Abstract
Systemic abnormalities often occur in patients with liver disease. In particular, cardiopulmonary or renal diseases accompanied by advanced liver disease can be serious and may determine the quality of life and prognosis of patients. Therefore, both hepatologists and non-hepatologists should pay attention to such abnormalities in the management of patients with liver diseases.
Keywords: Systemic abnormality, Risk of surgery, Drug dosage, Liver disease
INTRODUCTION
Systemic abnormalities are often seen in liver disease, possibly because of the following characteristics of the liver as an organ[1]. (1) The liver is the key organ for the metabolism of protein, carbohydrate and fat. The liver produces many proteins, including coagulation factors. (2) The liver is a major organ for drug metabolism and the removal of hormones and other substances. (3) The liver is frequently invaded by various pathogens, and hepatitis virus infection causes not only serious liver diseases, but also exhibits extrahepatic manifestations through either immunological or non-immunological mechanisms. (4) The liver has a high blood flow supplied from the portal vein and the hepatic artery, leading to changes in portal, systemic or cardiopulmonary circulation in severe or advanced liver disease. (5) The liver is a major hematopoietic organ in the fetus and produces hematopoietic growth factors such as thrombopoietin[2].
In this review, in addition to systemic abnormalities often found in liver disease, dosage adjustment of drugs and risks of surgery in patients with liver disease are summarized. These factors should always be considered in the diagnosis or management of patients with liver disease.
CARDIOPULMONARY SYSTEM IN LIVER DISEASES
Patients with advanced liver disease have mild hypoxemia attributable to an alteration in ventilation-perfusion matching[3]. When a patient with liver disease exhibits dyspnea in the absence of detectable primary cardiopulmonary disease, hepatopulmonary syndrome (HPS) or portopulmonary hypertension (PPHTN) should be considered[4]. Clinical features and diagnostic criteria for HPS and PPHTN are shown in Table 1.
Table 1.
Difference between HPS and PPHTN modified from Rodríguez-Roisin et al[4]
| HPS | PPHTN | |
| Prevalence | 11%-32% of patients with liver cirrhosis | 2% of patients with portal hypertension |
| Pathogenesis | Increased intrapulmonary shunting | Unknown |
| Intrapulmonary vascular dilatations | (+) | (-) |
| Pulmonary arterial hypertension | (-) | (+) |
| Symptom | Dyspnea, platypnea | Dyspnea on exertion, syncope, chest pain |
| Clinical manifestations | Cyanosis | No cyanosis |
| Orthodeoxia | Accentuated pulmonary component of IIs | |
| Spider nevi | Systolic murmur, edema | |
| ECG findings | None | RVH, RBBB, right axis deviation |
| Arterial blood gas levels | Moderate-to-severe hypoxemia (< 60-80 mmHg) | No/mild hypoxemia |
| Chest radiography | Normal | Cardiomegaly, hilar enlargement |
| CEE | Positive finding; left atrial opacification for > 3-6 heart beats after right atrial opacification | Usually negative finding |
| 99mTcMAA shunting index | ≥ 6% | < 6% |
| Pulmonary hemodynamics | Normal/low PVR | Elevated PVR mPAP > 25 mmHg at rest or > 30 mmHg with exercise |
| OLT | Indicated in severe stages | Only indicated in mild-to-moderate stages |
HPS: Hepatopulmonary syndrome; PPHTN: Portopulmonary hypertension; RVH: Right ventricle hypertrophy; IIs: Second heart sound; ECG: Electrocardiography; RBBB: Right bundle-branch block; AaPO2: Alveolar arterial oxygen gradient; CEE: Contrast-enhanced echocardiography;
TcMAA: Technetium-99m-labelled macroaggregated albumin; PVR: Pulmonary vascular resistance; mPAP: Mean pulmonary artery pressure; OLT: Orthotopic liver transplantation.
Hepatopulmonary syndrome
HPS is defined as the triad of liver disease, pulmonary gas exchange abnormalities leading to arterial deoxygenation and evidence of intrapulmonary vascular dilatation (IPVD)[4–7]. Although the mechanism for the development of HPS is complex and unclear, ventilation-perfusion mismatch and enhanced production of nitric oxide in the lung have been proposed as the predominant mechanisms[7].
A diagnosis of HPS requires demonstration of IPVD and an increased alveolar-arterial oxygen difference (AaDO2) (> 15 mmHg[8] or > 20 mmHg[6,9]) on breathing of air at room temperature and pressure. In order to diagnose IPVD, trans-thoracic contrast-enhanced echocardiography[4], technetium-99m-labeled macroaggregated albumin scanning[10] and pulmonary arteriography are useful. HPS is reported to occur in 11%-32% of patients with chronic liver disease, mainly among those with liver cirrhosis[11–15]. HPS patients who experience severe hypoxemia at rest should receive continuous long-term low-flow oxygen therapy, although no data on its long-term effectiveness is available. Complete resolution of HPS following orthotopic liver transplantation (OLT) has been observed in > 80% of reported cases[16]. At present, OLT appears to be the most effective therapy for patients with HPS.
Portopulmonary hypertension
PPHTN refers to pulmonary arterial hypertension that is associated with portal hypertension. PPHTN should be suspected in hypoxemic patients without pulmonary vascular dilatation[4,7,17]. The most common symptoms are dyspnea on exertion, syncope and chest pain. Although the cause of PPHTN is unknown, it has been reported that vasoactive substances could reach the pulmonary circulation at abnormally high concentrations owing to portosystemic shunts or decreased hepatic metabolism, leading to the pathological pulmonary vascular lesions exhibited in PPHTN[4,18]. Severe PPHTN is a contraindication of OLT, which can result in peri-operative death from acute right ventricular failure[19,20]. Therefore, it is very important to distinguish between HPS and PPHTN in patients who are candidates for liver transplantation[4,7,21].
Idiopathic pulmonary fibrosis
Chronic hepatitis C virus (HCV) infection has been associated with a variety of extrahepatic complications, and an association between idiopathic pulmonary fibrosis (IPF) and HCV infection has been reported[22]. Studies undertaken in Japan and Italy suggest that the incidence of anti-HCV antibody positivity in patients with IPF is significantly higher than in those without IPF[22,23]. Arase et al[24] reported that the cumulative rate of IPF development is 0.9% at 20 years after HCV infection, which is significantly higher than that after hepatitis B virus (HBV) infection.
Coronary artery disease
It has been reported that atherosclerosis-related coronary artery disease (CAD) is less frequent in patients with liver cirrhosis than in controls matched with cirrhotic patients according to age, sex or cigarette smoking[25–28]. The prevalence of myocardial infarction was found to be significantly lower in cirrhotic patients than that in non-cirrhotic patients (1.7% vs 6.4%)[28]. The mechanisms of this protective effect on coronary atherosclerosis are unclear, but it may be associated with liver-related cholesterol metabolism and hematologic changes in cirrhotic patients. In contrast to the reported effect of cirrhosis on CAD, Alyan et al[29] demonstrated that HCV infection is an independent predictor of increased coronary atherosclerosis when patients with severe liver disease and cirrhosis are excluded from the analysis. It is thus suggested that the incidence of coronary atherosclerosis in patients with liver disease could be influenced by the severity of the liver disease and by hepatitis virus infection.
Recently, nonalcoholic steatohepatitis (NASH) has become well known as one of the leading causes of cirrhosis, and NASH is known to be strongly related to insulin resistance[30]. Kadayifci et al[31] reported that the prevalence of all CAD risk factors and metabolic syndrome was significantly higher in NASH-related cirrhotic patients than in cirrhotic patients with other etiologies.
High cardiac output and increased circulatory volume
In patients with advanced cirrhosis, cardiac output and circulatory volume are increased because systemic vascular peripheral resistance is reduced and oxygen consumption is decreased by arteriovenous shunting[32,33]. However, it is also known that these circulatory changes rarely result in heart failure.
Cardiomyopathy
Dilated and hypertrophic cardiomyopathies associated with HCV infection have been reported[34–36]. Although HCV has been isolated from the myocardium of patients with myocarditis and cardiomyopathy[37], there are conflicting reports on the incidence of HCV infection in these patients[38–41]. International epidemiological studies examining the incidence of HCV infection in patients with non-ischemic cardiomyopathy should be conducted. Some HLA haplotypes have been identified in a particular population of patients with HCV-associated cardiomyopathy, suggesting that genetic predisposition may be involved in the development of the disease[42]. Although the mechanisms of myocardial damage by HCV have not been characterized, HCV core protein may damage the myocardium either directly or indirectly through immunological mechanisms[43–45]. The treatment of chronic hepatitis C with interferon is sometimes contraindicated in patients with myocardial dysfunction[46]. Therefore, understanding and assessing cardiomyopathy as an extrahepatic manifestation of HCV infection is important.
ENDOCRINE SYSTEM IN LIVER DISEASES
Because the liver is involved in the synthesis and metabolism of many kinds of hormones, various abnormalities of circulating hormone levels are found in patients with advanced liver disease. HCV infection is known to be linked to some endocrine disorders.
Diabetes mellitus
Type-2 diabetes mellitus is present in more patients with chronic HCV infection than in those with HBV infection (21% and 12% in the United States, 23.6% and 9.4% in the United Kingdom, respectively)[47–50]. The association between HCV infection and diabetes mellitus is thought to be responsible for insulin resistance, which is shown to be directly induced by HCV core proteins[51]. Insulin resistance is also reported to be closely associated with the progression of fibrosis[52]. Diabetes mellitus is considered to increase the risk of the development of hepatocellular carcinoma and to increase mortality[53,54]. The monitoring of blood glucose should be performed on patients with chronic HCV infection, and in particular on those with advanced fibrosis, and appropriate treatment may be required when diabetes mellitus is diagnosed. The treatment of diabetes should be performed carefully owing to existing liver damage and hepatotoxicity of oral hypoglycemic drugs, particularly in cirrhotic patients. Oral hypoglycemic drugs, like biguanides which improve insulin resistance, for advanced liver diseases may be useful because insulin resistance is considered to be the main cause of glucose intolerance in patients with advanced liver disease[55]. α-glucosidase inhibitors may also be effective, because they reduce the absorption of glucose from the intestine and improve postprandial hyperglycemia[56]. Insulin treatment should be carefully performed in cirrhotic patients, because insulin resistance is increased and the metabolism of insulin decreases as liver diseases advance[55].
Thyroid disease
Thyroid disease often accompanies chronic HCV infection, particularly in elderly women (13% in patients with HCV infection)[56]. Anti-thyroid autoantibodies are also found in patients with chronic HCV infection (0% in men and 14.7% in women)[57]. Interferon-α therapy has been independently associated with the development of thyroid disorders[58,59]. Therefore, thyroid function or anti-thyroid autoantibodies should be evaluated before and during anti-viral treatment with interferon. Furthermore, thyroid cancer is also reported in patients with chronic HCV infection, particularly in those with thyroid autoimmunity[60,61].
Nonalcoholic fatty liver disease (NAFLD)
NAFLD is the most common cause of chronic liver disease in the western world, and NAFLD is known to be accompanied by type-2 diabetes and hyperlipidemia in about 30% of patients[62]. In addition, endocrine disorders such as hypopituitarism or hypothyroidism have been associated with NAFLD (the prevalence is reported to be 2.3% and 15% in NAFLD cases, respectively), although the precise mechanism for this association is unknown[62–64]. Patients with NAFLD accompanied by hypopituitarism may be susceptible to central obesity, dyslipidemia and insulin resistance, leading to disease progression.
HEMATOLOGICAL ABNORMALITIES IN LIVER DISEASES
Erythrocyte abnormalities
About 50%-75% of patients with chronic liver disease develop anemia by various mechanisms[65] including hemodilution, hypersplenism, myelosuppression caused by viral infection or hemolysis (by either immunological or non-immunological mechanisms). Alcoholism has a close association with low dietary intake of folate and vitamin B12, and is known to inhibit the absorption of both nutrients from the gut leading to macrocytic anemia[66,67]. Alcoholic liver cirrhosis is associated with spur cell hemolytic anemia[68], although a recent report revealed that this can also be induced by NASH[69]. Other anemia-causing factors found in liver diseases include hemolysis in Wilson disease, alcoholic liver disease (Zieve syndrome), pregnancy (HELLP syndrome) and myelosuppression followed by viral hepatitis[65].
Leukocyte abnormalities
Leukocytopenia, especially neutropenia, is often found in advanced chronic liver disease. Although splenic sequestration of leukocytes due to hypersplenism or serum hematopoietic progenitor inhibitory factors has been thought to be the main mechanism causing leukocytopenia[70,71], a shortened neutrophil lifespan caused by increased apoptosis may also be responsible[72]. Lymphocytopenia is also often found in patients with liver cirrhosis, possibly due to malnutrition[73]. In contrast, HCV infection can cause monoclonal proliferation of B lymphocytes leading to the induction of various autoimmune disorders[74], and an increased association with the development of non-Hodgkin’s lymphoma has been reported[75]. Interestingly, the functional maturation of B lymphocytes has been proven to occur in the livers of patients with hepatitis C[76].
Platelet abnormalities
Patients with chronic liver disease show a reduction in both number and function of platelets[65], and platelet count is shown to decrease with disease progression, especially in the case of HCV infection. The aspartate aminotransferase to platelet ratio index has recently been reported to be a useful predictor of the progression of liver fibrosis in HBV infection[77] and recurrent HCV infection after liver transplantation[78]. The mechanisms responsible for the decrease in platelet count in chronic liver disease include splenic platelet sequestration due to hypersplenism[79] and a reduced level or activity of thrombopoietin[80], which is a hematopoietic factor for thrombocytes produced by mature hepatocytes[2]. In chronic HCV infection, bone marrow suppression by HCV itself[81], or immune-mediated destruction of platelets through production of anti-platelet antibody or formation of immune complexes[81], has been reported to be the cause of thrombocytopenia. Because platelet-associated immunoglobulin (Ig) G is found in as many as 88% of hepatitis C patients[82], it is not unusual for hepatitis C patients with thrombocytopenia to be diagnosed with idiopathic thrombocytopenic purpura.
