nuclear in spermatogonia but localizes to the nuclear rim in early spermatocytes, suggesting that it might function both in the development of spermatogonia and in meiosis (Wang and Pan, 2007). To elucidate the role of Nxf2 in spermatogenesis, we generated a floxed Nxf2 conditional allele (Nxf2fl) in mice using the Cre-loxP strategy (Fig. 1a). As expected, both Nxf2fl/Y males and Nxf2fl/fl females were fertile. Nxf2 floxed mice were crossed with TNAP-Cre mice that express Cre recombinase exclusively in primordial germ cells, resulting in Nxf2-/Y male mice that lack exons 3-11 in the germline (Lomeli et al., 2000). Western blot analysis showed that the NXF2 protein was absent in Nxf2-/Y testis. In contrast, the abundance of NXF1 was not affected in Nxf2-/Y testis (Fig. 1b). In addition, real-time PCR analysis revealed that the expression level of Nxf3 and Nxf7 in testes was comparable between wild type and Nxf2-/Y 2-month-old inbred C57BL/6J mice (data not shown), indicating that disruption of Nxf2 did not affect gene expression levels of other Nxf genes.
Meiotic arrest in mixed C57BL/6 × 129 Nxf2-/Y males
The Nxf2 mutant mice were generated in a C57BL/6 and 129 mixed mouse strain. Both Nxf2-/Y males and Nxf2-/- females were viable and appeared to be grossly normal. While Nxf2-/-females were fertile, Nxf2-/Y males had incomplete penetrance of sterility (Fig. 1c). Of adult Nxf2-/Y males, 29% (35 out of 121) had sharply reduced testis weight (∼ 65 mg). The remaining Nxf2-/Y males had testes of normal weight (∼ 160 mg) with apparently normal spermatogenesis and were fertile.
Histological analysis of small Nxf2-deficient testes revealed meiotic arrest and abnormal chromosome segregation. While wild type testis contained a full spectrum of spermatogenic cells (Fig. 1d), Nxf2-deficient tubules lacked post-meiotic germ cells, showing a block in late meiosis (Fig. 1e). In wild type anaphase spermatocytes, two sets of chromosomes migrated synchronously toward opposite poles (Fig. 1f). However, in Nxf2-deficient anaphase cells, chromosome segregation was chaotic (Fig. 1g).
Chromosome mis-segregation in Nxf2-deficient spermatocytes
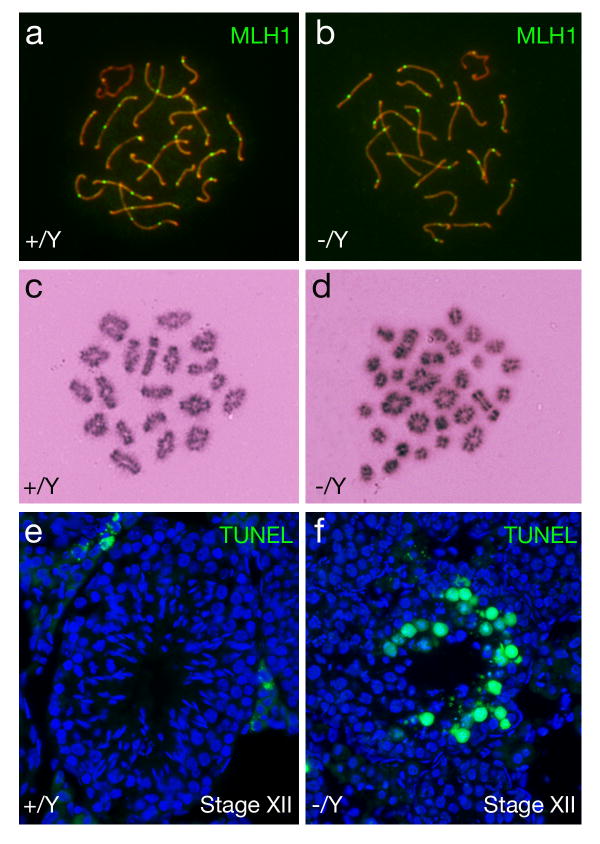
We focused our analysis on the small testes from Nxf2-/Y mice, which always exhibited meiotic arrest. Immunostaining of surface spread nuclei of spermatocytes with anti-SYCP1 and anti-SYCP2 antibodies revealed that chromosomal synapsis appeared to be normal in Nxf2-deficient spermatocytes as judged by the formation of the synaptonemal complex (data not shown). We then measured the number of meiotic crossovers in pachytene spermatocytes. MLH1 marks the site of crossovers at the mid-to-late pachytene stage (Baker et al., 1996; Edelmann et al., 1996). We found that the number of MLH1 foci (21.0 ± 3.7, n = 42 nuclei) in Nxf2-deficient spermatocytes was close to that (21.3 ± 2.4, n = 34 nuclei) in the wild type and that the distribution of MLH1 foci was normal (Fig. 2a, b). Interestingly, analysis of metaphase I spermatocytes showed that the number of bivalent chromosomes (15.2 ± 2.9 bivalents/cell) in Nxf2-/Y mice was greatly reduced in comparison with wild type (20 bivalents/cell) (Fig. 2c, d). The presence of univalent chromosomes leads to chromosome non-disjunction at the subsequent anaphase I stage and thus triggers the spindle assembly checkpoint (Eaker et al., 2002; Li and Nicklas, 1995). We found abnormal chromosome segregation in spermatocytes (Fig. 1g) and massive apoptosis of germ cells in Nxf2-deficient Stage XII tubules (containing metaphase I and anaphase I spermatocytes) (Fig. 2f). These studies show that Nxf2 is not essential for crossover formation but is required for proper chromosome segregation during male meiosis.
