Abstract
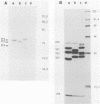
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting were used to compare the capsid proteins of 19 antigenic variants of echovirus type 25 wild-type strains isolated in France between 1976 and 1987 with those of the prototype JV-4 reference strain isolated in 1957. Immunoblots were developed by using polyclonal sera from rabbits and mice immunized with the reference strain. Immunoblotting patterns revealed reactivity only against viral protein VP1 for sera from both animals. Comparative immunoblotting patterns showed differences in the electrophoretic mobilities of viral protein VP1, especially for the Montpellier 76.1262 wild-type strain. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of [35S]methioinine-labeled viral polypeptides revealed that the two variant strains, Montpellier 76.1262 and Thionville 86.222, exhibited significant and reproducible shifts in the relative mobilities of VP1 and VP3 and, to a lesser extent, in those of VP0 and VP2. The relative mobility of VP4 seemed very similar for the JV-4 reference strain and the two variants. Interestingly, the structural differences in VP1 and VP3 of Montpellier 76.1262 were not correlated with the pattern of neutralization by monoclonal antibodies, unlike in our previous study, in which this strain differed from the prototype strain in only two epitopes. We concluded that, in addition to the heterogeneity of their biological and antigenic properties that we observed previously, echovirus type 25 wild-type strains may exhibit differences in their structural proteins.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beneke T. W., Habermehl K. O., Diefenthal W., Buchholz M. Iodination of poliovirus capsid proteins. J Gen Virol. 1977 Feb;34(2):387–390. doi: 10.1099/0022-1317-34-2-387. [DOI] [PubMed] [Google Scholar]
- Emini E. A., Jameson B. A., Lewis A. J., Larsen G. R., Wimmer E. Poliovirus neutralization epitopes: analysis and localization with neutralizing monoclonal antibodies. J Virol. 1982 Sep;43(3):997–1005. doi: 10.1128/jvi.43.3.997-1005.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. M., Minor P. D., Schild G. S., Almond J. W. Critical role of an eight-amino acid sequence of VP1 in neutralization of poliovirus type 3. Nature. 1983 Aug 4;304(5925):459–462. doi: 10.1038/304459a0. [DOI] [PubMed] [Google Scholar]
- Fuchs-Beraud F., Aymard M. The use of a nitrocellulose-enzyme immunoassay for the rapid screening of monoclonal antibodies to human enteroviruses. J Biol Stand. 1989 Jan;17(1):1–7. doi: 10.1016/0092-1157(89)90022-x. [DOI] [PubMed] [Google Scholar]
- Hayase Y., Toya K., Tanaka T., Tobita K. Protein analysis of newly isolated variants of echovirus type 18 by electrophoresis and western blotting. J Med Virol. 1990 Nov;32(3):143–147. doi: 10.1002/jmv.1890320303. [DOI] [PubMed] [Google Scholar]
- Kew O. M., Nottay B. K., Hatch M. H., Nakano J. H., Obijeski J. F. Multiple genetic changes can occur in the oral poliovaccines upon replication in humans. J Gen Virol. 1981 Oct;56(Pt 2):337–347. doi: 10.1099/0022-1317-56-2-337. [DOI] [PubMed] [Google Scholar]
- LIM K. A., BENYESH-MELNICK M. Typing of viruses by combinations of antiserum pools. Application to typing of enteroviruses (Coxsackie and ECHO). J Immunol. 1960 Mar;84:309–317. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Kobayashi T., Kimura Y. Isolation and preliminary characterization of antigenic variant of echovirus type 11. J Med Virol. 1990 Aug;31(4):253–258. doi: 10.1002/jmv.1890310403. [DOI] [PubMed] [Google Scholar]
- Minor P. D., Schild G. C., Bootman J., Evans D. M., Ferguson M., Reeve P., Spitz M., Stanway G., Cann A. J., Hauptmann R. Location and primary structure of a major antigenic site for poliovirus neutralization. Nature. 1983 Feb 24;301(5902):674–679. doi: 10.1038/301674a0. [DOI] [PubMed] [Google Scholar]
- Minor P. D., Schild G. C., Ferguson M., Mackay A., Magrath D. I., John A., Yates J. P., Spitz M. Genetic and antigenic variation in type 3 polioviruses: characterization of strains by monoclonal antibodies and T1 oligonucleotide mapping. J Gen Virol. 1982 Aug;61(Pt 2):167–176. doi: 10.1099/0022-1317-61-2-167. [DOI] [PubMed] [Google Scholar]
- Peigue-Lafeuille H., Fuchs F., Gharabaghi F., Chambon M., Aymard M. Impact on routine diagnosis of echovirus infections of intratypic differentiation and antigenic variation in echovirus type 25 studied by using monoclonal antibodies. J Clin Microbiol. 1990 Oct;28(10):2291–2296. doi: 10.1128/jcm.28.10.2291-2296.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwirth B., Eggers H. J. Biochemistry and pathogenicity of echovirus 9. I. Characterization of the virus particles of strains Barty and Hill. Virology. 1982 Nov;123(1):102–112. doi: 10.1016/0042-6822(82)90298-7. [DOI] [PubMed] [Google Scholar]
- Thorpe R., Minor P. D., Mackay A., Schild G. C., Spitz M. Immunochemical studies of polioviruses: identification of immunoreactive virus capsid polypeptides. J Gen Virol. 1982 Dec;63(2):487–492. doi: 10.1099/0022-1317-63-2-487. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetz K., Habermehl K. O. Topographical studies on poliovirus capsid proteins by chemical modification and cross-linking with bifunctional reagents. J Gen Virol. 1979 Aug;44(2):525–534. doi: 10.1099/0022-1317-44-2-525. [DOI] [PubMed] [Google Scholar]