Abstract
The present study aims to investigate the mechanism of Src kinase activation during hypoxia and tests the hypothesis that the hypoxia-induced activation of Src kinase, as determined by Src kinase phosphorylation, in the cerebral cortical membranes of newborn piglets is mediated by NO derived from neuronal nitric oxide synthase (nNOS). Fifteen piglets were divided into normoxic (Nx, n=5), hypoxic (Hx, n=5) and hypoxic-treated with nNOS inhibitor I (Hx-nNOSi) groups. Hypoxia was induced by decreasing FiO2 to 0.06 for 1 hr. nNOS inhibitor I (selectivity >2500 vs eNOS and >500 vs iNOS) was administered (0.4 mg/kg, i.v.) 30 min prior to hypoxia. Cortical membranes were isolated and phosphorylation of Src kinase was determined by Western blot analysis. Src kinase activity was determined by radioactive assay using immunopurified enzyme. Membrane proteins were separated by 12% SDS-PAGE and probed with anti-phospho (pTyr418)-Src kinase antibody. Protein bands were detected, analyzed by densitometry and expressed as absorbance (ODxmm2). Density (ODxmm2) of phosphorylated Src kinase was 111.7±21.1 in Nx, 234.5±23.8 in Hx (p< 0.05 vs Nx) and 104.7±18.1 in Hx-nNOSi (p< 0.05 vs Hx, p=NS vs Nx). Src kinase activity (pmols/mg protein/hr) was 2472±75 in Nx, 4556±358 in Hx (p< 0.05 vs Nx) and 2259 207 in Hx-nNOSi (p<0.05 vs Hx, p=NS vs.Nx). The data show that pretreatment with nNOS inhibitor prevents the hypoxia-induced increase in tyrosine phosphorylation and the activity of Src kinase. We conclude that the mechanism of hypoxia-induced increased activation of Src kinase is mediated by nNOS derived NO. We propose that NO mediated inhibition of protein tyrosine phosphatases SH-PTP-1 and SH-PTP-2 leads to increased tyrosine phosphorylation and activation of Src kinase in the cerebral cortex of newborn piglets.
Keywords: Src kinase activity, Tyrosine phosphorylation, nNOS, nNOSi, hypoxia, brain
INTRODUCTION
Based on the human genome, potentially 90 genes encode protein tyrosine kinases whose functions are controlled by 107 genes that encode protein tyrosine phosphatases [2, 18]. Protein tyrosine kinases mediate signal transduction and control many critical processes, such as transcription, cell death progression, differentiation, immune response, intercellular communication and programmed cell death [13, 24]. Protein tyrosine kinases (PTK) are primarily divided into two classes: the receptor PTK and the non-receptor PTK. The receptor PTK such as EGFR kinase contains an extracellular ligand binding domain, a transmembrane domain and an intracellular protein tyrosine kinase domain. The non-receptor PTK such as Src kinase lacks the transmembrane domain and functions down stream of receptor tyrosine kinases. Src kinase associates with the plasma membrane [29]. Protein tyrosine phosphatases regulate the activation of PTK by dephosphorylating tyrosine residues.
Src protein tyrosine kinase is the first member of the Src family of non-receptor tyrosine kinase. The prototype member of the Src family was identified as the transforming protein (v-Src) of the oncogenic retrovirus. The Src protein possesses tyrosine kinase activity. At least 10 proteins contain structural features similar to Src and have amino acid sequence homology: Fyn, Yes, Yrk, Blk, Fgr, Hck, Lck, Lyn and Frk/Rak and Lyk/Bsk. We focused on the first member: the Src kinase which is expressed ubiquitously and present in neurons at 500 fold higher than other cell types. Src kinase has six distinct functional regions (a) the Src (SH)4 domain, (b) the unique region, (c) the SH3 domain, (d) the SH2 domain, (e) the catalytic domain, and (f) a short negative regulatory tail. The SH3 and SH2 domains repress the kinase activity by interacting with amino acids within the catalytic domain. SH2 domain interacts with pTyr527 and adjacent residue in the negative regulatory tail. Tyr527 is the primary site of tyrosine phosphorylation. Dephosphorylation of Tyr527 leads to activation of Src activity. However, the phosphorylation at Tyr416 within the catalytic domain of Src is critical for kinase activity. Thus phosphorylation at Tyr416 and dephosphorylation at Tyr527 are proposed mechanisms of Src activation. Cytoplasmic protein tyrosine phosphatases SH-PTP-1 and SH-PTP-2 contain two SH2 (Src homology) domains or phosphotyrosine binding domains that help recognizing specific phosphorylated tyrosine on EGFR kinase or Src kinase. Both SH-PTP-1 and SH-PTP-2 are known to dephosphorylate Src kinase. Therefore, nitric oxide generated during hypoxia may result in inactivation of cytoplasmic SH-PTP-1 and SH-PTP-2 leading to increased activation of Src kinase.