Fig. 2.
Analyses of crossovers, bivalent formation, and apoptosis in Nxf2-deficient spermatocytes from mice of mixed (C57BL/6 × 129) backgrounds. Adult Nxf2-deficient mice with small testes (∼60 mg) were analyzed. (a, b) The number of MLH1 foci is comparable between wild type and Nxf2-deficient pachytene spermatocytes. (c) 20 bivalents in wild type metaphase I spermatocytes. (d) Prevalence of univalent chromosomes in Nxf2-deficient metaphase I spermatocytes. Of 30 chromatin masses, 10 are bivalent and 20 univalent. (e) TUNEL analysis of wild type tubules. Apoptotic cells are absent in stage XII tubules. (f) Massive apoptosis of germ cells (presumably anaphase I spermatocytes) in Nxf2-/Y Stage XII tubules.
Reduced sperm production and impaired sperm motility in Nxf2-/Y mice
To dissect the possible effect of the mixed genetic background on the mutant phenotype, we backcrossed the Nxf2 mutant allele to the inbred C57BL/6J mouse strain for more than ten generations. All subsequent studies were performed with C57BL/6J mice. We found that Nxf2-/-females were fertile, but Nxf2-/Y males exhibited impaired fertility. Six males of each genotype (Nxf2-/Y and wild type littermate) were subject to mating test. All six wild type males continued to sire offspring at an expected frequency. In contrast, two Nxf2-/Y males never sired any offspring and the other four Nxf2-Y males sired one to three litters initially before becoming infertile. The litter size (average ± standard deviation) sired by Nxf2-/Y males (4.3 ± 2.0, n=6 litters) was sharply reduced in comparison to that of wild-type littermate controls (7.3 ± 2.3, n=40 litters) (P < 0.0055). While the body weight of 2-month-old Nxf2-/Y males was similar to that of wild type littermates, the testis weight of Nxf2-/Y males was reduced by 30% (Table 1). Cauda epididymides from Nxf2-/Y males contained half the number of sperm compared to controls (Table 1). Analysis of sperm motility by computer-assisted sperm analysis revealed that in Nxf2-/Y mice, the percentage of motile sperm was significantly decreased compared to wild type (Table 2). Thus, loss of Nxf2 function impairs sperm function in the C57BL/6J background.
Table 1.
Testis weight and sperm production in Nxf2+/Y and Nxf2-/Y micea
| Genotype | ||||
|---|---|---|---|---|
| +/Y (n= 7b) | -/Y (n= 7b) | Ratio of -/Y to +/Y | P value | |
| Body weight (g) | 24.0±2.7 | 25.1±1.0 | 1.04 | p < 0.35 |
| Testicular weight (mg) | 166±15 | 118±8 | 0.71 | p < 0.0001* |
| Sperm/cauda (106) | 6.95±2.11 | 3.65±0.43 | 0.52 | p < 0.0016* |
Mice from the C57BL/6J background were used at 2 months of age.
7 mice (n=7) for each genotype were used.
Values were statistically significant (Student's t-test).
Table 2.
Motility of sperm from Nxf2+/Y and Nxf2-/Y micea
| Genotype | +/Y | -/Y |
|---|---|---|
| Motility (%) | 56.3 ± 4.9 | 29.7 ± 1.5 * |
| VAP (μm/s) | 126.8 ± 15.2 | 137.8 ± 27.0 |
| VSL (μm/s) | 75.5 ± 16.3 | 74.0 ± 21.2 |
| VCL (μm/s) | 257.3 ± 42.9 | 263.6 ± 96.4 |
| ALH (μm) | 14.6 ± 2.1 | 10.5 ± 5.7 |
| BCF (Hz) | 16.2 ± 0.8 | 14.3 ± 3.5 |
| STR (%) | 44.0 ± 3.0 | 23.3 ± 5.1 * |
| LIN (%) | 56.7 ± 32.7 | 41.3 ± 13.9 |
Values represent means ± standard deviations. Three adult mice of each genotype on the C57BL6/J inbred background were analyzed. Asterisks indicate statistically significant differences (p<0.05). Abbreviations: VAP, average path velocity; VSL, straight-line velocity; VCL, curvilinear velocity; ALH, amplitude of lateral head displacement; BCF, beat-cross frequency; STR, straightness; LIN, linearity.