With regard to platelet function, deficiency in platelet aggregation[83] or platelet-vessel wall interaction[84] has been reported in patients with cirrhosis, resulting in a tendency to bleed profusely.
Recently, the platelet count to spleen diameter ratio has proven to be effective for ruling out the presence of esophageal varices[85]. Therefore, platelet count is an important parameter for assessing disease progression and the presence of complications in advanced liver disease.
Abnormal coagulation
Because most coagulation factors are only synthesized in the liver[86], liver damage can easily lead to abnormal coagulation or a tendency to bleed profusely. Moreover, disseminated intravascular coagulation (DIC) is often seen in seriously ill patients with conditions including sepsis, malignancies and liver failure. Since both liver failure and DIC present with prolonged prothrombin time (PT), it is sometimes difficult to distinguish the two. It has been reported that a decrease in factor VIII (not synthesized in the liver) and decreasing fibrinogen levels and platelet counts over time could indicate DIC accompanying liver failure rather than liver failure alone[87]. Levels of factor V are also reported to be useful for distinguishing the two conditions, since they are < 10% of normal levels in DIC and 10%-40% of normal levels in cirrhosis[87]. Vitamin K deficiency can also cause prolonged PT, but in contrast to liver failure and DIC, it leads to near normal levels of factor V[87].
In addition to prolonged PT, liver diseases are associated with hyperfibrinolysis[88], dysfibrinogenemia[89], endothelial dysfunction[90] and low count and/or decreased function of platelets[81,83], which all increase the risk of profuse bleeding in patients with advanced liver diseases.
GASTROENTEROLOGICAL ABNORMALITIES IN LIVER DISEASES
The liver has a unique anatomical characteristic in that a large amount of blood is supplied directly to the organ from the intestines via the portal vein. Consequently, various complications of the gastrointestinal tract are seen in advanced liver diseases.
Portal hypertensive gastropathy and esophageal varices
In a recent large-scale study, 37% of patients with HCV infection and advanced fibrosis in the liver were found to have portal hypertensive gastropathy (PHG)[91]. Biochemical markers of liver disease severity such as low serum albumin, high bilirubin or low platelet count may be correlated with the prevalence of PHG[91]. The presence of PHG may be predictive of esophageal varices[91], and low platelet count could be an indicator of the development of varices[92]. Some previous reports suggested that patients with primary biliary cirrhosis (PBC) may develop esophageal varices at a relatively early stage of the disease when other symptoms related to cirrhosis are not exhibited[93,94]. In recent studies, it has been recommended that PBC patients with a decreased platelet count (140 000-200 000/mm3) should be screened for esophageal varices[95,96]. Nonselective beta-blockers may be effective for the treatment of PHG[92].
Pancreatic and biliary cancer
In a recent report, it was found that past exposure to HBV may be associated with the development of pancreatic or biliary tract cancer[97,98]. Anti-HBc antibody tests were positive in 7.6% of pancreatic cancer cases and 3.2% of controls[97], and a 2.4-fold increased risk of extrahepatic bile duct cancer in chronic HBV infection was reported[98]. HBV DNA integration in these tissues may have a pathogenetic influence. However, it is debatable whether the risk of biliary or pancreatic cancers is increased by HCV infection[98,99].
SKIN LESIONS IN LIVER DISEASES
Vascular spiders and palmar erythema are well known as skin lesions in patients with liver cirrhosis[100]. These skin lesions are thought to be related to excess estrogen, although the level of serum estrogen has been reported to be normal in patients with liver cirrhosis[100,101].
Chronic hepatitis infection is thought to be associated with various extrahepatic manifestations such as cutaneous lesions[102,103], including mixed cryoglobulinemia, porphyria cutanea tarda (PCT), lichen planus (LP), pruritus and urticaria. When these skin lesions are found, hepatitis virus infection should be considered.
Mixed cryoglobulinemia
Mixed cryoglobulin contains both a polyclonal IgG and a monoclonal IgM rheumatoid factor, and about 80% of mixed cryoglobulinemia is associated with HCV infection. Diagnosis of mixed cryoglobulinemia is made by skin palpable purpura, low serum complement levels and detection of circulating cryoglobulin[104–107]. Palpable purpura is a major finding suggestive of vasculitis. It is reported that a reduction in HCV load by treatment with interferon[108–110] could decrease serum cryoglobulin levels and improve skin lesions.
Porphyria cutanea tarda
PCT is caused by reduced activity of the enzyme uroporphyrinogen decarboxylase, and exposure to the sun can induce the development of skin erythema, vesicles and bullae[111]. A strong association between sporadic PCT and HCV infection has been reported, and a systematic review showed HCV infection in about 50% of patients with sporadic PCT[112]. Chronic HCV infection may impair porphyrin metabolism and cause sporadic PCT, but the mechanism for this is unclear.
Lichen planus
Although LP is a relatively rare skin disorder, it can be seen in patients with chronic liver diseases, particularly those with HCV infection[113]. It has been reported that anti-HCV antibodies are present in 10%-40% of these patients, but the relationship between HCV infection and LP is still uncertain[113,114]. During interferon treatment for chronic HCV infection, the development or exacerbation of LP has been reported[115]. In contrast, the improvement of oral LP in HCV-infected patients treated with interferon has also been reported[116].
Gianotti-crosti syndrome (GCS)
GCS is characterized by a symmetric papular eruption, which is mainly observed on the cheeks, buttocks and extensor surfaces of the forearms and legs[117]. GCS usually occurs in association with several viral infections, and acute HBV infection has been reported to be one of the most common causes of GCS in infants and young children[118,119]. It is, however, reported that GCS rarely occurs in adults[120].
RENAL DISEASES ASSOCIATED WITH LIVER DISEASES
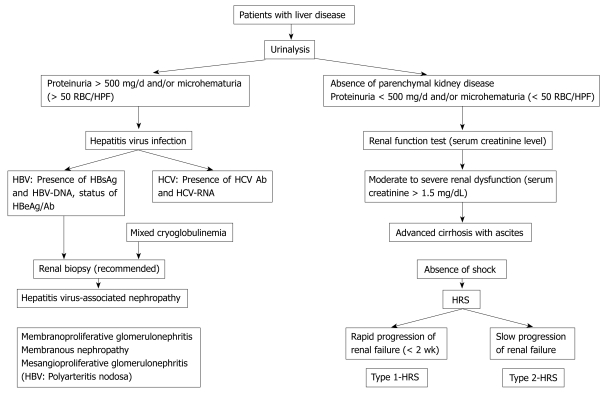
Renal diseases in patients with liver disease can be classified into two major categories by etiology. One is hepatitis virus-associated nephropathy including membranous nephropathy, membranoproliferative glomerulonephritis (MPGN) and mesangioproliferative glomerulonephritis[121–123]. The other is hepato-renal syndrome (HRS), which is a serious complication of advanced liver cirrhosis[124]. When a patient with chronic liver disease has proteinuria and/or hematuria, hepatitis virus-associated nephropathy should be considered (Figure 1)[122].
Figure 1.
Diagnostic strategy for renal disorders found in patients with liver disease. RBC: Red blood cell; HPF: High power field; HBV: Hepatitis B virus; HCV: Hepatitis C virus.
Hepatitis virus-associated nephropathy
HBV infection may be directly associated with a variety of renal diseases, including membranous nephropathy and MPGN[121,125]. The diagnosis is based on an assessment of the status of HBV replication (HBeAg/Ab and HBV DNA levels)[126], laboratory findings (urinalysis and liver function test) and a kidney biopsy, although it is sometimes difficult to detect the deposition of viral antigens in the kidney by routine immunohistochemical analysis. HBV-associated nephrotic syndrome due to membranous nephropathy is not uncommon in children, and spontaneous recovery has been reported, which is often associated with seroconversion of HBeAg to anti-HBe[127]. In adults, on the other hand, spontaneous resolution is relatively uncommon and antiviral therapy may be effective[128].
HBV-related MPGN is characterized by the deposition of antigen-antibody complexes in the mesangium and subendothelial space. Antiviral therapy with interferon[129,130] or lamivudine[131] has been reported to induce remission in HBV-associated MPGN.
HCV infection is more often associated with renal diseases such as mixed cryoglobulinemia, MPGN and membranous nephropathy[122] than is HBV infection. The prevalence of MPGN in patients with HCV infection is higher than that of patients with HBV infection[123]. A high incidence of mixed cryoglobulinemia (35%-90%) has been reported in patients with HCV infection[132–134]. Mixed cryoglobulinemia is a systemic vasculitis and can frequently cause renal disease. MPGN associated with mixed cryoglobulinemia is the predominant type of glomerulonephritis, and the incidence of MPGN in patients with mixed cryoglobulinemia is approximately 30%[123,135,136]. The pathogenesis of HCV-related cryoglobulinemic MPGN is unknown, but the glomerular damage may be caused by the deposition of immune complexes of HCV, and IgG and IgM rheumatoid factors[135]. The clinical manifestations of renal diseases may include hematuria, nephritic range proteinuria and renal insufficiency. HCV-infected patients should be screened for proteinuria, hematuria, hypertension and renal function, as well as for cryoglobulinemia, complement and rheumatoid factors[124]. A kidney biopsy should be performed when significant proteinuria and/or impaired renal function are observed (Figure 1).
There have been many reports on beneficial responses of patients with HCV-induced renal disease to antiviral therapy with interferon[137–139]. Improvements in both serum cryoglobulin levels and plasma creatinine concentration have been reported in patients who exhibited undetectable levels of serum HCV RNA after interferon therapy. A recently developed combination therapy involving pegylated interferon and ribavirin improved the rate of sustained virologic clearance. It has also been reported that this combination regimen improved HCV-associated mixed cryoglobulinemia[140,141], although the efficacy of ribavirin is limited when renal insufficiency is complicated. Recently, the effectiveness of anti-CD20 chimeric monoclonal antibody (rituximab) in the treatment of cryoglobulinemic glomerulonephritis has been reported[142].
HRS
HRS involves renal failure in patients with severe liver disease in the absence of any identifiable renal pathology[124,143,144]. The incidence of HRS in patients with cirrhosis and ascites is 18% and 39% after 1 and 5 years of follow-up, respectively[145]. New criteria for a diagnosis of HRS were reported by the International Ascites Club in 2007 (Table 2)[146]. HRS may be classified into two types: type-1 HRS is characterized by a rapid progression of renal failure (within 2 wk) with the mortality rate at 2 wk being about 80%. In contrast, the degree of renal failure is less severe in patients with type-2 HRS, and median survival is around 4-6 mo[144]. Type-1 HRS is often induced by a precipitating event, in particular spontaneous bacterial peritonitis[147]. HRS is related to renal vasoconstriction following a reduction of effective circulating volume due to peripheral vasodilation[148]. OLT is the ideal treatment for cirrhotic patients with HRS, but the survival rate after OLT is lower in those patients than in patients without HRS (60% vs 70%-80% at three years)[149]. The combined use of vasoconstrictors and albumin is one of the most useful options in treatment of patients with HRS[150].
Table 2.
New diagnostic criteria for hepatorenal syndrome in cirrhosis[146]
| Cirrhosis with ascites |
| Serum creatinine > 133 μmol/L (1.5 mg/dL) |
| No improvement of serum creatinine level (decrease to ≤ 133 μmol/L) after at least 2 d with diuretic withdrawal and volume expansion with albumin. The recommended dose of albumin is 1 g/kg of body weight per day up to a maximum of 100 g/d |
| Absence of shock |
| No current or recent treatment with nephrotoxic drugs |
| Absence of parenchymal kidney disease as indicated by proteinuria > 500 mg/d, microhematuria (> 50 red blood cells per high-power field) and/or abnormal renal ultrasonography |
The diagnostic strategy for hepatitis virus-associated nephropathy and HRS is shown in Figure 1.
NUTRITIONAL ABNORMALITIES IN LIVER DISEASES
Protein-energy malnutrition is often found in patients with liver cirrhosis, and is reported to have an incidence as high as 30%-90%[151]. Malnutrition has been reported to be associated with survival and surgical outcome in cirrhotic patients, and nutritional intervention such as supplementation of branched-chain amino acids (BCAA) could improve patient outcome[152,153]. Hypermetabolism, which is indicated by increased resting energy expenditure, has been reported to be associated with malnutrition, and the measurement of energy metabolism could thus be used to predict the prognosis of liver cirrhosis[154,155]. Hypermetabolism may be explained by increased α-adrenergic activity[156], and further investigation is required to determine whether the administration of beta-blockers is effective for the treatment of malnutrition in patients with liver cirrhosis.
The administration of BCAA has been reported to not only reduce malnutrition and improve energy metabolism, but also to improve the liver function and quality of life of patients with cirrhosis[157–159]. However, BCCA supplementation may be harmful because glucose intolerance can be exhibited by cirrhotic livers[55]. The administration of BCAA and α-glucosidase inhibitors in combination may improve the therapeutic effects[160].
In addition, hepatic glycogen stores decrease in patients with liver cirrhosis because of liver atrophy, leading to the development of a severe catabolic state after fasting. Late evening snacks including BCAA are recommended in order to avoid such problems during the night-time[158,159].