Oxygen free radical generation, lipid peroxidation and cell membrane dysfunction in the hypoxic brain can be reduced or prevented by using inhibitors of NOS such as N-nitro-L-arginine (NNLA) [25]. Administration of a NOS inhibitor or a selective inhibitor of nNOS prior to hypoxia prevented the hypoxia- increase in Ca++/calmodulin–dependent protein kinase (CaM kinase IV) activity in neuronal nuclei, increase in cyclic AMP response element binding (CREB) protein phosphorylation, increased expression of cell death promoter protein Bax, activation of poly(ADP-ribose) polymerase, caspase-3 activation and damage to nuclear DNA [33, 21, 34, 22, 27, 23]. nNOS mRNA up-regulation represents a response to stress conditions including hypoxia [9, 10] and ischemia [30]. These studies provide compelling evidence for the role of NO generation and nNOS activation in hypoxic neuronal death.
The present study specifically focuses on investigating the effect of hypoxia on activation of Src kinase and phosphorylation of Src kinase active site (Tyr416) and the mechanism of hypoxia-induced activation of Src kinase in the cerebral cortex of newborn piglets. In the present study we have tested the hypothesis that hypoxia results in increased activation and phosphorylation of Src kinase in the cerebral cortex of newborn piglets and that the activation is mediated by nitric oxide (NO) derived from nNOS. Therefore, administration of a highly selective nNOS inhibitor, prior to hypoxia, will prevent the increased activation and phosphorylation of Src kinase active site in the cerebral cortex of newborn piglets.
Studies were performed on 3–5 day old Yorkshire piglets obtained from the Willow Glenn Farm, Strausburg, PA. The experimental animal protocol was approved by the Institutional Animal Care and Use Committee of Drexel University. Newborn piglets were randomly divided into 3 groups: normoxic (n = 5), hypoxic (n = 5), and hypoxic with nNOS inhibitor [(4s)-N(4-amino-5(aminoethyl)aminopentyl-N′-nitroguanidine, hypoxic-nNOSi n=5]. nNOSi (0.4mg/Kg) was administered i.v. 30 min prior to hypoxia. This nNOS inhibitor is highly selective for nNOS (selectivity >2500 fold vs. eNOS and >500 fold vs. iNOS). The animals were ventilated for 1 hr under either normoxic condition (FiO2 = 0.21) or hypoxic condition; hypoxia was induced by lowering the FiO2 to 0.06 for 60 min. At the end of the experimental period, the animal was sacrificed; the cortical tissue was removed and placed either in homogenization buffer for isolation of cortical cell membranes or in liquid nitrogen, and then stored at −80°C for biochemical studies.
Cerebral cortical tissue was homogenized in 10 vol of buffer (1% Triton X-100, 1% deoxycholic acid, 0.1% SDS, 158 mM NaCl, 10 mM Tris-HCl buffer, pH 7.0, 1 mM EGTA, 1μg/ml each of aprotinin, leupeptin and pepstatin, 1mM sodium orthovanadate (SOV), 0.1 mM phenylmethylsulfonyfluoride (PMSF) and 0.1% IGEPAL. The homogenate was centrifuged at 1000g for 10 min followed by at 40000 g for 60 min and the pellet homogenized in a medium containing (1:1) homogenization buffer and assay buffer (25 mM Tris, pH 7.0, 1 mM EDTA-Na, 0.1%Tween-20, 125 mM MgCl2, 25 mM MnCl2, 2mM DTT, 0.2 mM SOV and 2mM EGTA) and used as membrane fraction. Protein was determined by the method of Lowry et al., [17]. 100μl protein A agarose beads were washed and incubated with 4 μg of anti-Src kinase (rabbit purified polyclonal IgG) for 1 hr at 4°C. Samples were centrifuged at 14,000 g followed by 2 washes. 400 μg protein was incubated with the antibody-bead complex for 2 hr at 4°C and washed 5 times with the reaction buffer.