To address whether reduced sperm production in Nxf2-/Y males is caused by defects in meiosis, we analyzed spermatocytes from adult (2-month-old) mice by surface spread analysis. The relative percentage of each stage of spermatocytes was comparable between Nxf2-/Y and wild type mice (data not shown). Chromosomal analysis of Nxf2-deficient metaphase I spermatocytes (50 cells examined) by Giemsa staining revealed no univalent chromosomes. Collectively, these results showed that in the C57BL/6J mouse strain, Nxf2 is not essential for the progression of meiosis.
FMR1 interacts with NXF2 in testis and both proteins co-localize at the perinuclear region in early germ cells (Lai et al., 2006). We examined the subcellular localization of FMR1 in Nxf2-deficient testis (Supplementary Fig. S1). FMR1 was still localized to the perinuclear region in Nxf2-deficient germ cells, suggesting that FMR1 localization is independent of NXF2.
Reduced spermatogonial proliferation in Nxf2-/Y mice
The Nxf2 gene was initially cloned from mouse spermatogonia (Wang et al., 2001). The NXF2 protein is abundantly expressed in the nuclei of spermatogonia, suggesting that it might be involved in the proliferation and/or differentiation of spermatogonia (Wang and Pan, 2007). However, spermatogonia were present in the Nxf2-deficient testis from young mice, showing that Nxf2 is not essential for the survival of spermatogonia. We first examined the number of spermatogonia in postnatal day 4 testes, where spermatogonia have characteristically large nuclei with little heterochromatin. The number of spermatogonia was similar between wild type (88.9 ± 19.9 spermatogonia/100 tubule cross-sections) and Nxf2-/Y (91.6 ± 10.6) mice at postnatal day 4.
We next performed BrdU incorporation assays to measure the proliferation rate of spermatogonia in adult mice (2-3 months-old). Wild type and Nxf2-/Y males were injected intraperitoneally with BrdU two hours prior to euthanasia. Single cells were prepared from testes and were double immunostained with anti-TEX17 and anti-BrdU antibodies. TEX17 is specifically expressed in spermatogonia and thus is a marker of spermatogonia (Wang et al., 2005). We found that the percentage of BrdU-positive spermatogonia (24.9 ± 3.8%) in Nxf2-/Y testis was significantly lower than that (33.3 ± 2.0%) of wild type (p < 0.028), suggesting that Nxf2 promotes proliferation of spermatogonia in adult mice.
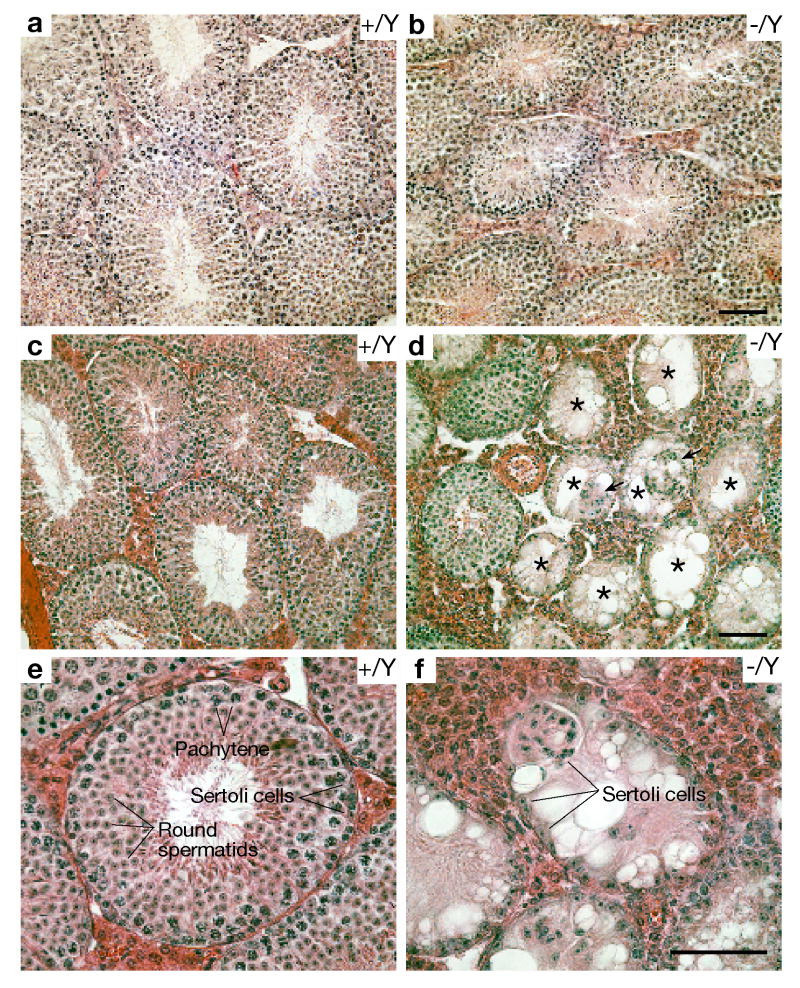
Histological analysis of testes from 2.5-month-old C57BL/6J Nxf2-/Y mice revealed abnormal spermatogenesis (Fig. 3). Consistent with reduced testis weight, the diameter of seminiferous tubules in the Nxf2-/Y mice (Fig. 3b) was smaller than that in the wild type (Fig. 3a). The Nxf2-/Y testis exhibited no spermatogenetic arrest, evident from the presence of spermatogonia, spermatocytes, and spermatids. However, the number of germ cells in Nxf2-/Y tubules was clearly lower than that in the wild type, suggesting that spermatogenesis is impaired in 2.5-month-old Nxf2-/Y mice (Fig. 3b).
Fig. 3.
