Abstract
Eosinophilic colitis (EC) is a rare form of primary eosinophilic gastrointestinal disease with a bimodal peak of prevalence in neonates and young adults. EC remains a little understood condition in contrast to the increasingly recognized eosinophilic esophagitis. Clinical presentation of EC is highly variable according to mucosal, transmural, or serosal predominance of inflammation. EC has a broad differential diagnosis because colon tissue eosinophilia often occurs in parasitic infection, drug-induced allergic reactions, inflammatory bowel disease, and various connective tissue disorders, which require thorough searching for secondary causes that may be specifically treated with antibiotics or dietary and drug elimination. Like eosinophilic gastrointestinal disease involving other segments of the gastrointestinal tract, EC responds very well to steroids that may be spared by using antihistamines, leukotriene inhibitors and biologics.
Keywords: Eosinophilia, Colitis, Gastrointestinal disease, Ascites
INTRODUCTION
Primary eosinophilic gastrointestinal disease (EGID), originally described by Kaijser in 1937[1], is a rare spectrum of gastrointestinal disorders characterized by inflammation rich in eosinophils, without evidence of known causes for eosinophilia, such as parasitic infection, drug reaction, or malignancy[2]. The disease can affect any segment or combination of segments of the gastrointestinal tract from the esophagus to the rectum, giving rise to various clinical presentations including eosinophilic esophagitis (EE), eosinophilic gastritis, eosinophilic gastroenteritis, and eosinophilic colitis (EC). Since secondary eosinophilic inflammation may occur in numerous gastrointestinal disorders such as IgE-mediated food allergy, gastroesophageal reflux disease, and inflammatory bowel disease, the true incidence and prevalence of primary EGID remains largely unknown. A recently established world-wide-web registry found that EGID mainly affects the pediatric population, although it has been reported in patients up to 68 years of age[3]. In the past few years, EE has been increasingly recognized as a distinct condition that affects about 1% of the population, and accounts for dysphagia and food impaction that remain non-responsive to traditional anti-reflux management, both in pediatric and adult gastroenterology[4]. Accordingly, several excellent reviews on EE have recently been published[4–6]. In contrast, EC represents the least frequent manifestation of EGID whether or not it presents with disease in other segments of the gastrointestinal tract[3]. EC appears to have a bimodal distribution that affects neonates with a relatively high prevalence and a separate group of young adults with no gender preference[2].
CLINICAL PRESENTATION
EGID in general has three hallmarks including peripheral eosinophilia (typically in the range of 5% to 35%), segmental eosinophilic infiltration of the gastrointestinal tract, and functional abnormalities[7,8]. Importantly, up to 23% of patients with primary EGID have no peripheral eosinophilia[7]. Symptoms and signs of EGID are usually non-specific and, depending on the affected segment, include abdominal pain, nausea, vomiting, diarrhea, gastrointestinal bleeding, obstruction, malabsorption, weight loss, and ascites. In 1970, Klein et al[9] subdivided the disease based on the layer of intestinal wall most extensively infiltrated by eosinophils, to distinguish mucosa-predominant, muscularis-propria-predominant, and serosa-predominant forms of EGID.
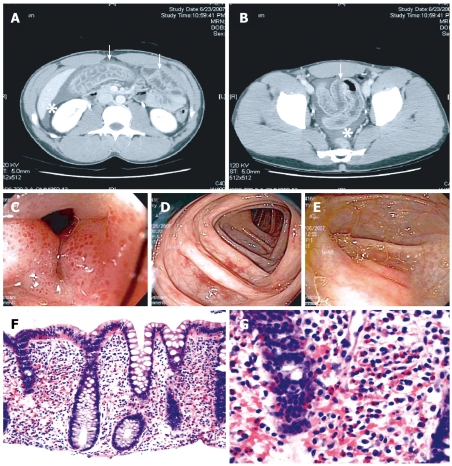
The above classification provides good correlation of the physical symptoms and signs with the pathological findings, and it is also applicable to EC. Thus, mucosa-predominant disease shows evidence of mucosal dysfunction, such as protein-losing enteropathy, malabsorption, and diarrhea. Transmural disease is recognized by symptoms of intestinal obstruction and bowel wall thickening on imaging studies. Finally, serosal involvement is distinguished by the presence of eosinophilic ascites, with up to 88% eosinophils seen on fluid analysis[10]. Accordingly, while mucosal EC results in diarrhea[11], the transmural form has been associated with volvulus[12], intussusception[13,14], and even perforation[15,16], and involvement of the intestinal serosa may manifest with ascites[17], which was also illustrated by a case that we have encountered recently (Figure 1).
Figure 1.
Diagnostic findings in EC. Representative images from a case of a previously healthy 30-year-old man with recurring episodes of abdominal pain, non-bloody diarrhea, and peripheral eosinophilia; extensive workup confirming EC by exclusion; and excellent response to short-term steroid therapy. A and B: Abdominal CT shows circumferential colon wall thickening (arrows) and moderate ascites (asterisks); C-E: Colonoscopy reveals patchy areas in the colon with mucosal edema and punctate erythema; F and G: Histology indicates markedly increased tissue eosinophilia in all examined segments of the colon. HE stains, magnification 100 × and 400 ×, respectively.