The Src kinase activity was performed according to Alessi et al, [3], by 33P incorporation into a specific peptide substrate for 15 min at 30°C in a 80 μl medium containing immunocomplex, 25 mM Tris, pH 7.0, 1 mM EDTA, 125 mM MgCl2, 25 mM MnCl2, 2 mM DTT, 0.2 mM SOV, 2 mM EGTA, 150 μM tyrosine kinase peptide substrate [Lys 19] cdc2(6–20)-NH2, and 50 μM 33P-ATP (0.5μCi). The reaction mixture was pelleted and 30 μl supernatant spotted on P 81 filters, washed 3 times with 0.75% phosphoric acid, dried and the radioactivity counted. The data (tubes with substrate – without the substrate) provided the Src kinase activity which was expressed as pmoles/mg protein/hr.
The membrane protein was solubilized and brought to a final concentration of 1 μg/μl in a modified RIPA buffer (50 mM Tris–HCl, pH 7.4, 1 mM EDTA, 150 mM NaCl, 1% NP-40, 0.25% sodium deoxycholate, 1 mM PMSF, 1 mM Na3VO4, 1 mM NaF, and 1 μg/ml each of aprotinine, leupeptin and pepstatin). Then 5 μl of Laemmli buffer (100 mM Tris–HCl pH 6.8, 200 mM dithiothreitol, 4% SDS, 0.2% bromophenol blue, 20% glycerol) was added to each 20 μg of membrane protein. Equal protein amounts of each sample was separated by using 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE). The proteins were electrically transferred to nitrocellulose membranes and probed with primary antibodies directed against Src kinase and anti-phospho (pTyr 418)-Src kinase antibody. Immunoreactivity was detected with horseradish peroxidase conjugated secondary antibody. Specific complexes were detected by enhanced chemiluminescence using the ECL detection system and analyzed by imaging densitometry using Quantity One Software. The data are expressed as optical density (OD)×mm2. ATP and phosphocreatine concentrations were determined according to the method of Lamprecht et al, [15].
Statistical analysis of the data was performed using one way analysis of variance ANOVA to compare normoxic, hypoxic, and hypoxic-nNOSi groups. A p value of less than 0.05 was considered statistically significant. All values are presented as mean ± standard deviation (SD).
Cerebral cortical tissue hypoxia in newborn piglets was documented by determining the levels of ATP and PCr in the cerebral cortical tissue. The level of ATP (μmoles/g brain) decreased from 4.35±0.21in Nx to 1.43 ±0.28 in Hx (p<0.05 vs Nx), and 1.73± 0.33 in Hx-nNOSi (p<0.05, vs Nx, p=NS vs Hx). PCr level (μmoles/g brain) decreased from 3.80±0.26 in Nx to 0.96 ±0.20 in Hx (p<0.05 vs Nx), and 1.09±0.39 in Hx+nNOSi (p<0.05 vs Nx, p=NS vs Hx). The level of high energy phosphates decreased significantly in the hypoxic group as compared to normoxic and the data demonstrate that cerebral tissue hypoxia was achieved in the hypoxic group. In addition, these results demonstrate that the level of cerebral tissue high energy phosphates, ATP and PCr, were comparable in the hypoxic and hypoxic-treated with nNOSi groups.