Age-dependent loss of spermatogonia in C57BL/6J Nxf2-/Y mice. (a, b) Histological analysis of testes from 2.5-month-old mice. The diameter of seminiferous tubules is smaller in Nxf2-/Y mice (b) than in wild type (a). (c, d) Histological analysis of testes from 9-month-old mice at medium magnification (200×). Sertoli cell-only (SCO) tubules (indicated by asterisks) are prevalent in Nxf2-/Y mice but not in wild type. Groups of Sertoli cells indicated by arrows are sloughed off the epithelium. (e, f) Histological analysis of testes from 9-month-old mice at high magnification (400×). Note the absence of germ cells in the Nxf2-deficient SCO tubule. Scale bars: 50 μm.
Loss of spermatogonia in aged Nxf2-/Y mice
We next determined the long-term effect of reduced spermatogonial proliferation on spermatogenesis (Fig. 3c-f). In testes of older (9-month old) Nxf2-/Y mice, 26% of seminiferous tubules were “Sertoli cell only” (SCO) tubules that are devoid of germ cells (Fig. 3d, f). In contrast, SCO tubules were very rare in testes from older (9 month old) wild type and young (2.5-month old) Nxf2-/Y mice (∼1%). Age-dependent depletion of spermatogenesis in mutant mice suggests that Nxf2 promotes the self-renewal and/or survival of spermatogonial stem cells.
Transcript profiling of Nxf2-/Y testes
Inactivation of Mex67 in yeast blocks nuclear export of bulk poly(A)+ RNA (Segref et al., 1997). To determine whether disruption of Nxf2 leads to nuclear accumulation of poly(A)+ RNA in germ cells, we performed in situ hybridization on Nxf2-/Y and wild type testis sections with an oligo(dT)45-Cy3 probe (Herold et al., 2001). This analysis did not reveal increased abundance of poly(A)+ RNA in the nuclei of Nxf2-deficient germ cells (data not shown), indicating that nuclear export of bulk cellular mRNA can occur in the absence of Nxf2.
To systematically identify genes with altered transcript abundance in Nxf2-/Y testes, we performed microarray analysis of testes from post-natal day 21 mice using Affymetrix Mouse Genome 430 2.0 GeneChips. At post-natal day 21, the weight of Nxf2-/Y testes (29.3 ± 4.5 mg) was not significantly different from that of wild type (30.4 ± 5.5 mg). With an expression cutoff of two-fold change or greater, our microarray analysis identified 331 genes that were down regulated in Nxf2-/Y testes (Supplementary Table S1). We noted that most genes with altered abundance are specifically or preferentially expressed in spermatids such as Odf1, Txndc8, and Hils1. Real-time PCR analysis showed that these genes are only slightly down regulated in adult Nxf2-/Y testes (Supplementary Table S2), suggesting that the dramatically decreased abundance of these genes in post-natal day 21 Nxf2-/Y testes could be due to delayed appearance of spermatids in the mutant testes.
Discussion
NXF2 exhibits two distinct localization patterns in germ cells: nuclear localization in spermatogonia and nuclear rim (envelope) localization in early spermatocytes, suggesting that NXF2 might have a dual function in spermatogenesis: development of spermatogonia and regulation of meiosis (Wang and Pan, 2007). In support of this prediction, disruption of Nxf2 leads to age-dependent loss of spermatogonia and defects in male meiosis.