NERVOUS SYSTEM IN LIVER DISEASES
Liver diseases frequently affect the nervous system.
Hepatic encephalopathy
Hepatic encephalopathy (HE) is a major complication of chronic liver diseases with neuropsychiatric manifestations ranging from sleep disturbance to deep coma. The swelling of astrocytes and oxidative stress induced by ammonia, inflammatory cytokines, benzodiazepines and hyponatremia have been regarded as essential causes of HE[161]. Psychometric tests such as the number connection test may be effective for assessing HE[162], and neuropsychiatric function should be carefully evaluated in patients with chronic liver diseases including alcoholic liver disease and Wilson disease (WD). Minimal hepatic encephalopathy (MHE) occurs in 30%-80% of cirrhotic patients, which could be a serious problem because of the associated impaired quality of life[161,163]. For the management of patients with HE, removal of precipitating causes, such as gastrointestinal bleeding, excessive protein intake, hypokalemic alkalosis, infection, constipation (which can all increase blood ammonia levels), hypovolemia, hypoglycemia, hypoxia and the administration of sedatives, is important. Lactulose is the most effective therapy for HE so far, and has been reported to even be effective for patients with MHE[164].
Polyneuropathy and neurocognitive dysfunction
Nervous system disorders such as neuropathy, fatigue and depression are often associated with chronic HCV infection, even without advanced cirrhosis[165–167]. Mixed cryoglobulinemia in HCV causes peripheral neuropathy as a moderate axonal sensory polyneuropathy[168], and chronic sensory polyneuropathies were found in 13% of HCV infected patients with cryoglobulinemia in southern Italy[169]. In addition, paresthesia has been found in about 20% of patients with chronic HCV infection, particularly among those with cryoglobulinemia[170,171]. The therapeutic response to antiviral treatment for neuropathies is generally unsatisfactory[172]. Furthermore, fatigue or depression is found in many patients with chronic HCV infection, with incidences of about 50% and 35%, respectively[173–175]. These neurocognitive dysfunctions have been characterized epidemiologically or pathophysiologically in chronic HCV infection[166,167,176], and may be explained by the neuroinvasion of HCV, because HCV has been reported to be found in monocytes/microglia of the central nervous system[176,177]. However, antiviral treatment with interferon, particularly interferon-α, is known to exacerbate depression[178].
Guillain-Barre syndrome
Guillain-Barre syndrome accompanying hepatitis virus infection, which is primarily associated with HBV and rarely with HCV or hepatitis A virus, has also been reported[179–181]. Immune complexes have been found in the serum of these patients, which may be the cause of the development of the disease.
Autonomic and sensory nerve dysfunction
Autonomic and sensory nerve dysfunction presenting as reduced 24 h heart rate variability and lower current perception threshold has also been reported to be common in patients with PBC, particularly those suffering a severe form of the disease for a long period[182]. Peripheral sensory nerve dysfunction may contribute to hyperesthesia, leading to the itching that is a characteristic symptom of PBC[182].
Wilson disease
WD is an autosomal recessive inherited disorder of copper metabolism, resulting in excessive accumulation of copper in virtually all organs, especially in the liver. The clinical spectrum of liver diseases in WD is broad from asymptomatic with only mild biochemical abnormalities, chronic active hepatitis, liver cirrhosis to fulminant hepatitis[183]. Copper accumulation is also remarkable in the cornea (Kayser-Fleischer rings) and brain in patients with WD. The patients show various neuropsychiatric symptoms such as dysarthria, dyspraxia, ataxia, a tremor-rigidity syndrome and psychoses, and progressive extrapyramidal neurological disorder is the typical sign of neurologic WD[183,184]. The initial neurological symptoms usually develop in mid-teenage years or in the twenties and are frequently misdiagnosed as behavioral problems associated with puberty[184].
BONE DISEASE ASSOCIATED WITH LIVER DISEASES
Metabolic bone disease is a common complication of chronic liver disease, particularly in patients with end-stage liver disease due to cholestatic disorders such as PBC. It has been reported that osteoporosis occurs in approximately 20%-30% of patients with PBC[185,186], and older age and severity of the disease were the main risk factors for osteoporosis[187]. Moreover, there were 2-fold relative increases in the risk of bone fractures in patients with PBC compared with the general population[188]. Although the mechanisms responsible for osteoporosis in liver diseases are not well understood, cirrhotic patients show reduced osteoid thickness, osteoblast surfaces and bone formation rate, suggesting the presence of an osteoblast defect[189].
In post-liver transplantation populations, osteoporosis is known to be a major complication[190]. Several studies suggest that bone loss is remarkable within the first year after liver transplantation. The etiology is multifactorial, consisting of both preexisting low bone mineral density associated with underlying liver disease and post-transplantation factors[190]. High-dose corticosteroids and immunosuppressive agents such as cyclosporine A are thought to contribute to bone loss[191]. Although few prospective studies are available, vitamin D and calcium supplementation are generally recommended for those patients[192].
ARTHROPATHY IN LIVER DISEASES
Arthralgia or arthritis is often seen in patients with liver diseases, and it is not rare that arthropathy may be the first presentation of the disease[193]. In acute viral hepatitis, especially in HBV infection, patients may show polyarthralgia or polyarthritis during the prodromal period and immune complex is thought to be responsible for the pathogenesis[194]. Similar arthropathies are seen in patients with chronic hepatitis C, autoimmune hepatitis, PBC, hemochromatosis or WD[193]. Arthralgia or arthritis is reported to be the most common extrahepatic manifestation in patients with hemochromatosis, autoimmune hepatitis and PBC. Polyarthralgia is also the most common symptom of mixed cryoglobulinemia[195], which often occurs secondary to HCV infection.
SEXUAL DYSFUNCTION IN LIVER DISEASES
Erectile dysfunction (ED) is frequently problematic in patients with chronic liver diseases such as hemochromatosis[196], alcoholic liver disease[197] or liver transplant patients[198], leading to worsening of quality of life in those patients. Hypogonadism or protein malnutrition found in patients with advanced liver disease may induce ED[199,200]. Removal of causative factors such as iron or ethanol, or administration of testosterone may improve ED in such patients[197,198]. ED is also more frequent in patients with chronic HCV infection than in control subjects (39% vs 14%, respectively) probably due to a direct effect of HCV on neurovascular and hormonal systems (i.e. low testosterone levels)[201]. Successful antiviral treatment such as interferon plus ribavirin may improve such sexual dysfunction in patients with chronic HCV infection[202].
DOSAGE ADJUSTMENT OF DRUGS IN LIVER DISEASES
The liver is the main organ of biotransformation and elimination of drugs. Thus, liver diseases could affect drug metabolism, resulting in abnormally high concentrations of drugs in the body.
Drug elimination by the liver may be determined mainly by first-pass effect, hepatic metabolism and biliary extraction. In addition, since the liver produces most plasma proteins, decreased liver function could influence the binding of drugs to plasma proteins, leading to changes in the distribution and elimination of such drugs[203].
The first-pass effect of each drug is variable and drugs with high first-pass effects are listed in Table 3[204]. The serum concentration of these drugs could easily be elevated by a decrease in hepatic blood flow (especially portal blood flow) or total hepatocyte mass. For drugs with a high first-pass effect, both initial dose and maintenance dose should be reduced in cirrhotic patients if the drug is orally administered.
Table 3.
Drugs that may need to be administered at reduced doses in patients with liver cirrhosis[187]
| Drugs with high first-pass effect |
Drugs metabolized mainly by |
||
| CYP 1A2 | CYP3A4 | CYP2C9 | |
| Amitriptyline | Acetaminophen | Quinidine | Diclofenac |
| Bromocriptine | Caffeine | Amiodarone | Ibuprofen |
| Diltiazem | Mexiletine | Lidocaine | Mefenamic acid |
| Flumazenil | (R)-Warfarin | Midazolam | Tolbutamide |
| Fluorouracil | Imipramine | Diazepam | Phenytoin |
| Imipramine | Theophylline | Amitriptyline | Phenobarbital |
| Isosorbide dinitrate | Propranolol | Imipramine | (S)-Warfarin |
| Labetalol | Tamoxifen | Carbamazepine | Losartan |
| Lidocaine | Estradiol | (R)-Warfarin | Piroxicam |
| Morphine | Erythromycin | ||
| Nifedipine | Clarithromycin | ||
| Pentazocine | |||
| Propranolol | |||
| Verapamil | |||
Drug metabolism in the liver largely depends on the activity of the cytochrome P (CYP) 450 enzymes, which is known to be affected in different ways in patients with cirrhosis. The activities of CYP3A, 1A and 2C19 are reported to be substantially reduced, whereas those of CYP2D6, 2C9, 2B and 2E1 are also reduced, but to a lesser extent[205]. The severity of liver cirrhosis is estimated using Child-Pugh (C-P) classification, and patients with C-P grade A show mild to moderate deterioration of CYP activities. On the other hand, patients with C-P grade B or C are shown to have prominent reduction in CYP activity. Therefore, the doses of drugs mainly metabolized by CYP 3A, 1A or 2C19 in the liver may need to be reduced in these patients (Table 3)[206]. Moreover, cirrhotic patients often have impaired renal function, despite a normal serum creatinine level[207]. Therefore, creatinine clearance should be measured or estimated in cirrhotic patients to determine the appropriate dose of drugs with predominant renal excretion.
RISK OF SURGERY IN PATIENTS WITH LIVER DISEASES
Patients with liver diseases face relatively high risks during surgery, and these risks could be increased according to the progression of liver disease. A decrease in hepatic blood flow during anesthesia or surgery is thought to be mainly responsible for postoperative liver damage, and Cowan et al[208] reported that a major reduction in hepatic blood flow occurs after the induction of anesthesia, but not during or after surgery.
In acute viral hepatitis, as well as acute alcoholic hepatitis, the risk of surgery might be extremely high. Therefore, surgery should be avoided unless the situation is life-threatening[209]. In contrast, surgery on patients with chronic hepatitis can generally be considered safe[210], and there have been no deaths or complications reported in patients with chronic hepatitis C undergoing laparoscopic cholecystectomy[211]. In patients with liver cirrhosis, complications and mortality rates of surgery are high[212], especially if one or more of the following factors apply to the patient: elevated bilirubin, prolonged PT, ascites, decreased albumin, encephalopathy, portal hypertension, elevated creatinine concentration, intraoperative hypotension and emergent surgery[213]. The outcome of surgery also depends on the invasiveness or duration of the operation[214]. There are two scores for the assessment of the progression of liver cirrhosis: the model for end-stage liver disease (MELD) score and the C-P grade[215]. Both scores are useful for assessing the risk of surgery, and C-P classification was reported to be useful in stratifying the risk of death. Two studies showed that patients in class A had mortality rates of about 10%, those in grade B had mortality rates of around 30%, and those in grade C had mortality rates above 70%[216,217]. A recent study in Italy also reported similar mortality rates of surgery for patients with liver cirrhosis (C-P grade A; 7.1%, C-P grade B; 23%, C-P grade C; 84%)[218]. Teh et al[219] recently reported that MELD score, age and American Society of Anesthesiologists class are important predictors for the risk of postoperative mortality in patients with cirrhosis. Interestingly, they also reported that the risk was not associated with the type of surgery performed. For MELD, operation risks increase according to the score, and one report showed that a MELD score ≥ 14 or plasma hemoglobin levels < 10 g/dL were independent predictors of poor outcomes in patients undergoing abdominal surgery excluding hepatic surgery[220]. For patients undergoing laparoscopic cholecystectomy, a preoperative MELD score of 8 was linked to high morbidity, and was suggested as the cutoff value for avoiding the operation in patients with liver cirrhosis[221]. However, Schiff et al[222] recently reported that preoperative platelet levels and PT (international normalized ratio) are more important factors for the safety of cholecystectomy than C-P grade.
In patients undergoing cardiac surgery, the risk of mortality or complications may be high when a cardiopulmonary bypass is performed on patients with chronic liver disease[223]. In general, the mortality of cirrhotic patients with C-P grade A is low, but that of patients exhibiting C-P grade B for a long period and that of all patients with C-P grade C is high, especially when open heart surgery is performed. Therefore, open heart surgery should be avoided for patients with C-P grade C, and cardiac operation on the beating heart is recommended for patients with C-P grade B[224]. The results of another study led to the recommendation that patients with a C-P score > 7 avoid cardiac surgery involving cardiopulmonary bypass[225].
In summary, surgery should be avoided for patients with acute hepatitis. However, surgery is generally safe for patients with chronic hepatitis and cirrhotic patients with C-P grade A. The risks are elevated for cirrhotic patients with C-P grade B or C, or patients with a MELD score of ≥ 8, though this might vary according to the type of surgery performed. Other predictive factors for safe surgery are platelet count and PT, both of which are markers for the tendency to bleed profusely and for the progression of liver disease.
CONCLUSION
Liver diseases often cause systemic abnormalities, and it is not uncommon that these complications, rather than the liver disease itself, determine the quality of life and prognosis of patients. Therefore, both hepatologists and non-hepatologists should always pay attention to the abnormalities caused by liver diseases, and should be concerned with their management in addition to the actual treatment of the liver disease itself.