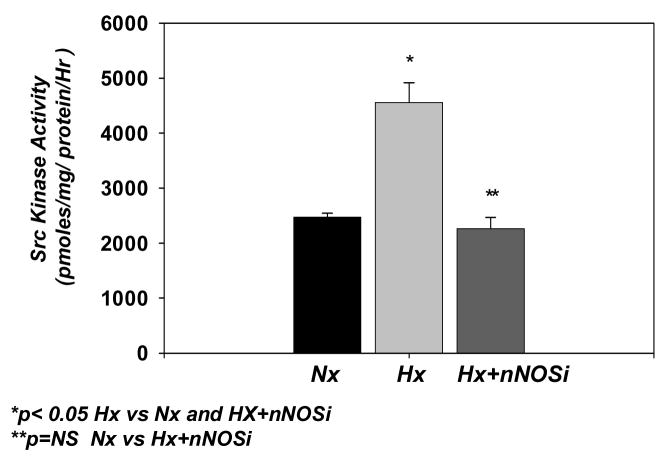
Src kinase activity in the cerebral cortical membranes of the normoxic, hypoxic and hypoxic-treated with nNOSi groups is shown in Fig 1. Src kinase activity in the cortical membrane fraction (pmol/mg protein/hr) increased from 2472 ± 75 in the normoxic to 4556 ± 358 in the hypoxic group (p<0.05 vs Nx and Hx-nNOSi). The activity in the hypoxic-treated with nNOS inhibitor was 2259 ± 207 (p<0.05 vs Hx, p=NS vs Nx). These results demonstrate that hypoxia results in increased activity of Src kinase and administration of nNOS inhibitor prevents the hypoxia-induced increase in Src kinase activity in the cerebral cortex of newborn piglets.
Figure 1.
Effect of hypoxia on the Src kinase activity in cortical membranes of the cerebral cortex of normoxic, hypoxic and hypoxic-nNOSi newborn piglets. Src kinase activity (pmoles/mg protein/hr) is presented on Y-axis. The data are expressed as mean ± SD.
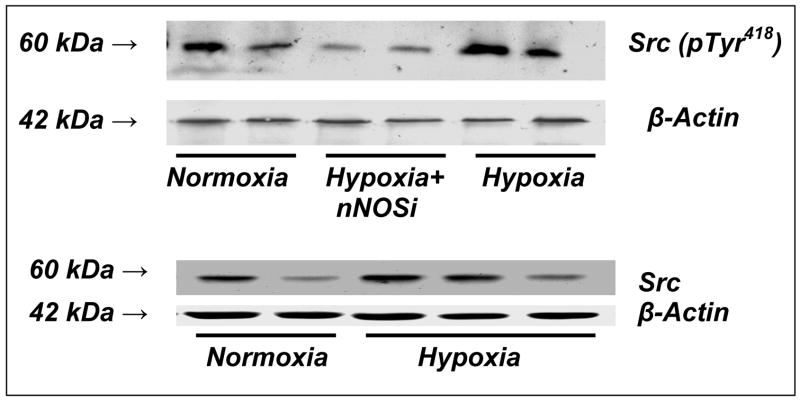
Representative Western blots of phospho (pTyr 418)-Src kinase for normoxic, hypoxic and hypoxic-nNOSi groups are shown in Figure 2 The results show an increased expression of phoshorylated Src kinase in the Hx group indicating increased level of phosphorylated Src in the cerebral cortex during hypoxia. Src kinase protein expression did not change during hypoxia.
Figure 2.
Representative western blots of phosphorylated Src kinase at Tyr418 in cortical membranes of the cerebral cortex of normoxic, hypoxic and hypoxic-nNOS newborn piglets. Western blot analysis was performed using anti-phospho (pTyr 418)-Src kinase, anti-Src kinase (Santa Cruz biotechnology, CA) and anti-actin antibody (Chemicon). For phsophorylated Src kinase- Lanes 1 and 2 represent normoxic; lanes 3 and 4 represent hypoxic-nNOSi; and lanes 5 and 6 represent hypoxic piglets. For Src kinase- Lanes 1 and 2 represent normoxic and lanes 3,4 and 5 represent hypoxic.
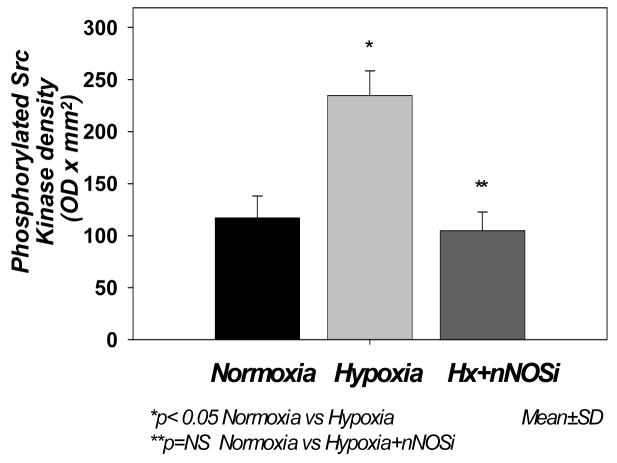
The data (Fig 3) show that the density (expressed as optical density × mm2) of the phosphorylated (pTyr 418) Src kinase active site was 111.78 ± 21.13 in Nx, 234.52 ± 23.82 in Hx (p< 0.05 vs Nx and Hx-nNOSi) and 104.76 ± 18.15 in Hx-nNOSi, (p<0.05 vs Hx). The data show that hypoxia resulted in increased phosphorylation of Src kinase active site and the phosphorylation of Src kinase active site is prevented by nNOS inhibitor, indicating that the hypoxia-induced increased activation of Src kinase is mediated by nNOS-derived NO.