Since spermatogonial stem cells (SSCs) replenish spermatogenesis, males produce sperm through lifetime (Brinster, 2007; de Rooij, 1998). Spermatogonial stem cells undergo self-renewal, proliferation, and differentiation. Eventually, differentiating spermatogonia enter meiosis to produce sperm. Thus, the number of spermatogonial stem cells and the rate of spermatogonial proliferation affect the ultimate sperm output. Disruption of Nxf2 in C57BL/6J inbred mice resulted in a paucity of germ cells in the testis and reduced sperm output in the epididymis, but no meiotic block. BrdU incorporation experiments showed that the proliferation of spermatogonia in Nxf2-/Y testes was significantly reduced. These data suggest that the reduction in testis weight and sperm output in Nxf2-/Y mice might be due to a decreased population of spermatogonia – pre-meiotic germ cells. Disruption of genes (for example, Plzf and Bcl6b) involved in the self-renewal and survival of SSCs causes a progressive loss of SSCs with age (Buaas et al., 2004; Costoya et al., 2004; Oatley et al., 2006). We found that a substantial percentage of seminiferous tubules lacked germ cells in 9-month-old Nxf2-/Y mice but not in young adult mutant mice, suggesting that Nxf2 plays a role in the maintenance of spermatogonial stem cells.
The effect of Nxf2 ablation on meiosis depends on the mouse strain. In mice of mixed (C57BL/6 × 129) genetic background, nearly one-third of Nxf2-/Y males displayed meiotic arrest, whereas the remaining mutant males had normal spermatogenesis and were fertile. However, in C57BL/6J inbred Nxf2-/Y males, meiotic arrest was never observed and meiotic progression appeared to be normal. All C57BL/6J inbred Nxf2-/Y males had a reduction in testis weight, sperm count, and sperm motility. Variable phenotypic expression of mouse mutants has been observed for many genes such as Scmh1 and Dazl (Lin and Page, 2005; Saunders et al., 2003; Takada et al., 2007). The incomplete penetrance of meiotic defects in Nxf2-/Y mice might be attributed to compensatory effects by other Nxf genes such as the ubiquitously expressed Nxf1 gene.
Nxf2 could regulate spermatogenesis through two possible mechanisms: a) nuclear retention/export of specific transcripts and b) RNA trafficking and translational control after nuclear export. Most cellular mRNAs exit the nucleus through the NXF1/Mex67-mediated pathway. However, the identity of mRNA substrates for other NXF homologues in metazoans remains unknown except for CeNXF-2. In C. elegans, CeNXF-2 is required for nuclear retention of tra-2 mRNA in the absence of TRA-1 (Kuersten et al., 2004). TRA-1 and TRA-2 are involved in determination of female cell fate in C. elegans. The nuclear export of tra-2 mRNA is mediated through the TRE element in its 3′UTR in a leptomycin B (inhibitor of Crm1)-sensitive manner. CeNXF-2 and REF-1 specifically bind to the TRE to cause the nuclear retention of tra-2 mRNA by blocking NXF1-mediated nuclear export. Binding of TRA-1 to the TRE could displace CeNXF-2, resulting in the nuclear export of tra-2 mRNA via an unknown Crm1-dependent factor. These studies demonstrate that CeNXF-2 together with other proteins influences the choice of nuclear export pathways for tra-2 mRNA (Kuersten et al., 2004). It is not clear whether CeNXF-2 is the orthologue of mouse NXF2, since CeNXF-2 lacks the nuclear pore complex-binding domain that is present in mouse NXF2 (as well as in NXF1). We predict that given its nuclear localization in spermatogonia, mouse NXF2 could be involved in nuclear retention of certain gene transcripts in spermatogonia.
NXF2 could also be involved in mRNA trafficking and translational control in the cytoplasm through its interaction with various cytoplasmic proteins including the kinesin KIF17, the microtubule-associated protein MAP1B, and the RNA-binding translational regulator FMR1 (Lai et al., 2006; Takano et al., 2007; Tretyakova et al., 2005; Zhang et al., 2007). KIF17 has also been shown to be associated with ACT (activator of CREM in the testis) and TB-RBP (testis brain RNA-binding protein) (Chennathukuzhi et al., 2003; Macho et al., 2002). Macroorchidism (enlarged testis) is a prominent symptom of Fragile X syndrome in humans and the Fmr1 knockout mice (Bakker et al., 1994). Although FMR1 is expressed in spermatogonia but not in Sertoli cells in the testis, macroorchidism in the Fmr1 knockout is caused by increased proliferation of Sertoli cells (Slegtenhorst-Eegdeman et al., 1998). NXF2 and its interacting proteins (KIF17, MAP1B, and FMR1) are associated with RNA-containing granules in transfected neurons, indicating a role in translational control.