Peer reviewers: Akihito Tsubota, Assistant Professor, Institute of Clinical Medicine and Research, Jikei University School of Medicine, 163-1 Kashiwa-shita, Kashiwa, Chiba 277-8567, Japan; Peter Ferenci, Professor, Department of Internal Medicine IV/Division of Gastroenterology and Hepatology, Waehringer Guertel 18-20, Vienna A-1090, Austria
S- Editor Tian L L- Editor O’Neill M E- Editor Yin DH
References
- 1.Guyton AC, Hall JE. The liver as an organ. In: AC Guyton, JE Hall., editors. Textbook of Medical Physiology. 11th ed. Elsevier Saunders: Pennsylvania PA; 2006. pp. 859–864. [Google Scholar]
- 2.Shimada Y, Kato T, Ogami K, Horie K, Kokubo A, Kudo Y, Maeda E, Sohma Y, Akahori H, Kawamura K. Production of thrombopoietin (TPO) by rat hepatocytes and hepatoma cell lines. Exp Hematol. 1995;23:1388–1396. [PubMed] [Google Scholar]
- 3.Naeije R, Melot C, Hallemans R, Mols P, Lejeune P. Pulmonary hemodynamics in liver cirrhosis. Semin Respir Med. 1985;7:164–170. [Google Scholar]
- 4.Rodríguez-Roisin R, Krowka MJ, Herve P, Fallon MB. Pulmonary-Hepatic vascular Disorders (PHD) Eur Respir J. 2004;24:861–880. doi: 10.1183/09031936.04.00010904. [DOI] [PubMed] [Google Scholar]
- 5.Berthelot P, Walker JG, Sherlock S, Reid L. Arterial changes in the lungs in cirrhosis of the liver--lung spider nevi. N Engl J Med. 1966;274:291–298. doi: 10.1056/NEJM196602102740601. [DOI] [PubMed] [Google Scholar]
- 6.Schenk P, Fuhrmann V, Madl C, Funk G, Lehr S, Kandel O, Müller C. Hepatopulmonary syndrome: prevalence and predictive value of various cut offs for arterial oxygenation and their clinical consequences. Gut. 2002;51:853–859. doi: 10.1136/gut.51.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodríguez-Roisin R, Krowka MJ. Hepatopulmonary syndrome--a liver-induced lung vascular disorder. N Engl J Med. 2008;358:2378–2387. doi: 10.1056/NEJMra0707185. [DOI] [PubMed] [Google Scholar]
- 8.Rolla G, Brussino L, Colagrande P, Dutto L, Polizzi S, Scappaticci E, Bergerone S, Morello M, Marzano A, Martinasso G, et al. Exhaled nitric oxide and oxygenation abnormalities in hepatic cirrhosis. Hepatology. 1997;26:842–847. doi: 10.1053/jhep.1997.v26.pm0009328302. [DOI] [PubMed] [Google Scholar]
- 9.Castro M, Krowka MJ. Hepatopulmonary syndrome. A pulmonary vascular complication of liver disease. Clin Chest Med. 1996;17:35–48. doi: 10.1016/s0272-5231(05)70297-5. [DOI] [PubMed] [Google Scholar]
- 10.Krowka MJ, Wiseman GA, Burnett OL, Spivey JR, Therneau T, Porayko MK, Wiesner RH. Hepatopulmonary syndrome: a prospective study of relationships between severity of liver disease, PaO(2) response to 100% oxygen, and brain uptake after (99m)Tc MAA lung scanning. Chest. 2000;118:615–624. doi: 10.1378/chest.118.3.615. [DOI] [PubMed] [Google Scholar]
- 11.Stoller JK, Lange PA, Westveer MK, Carey WD, Vogt D, Henderson JM. Prevalence and reversibility of the hepatopulmonary syndrome after liver transplantation. The Cleveland Clinic experience. West J Med. 1995;163:133–138. [PMC free article] [PubMed] [Google Scholar]
- 12.Abrams GA, Jaffe CC, Hoffer PB, Binder HJ, Fallon MB. Diagnostic utility of contrast echocardiography and lung perfusion scan in patients with hepatopulmonary syndrome. Gastroenterology. 1995;109:1283–1288. doi: 10.1016/0016-5085(95)90589-8. [DOI] [PubMed] [Google Scholar]
- 13.Gupta D, Vijaya DR, Gupta R, Dhiman RK, Bhargava M, Verma J, Chawla YK. Prevalence of hepatopulmonary syndrome in cirrhosis and extrahepatic portal venous obstruction. Am J Gastroenterol. 2001;96:3395–3399. doi: 10.1111/j.1572-0241.2001.05274.x. [DOI] [PubMed] [Google Scholar]
- 14.Mandell MS. Hepatopulmonary syndrome and portopulmonary hypertension in the model for end-stage liver disease (MELD) era. Liver Transpl. 2004;10:S54–S58. doi: 10.1002/lt.20260. [DOI] [PubMed] [Google Scholar]
- 15.Rodríquez-Roisin R, Krowka MJ, Hervé P, Fallon MB. Highlights of the ERS Task Force on pulmonary-hepatic vascular disorders (PHD) J Hepatol. 2005;42:924–927. doi: 10.1016/j.jhep.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Battaglia SE, Pretto JJ, Irving LB, Jones RM, Angus PW. Resolution of gas exchange abnormalities and intrapulmonary shunting following liver transplantation. Hepatology. 1997;25:1228–1232. doi: 10.1002/hep.510250527. [DOI] [PubMed] [Google Scholar]
- 17.Colle IO, Moreau R, Godinho E, Belghiti J, Ettori F, Cohen-Solal A, Mal H, Bernuau J, Marty J, Lebrec D, et al. Diagnosis of portopulmonary hypertension in candidates for liver transplantation: a prospective study. Hepatology. 2003;37:401–409. doi: 10.1053/jhep.2003.50060. [DOI] [PubMed] [Google Scholar]
- 18.Krowka MJ. Portopulmonary hypertension: diagnostic advances and caveats. Liver Transpl. 2003;9:1336–1337. doi: 10.1002/lt.500091215. [DOI] [PubMed] [Google Scholar]
- 19.Ramsay MA, Simpson BR, Nguyen AT, Ramsay KJ, East C, Klintmalm GB. Severe pulmonary hypertension in liver transplant candidates. Liver Transpl Surg. 1997;3:494–500. doi: 10.1002/lt.500030503. [DOI] [PubMed] [Google Scholar]
- 20.Hervé P, Lebrec D, Brenot F, Simonneau G, Humbert M, Sitbon O, Duroux P. Pulmonary vascular disorders in portal hypertension. Eur Respir J. 1998;11:1153–1166. doi: 10.1183/09031936.98.11051153. [DOI] [PubMed] [Google Scholar]
- 21.Krowka MJ, Plevak DJ, Findlay JY, Rosen CB, Wiesner RH, Krom RA. Pulmonary hemodynamics and perioperative cardiopulmonary-related mortality in patients with portopulmonary hypertension undergoing liver transplantation. Liver Transpl. 2000;6:443–450. doi: 10.1053/jlts.2000.6356. [DOI] [PubMed] [Google Scholar]
- 22.Ueda T, Ohta K, Suzuki N, Yamaguchi M, Hirai K, Horiuchi T, Watanabe J, Miyamoto T, Ito K. Idiopathic pulmonary fibrosis and high prevalence of serum antibodies to hepatitis C virus. Am Rev Respir Dis. 1992;146:266–268. doi: 10.1164/ajrccm/146.1.266. [DOI] [PubMed] [Google Scholar]
- 23.Meliconi R, Andreone P, Fasano L, Galli S, Pacilli A, Miniero R, Fabbri M, Solforosi L, Bernardi M. Incidence of hepatitis C virus infection in Italian patients with idiopathic pulmonary fibrosis. Thorax. 1996;51:315–317. doi: 10.1136/thx.51.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arase Y, Suzuki F, Suzuki Y, Akuta N, Kobayashi M, Kawamura Y, Yatsuji H, Sezaki H, Hosaka T, Hirakawa M, et al. Hepatitis C virus enhances incidence of idiopathic pulmonary fibrosis. World J Gastroenterol. 2008;14:5880–5886. doi: 10.3748/wjg.14.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howell WL, Manion WC. The low incidence of myocardial infarction in patients with portal cirrhosis of the liver: A review of 639 cases of cirrhosis of the liver from 17,731 autopsies. Am Heart J. 1960;60:341–344. doi: 10.1016/0002-8703(60)90192-7. [DOI] [PubMed] [Google Scholar]
- 26.Vanĕcek R. Atherosclerosis and cirrhosis of the liver. Bull World Health Organ. 1976;53:567–570. [PMC free article] [PubMed] [Google Scholar]
- 27.Marchesini G, Ronchi M, Forlani G, Bugianesi E, Bianchi G, Fabbri A, Zoli M, Melchionda N. Cardiovascular disease in cirrhosis--a point-prevalence study in relation to glucose tolerance. Am J Gastroenterol. 1999;94:655–662. doi: 10.1111/j.1572-0241.1999.00931.x. [DOI] [PubMed] [Google Scholar]
- 28.Berzigotti A, Bonfiglioli A, Muscari A, Bianchi G, Libassi S, Bernardi M, Zoli M. Reduced prevalence of ischemic events and abnormal supraortic flow patterns in patients with liver cirrhosis. Liver Int. 2005;25:331–336. doi: 10.1111/j.1478-3231.2005.01002.x. [DOI] [PubMed] [Google Scholar]
- 29.Alyan O, Kacmaz F, Ozdemir O, Deveci B, Astan R, Celebi AS, Ilkay E. Hepatitis C infection is associated with increased coronary artery atherosclerosis defined by modified Reardon severity score system. Circ J. 2008;72:1960–1965. doi: 10.1253/circj.cj-08-0459. [DOI] [PubMed] [Google Scholar]
- 30.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 31.Kadayifci A, Tan V, Ursell PC, Merriman RB, Bass NM. Clinical and pathologic risk factors for atherosclerosis in cirrhosis: a comparison between NASH-related cirrhosis and cirrhosis due to other aetiologies. J Hepatol. 2008;49:595–599. doi: 10.1016/j.jhep.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 32.Kowalski HJ, Abelmann WH. The cardiac output at rest in Laennec's cirrhosis. J Clin Invest. 1953;32:1025–1033. doi: 10.1172/JCI102813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murray JF, Dawson AM, Sherlock S. Circulatory changes in chronic liver disease. Am J Med. 1958;24:358–367. doi: 10.1016/0002-9343(58)90322-x. [DOI] [PubMed] [Google Scholar]
- 34.Matsumori A, Matoba Y, Sasayama S. Dilated cardiomyopathy associated with hepatitis C virus infection. Circulation. 1995;92:2519–2525. doi: 10.1161/01.cir.92.9.2519. [DOI] [PubMed] [Google Scholar]
- 35.Teragaki M, Nishiguchi S, Takeuchi K, Yoshiyama M, Akioka K, Yoshikawa J. Prevalence of hepatitis C virus infection among patients with hypertrophic cardiomyopathy. Heart Vessels. 2003;18:167–170. doi: 10.1007/s00380-003-0705-0. [DOI] [PubMed] [Google Scholar]
- 36.Matsumori A. Hepatitis C virus infection and cardiomyopathies. Circ Res. 2005;96:144–147. doi: 10.1161/01.RES.0000156077.54903.67. [DOI] [PubMed] [Google Scholar]
- 37.Matsumori A, Matoba Y, Nishio R, Shioi T, Ono K, Sasayama S. Detection of hepatitis C virus RNA from the heart of patients with hypertrophic cardiomyopathy. Biochem Biophys Res Commun. 1996;222:678–682. doi: 10.1006/bbrc.1996.0803. [DOI] [PubMed] [Google Scholar]
- 38.Matsumori A, Ohashi N, Hasegawa K, Sasayama S, Eto T, Imaizumi T, Izumi T, Kawamura K, Kawana M, Kimura A, et al. Hepatitis C virus infection and heart diseases: a multicenter study in Japan. Jpn Circ J. 1998;62:389–391. doi: 10.1253/jcj.62.389. [DOI] [PubMed] [Google Scholar]
- 39.Prati D, Poli F, Farma E, Picone A, Porta E, De Mattei C, Zanella A, Scalamogna M, Gamba A, Gronda E, et al. Multicenter study on hepatitis C virus infection in patients with dilated cardiomyopathy. North Italy Transplant Program (NITP) J Med Virol. 1999;58:116–120. doi: 10.1002/(sici)1096-9071(199906)58:2<116::aid-jmv3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 40.Grumbach IM, Heermann K, Figulla HR. Low prevalence of hepatitis C virus antibodies and RNA in patients with myocarditis and dilated cardiomyopathy. Cardiology. 1998;90:75–78. doi: 10.1159/000006822. [DOI] [PubMed] [Google Scholar]
- 41.Dalekos GN, Achenbach K, Christodoulou D, Liapi GK, Zervou EK, Sideris DA, Tsianos EV. Idiopathic dilated cardiomyopathy: lack of association with hepatitis C virus infection. Heart. 1998;80:270–275. doi: 10.1136/hrt.80.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shichi D, Matsumori A, Naruse TK, Inoko H, Kimura A. HLA-DPbeta chain may confer the susceptibility to hepatitis C virus-associated hypertrophic cardiomyopathy. Int J Immunogenet. 2008;35:37–43. doi: 10.1111/j.1744-313X.2007.00733.x. [DOI] [PubMed] [Google Scholar]
- 43.Bristow MR. Tumor necrosis factor-alpha and cardiomyopathy. Circulation. 1998;97:1340–1341. doi: 10.1161/01.cir.97.14.1340. [DOI] [PubMed] [Google Scholar]