Figure 3.
Effect of hypoxia on the expression phosphorylation of Src kinase at Tyr418 in cortical membranes of the cerebral cortex of normoxic, hypoxic and hypoxic-nNOS newborn piglets. The protein density (OD × mm2) is presented on Y-axis. The data are expressed as mean ± SD.
Cerebral hypoxia results in increased nuclear Ca++ influx and increased activity of CaM kinase IV which is predominantly located in the nucleus [6, 33]. Hypoxia also results in increased phosphorylation of cyclic AMP response element binding (CREB) protein and increased expression of proapototic protein Bax [21, 34]. In addition, hypoxia results in increased fragmentation of nuclear DNA [1]. Furthermore, hypoxia results in increased generation of NO [20] and administration of nitric oxide synthase inhibitor prevented the hypoxia-induced CREB phosphorylation, Bax protein expression and DNA fragmentation. We observed that hypoxia results in SH-PTP inactivation. The present study investigated the effect of hypoxia on Src kinase activation and phosphorylation of Src kinase active site and tested the hypothesis that cerebral hypoxia results in increased activation and phosphorylation of Src kinase and the hypoxia-induced activation of Src kinase is mediated by nitric oxide derived from nNOS.
The results of the present study show that cerebral hypoxia results in increased tyrosine phosphorylation of Src kinase at its active site as well as increased activity of Src kinase in the cortical membrane fraction of the cerebral cortex of newborn piglets. The results also show that hypoxia resulted in increased activity of Src kinase in the cortical membranes. Administration of a highly selective nNOS inhibitor prevented the hypoxia –induced increased phosphorylation of Src kinase active site, an index of Src kinase activation as well as the activity of Src kinase in the cortical membranes of newborn piglets. These results demonstrate that nitric oxide derived from nNOS mediates the activation and phosphorylation at the active site of Src kinase.
We propose the mechanism that nitric oxide free radicals generated during hypoxia result in activation of Src kinase by inactivating protein tyrosine phosphatases, SH-PTP-1 and SH-PTP-2. Since all protein tyrosine phosphatases contain a cysteine residue at their active site, enzyme activity can be affected by redox mechanisms [4, 16, 31]. Under hypoxic conditions, NO free radicals are generated which can combine with superoxide radicals to produce peroxynitrite. The reaction between NO free radical and superoxide to form peroxynitrite is favored over the reaction between superoxide and superoxide dismutase [5, 11]. Hypoxia-induced nitration of NMDA receptor subunits indicates formation of peroxynitrite during hypoxia [32]. Therefore, we propose that during hypoxia NO-mediated peroxynitrite-dependent inactivation of SH-PTP-1 and SH-PTP-2 leads to increased tyrosine phosphorylation of Src kinase and its increased activation.
The results of the present study raise some very fundamental questions regarding the role of Src kinase in cell proliferation and cell death. The increased activation of Src kinase is known in cases of a variety of cancer conditions including breast cancer and glioblastoma [19]. As shown in this study, cerebral hypoxia leads to increased activation and tyrosine phosphorylation of Src kinase in the cerebral cortex of newborn piglets. Hypoxia is also known to result in cell death in the hypoxic brain. Therefore, the role of Src kinase activation in leading to both the cell proliferation and cell death needs serious investigation and evaluation. In addition, Src kinase may potentially be a target that mediates both the cell survival and cell death in the cancerous tissue and the hypoxic brain, respectively. Increased tyrosine phosphorylation of several molecules following hypoxia/ischemia has been observed [8, 12].