Transcript profiling of wild type and Nxf2-deficient testes failed to identify specific mRNA substrates for NXF2. Disruption of Nxf2 might affect the translation of NXF2-regulated mRNA but not the abundance. In this case, the mRNA substrates could be identified by crosslinking and immunoprecipitation with NXF2 antibody followed by microarray profiling or direct sequencing of associated RNAs.
Our genetic study of Nxf2 in mice has important implications for male infertility in humans. Infertility affects 15% of couples worldwide (Matzuk and Lamb, 2002). The human NXF2 is also a testis-specific gene located on the X chromosome. A recent study of 65 infertile men with Sertoli cell-only syndrome did not identify mutations in NXF2 (Stouffs et al., 2008). Disruption of Nxf2 causes sharply reduced sperm output in mice. Therefore, mutations in the human NXF2 gene could be found in infertile men with oligozoospermia (reduced sperm count) rather than azoospermia (no sperm in semen).
Supplementary Material
Acknowledgments
We thank J. Saionz for technical support, C. Heyting for anti-SYCP1 antibody, D. Baldwin for microarray experiments, and J. Tobias for microarray data analysis. We thank F. Yang and K. Zheng for critical reading of the manuscript. We are grateful to the two anonymous reviewers for valuable comments. This work is supported by a seed grant from Penn Genome Frontiers Institute (PGFI) and an NIH/NIGMS grant RO1GM076327 (PJW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bachi A, Braun IC, Rodrigues JP, Pante N, Ribbeck K, von Kobbe C, Kutay U, Wilm M, Gorlich D, Carmo-Fonseca M, Izaurralde E. The C-Terminal Domain of TAP Interacts with the Nuclear Pore Complex and Promotes Export of Specific CTE-Bearing RNA Substrates. RNA. 2000;6:136–158. doi: 10.1017/s1355838200991994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SM, Plug AW, Prolla TA, Bronner CE, Harris AC, Yao X, Christie DM, Monell C, Arnheim N, Bradley A, Ashley T, Liskay RM. Involvement of Mouse Mlh1 in DNA Mismatch Repair and Meiotic Crossing Over. Nat Genet. 1996;13:336–342. doi: 10.1038/ng0796-336. [DOI] [PubMed] [Google Scholar]
- Bakker CE, Verheij CE, Willemsen R, van der Helm R, Oerlemans F, Vermeij M, Bygrave A, Hoogeveen AT, Oostra BA, Reyniers E, De Boulle K, D'Hooge R, Cras P, van Velzen D, Nagels G, Martin J, De Deyn P, Darby JK, Willems PJ. Fmr1 Knockout Mice: A Model to Study Fragile X Mental Retardation the Dutch-Belgian Fragile X Consortium. Cell. 1994;78:23–33. [PubMed] [Google Scholar]
- Brinster RL. Male Germline Stem Cells: From Mice to Men. Science. 2007;316:404–405. doi: 10.1126/science.1137741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buaas FW, Kirsh AL, Sharma M, McLean DJ, Morris JL, Griswold MD, de Rooij DG, Braun RE. Plzf is Required in Adult Male Germ Cells for Stem Cell Self-Renewal. Nat Genet. 2004;36:647–652. doi: 10.1038/ng1366. [DOI] [PubMed] [Google Scholar]
- Chennathukuzhi V, Morales CR, El-Alfy M, Hecht NB. The Kinesin KIF17b and RNA-Binding Protein TB-RBP Transport Specific cAMP-Responsive Element Modulator-Regulated mRNAs in Male Germ Cells. Proc Natl Acad Sci U S A. 2003;100:15566–15571. doi: 10.1073/pnas.2536695100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costoya JA, Hobbs RM, Barna M, Cattoretti G, Manova K, Sukhwani M, Orwig KE, Wolgemuth DJ, Pandolfi PP. Essential Role of Plzf in Maintenance of Spermatogonial Stem Cells. Nat Genet. 2004;36:653–659. doi: 10.1038/ng1367. [DOI] [PubMed] [Google Scholar]
- de Rooij DG. Stem Cells in the Testis. Int J Exp Pathol. 1998;79:67–80. doi: 10.1046/j.1365-2613.1998.t01-1-00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaker S, Cobb J, Pyle A, Handel MA. Meiotic Prophase Abnormalities and Metaphase Cell Death in MLH1-Deficient Mouse Spermatocytes: Insights into Regulation of Spermatogenic Progress. Dev Biol. 2002;249:85–95. doi: 10.1006/dbio.2002.0708. [DOI] [PubMed] [Google Scholar]