- 44.Zhu N, Khoshnan A, Schneider R, Matsumoto M, Dennert G, Ware C, Lai MM. Hepatitis C virus core protein binds to the cytoplasmic domain of tumor necrosis factor (TNF) receptor 1 and enhances TNF-induced apoptosis. J Virol. 1998;72:3691–3697. doi: 10.1128/jvi.72.5.3691-3697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Omura T, Yoshiyama M, Hayashi T, Nishiguchi S, Kaito M, Horiike S, Fukuda K, Inamoto S, Kitaura Y, Nakamura Y, et al. Core protein of hepatitis C virus induces cardiomyopathy. Circ Res. 2005;96:148–150. doi: 10.1161/01.RES.0000154263.70223.13. [DOI] [PubMed] [Google Scholar]
- 46.Zimmerman S, Adkins D, Graham M, Petruska P, Bowers C, Vrahnos D, Spitzer G. Irreversible, severe congestive cardiomyopathy occurring in association with interferon alpha therapy. Cancer Biother. 1994;9:291–299. doi: 10.1089/cbr.1994.9.291. [DOI] [PubMed] [Google Scholar]
- 47.Mason AL, Lau JY, Hoang N, Qian K, Alexander GJ, Xu L, Guo L, Jacob S, Regenstein FG, Zimmerman R, et al. Association of diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;29:328–333. doi: 10.1002/hep.510290235. [DOI] [PubMed] [Google Scholar]
- 48.Caronia S, Taylor K, Pagliaro L, Carr C, Palazzo U, Petrik J, O'Rahilly S, Shore S, Tom BD, Alexander GJ. Further evidence for an association between non-insulin-dependent diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;30:1059–1063. doi: 10.1002/hep.510300416. [DOI] [PubMed] [Google Scholar]
- 49.Mehta SH, Brancati FL, Sulkowski MS, Strathdee SA, Szklo M, Thomas DL. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Intern Med. 2000;133:592–599. doi: 10.7326/0003-4819-133-8-200010170-00009. [DOI] [PubMed] [Google Scholar]
- 50.Mehta SH, Brancati FL, Strathdee SA, Pankow JS, Netski D, Coresh J, Szklo M, Thomas DL. Hepatitis C virus infection and incident type 2 diabetes. Hepatology. 2003;38:50–56. doi: 10.1053/jhep.2003.50291. [DOI] [PubMed] [Google Scholar]
- 51.Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Tsukamoto K, Kimura S, Moriya K, Koike K. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology. 2004;126:840–848. doi: 10.1053/j.gastro.2003.11.056. [DOI] [PubMed] [Google Scholar]
- 52.Moucari R, Asselah T, Cazals-Hatem D, Voitot H, Boyer N, Ripault MP, Sobesky R, Martinot-Peignoux M, Maylin S, Nicolas-Chanoine MH, et al. Insulin resistance in chronic hepatitis C: association with genotypes 1 and 4, serum HCV RNA level, and liver fibrosis. Gastroenterology. 2008;134:416–423. doi: 10.1053/j.gastro.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 53.Hickman IJ, Macdonald GA. Impact of diabetes on the severity of liver disease. Am J Med. 2007;120:829–834. doi: 10.1016/j.amjmed.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 54.El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460–468. doi: 10.1053/j.gastro.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 55.Garcia-Compean D, Jaquez-Quintana JO, Gonzalez-Gonzalez JA, Maldonado-Garza H. Liver cirrhosis and diabetes: risk factors, pathophysiology, clinical implications and management. World J Gastroenterol. 2009;15:280–288. doi: 10.3748/wjg.15.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Antonelli A, Ferri C, Pampana A, Fallahi P, Nesti C, Pasquini M, Marchi S, Ferrannini E. Thyroid disorders in chronic hepatitis C. Am J Med. 2004;117:10–13. doi: 10.1016/j.amjmed.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 57.Marazuela M, García-Buey L, González-Fernández B, García-Monzón C, Arranz A, Borque MJ, Moreno-Otero R. Thyroid autoimmune disorders in patients with chronic hepatitis C before and during interferon-alpha therapy. Clin Endocrinol (Oxf) 1996;44:635–642. doi: 10.1046/j.1365-2265.1996.751768.x. [DOI] [PubMed] [Google Scholar]
- 58.Preziati D, La Rosa L, Covini G, Marcelli R, Rescalli S, Persani L, Del Ninno E, Meroni PL, Colombo M, Beck-Peccoz P. Autoimmunity and thyroid function in patients with chronic active hepatitis treated with recombinant interferon alpha-2a. Eur J Endocrinol. 1995;132:587–593. doi: 10.1530/eje.0.1320587. [DOI] [PubMed] [Google Scholar]
- 59.Fernandez-Soto L, Gonzalez A, Escobar-Jimenez F, Vazquez R, Ocete E, Olea N, Salmeron J. Increased risk of autoimmune thyroid disease in hepatitis C vs hepatitis B before, during, and after discontinuing interferon therapy. Arch Intern Med. 1998;158:1445–1448. doi: 10.1001/archinte.158.13.1445. [DOI] [PubMed] [Google Scholar]
- 60.Antonelli A, Ferri C, Fallahi P. Thyroid cancer in patients with hepatitis C infection. JAMA. 1999;281:1588. doi: 10.1001/jama.281.17.1588. [DOI] [PubMed] [Google Scholar]
- 61.Antonelli A, Ferri C, Fallahi P, Nesti C, Zignego AL, Maccheroni M. Thyroid cancer in HCV-related mixed cryoglobulinemia patients. Clin Exp Rheumatol. 2002;20:693–696. [PubMed] [Google Scholar]
- 62.Vuppalanchi R, Chalasani N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Selected practical issues in their evaluation and management. Hepatology. 2009;49:306–317. doi: 10.1002/hep.22603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Adams LA, Feldstein A, Lindor KD, Angulo P. Nonalcoholic fatty liver disease among patients with hypothalamic and pituitary dysfunction. Hepatology. 2004;39:909–914. doi: 10.1002/hep.20140. [DOI] [PubMed] [Google Scholar]
- 64.Liangpunsakul S, Chalasani N. Is hypothyroidism a risk factor for non-alcoholic steatohepatitis? J Clin Gastroenterol. 2003;37:340–343. doi: 10.1097/00004836-200310000-00014. [DOI] [PubMed] [Google Scholar]
- 65.Senzolo M, Burroughs AK. Haematological abnormalities in liver disease. In: J Rodés, JP Benhaumou, AT Blei, J Reichen, M Rizzetto, et al., editors. Textbook of Hepatology. 3rd ed. Blackwell Sci Pub: Oxford; 2007. pp. 1767–1779. [Google Scholar]
- 66.Hamid A, Wani NA, Rana S, Vaiphei K, Mahmood A, Kaur J. Down-regulation of reduced folate carrier may result in folate malabsorption across intestinal brush border membrane during experimental alcoholism. FEBS J. 2007;274:6317–6328. doi: 10.1111/j.1742-4658.2007.06150.x. [DOI] [PubMed] [Google Scholar]
- 67.Lambert D, Benhayoun S, Adjalla C, Gélot MM, Renkes P, Gérard P, Felden F, Belleville F, Gaucher P, Guéant JL, et al. Alcoholic cirrhosis and cobalamin metabolism. Digestion. 1997;58:64–71. doi: 10.1159/000201425. [DOI] [PubMed] [Google Scholar]
- 68.Arienti G, Carlini E, Scionti L, Puxeddu E, Brunetti P. Liver alcoholic cirrhosis and spur-cell (acanthocytic) anaemia. A study of erythrocyte ghost composition and fluidity. Scand J Gastroenterol. 1995;30:1204–1209. doi: 10.3109/00365529509101632. [DOI] [PubMed] [Google Scholar]
- 69.Haruta I, Hashimoto E, Kabutake A, Taniai M, Tokushige K, Shiratori K. Spur cell anemia associated with a cirrhotic non-alcoholic steatohepatitis patient. Hepatol Res. 2007;37:482–485. doi: 10.1111/j.1872-034X.2007.00037.x. [DOI] [PubMed] [Google Scholar]
- 70.Uchida T, Kariyone S. Intravascular granulocyte kinetics and spleen size in patients with neutropenia and chronic splenomegaly. J Lab Clin Med. 1973;82:9–19. [PubMed] [Google Scholar]
- 71.Ohki I, Dan K, Kuriya S, Nomura T. A study on the mechanism of anemia and leukopenia in liver cirrhosis. Jpn J Med. 1988;27:155–159. doi: 10.2169/internalmedicine1962.27.155. [DOI] [PubMed] [Google Scholar]
- 72.Kusaba N, Kumashiro R, Ogata H, Sata M, Tanikawa K. In vitro study of neutrophil apoptosis in liver cirrhosis. Intern Med. 1998;37:11–17. doi: 10.2169/internalmedicine.37.11. [DOI] [PubMed] [Google Scholar]
- 73.O'Keefe SJ, El-Zayadi AR, Carraher TE, Davis M, Williams R. Malnutrition and immuno-incompetence in patients with liver disease. Lancet. 1980;2:615–617. doi: 10.1016/s0140-6736(80)90284-6. [DOI] [PubMed] [Google Scholar]
- 74.Landau DA, Saadoun D, Calabrese LH, Cacoub P. The pathophysiology of HCV induced B-cell clonal disorders. Autoimmun Rev. 2007;6:581–587. doi: 10.1016/j.autrev.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 75.Schöllkopf C, Smedby KE, Hjalgrim H, Rostgaard K, Panum I, Vinner L, Chang ET, Glimelius B, Porwit A, Sundström C, et al. Hepatitis C infection and risk of malignant lymphoma. Int J Cancer. 2008;122:1885–1890. doi: 10.1002/ijc.23416. [DOI] [PubMed] [Google Scholar]
- 76.Murakami J, Shimizu Y, Kashii Y, Kato T, Minemura M, Okada K, Nambu S, Takahara T, Higuchi K, Maeda Y, et al. Functional B-cell response in intrahepatic lymphoid follicles in chronic hepatitis C. Hepatology. 1999;30:143–150. doi: 10.1002/hep.510300107. [DOI] [PubMed] [Google Scholar]
- 77.Kim BK, Kim SA, Park YN, Cheong JY, Kim HS, Park JY, Cho SW, Han KH, Chon CY, Moon YM, et al. Noninvasive models to predict liver cirrhosis in patients with chronic hepatitis B. Liver Int. 2007;27:969–976. doi: 10.1111/j.1478-3231.2007.01519.x. [DOI] [PubMed] [Google Scholar]
- 78.Toniutto P, Fabris C, Bitetto D, Falleti E, Avellini C, Rossi E, Smirne C, Minisini R, Pirisi M. Role of AST to platelet ratio index in the detection of liver fibrosis in patients with recurrent hepatitis C after liver transplantation. J Gastroenterol Hepatol. 2007;22:1904–1908. doi: 10.1111/j.1440-1746.2006.04628.x. [DOI] [PubMed] [Google Scholar]
- 79.Afdhal N, McHutchison J, Brown R, Jacobson I, Manns M, Poordad F, Weksler B, Esteban R. Thrombocytopenia associated with chronic liver disease. J Hepatol. 2008;48:1000–1007. doi: 10.1016/j.jhep.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 80.Eissa LA, Gad LS, Rabie AM, El-Gayar AM. Thrombopoietin level in patients with chronic liver diseases. Ann Hepatol. 2008;7:235–244. [PubMed] [Google Scholar]
- 81.Weksler BB. Review article: the pathophysiology of thrombocytopenia in hepatitis C virus infection and chronic liver disease. Aliment Pharmacol Ther. 2007;26 Suppl 1:13–19. doi: 10.1111/j.1365-2036.2007.03512.x. [DOI] [PubMed] [Google Scholar]
- 82.Nagamine T, Ohtuka T, Takehara K, Arai T, Takagi H, Mori M. Thrombocytopenia associated with hepatitis C viral infection. J Hepatol. 1996;24:135–140. doi: 10.1016/s0168-8278(96)80021-3. [DOI] [PubMed] [Google Scholar]
- 83.Escolar G, Cases A, Viñas M, Pino M, Calls J, Cirera I, Ordinas A. Evaluation of acquired platelet dysfunctions in uremic and cirrhotic patients using the platelet function analyzer (PFA-100 ): influence of hematocrit elevation. Haematologica. 1999;84:614–619. [PubMed] [Google Scholar]
- 84.Tripodi A. Hemostasis abnormalities in liver cirrhosis: myth or reality? Pol Arch Med Wewn. 2008;118:445–448. [PubMed] [Google Scholar]
- 85.Giannini E, Botta F, Borro P, Risso D, Romagnoli P, Fasoli A, Mele MR, Testa E, Mansi C, Savarino V, et al. Platelet count/spleen diameter ratio: proposal and validation of a non-invasive parameter to predict the presence of oesophageal varices in patients with liver cirrhosis. Gut. 2003;52:1200–1205. doi: 10.1136/gut.52.8.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Peck-Radosavljevic M. Review article: coagulation disorders in chronic liver disease. Aliment Pharmacol Ther. 2007;26 Suppl 1:21–28. doi: 10.1111/j.1365-2036.2007.03509.x. [DOI] [PubMed] [Google Scholar]
- 87.Caldwell SH, Northup PG, Sundaram V. Haemostasis in liver disease. In: J Rodés, JP Benhaumou, AT Blei, J Reichen, M Rizzetto, et al., editors. Textbook of Hepatology. 3rd ed. Blackwell Sci Pub: Oxford; 2007. pp. 1780–1797. [Google Scholar]
- 88.Ferro D, Celestini A, Violi F. Hyperfibrinolysis in liver disease. Clin Liver Dis. 2009;13:21–31. doi: 10.1016/j.cld.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 89.Kelly DA, Summerfield JA. Hemostasis in liver disease. Semin Liver Dis. 1987;7:182–191. doi: 10.1055/s-2008-1040575. [DOI] [PubMed] [Google Scholar]