Hypoxia results in increased expression of proapoptotic protein Bax in neuronal nuclei, mitochondria and cytosol [7, 28]. Administration of a NOS inhibitor prior to hypoxia prevents the hypoxia-induced increase in Bax expression as well as DNA fragmentation indicating that the increased expression of proapoptotic protein Bax and the nuclear DNA fragmentation are mediated by nitric oxide [33]. These results demonstrate that hypoxia results in activating cell death mechanism that is mediated by NO.
Overexpression of Bax or an increase in the ratio of Bax to Bcl-2, leads to programmed cell death [26]. After 10 min of global ischemia, there were high levels of Bax, low levels of Bcl-2, and DNA-strand breaks in the same population of neurons found to be degenerating morphologically [14]. Neurons with elevated Bax levels almost uniformly had morphologic evidence of ischemic degeneration with apoptotic features including nuclear DNA fragmentation [28]. NO can play a central role in hypoxia-induced neuronal death by both the necrotic as well as programmed cell death mechanisms.
The results of the present study demonstrate that hypoxia results in increased tyrosine phosphorylation and increased activation of Src kinase. We propose that increased activation of Src kinase results in increased tyrosine phosphorylation of nNOS and increased tyrosine phosphorylation of calmodulin, an activator of nNOS, at Tyr99. The Tyr99 phosphorylated calmodulin may bind with increased affinity with the enzyme as well as non-phosphorylated camodulin may bind with increased affinity with the tyrosine phosphorylated enzyme and lead to increased nNOS activity. Subsequently, increased activation of nNOS during hypoxia results in increased generation of nitric oxide. We have demonstrated that hypoxia results in increased generation of nitric oxide and increased nitration of NMDA receptor subunits in the cerebral cortical tissue. Thus increasd activation of nNOS leading to increased generation of NO during hypoxia leads to increased activation of Src kinase by inhibiting protein tyrosine phosphatases SH-PTP-1 and SH-PTP-2.. Thus nNOS activation leads to Src kinase activation that subsequently activates nNOS. Thus this perpetual cycle of nNOS activation → Src kinase activation → nNOS activation goes on in the hypoxic brain resulting in hypoxic brain injury.
In summary: These results show that cerebral tissue hypoxic results in increased activity and increased phosphorylation of Src kinase at its active site Tyr418 in the cerebral cortex of newborn piglets. In addition, administration of nNOSi, a highly selective inhibitor of nNOS, prior to hypoxia prevented the hypoxia-induced increased activity and phosphorylation of EGFR kinase active site Tyr418, an index of Src kinase activation. We conclude that Src kinase activation during hypoxia is mediated by nitric oxide derived from nNOS. We propose that nNOS-derived NO, by inhibiting protein tyrosine phosphatases (SH-PTP-1 and SH-PTP-2) mediates the increased tyrosine phosphorylation of Src kinase active site and the activation of Src kinase that regulates cell proliferation and cell death.
Acknowledgments
This research work was supported by The National Institutes of Health’s grants NIH-HD-38079 and NIH-HD-20337.
This study was supported by the National Institute of Health grants HD-38079 (OPM) and HD-20337 (MDP). The authors thank Mrs. Anli Zhu for her expert technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akhter WA, Ashraf QM, Zanelli SA, Mishra OP, Delivoria-Papadopoulos M. Effects of graded hypoxia on cerebral cortical genomic DNA fragmentation in newborn piglets. Biol Neonate. 2001;79:187–193. doi: 10.1159/000047089. [DOI] [PubMed] [Google Scholar]