- Edelmann W, Cohen PE, Kane M, Lau K, Morrow B, Bennett S, Umar A, Kunkel T, Cattoretti G, Chaganti R, Pollard JW, Kolodner RD, Kucherlapati R. Meiotic Pachytene Arrest in MLH1-Deficient Mice. Cell. 1996;85:1125–1134. doi: 10.1016/s0092-8674(00)81312-4. [DOI] [PubMed] [Google Scholar]
- Gruter P, Tabernero C, von Kobbe C, Schmitt C, Saavedra C, Bachi A, Wilm M, Felber BK, Izaurralde E. TAP, the Human Homolog of Mex67p, Mediates CTE-Dependent RNA Export from the Nucleus. Mol Cell. 1998;1:649–659. doi: 10.1016/s1097-2765(00)80065-9. [DOI] [PubMed] [Google Scholar]
- Herold A, Klymenko T, Izaurralde E. NXF1/p15 Heterodimers are Essential for mRNA Nuclear Export in Drosophila. RNA. 2001;7:1768–1780. [PMC free article] [PubMed] [Google Scholar]
- Herold A, Suyama M, Rodrigues JP, Braun IC, Kutay U, Carmo-Fonseca M, Bork P, Izaurralde E. TAP (NXF1) Belongs to a Multigene Family of Putative RNA Export Factors with a Conserved Modular Architecture. Mol Cell Biol. 2000;20:8996–9008. doi: 10.1128/mcb.20.23.8996-9008.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Cullen BR. The Human Tap Protein is a Nuclear mRNA Export Factor that Contains Novel RNA-Binding and Nucleocytoplasmic Transport Sequences. Genes Dev. 1999;13:1126–1139. doi: 10.1101/gad.13.9.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katahira J, Strasser K, Podtelejnikov A, Mann M, Jung JU, Hurt E. The Mex67p-Mediated Nuclear mRNA Export Pathway is Conserved from Yeast to Human. EMBO J. 1999;18:2593–2609. doi: 10.1093/emboj/18.9.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehler J, Tolkunova E, Koschorz B, Pesce M, Gentile L, Boiani M, Lomeli H, Nagy A, McLaughlin KJ, Scholer HR, Tomilin A. Oct4 is Required for Primordial Germ Cell Survival. EMBO Rep. 2004;5:1078–1083. doi: 10.1038/sj.embor.7400279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuersten S, Segal SP, Verheyden J, LaMartina SM, Goodwin EB. NXF-2, REF-1, and REF-2 Affect the Choice of Nuclear Export Pathway for Tra-2 mRNA in C. Elegans. Mol Cell. 2004;14:599–610. doi: 10.1016/j.molcel.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Lai D, Sakkas D, Huang Y. The Fragile X Mental Retardation Protein Interacts with a Distinct mRNA Nuclear Export Factor NXF2. RNA. 2006;12:1446–1449. doi: 10.1261/rna.94306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MA, Storey BT. Bicarbonate is Essential for Fertilization of Mouse Eggs: Mouse Sperm Require it to Undergo the Acrosome Reaction. Biol Reprod. 1986;34:349–356. doi: 10.1095/biolreprod34.2.349. [DOI] [PubMed] [Google Scholar]
- Li X, Nicklas RB. Mitotic Forces Control a Cell-Cycle Checkpoint. Nature. 1995;373:630–632. doi: 10.1038/373630a0. [DOI] [PubMed] [Google Scholar]
- Lin Y, Page DC. Dazl Deficiency Leads to Embryonic Arrest of Germ Cell Development in XY C57BL/6 Mice. Dev Biol. 2005;288:309–316. doi: 10.1016/j.ydbio.2005.06.032. [DOI] [PubMed] [Google Scholar]
- Lomeli H, Ramos-Mejia V, Gertsenstein M, Lobe CG, Nagy A. Targeted Insertion of Cre Recombinase into the TNAP Gene: Excision in Primordial Germ Cells. Genesis. 2000;26:116–117. [PubMed] [Google Scholar]
- Macho B, Brancorsini S, Fimia GM, Setou M, Hirokawa N, Sassone-Corsi P. CREM-Dependent Transcription in Male Germ Cells Controlled by a Kinesin. Science. 2002;298:2388–2390. doi: 10.1126/science.1077265. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Lamb DJ. Genetic Dissection of Mammalian Fertility Pathways. Nat Cell Biol. 2002;4(Suppl):s41–9. doi: 10.1038/ncb-nm-fertilityS41. [DOI] [PubMed] [Google Scholar]