- 90.Iwakiri Y, Groszmann RJ. Vascular endothelial dysfunction in cirrhosis. J Hepatol. 2007;46:927–934. doi: 10.1016/j.jhep.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 91.Fontana RJ, Sanyal AJ, Mehta S, Doherty MC, Neuschwander-Tetri BA, Everson GT, Kahn JA, Malet PF, Sheikh MY, Chung RT, et al. Portal hypertensive gastropathy in chronic hepatitis C patients with bridging fibrosis and compensated cirrhosis: results from the HALT-C trial. Am J Gastroenterol. 2006;101:983–992. doi: 10.1111/j.1572-0241.2006.00461.x. [DOI] [PubMed] [Google Scholar]
- 92.Sanyal AJ, Fontana RJ, Di Bisceglie AM, Everhart JE, Doherty MC, Everson GT, Donovan JA, Malet PF, Mehta S, Sheikh MY, Reid AE, Ghany MG, Gretch DR, et al. The prevalence and risk factors associated with esophageal varices in subjects with hepatitis C and advanced fibrosis. Gastrointest Endosc. 2006;64:855–864. doi: 10.1016/j.gie.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 93.Zeegen R, Stansfeld AG, Dawson AM, Hunt AH. Bleeding oesophageal varices as the presenting feature in primary bilirary cirrhosis. Lancet. 1969;2:9–12. doi: 10.1016/s0140-6736(69)92595-1. [DOI] [PubMed] [Google Scholar]
- 94.Gores GJ, Wiesner RH, Dickson ER, Zinsmeister AR, Jorgensen RA, Langworthy A. Prospective evaluation of esophageal varices in primary biliary cirrhosis: development, natural history, and influence on survival. Gastroenterology. 1989;96:1552–1559. doi: 10.1016/0016-5085(89)90526-x. [DOI] [PubMed] [Google Scholar]
- 95.Bressler B, Pinto R, El-Ashry D, Heathcote EJ. Which patients with primary biliary cirrhosis or primary sclerosing cholangitis should undergo endoscopic screening for oesophageal varices detection? Gut. 2005;54:407–410. doi: 10.1136/gut.2004.040832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Levy C, Zein CO, Gomez J, Soldevila-Pico C, Firpi R, Morelli G, Nelson D. Prevalence and predictors of esophageal varices in patients with primary biliary cirrhosis. Clin Gastroenterol Hepatol. 2007;5:803–808. doi: 10.1016/j.cgh.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 97.Hassan MM, Li D, El-Deeb AS, Wolff RA, Bondy ML, Davila M, Abbruzzese JL. Association between hepatitis B virus and pancreatic cancer. J Clin Oncol. 2008;26:4557–4562. doi: 10.1200/JCO.2008.17.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hsing AW, Zhang M, Rashid A, McGlynn KA, Wang BS, Niwa S, Ortiz-Conde BA, Goedert JJ, Fraumeni JF Jr, O'Brien TR, et al. Hepatitis B and C virus infection and the risk of biliary tract cancer: a population-based study in China. Int J Cancer. 2008;122:1849–1853. doi: 10.1002/ijc.23251. [DOI] [PubMed] [Google Scholar]
- 99.El-Serag HB, Engels EA, Landgren O, Chiao E, Henderson L, Amaratunge HC, Giordano TP. Risk of hepatobiliary and pancreatic cancers after hepatitis C virus infection: A population-based study of U.S. veterans. Hepatology. 2009;49:116–123. doi: 10.1002/hep.22606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sherlock S, Dooley J. Hepatocellular failure. In: S Sherlock, J Dooley, et al., editors. Diseases of the liver and biliary system, 11th edition. Blackwell Sci Pub: Oxford; 2002. pp. 87–89. [Google Scholar]
- 101.Pirovino M, Linder R, Boss C, Köchli HP, Mahler F. Cutaneous spider nevi in liver cirrhosis: capillary microscopical and hormonal investigations. Klin Wochenschr. 1988;66:298–302. doi: 10.1007/BF01727516. [DOI] [PubMed] [Google Scholar]
- 102.Gumber SC, Chopra S. Hepatitis C: a multifaceted disease. Review of extrahepatic manifestations. Ann Intern Med. 1995;123:615–620. doi: 10.7326/0003-4819-123-8-199510150-00008. [DOI] [PubMed] [Google Scholar]
- 103.Zignego AL, Ferri C, Pileri SA, Caini P, Bianchi FB. Extrahepatic manifestations of Hepatitis C Virus infection: a general overview and guidelines for a clinical approach. Dig Liver Dis. 2007;39:2–17. doi: 10.1016/j.dld.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 104.Agnello V, Chung RT, Kaplan LM. A role for hepatitis C virus infection in type II cryoglobulinemia. N Engl J Med. 1992;327:1490–1495. doi: 10.1056/NEJM199211193272104. [DOI] [PubMed] [Google Scholar]
- 105.Misiani R, Bellavita P, Fenili D, Borelli G, Marchesi D, Massazza M, Vendramin G, Comotti B, Tanzi E, Scudeller G. Hepatitis C virus infection in patients with essential mixed cryoglobulinemia. Ann Intern Med. 1992;117:573–577. doi: 10.7326/0003-4819-117-7-573. [DOI] [PubMed] [Google Scholar]
- 106.Sasso EH. The rheumatoid factor response in the etiology of mixed cryoglobulins associated with hepatitis C virus infection. Ann Med Interne (Paris) 2000;151:30–40. [PubMed] [Google Scholar]
- 107.Ferri C. Mixed cryoglobulinemia. Orphanet J Rare Dis. 2008;3:25. doi: 10.1186/1750-1172-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ferri C, Marzo E, Longombardo G, Lombardini F, La Civita L, Vanacore R, Liberati AM, Gerli R, Greco F, Moretti A. Interferon-alpha in mixed cryoglobulinemia patients: a randomized, crossover-controlled trial. Blood. 1993;81:1132–1136. [PubMed] [Google Scholar]
- 109.Misiani R, Bellavita P, Fenili D, Vicari O, Marchesi D, Sironi PL, Zilio P, Vernocchi A, Massazza M, Vendramin G. Interferon alfa-2a therapy in cryoglobulinemia associated with hepatitis C virus. N Engl J Med. 1994;330:751–756. doi: 10.1056/NEJM199403173301104. [DOI] [PubMed] [Google Scholar]
- 110.Zuckerman E, Keren D, Slobodin G, Rosner I, Rozenbaum M, Toubi E, Sabo E, Tsykounov I, Naschitz JE, Yeshurun D. Treatment of refractory, symptomatic, hepatitis C virus related mixed cryoglobulinemia with ribavirin and interferon-alpha. J Rheumatol. 2000;27:2172–2178. [PubMed] [Google Scholar]
- 111.de Verneuil H, Aitken G, Nordmann Y. Familial and sporadic porphyria cutanea: two different diseases. Hum Genet. 1978;44:145–151. doi: 10.1007/BF00295407. [DOI] [PubMed] [Google Scholar]
- 112.Gisbert JP, García-Buey L, Pajares JM, Moreno-Otero R. Prevalence of hepatitis C virus infection in porphyria cutanea tarda: systematic review and meta-analysis. J Hepatol. 2003;39:620–627. doi: 10.1016/s0168-8278(03)00346-5. [DOI] [PubMed] [Google Scholar]
- 113.Carrozzo M, Pellicano R. Lichen planus and hepatitis C virus infection: an updated critical review. Minerva. Gastroenterol Dietol. 2008;54:65–74. [PubMed] [Google Scholar]
- 114.Nagao Y, Sata M, Fukuizumi K, Ryu F, Ueno T. High incidence of oral lichen planus in an HCV hyperendemic area. Gastroenterology. 2000;119:882–883. doi: 10.1053/gast.2000.17936. [DOI] [PubMed] [Google Scholar]
- 115.Protzer U, Ochsendorf FR, Leopolder-Ochsendorf A, Holtermüller KH. Exacerbation of lichen planus during interferon alfa-2a therapy for chronic active hepatitis C. Gastroenterology. 1993;104:903–905. doi: 10.1016/0016-5085(93)91029-h. [DOI] [PubMed] [Google Scholar]
- 116.Nagao Y, Sata M, Suzuki H, Kameyama T, Ueno T. Histological improvement of oral Lichen planus in patients with chronic hepatitis C treated with interferon. Gastroenterology. 1999;117:283–284. doi: 10.1016/s0016-5085(99)70595-0. [DOI] [PubMed] [Google Scholar]
- 117.Gianotti F. Papular acrodermatitis of childhood. An Australia antigen disease. Arch Dis Child. 1973;48:794–799. doi: 10.1136/adc.48.10.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Caputo R, Gelmetti C, Ermacora E, Gianni E, Silvestri A. Gianotti-Crosti syndrome: a retrospective analysis of 308 cases. J Am Acad Dermatol. 1992;26:207–210. doi: 10.1016/0190-9622(92)70028-e. [DOI] [PubMed] [Google Scholar]
- 119.Toda G, Ishimaru Y, Mayumi M, Oda T. Infantile papular acrodermatitis (Gianotti's disease) and intrafamilial occurence of acute hepatitis B with jaundice: age dependency of clinical manifestations of hepatitis B virus infection. J Infect Dis. 1978;138:211–216. doi: 10.1093/infdis/138.2.211. [DOI] [PubMed] [Google Scholar]
- 120.Turhan V, Ardic N, Besirbellioglu B, Dogru T. Gianotti-Crosti syndrome associated with HBV infection in an adult. Ir J Med Sci. 2005;174:92–94. doi: 10.1007/BF03169157. [DOI] [PubMed] [Google Scholar]
- 121.Johnson RJ, Couser WG. Hepatitis B infection and renal disease: clinical, immunopathogenetic and therapeutic considerations. Kidney Int. 1990;37:663–676. doi: 10.1038/ki.1990.32. [DOI] [PubMed] [Google Scholar]
- 122.Coccoli R, Esposito R, Cianciaruso B, Pota A, Visciano B, Annecchini R, Parrilli G. Hepatitis C and kidney disease. Dig Liver Dis. 2007;39 Suppl 1:S83–S85. doi: 10.1016/s1590-8658(07)80017-x. [DOI] [PubMed] [Google Scholar]
- 123.Agnello V, De Rosa FG. Extrahepatic disease manifestations of HCV infection: some current issues. J Hepatol. 2004;40:341–352. doi: 10.1016/j.jhep.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 124.Angeli P, Merkel C. Pathogenesis and management of hepatorenal syndrome in patients with cirrhosis. J Hepatol. 2008;48 Suppl 1:S93–S103. doi: 10.1016/j.jhep.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 125.Levy M, Chen N. Worldwide perspective of hepatitis B-associated glomerulonephritis in the 80s. Kidney Int Suppl. 1991;35:S24–S33. [PubMed] [Google Scholar]
- 126.Lai KN, Ho RT, Tam JS, Lai FM. Detection of hepatitis B virus DNA and RNA in kidneys of HBV related glomerulonephritis. Kidney Int. 1996;50:1965–1977. doi: 10.1038/ki.1996.519. [DOI] [PubMed] [Google Scholar]
- 127.Gilbert RD, Wiggelinkhuizen J. The clinical course of hepatitis B virus-associated nephropathy. Pediatr Nephrol. 1994;8:11–14. doi: 10.1007/BF00868249. [DOI] [PubMed] [Google Scholar]
- 128.Lai KN, Li PK, Lui SF, Au TC, Tam JS, Tong KL, Lai FM. Membranous nephropathy related to hepatitis B virus in adults. N Engl J Med. 1991;324:1457–1463. doi: 10.1056/NEJM199105233242103. [DOI] [PubMed] [Google Scholar]
- 129.Conjeevaram HS, Hoofnagle JH, Austin HA, Park Y, Fried MW, Di Bisceglie AM. Long-term outcome of hepatitis B virus-related glomerulonephritis after therapy with interferon alfa. Gastroenterology. 1995;109:540–546. doi: 10.1016/0016-5085(95)90343-7. [DOI] [PubMed] [Google Scholar]
- 130.Abbas NA, Pitt MA, Green AT, Solomon LR. Successful treatment of hepatitis B virus (HBV)-associated membranoproliferative glomerulonephritis (MPGN) with alpha interferon. Nephrol Dial Transplant. 1999;14:1272–1275. doi: 10.1093/ndt/14.5.1272. [DOI] [PubMed] [Google Scholar]
- 131.Wen YK, Chen ML. Remission of hepatitis B virus-associated membranoproliferative glomerulonephritis in a cirrhotic patient after lamivudine therapy. Clin Nephrol. 2006;65:211–215. doi: 10.5414/cnp65211. [DOI] [PubMed] [Google Scholar]
- 132.Pawlotsky JM, Ben Yahia M, Andre C, Voisin MC, Intrator L, Roudot-Thoraval F, Deforges L, Duvoux C, Zafrani ES, Duval J. Immunological disorders in C virus chronic active hepatitis: a prospective case-control study. Hepatology. 1994;19:841–848. [PubMed] [Google Scholar]
- 133.Tarantino A, Campise M, Banfi G, Confalonieri R, Bucci A, Montoli A, Colasanti G, Damilano I, D'Amico G, Minetti L. Long-term predictors of survival in essential mixed cryoglobulinemic glomerulonephritis. Kidney Int. 1995;47:618–623. doi: 10.1038/ki.1995.78. [DOI] [PubMed] [Google Scholar]