- 2.Alanso A, Sasin J, Bottini N, Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J, Mustalin T. Protein tyrosine phosphatases in human genome. Cell. 2002;117:699–711. doi: 10.1016/j.cell.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 3.Alessi DR, Cohen P, Ashworth A, Cowley S, Leevers SJ, Marshall CJ. Assay and expression of mitogen-activated protein kinase, MAP kinase kinase. Methods Enzymol. 1995;255:289. doi: 10.1016/s0076-6879(95)55031-3. [DOI] [PubMed] [Google Scholar]
- 4.Barret WE, Degnore JP, Keng YF, Zang ZY, Yim MB, Chock PB. Roles of superoxide radical anion in signal transduction mediated by reversible regulation of protein-tyrosine phosphatase IB. J Biol Chem. 1999;49:34543–34546. doi: 10.1074/jbc.274.49.34543. [DOI] [PubMed] [Google Scholar]
- 5.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical by peroxynitrite: implications for enthothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delivoria-Papadopoulos M, Akhter WA, Mishra OP. Hypoxia-induced Ca++-influx in cerebral cortical neuronal nuclei of newborn piglets. Neurosci Lett. 2003;342:119–123. doi: 10.1016/s0304-3940(03)00256-8. [DOI] [PubMed] [Google Scholar]
- 7.Delivoria-Papadopoulos M, Ashraf QM, Mishra OP. Effect of hypoxia on expression of apoptotic proteins in nuclear, mitochondrial and cytosolic fractions of the cerebral cortex of newborn piglets: The role of nuclear Ca++-influx. Neurochem Res. 2008;33:1196–204. doi: 10.1007/s11064-007-9568-6. [DOI] [PubMed] [Google Scholar]
- 8.Du CP, Gao J, Tai JM, Liu Y, Qi J, Wang W, Hou XY. Increased tyrosine phosphorylation of PSD-95 by Src family kinases after brain ischemia. Biochem J. 2009;417:277–285. doi: 10.1042/BJ20080004. [DOI] [PubMed] [Google Scholar]
- 9.Forstermann U, Boissel JP, Kleinert H. Expressional control of the ‘constitutive” isoforms of nitric oxide synthase (NOS I and NOS III) FASEB J. 1998;12:773–790. [PubMed] [Google Scholar]
- 10.Guo Y, Ward ME, Beasjours S, Mori M, Hussain SNA. Regulation of cerebellar nitric oxide production in response to prolonged in vivo hypoxia. J Neurosci Res. 1997;49:89–97. [PubMed] [Google Scholar]
- 11.Ischiropoulos H, Zhu L, Beckman JS. Peroxynitrite formation from macrophage-derived nitric oxide. Arch Biochem Biophys. 1992;298:446–451. doi: 10.1016/0003-9861(92)90433-w. [DOI] [PubMed] [Google Scholar]
- 12.Jiang X, Mu D, Biran V, Faustino J, Chang S, Rincon CM, Sheldon RA, Ferriero DM. Activated Src kinases interact with the N-methyl-D-aspartate receptor after neonatal brain ischemia. Ann Neurol. 2008;63:632–641. doi: 10.1002/ana.21365. [DOI] [PubMed] [Google Scholar]
- 13.Johnson GL, Lapadat R. Mitogen activated protein kinase pathways mediated by ERK, JNK and p38 protein kinases. Science. 2004;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 14.Krajewski S, Mal JK, Krajewska M, Sikorska M, Mossakowski MJ. Upregulation of Bax protein levels in neurons following cerebral ischemia. J Neurosci. 1995;15:6364–6376. doi: 10.1523/JNEUROSCI.15-10-06364.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamprecht W, Stein P, Heinz F, Weissner H. Creatine phosphate. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. Vol. 4. Academic Press; New York: 1974. pp. 1777–1781. [Google Scholar]
- 16.Lee SR, Kwan KS, Kim SR, Rhu SA. Reversible inactivation of protein-tyrosine phosphatase IB in A431 cells stimulated with epidermal growth factor. J Biol Chem. 1998;273:15366–15372. doi: 10.1074/jbc.273.25.15366. [DOI] [PubMed] [Google Scholar]
- 17.Lowry O, Rosenbrough NJ, Farr A, Randall RJ. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 18.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 19.Milano V, Piao Y, LaFortune T, DeGroot J. Dasatinib-induced autophagy is enhanced in combination with temozolomide in glioma. Mol J Cancer Ther. 2009;8:394–406. doi: 10.1158/1535-7163.MCT-08-0669. [DOI] [PubMed] [Google Scholar]