- Oatley JM, Avarbock MR, Telaranta AI, Fearon DT, Brinster RL. Identifying Genes Important for Spermatogonial Stem Cell Self-Renewal and Survival. Proc Natl Acad Sci U S A. 2006;103:9524–9529. doi: 10.1073/pnas.0603332103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Takeda E, Takano K, Yomogida K, Katahira J, Yoneda Y. Molecular Cloning and Functional Characterization of Mouse Nxf Family Gene Products. Genomics. 2005;85:641–653. doi: 10.1016/j.ygeno.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Saunders PT, Turner JM, Ruggiu M, Taggart M, Burgoyne PS, Elliott D, Cooke HJ. Absence of mDazl Produces a Final Block on Germ Cell Development at Meiosis. Reproduction. 2003;126:589–597. doi: 10.1530/rep.0.1260589. [DOI] [PubMed] [Google Scholar]
- Segref A, Sharma K, Doye V, Hellwig A, Huber J, Luhrmann R, Hurt E. Mex67p, a Novel Factor for Nuclear mRNA Export, Binds to both Poly(A)+ RNA and Nuclear Pores. EMBO J. 1997;16:3256–3271. doi: 10.1093/emboj/16.11.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slegtenhorst-Eegdeman KE, de Rooij DG, Verhoef-Post M, van de Kant HJ, Bakker CE, Oostra BA, Grootegoed JA, Themmen AP. Macroorchidism in FMR1 Knockout Mice is Caused by Increased Sertoli Cell Proliferation during Testicular Development. Endocrinology. 1998;139:156–162. doi: 10.1210/endo.139.1.5706. [DOI] [PubMed] [Google Scholar]
- Stouffs K, Tournaye H, Van der Elst J, Liebaers I, Lissens W. Is there a Role for the Nuclear Export Factor 2 Gene in Male Infertility? Fertil Steril. 2008;90:1787–1791. doi: 10.1016/j.fertnstert.2007.08.071. [DOI] [PubMed] [Google Scholar]
- Takada Y, Isono K, Shinga J, Turner JM, Kitamura H, Ohara O, Watanabe G, Singh PB, Kamijo T, Jenuwein T, Burgoyne PS, Koseki H. Mammalian Polycomb Scmh1 Mediates Exclusion of Polycomb Complexes from the XY Body in the Pachytene Spermatocytes. Development. 2007;134:579–590. doi: 10.1242/dev.02747. [DOI] [PubMed] [Google Scholar]
- Takano K, Miki T, Katahira J, Yoneda Y. NXF2 is Involved in Cytoplasmic mRNA Dynamics through Interactions with Motor Proteins. Nucleic Acids Res. 2007;35:2513–2521. doi: 10.1093/nar/gkm125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W, Zolotukhin AS, Tretyakova I, Bear J, Lindtner S, Smulevitch SV, Felber BK. Identification and Characterization of the Mouse Nuclear Export Factor (Nxf) Family Members. Nucleic Acids Res. 2005;33:3855–3865. doi: 10.1093/nar/gki706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretyakova I, Zolotukhin AS, Tan W, Bear J, Propst F, Ruthel G, Felber BK. Nuclear Export Factor Family Protein Participates in Cytoplasmic mRNA Trafficking. J Biol Chem. 2005;280:31981–31990. doi: 10.1074/jbc.M502736200. [DOI] [PubMed] [Google Scholar]
- Wang PJ, McCarrey JR, Yang F, Page DC. An Abundance of X-Linked Genes Expressed in Spermatogonia. Nat Genet. 2001;27:422–426. doi: 10.1038/86927. [DOI] [PubMed] [Google Scholar]
- Wang PJ, Page DC, McCarrey JR. Differential Expression of Sex-Linked and Autosomal Germ-Cell-Specific Genes during Spermatogenesis in the Mouse. Hum Mol Genet. 2005;14:2911–2918. doi: 10.1093/hmg/ddi322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PJ, Pan J. The Role of Spermatogonially Expressed Germ Cell-Specific Genes in Mammalian Meiosis. Chromosome Res. 2007;15:623–632. doi: 10.1007/s10577-007-1141-2. [DOI] [PubMed] [Google Scholar]
- Yang F, Gell K, van der Heijden GW, Eckardt S, Leu NA, Page DC, Benavente R, Her C, Hoog C, McLaughlin KJ, Wang PJ. Meiotic Failure in Male Mice Lacking an X-Linked Factor. Genes Dev. 2008;22:682–691. doi: 10.1101/gad.1613608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Bogerd HP, Wang PJ, Page DC, Cullen BR. Two Closely Related Human Nuclear Export Factors Utilize Entirely Distinct Export Pathways. Mol Cell. 2001;8:397–406. doi: 10.1016/s1097-2765(01)00303-3. [DOI] [PubMed] [Google Scholar]
- Zhang M, Wang Q, Huang Y. Fragile X Mental Retardation Protein FMRP and the RNA Export Factor NXF2 Associate with and Destabilize Nxf1 mRNA in Neuronal Cells. Proc Natl Acad Sci U S A. 2007;104:10057–10062. doi: 10.1073/pnas.0700169104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.