- 134.D'Amico G. Renal involvement in hepatitis C infection: cryoglobulinemic glomerulonephritis. Kidney Int. 1998;54:650–671. doi: 10.1046/j.1523-1755.1998.00028.x. [DOI] [PubMed] [Google Scholar]
- 135.Horikoshi S, Okada T, Shirato I, Inokuchi S, Ohmuro H, Tomino Y, Koide H. Diffuse proliferative glomerulonephritis with hepatitis C virus-like particles in paramesangial dense deposits in a patient with chronic hepatitis C virus hepatitis. Nephron. 1993;64:462–464. doi: 10.1159/000187372. [DOI] [PubMed] [Google Scholar]
- 136.Arase Y, Ikeda K, Murashima N, Chayama K, Tsubota A, Koida I, Suzuki Y, Saitoh S, Kobayashi M, Kobayashi M, et al. Glomerulonephritis in autopsy cases with hepatitis C virus infection. Intern Med. 1998;37:836–840. doi: 10.2169/internalmedicine.37.836. [DOI] [PubMed] [Google Scholar]
- 137.Johnson RJ, Gretch DR, Couser WG, Alpers CE, Wilson J, Chung M, Hart J, Willson R. Hepatitis C virus-associated glomerulonephritis. Effect of alpha-interferon therapy. Kidney Int. 1994;46:1700–1704. doi: 10.1038/ki.1994.471. [DOI] [PubMed] [Google Scholar]
- 138.Fabrizi F, Lunghi G, Messa P, Martin P. Therapy of hepatitis C virus-associated glomerulonephritis: current approaches. J Nephrol. 2008;21:813–825. [PubMed] [Google Scholar]
- 139.Casato M, Agnello V, Pucillo LP, Knight GB, Leoni M, Del Vecchio S, Mazzilli C, Antonelli G, Bonomo L. Predictors of long-term response to high-dose interferon therapy in type II cryoglobulinemia associated with hepatitis C virus infection. Blood. 1997;90:3865–3873. [PubMed] [Google Scholar]
- 140.Loustaud-Ratti V, Liozon E, Karaaslan H, Alain S, Paraf F, Le Meur Y, Denis F, Vidal E. Interferon alpha and ribavirin for membranoproliferative glomerulonephritis and hepatitis C infection. Am J Med. 2002;113:516–519. doi: 10.1016/s0002-9343(02)01257-3. [DOI] [PubMed] [Google Scholar]
- 141.Bruchfeld A, Lindahl K, Ståhle L, Söderberg M, Schvarcz R. Interferon and ribavirin treatment in patients with hepatitis C-associated renal disease and renal insufficiency. Nephrol Dial Transplant. 2003;18:1573–1580. doi: 10.1093/ndt/gfg209. [DOI] [PubMed] [Google Scholar]
- 142.Kamar N, Rostaing L, Alric L. Treatment of hepatitis C-virus-related glomerulonephritis. Kidney Int. 2006;69:436–439. doi: 10.1038/sj.ki.5000142. [DOI] [PubMed] [Google Scholar]
- 143.Arroyo V, Guevara M, Ginès P. Hepatorenal syndrome in cirrhosis: pathogenesis and treatment. Gastroenterology. 2002;122:1658–1676. doi: 10.1053/gast.2002.33575. [DOI] [PubMed] [Google Scholar]
- 144.Ginès P, Guevara M, Arroyo V, Rodés J. Hepatorenal syndrome. Lancet. 2003;362:1819–1827. doi: 10.1016/S0140-6736(03)14903-3. [DOI] [PubMed] [Google Scholar]
- 145.Ginès A, Escorsell A, Ginès P, Saló J, Jiménez W, Inglada L, Navasa M, Clària J, Rimola A, Arroyo V. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology. 1993;105:229–236. doi: 10.1016/0016-5085(93)90031-7. [DOI] [PubMed] [Google Scholar]
- 146.Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310–1318. doi: 10.1136/gut.2006.107789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Follo A, Llovet JM, Navasa M, Planas R, Forns X, Francitorra A, Rimola A, Gassull MA, Arroyo V, Rodés J. Renal impairment after spontaneous bacterial peritonitis in cirrhosis: incidence, clinical course, predictive factors and prognosis. Hepatology. 1994;20:1495–1501. doi: 10.1002/hep.1840200619. [DOI] [PubMed] [Google Scholar]
- 148.Henriksen JH, Ring-Larsen H. Hepatorenal disorders: role of the sympathetic nervous system. Semin Liver Dis. 1994;14:35–43. doi: 10.1055/s-2007-1007296. [DOI] [PubMed] [Google Scholar]
- 149.Gonwa TA, Klintmalm GB, Levy M, Jennings LS, Goldstein RM, Husberg BS. Impact of pretransplant renal function on survival after liver transplantation. Transplantation. 1995;59:361–365. [PubMed] [Google Scholar]
- 150.Alessandria C, Venon WD, Marzano A, Barletti C, Fadda M, Rizzetto M. Renal failure in cirrhotic patients: role of terlipressin in clinical approach to hepatorenal syndrome type 2. Eur J Gastroenterol Hepatol. 2002;14:1363–1368. doi: 10.1097/00042737-200212000-00013. [DOI] [PubMed] [Google Scholar]
- 151.Müller MJ. Malnutrition in cirrhosis. J Hepatol. 1995;23 Suppl 1:31–35. [PubMed] [Google Scholar]
- 152.Merli M, Riggio O, Dally L. Does malnutrition affect survival in cirrhosis? PINC (Policentrica Italiana Nutrizione Cirrosi) Hepatology. 1996;23:1041–1046. doi: 10.1002/hep.510230516. [DOI] [PubMed] [Google Scholar]
- 153.Fan ST, Lo CM, Lai EC, Chu KM, Liu CL, Wong J. Perioperative nutritional support in patients undergoing hepatectomy for hepatocellular carcinoma. N Engl J Med. 1994;331:1547–1552. doi: 10.1056/NEJM199412083312303. [DOI] [PubMed] [Google Scholar]
- 154.Tajika M, Kato M, Mohri H, Miwa Y, Kato T, Ohnishi H, Moriwaki H. Prognostic value of energy metabolism in patients with viral liver cirrhosis. Nutrition. 2002;18:229–234. doi: 10.1016/s0899-9007(01)00754-7. [DOI] [PubMed] [Google Scholar]
- 155.Mathur S, Peng S, Gane EJ, McCall JL, Plank LD. Hypermetabolism predicts reduced transplant-free survival independent of MELD and Child-Pugh scores in liver cirrhosis. Nutrition. 2007;23:398–403. doi: 10.1016/j.nut.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 156.Müller MJ, Böttcher J, Selberg O, Weselmann S, Böker KH, Schwarze M, von zur Mühlen A, Manns MP. Hypermetabolism in clinically stable patients with liver cirrhosis. Am J Clin Nutr. 1999;69:1194–1201. doi: 10.1093/ajcn/69.6.1194. [DOI] [PubMed] [Google Scholar]
- 157.Moriwaki H, Miwa Y, Tajika M, Kato M, Fukushima H, Shiraki M. Branched-chain amino acids as a protein- and energy-source in liver cirrhosis. Biochem Biophys Res Commun. 2004;313:405–409. doi: 10.1016/j.bbrc.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 158.Nakaya Y, Okita K, Suzuki K, Moriwaki H, Kato A, Miwa Y, Shiraishi K, Okuda H, Onji M, Kanazawa H, et al. BCAA-enriched snack improves nutritional state of cirrhosis. Nutrition. 2007;23:113–120. doi: 10.1016/j.nut.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 159.Marchesini G, Bianchi G, Merli M, Amodio P, Panella C, Loguercio C, Rossi Fanelli F, Abbiati R. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: a double-blind, randomized trial. Gastroenterology. 2003;124:1792–1801. doi: 10.1016/s0016-5085(03)00323-8. [DOI] [PubMed] [Google Scholar]
- 160.Korenaga K, Korenaga M, Uchida K, Yamasaki T, Sakaida I. Effects of a late evening snack combined with alpha-glucosidase inhibitor on liver cirrhosis. Hepatol Res. 2008;38:1087–1097. doi: 10.1111/j.1872-034X.2008.00391.x. [DOI] [PubMed] [Google Scholar]
- 161.Häussinger D, Schliess F. Pathogenetic mechanisms of hepatic encephalopathy. Gut. 2008;57:1156–1165. doi: 10.1136/gut.2007.122176. [DOI] [PubMed] [Google Scholar]
- 162.Amodio P, Del Piccolo F, Marchetti P, Angeli P, Iemmolo R, Caregaro L, Merkel C, Gerunda G, Gatta A. Clinical features and survivial of cirrhotic patients with subclinical cognitive alterations detected by the number connection test and computerized psychometric tests. Hepatology. 1999;29:1662–1667. doi: 10.1002/hep.510290619. [DOI] [PubMed] [Google Scholar]
- 163.Bajaj JS. Minimal hepatic encephalopathy matters in daily life. World J Gastroenterol. 2008;14:3609–3615. doi: 10.3748/wjg.14.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Prasad S, Dhiman RK, Duseja A, Chawla YK, Sharma A, Agarwal R. Lactulose improves cognitive functions and health-related quality of life in patients with cirrhosis who have minimal hepatic encephalopathy. Hepatology. 2007;45:549–559. doi: 10.1002/hep.21533. [DOI] [PubMed] [Google Scholar]
- 165.Poynard T, Cacoub P, Ratziu V, Myers RP, Dezailles MH, Mercadier A, Ghillani P, Charlotte F, Piette JC, Moussalli J. Fatigue in patients with chronic hepatitis C. J Viral Hepat. 2002;9:295–303. doi: 10.1046/j.1365-2893.2002.00364.x. [DOI] [PubMed] [Google Scholar]
- 166.Weissenborn K, Ennen JC, Bokemeyer M, Ahl B, Wurster U, Tillmann H, Trebst C, Hecker H, Berding G. Monoaminergic neurotransmission is altered in hepatitis C virus infected patients with chronic fatigue and cognitive impairment. Gut. 2006;55:1624–1630. doi: 10.1136/gut.2005.080267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kramer L, Bauer E, Funk G, Hofer H, Jessner W, Steindl-Munda P, Wrba F, Madl C, Gangl A, Ferenci P. Subclinical impairment of brain function in chronic hepatitis C infection. J Hepatol. 2002;37:349–354. doi: 10.1016/s0168-8278(02)00172-1. [DOI] [PubMed] [Google Scholar]
- 168.Tembl JI, Ferrer JM, Sevilla MT, Lago A, Mayordomo F, Vilchez JJ. Neurologic complications associated with hepatitis C virus infection. Neurology. 1999;53:861–864. doi: 10.1212/wnl.53.4.861. [DOI] [PubMed] [Google Scholar]
- 169.Gemignani F, Melli G, Inglese C, Marbini A. Cryoglobulinemia is a frequent cause of peripheral neuropathy in undiagnosed referral patients. J Peripher Nerv Syst. 2002;7:59–64. doi: 10.1046/j.1529-8027.2002.02007.x. [DOI] [PubMed] [Google Scholar]
- 170.Stefanova-Petrova DV, Tzvetanska AH, Naumova EJ, Mihailova AP, Hadjiev EA, Dikova RP, Vukov MI, Tchernev KG. Chronic hepatitis C virus infection: prevalence of extrahepatic manifestations and association with cryoglobulinemia in Bulgarian patients. World J Gastroenterol. 2007;13:6518–6528. doi: 10.3748/wjg.v13.i48.6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cacoub P, Poynard T, Ghillani P, Charlotte F, Olivi M, Piette JC, Opolon P. Extrahepatic manifestations of chronic hepatitis C. MULTIVIRC Group. Multidepartment Virus C. Arthritis Rheum. 1999;42:2204–2212. doi: 10.1002/1529-0131(199910)42:10<2204::AID-ANR24>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 172.Heckmann JG, Kayser C, Heuss D, Manger B, Blum HE, Neundörfer B. Neurological manifestations of chronic hepatitis C. J Neurol. 1999;246:486–491. doi: 10.1007/s004150050388. [DOI] [PubMed] [Google Scholar]
- 173.Angelino AF, Treisman GJ. Evidence-informed assessment and treatment of depression in HCV and interferon-treated patients. Int Rev Psychiatry. 2005;17:471–476. doi: 10.1080/02646830500381567. [DOI] [PubMed] [Google Scholar]
- 174.Fireman M, Indest DW, Blackwell A, Whitehead AJ, Hauser P. Addressing tri-morbidity (hepatitis C, psychiatric disorders, and substance use): the importance of routine mental health screening as a component of a comanagement model of care. Clin Infect Dis. 2005;40 Suppl 5:S286–S291. doi: 10.1086/427442. [DOI] [PubMed] [Google Scholar]
- 175.McAndrews MP, Farcnik K, Carlen P, Damyanovich A, Mrkonjic M, Jones S, Heathcote EJ. Prevalence and significance of neurocognitive dysfunction in hepatitis C in the absence of correlated risk factors. Hepatology. 2005;41:801–808. doi: 10.1002/hep.20635. [DOI] [PubMed] [Google Scholar]
- 176.Laskus T, Radkowski M, Adair DM, Wilkinson J, Scheck AC, Rakela J. Emerging evidence of hepatitis C virus neuroinvasion. AIDS. 2005;19 Suppl 3:S140–S144. doi: 10.1097/01.aids.0000192083.41561.00. [DOI] [PubMed] [Google Scholar]
- 177.Wilkinson J, Radkowski M, Laskus T. Hepatitis C virus neuroinvasion: identification of infected cells. J Virol. 2009;83:1312–1319. doi: 10.1128/JVI.01890-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Asnis GM, De La Garza R 2nd. Interferon-induced depression in chronic hepatitis C: a review of its prevalence, risk factors, biology, and treatment approaches. J Clin Gastroenterol. 2006;40:322–335. doi: 10.1097/01.mcg.0000210099.36500.fe. [DOI] [PubMed] [Google Scholar]