- 20.Mishra OP, Zanelli S, Ohnishi ST, Delivoria-Papadopoulos M. Hypoxia-induced generation of nitric oxide free radicals in cerebral cortex of newborn guinea pigs. Neurochem Res. 2000;25:1559–1565. doi: 10.1023/a:1026610301978. [DOI] [PubMed] [Google Scholar]
- 21.Mishra OP, Ashraf QM, Delivoria-Papadopoulos M. Phosphorylation of cAMP response element binding (CREB) protein during hypoxia in cerebral cortex of newborn piglets and the effect of nitric oxide synthase inhibition. Neurosci. 2002;115:985–991. doi: 10.1016/s0306-4522(02)00275-0. [DOI] [PubMed] [Google Scholar]
- 22.Mishra OP, Akhter W, Ashraf QM, Delivoria-Papadopoulos M. Hypoxia-induced modification of poly(ADP-ribose)polymyrase and DNA polymyrase B activity in cerebral cortical nuclei of newborn piglets: Role of nitric oxice. Neurosci. 2003;119:1023–1032. doi: 10.1016/s0306-4522(03)00166-0. [DOI] [PubMed] [Google Scholar]
- 23.Mishra OP, Delivoria-Papadopoulos M. NO-mediated mechanisms of hypoxic brain injury in newborns. In: Mishra OP, editor. Mechanisms of Hypoxic Brain Injury in the Newborn and Potential Strtegies for Neuroprotection. Signpost Publishers; 2007. pp. 157–171. [Google Scholar]
- 24.Nobel MEM, Endicott JA, Johnson LN. Protein kinase inhibitors: Insights into drug design from structure. Science. 2004;303:1800–1805. doi: 10.1126/science.1095920. [DOI] [PubMed] [Google Scholar]
- 25.Numagami Y, Zubrow AB, Mishra OP, Delivoria-Papadopoulos M. Lipid free radical generation and brain cell membrane alteration following nitric oxide synthase inhibition during cerebral hypoxia in the newborn piglet. J Neurochem. 1997;69:1542–1547. doi: 10.1046/j.1471-4159.1997.69041542.x. [DOI] [PubMed] [Google Scholar]
- 26.Oltvai ZN, Milliman CM, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 27.Parikh NA, Katsetos CD, Ashraf QM, Haider SH, Legido A, Delivoria-Papadopoulos MM, Mishra OP. Hypoxia-induced caspase-3 activation and DNA fragmentation in cortical neurons of newborn piglets. Role of nitric oxide. Neurochem Res. 2003;28:1351–1357. doi: 10.1023/a:1024992214886. [DOI] [PubMed] [Google Scholar]
- 28.Reed JC. Mechanisms of Bcl-2 family protein function and dysfunction in health and disease. Behring Inst Mitt. 1996;97:72–100. [PubMed] [Google Scholar]
- 29.Donepudi M, Resh MD. c-Src trafficking and co-localization with the EGF receptor promotes EGF ligand-independent EGF receptor activation and signaling. Cell Signal. 2008;20:1359–1367. doi: 10.1016/j.cellsig.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samdani AF, Dawson TM, Dawson VL. Nitric oxide synthase in models of focal ischemia. Stroke. 1997;28:1283–1288. doi: 10.1161/01.str.28.6.1283. [DOI] [PubMed] [Google Scholar]
- 31.Takakure K, Beckman JS, MacMillan-Cron LA, Cron JP. Rapid and irreversible inactivation of protein tyrosine phosphatases PTPIB, CD45 and LAR by peroxynitrite. Arch Biochem Biophys. 1999;369:197–207. doi: 10.1006/abbi.1999.1374. [DOI] [PubMed] [Google Scholar]
- 32.Zanelli S, Ashraf QM, Mishra OP. Nitration is a mechanism of regulation of the NMDA receptor function during hypoxia. Neurosci Lett. 2002;112:869–877. doi: 10.1016/s0306-4522(02)00141-0. [DOI] [PubMed] [Google Scholar]
- 33.Zubrow AB, Delivoria-Papadopoulos M, Ashraf QM, Fritz KI, Mishra OP. Nitric Oxide-Mediated Ca2+/Calmodulin-Dependent Protein Kinase IV Activity During Hypoxia in Neuronal Nuclei from Newborn Piglets. Neurosci Lett. 2002;335:5–8. doi: 10.1016/s0304-3940(02)01138-2. [DOI] [PubMed] [Google Scholar]
- 34.Zubrow AB, Delivoria-Papadopoulos M, Ashraf QM, Ballesteros JR, Fritz KI, Mishra OP. Nitric Oxide-Mediated Expression of Bax Protein and DNA Fragmentation During Hypoxia in Neuronal Nuclei from Newborn Piglets. Brain Res. 2002;954:60–67. doi: 10.1016/s0006-8993(02)03342-5. [DOI] [PubMed] [Google Scholar]