- 179.Murthy JM. Guillain-Barre syndrome following specific viral infections--an appraisal. J Assoc Physicians India. 1994;42:27–29. [PubMed] [Google Scholar]
- 180.Lee DK, Do JK, Kim YJ. Guillain-Barré like syndrome associated with acute renal failure and thrombocytopenia following acute viral hepatitis A. J Korean Med Sci. 1997;12:151–156. doi: 10.3346/jkms.1997.12.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lacaille F, Zylberberg H, Hagège H, Roualdès B, Meyrignac C, Chousterman M, Girot R. Hepatitis C associated with Guillain-Barré syndrome. Liver. 1998;18:49–51. doi: 10.1111/j.1600-0676.1998.tb00126.x. [DOI] [PubMed] [Google Scholar]
- 182.Keresztes K, Istenes I, Folhoffer A, Lakatos PL, Horvath A, Csak T, Varga P, Kempler P, Szalay F. Autonomic and sensory nerve dysfunction in primary biliary cirrhosis. World J Gastroenterol. 2004;10:3039–3043. doi: 10.3748/wjg.v10.i20.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Schilsky ML, Tavill AS. Wilson disease. In: ER Schieff, MF Sorrell, WC Maddrey, et al., editors. Schiff’s Diseases of the liver. 9th ed. Lippincott Williams & Wilkins: Philadelphia; 2003. pp. 1169–1186. [Google Scholar]
- 184.Kitzberger R, Madl C, Ferenci P. Wilson disease. Metab Brain Dis. 2005;20:295–302. doi: 10.1007/s11011-005-7910-8. [DOI] [PubMed] [Google Scholar]
- 185.Menon KV, Angulo P, Weston S, Dickson ER, Lindor KD. Bone disease in primary biliary cirrhosis: independent indicators and rate of progression. J Hepatol. 2001;35:316–323. doi: 10.1016/s0168-8278(01)00144-1. [DOI] [PubMed] [Google Scholar]
- 186.Springer JE, Cole DE, Rubin LA, Cauch-Dudek K, Harewood L, Evrovski J, Peltekova VD, Heathcote EJ. Vitamin D-receptor genotypes as independent genetic predictors of decreased bone mineral density in primary biliary cirrhosis. Gastroenterology. 2000;118:145–151. doi: 10.1016/s0016-5085(00)70423-9. [DOI] [PubMed] [Google Scholar]
- 187.Guañabens N, Parés A, Ros I, Caballería L, Pons F, Vidal S, Monegal A, Peris P, Rodés J. Severity of cholestasis and advanced histological stage but not menopausal status are the major risk factors for osteoporosis in primary biliary cirrhosis. J Hepatol. 2005;42:573–577. doi: 10.1016/j.jhep.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 188.Solaymani-Dodaran M, Card TR, Aithal GP, West J. Fracture risk in people with primary biliary cirrhosis: a population-based cohort study. Gastroenterology. 2006;131:1752–1757. doi: 10.1053/j.gastro.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 189.Diamond TH, Stiel D, Lunzer M, McDowall D, Eckstein RP, Posen S. Hepatic osteodystrophy. Static and dynamic bone histomorphometry and serum bone Gla-protein in 80 patients with chronic liver disease. Gastroenterology. 1989;96:213–221. [PubMed] [Google Scholar]
- 190.Guichelaar MM, Kendall R, Malinchoc M, Hay JE. Bone mineral density before and after OLT: long-term follow-up and predictive factors. Liver Transpl. 2006;12:1390–1402. doi: 10.1002/lt.20874. [DOI] [PubMed] [Google Scholar]
- 191.Cvetkovic M, Mann GN, Romero DF, Liang XG, Ma Y, Jee WS, Epstein S. The deleterious effects of long-term cyclosporine A, cyclosporine G, and FK506 on bone mineral metabolism in vivo. Transplantation. 1994;57:1231–1237. doi: 10.1097/00007890-199404270-00016. [DOI] [PubMed] [Google Scholar]
- 192.Leslie WD, Bernstein CN, Leboff MS. AGA technical review on osteoporosis in hepatic disorders. Gastroenterology. 2003;125:941–966. doi: 10.1016/s0016-5085(03)01062-x. [DOI] [PubMed] [Google Scholar]
- 193.Chi ZC, Ma SZ. Rheumatologic manifestations of hepatic diseases. Hepatobiliary Pancreat Dis Int. 2003;2:32–37. [PubMed] [Google Scholar]
- 194.Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733–1745. doi: 10.1056/NEJM199712113372406. [DOI] [PubMed] [Google Scholar]
- 195.Sterling RK, Bralow S. Extrahepatic manifestations of hepatitis C virus. Curr Gastroenterol Rep. 2006;8:53–59. doi: 10.1007/s11894-006-0064-y. [DOI] [PubMed] [Google Scholar]
- 196.Kelly TM, Edwards CQ, Meikle AW, Kushner JP. Hypogonadism in hemochromatosis: reversal with iron depletion. Ann Intern Med. 1984;101:629–632. doi: 10.7326/0003-4819-101-5-629. [DOI] [PubMed] [Google Scholar]
- 197.Gluud C, Wantzin P, Eriksen J. No effect of oral testosterone treatment on sexual dysfunction in alcoholic cirrhotic men. Gastroenterology. 1988;95:1582–1587. doi: 10.1016/s0016-5085(88)80081-7. [DOI] [PubMed] [Google Scholar]
- 198.Huyghe E, Kamar N, Wagner F, Yeung SJ, Capietto AH, El-Kahwaji L, Muscari F, Plante P, Rostaing L. Erectile dysfunction in liver transplant patients. Am J Transplant. 2008;8:2580–2589. doi: 10.1111/j.1600-6143.2008.02424.x. [DOI] [PubMed] [Google Scholar]
- 199.Huyghe E, Kamar N, Wagner F, Capietto AH, El-Kahwaji L, Muscari F, Plante P, Rostaing L. Erectile dysfunction in end-stage liver disease men. J Sex Med. 2009;6:1395–1401. doi: 10.1111/j.1743-6109.2008.01169.x. [DOI] [PubMed] [Google Scholar]
- 200.Toda K, Miwa Y, Kuriyama S, Fukushima H, Shiraki M, Murakami N, Shimazaki M, Ito Y, Nakamura T, Sugihara J, et al. Erectile dysfunction in patients with chronic viral liver disease: its relevance to protein malnutrition. J Gastroenterol. 2005;40:894–900. doi: 10.1007/s00535-005-1634-8. [DOI] [PubMed] [Google Scholar]
- 201.Ferri C, Bertozzi MA, Zignego AL. Erectile dysfunction and hepatitis C virus infection. JAMA. 2002;288:698–699. doi: 10.1001/jama.288.6.698. [DOI] [PubMed] [Google Scholar]
- 202.Malaguarnera M, Vicari E, Calogero A, Cammalleri L, Di Fazio I, Gargante MP, Pennisi G, Risino C, Ranno S, Rampello L. Sexual dysfunction in chronic hepatitis C virus patients treated with interferon alpha and ribavirin. J Interferon Cytokine Res. 2008;28:603–609. doi: 10.1089/jir.2008.0021. [DOI] [PubMed] [Google Scholar]
- 203.Verbeeck RK. Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction. Eur J Clin Pharmacol. 2008;64:1147–1161. doi: 10.1007/s00228-008-0553-z. [DOI] [PubMed] [Google Scholar]
- 204.Larrey D, Pageaux GP. Prescribibg drugs in liver disease. In: J Rodés, JP Benhaumou, AT Blei, J Reichen, M Rizzetto, et al., editors. Textbook of Hepatology. 3rd ed. Blackwell Sci Pub: Oxford; 2007. pp. 1912–1921. [Google Scholar]
- 205.Villeneuve JP, Pichette V. Cytochrome P450 and liver diseases. Curr Drug Metab. 2004;5:273–282. doi: 10.2174/1389200043335531. [DOI] [PubMed] [Google Scholar]
- 206.Spray JW, Willett K, Chase D, Sindelar R, Connelly S. Dosage adjustment for hepatic dysfunction based on Child-Pugh scores. Am J Health Syst Pharm. 2007;64:690, 692–693. doi: 10.2146/ajhp060287. [DOI] [PubMed] [Google Scholar]
- 207.Orlando R, Mussap M, Plebani M, Piccoli P, De Martin S, Floreani M, Padrini R, Palatini P. Diagnostic value of plasma cystatin C as a glomerular filtration marker in decompensated liver cirrhosis. Clin Chem. 2002;48:850–858. [PubMed] [Google Scholar]
- 208.Cowan RE, Jackson BT, Grainger SL, Thompson RP. Effects of anesthetic agents and abdominal surgery on liver blood flow. Hepatology. 1991;14:1161–1166. [PubMed] [Google Scholar]
- 209.Powell-Jackson P, Greenway B, Williams R. Adverse effects of exploratory laparotomy in patients with unsuspected liver disease. Br J Surg. 1982;69:449–451. doi: 10.1002/bjs.1800690805. [DOI] [PubMed] [Google Scholar]
- 210.Runyon BA. Surgical procedures are well tolerated by patients with asymptomatic chronic hepatitis. J Clin Gastroenterol. 1986;8:542–544. doi: 10.1097/00004836-198610000-00010. [DOI] [PubMed] [Google Scholar]
- 211.O'Sullivan MJ, Evoy D, O'Donnell C, Rajpal PK, Cannon B, Kenny-Walsh L, Whelton MJ, Redmond HP, Kirwan WO. Gallstones and laparoscopic cholecystectomy in hepatitis C patients. Ir Med J. 2001;94:114–117. [PubMed] [Google Scholar]
- 212.Suman A, Carey WD. Assessing the risk of surgery in patients with liver disease. Cleve Clin J Med. 2006;73:398–404. doi: 10.3949/ccjm.73.4.398. [DOI] [PubMed] [Google Scholar]
- 213.Ziser A, Plevak DJ, Wiesner RH, Rakela J, Offord KP, Brown DL. Morbidity and mortality in cirrhotic patients undergoing anesthesia and surgery. Anesthesiology. 1999;90:42–53. doi: 10.1097/00000542-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 214.Keegan MT, Plevak DJ. Preoperative assessment of the patient with liver disease. Am J Gastroenterol. 2005;100:2116–2127. doi: 10.1111/j.1572-0241.2005.41453.x. [DOI] [PubMed] [Google Scholar]
- 215.Durand F, Valla D. Assessment of prognosis of cirrhosis. Semin Liver Dis. 2008;28:110–122. doi: 10.1055/s-2008-1040325. [DOI] [PubMed] [Google Scholar]
- 216.Mansour A, Watson W, Shayani V, Pickleman J. Abdominal operations in patients with cirrhosis: still a major surgical challenge. Surgery. 1997;122:730–735; discussion 735-736. doi: 10.1016/s0039-6060(97)90080-5. [DOI] [PubMed] [Google Scholar]
- 217.Garrison RN, Cryer HM, Howard DA, Polk HC Jr. Clarification of risk factors for abdominal operations in patients with hepatic cirrhosis. Ann Surg. 1984;199:648–655. doi: 10.1097/00000658-198406000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Franzetta M, Raimondo D, Giammanco M, Di Trapani B, Passariello P, Sammartano A, Di Gesù G. Prognostic factors of cirrhotic patients in extra-hepatic surgery. Minerva Chir. 2003;58:541–544. [PubMed] [Google Scholar]
- 219.Teh SH, Nagorney DM, Stevens SR, Offord KP, Therneau TM, Plevak DJ, Talwalkar JA, Kim WR, Kamath PS. Risk factors for mortality after surgery in patients with cirrhosis. Gastroenterology. 2007;132:1261–1269. doi: 10.1053/j.gastro.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 220.Befeler AS, Palmer DE, Hoffman M, Longo W, Solomon H, Di Bisceglie AM. The safety of intra-abdominal surgery in patients with cirrhosis: model for end-stage liver disease score is superior to Child-Turcotte-Pugh classification in predicting outcome. Arch Surg. 2005;140:650–654; discussion 655. doi: 10.1001/archsurg.140.7.650. [DOI] [PubMed] [Google Scholar]
- 221.Perkins L, Jeffries M, Patel T. Utility of preoperative scores for predicting morbidity after cholecystectomy in patients with cirrhosis. Clin Gastroenterol Hepatol. 2004;2:1123–1128. doi: 10.1016/s1542-3565(04)00547-6. [DOI] [PubMed] [Google Scholar]
- 222.Schiff J, Misra M, Rendon G, Rothschild J, Schwaitzberg S. Laparoscopic cholecystectomy in cirrhotic patients. Surg Endosc. 2005;19:1278–1281. doi: 10.1007/s00464-004-8823-z. [DOI] [PubMed] [Google Scholar]
- 223.An Y, Xiao YB, Zhong QJ. Open-heart surgery in patients with liver cirrhosis: indications, risk factors, and clinical outcomes. Eur Surg Res. 2007;39:67–74. doi: 10.1159/000099145. [DOI] [PubMed] [Google Scholar]
- 224.Kaplan M, Cimen S, Kut MS, Demirtas MM. Cardiac operations for patients with chronic liver disease. Heart Surg Forum. 2002;5:60–65. [PubMed] [Google Scholar]
- 225.Suman A, Barnes DS, Zein NN, Levinthal GN, Connor JT, Carey WD. Predicting outcome after cardiac surgery in patients with cirrhosis: a comparison of Child-Pugh and MELD scores. Clin Gastroenterol Hepatol. 2004;2:719–723. doi: 10.1016/s1542-3565(04)00296-4. [DOI] [PubMed] [Google Scholar]