DIAGNOSTIC CRITERIA AND DIFFERENTIAL DIAGNOSIS
The diagnosis of EGID is made from the presence of gastrointestinal symptoms, peripheral eosinophilia, endoscopic and histological findings, and eosinophilic ascites, with no well-defined causes of eosinophilia on thorough evaluation[8]. A multidisciplinary task force has recently reached consensus on the diagnostic criteria of EE, including the presence of more than 15 eosinophils per high-power field in the esophageal squamous mucosa[6]. No such consensus exists for EC, although most authors have used a diagnostic threshold of 20 eosinophils per high-power field. Of note, normal values for tissue eosinophils vary widely between different segments of the colon, ranging from < 10 eosinophils per high-power field in the rectum to > 30 in the cecum[5], thus location of the biopsy is critically important for interpretation of findings.
More or less prominent tissue eosinophilia in the colon may result from a number of conditions (Table 1) and EC remains therefore a diagnosis of exclusion. Colonoscopic biopsies obtained from patients with inflammatory bowel disease, in particular with Crohn’s colitis, often show severe tissue eosinophilia[18]. Parasitic infection of the colon with pinworms, roundworms, or whipworms may lead to marked eosinophilic infiltration, and repeated stool or serological testing may be needed to reveal this specific etiology[19–23]. Drug-induced EC has been described in response to clozapine[24], carbamazepine[25], rifampicin[26], non-steroidal anti-inflammatory agents[27,28], tacrolimus[29], and gold[30]. EC has also been associated with autoimmune connective tissue disease including scleroderma, dermatomyositis and polymyositis[11,31,32], as well as with allogeneic bone marrow transplantation[33] and the rare Tolosa-Hunt syndrome that features inflammatory ophthalmoparesis[34]. The idiopathic hypereosinophilic syndrome (HES) may also affect the colon, but this rare condition presents with sustained and marked peripheral eosinophilia with end-organ damage that extends beyond the gastrointestinal tract (e.g. heart and skin)[35].
Table 1.
Differential diagnosis of EC
| Differential diagnosis of EC |
| Parasitic colitis |
| Enterobius vermicularis[19,20] |
| Strongyloides stercoralis[21,22] |
| Trichuris trichiura[23] |
| Drug-induced colitis |
| Clozapine[24] |
| Carbamazepine[25] |
| Rifampicin[26] |
| Non-steroidal anti-inflammatory drugs[27,28] |
| Tacrolimus[29] |
| Gold[30] |
| HES[35,51] |
| Inflammatory bowel disease[18] |
| Allogeneic bone marrow transplantation[33] |
| Tolosa-Hunt syndrome[34] |
EC: Eosinophilic colitis; HES: Hypereosinophilic syndrome.
ETIOLOGY AND PATHOGENESIS
The etiology of primary EGID remains largely unknown. Several studies have suggested a relationship with specific food allergies; indeed, about 75% of affected patients have a history of allergy or atopy[2]. Cow’s milk and soy proteins are the foods most frequently implicated in the infantile form of EC, although the condition has been described in infants exclusively breast-fed or given protein hydrolysate formulas[2]. Even less is known about the potential causes of the adult form of primary EC. A case report by Inamura et al[36] has demonstrated accumulation of mast cells in the colon interstitium after immunohistochemical staining for mast cell tryptase, which suggests the pathogenic role of IgE, while other observations suggest that EC may not be an IgE-mediated disease. Thus, colonic T cells in an animal model have been shown to transfer oral antigen-induced diarrhea to naive mice through a STAT6-dependent mechanism[37]. Specific eosinophil chemoattractants, such as interleukin-5 and eotaxins, may also have a pathogenic role in EC[38]. While EE may develop without other gastrointestinal involvement when experimental animals are sensitized and challenged in the lung, direct exposure of the gastrointestinal mucosa seems to result in multisegmental disease[39].
TREATMENT
No prospective randomized controlled trials exist to date on specific therapy for EC or any other forms of primary or idiopathic EGID. Therapeutic efforts have been based on case reports and small case series. Corticosteroid therapy has formed the backbone for initial management, and it has proven to be the most effective instrument for symptom control in EC[2,40,41]. Up to 90% of cases will respond within 2 wk of treatment, when a slow taper is initiated. However, relapse is frequent and requires recurrent courses or leads to steroid dependence. A role for budesonide has been demonstrated, particularly in disease of the right colon and ileum[42]. It must be emphasized that efforts to rule out parasitic or drug-induced EC are important since empiric treatment with corticosteroids may aggravate the patient’s condition, or at least, it may be avoidable. The beneficial effect of elimination and elemental diets has been limited to cases with specific food allergies, especially in treating neonatal disease[43].
Approaches to avoid steroids by using alternative medications have been directed mostly to more prevalent forms of EGID, and expertise about their need and efficacy in EC has been limited. Antihistamine therapy in EGID appears to be gaining prominence. Ketotifen, an H1 antihistamine not yet available in US, has been shown to decrease symptoms as well as tissue eosinophilia[44,45]. The leukotriene inhibitor montelukast, an agent that blocks the action of potent eosinophil chemoattractant leukotriene D4, by competitively antagonizing its receptor expressed on eosinophils, has also been found to be helpful in EGID[46,47]. Mast cell stabilizers, such as cromolyn, are effective by inhibiting release of mast cell mediators such as histamine H1, platelet activating factor, and leukotoxin[48]. More recently, the role of biologics in EGID has also been studied, with favorable outcomes reported by using monoclonal antibodies targeting interleukin 5 (mepolizumab) and IgE (omalizumab)[49,50].
CONCLUSION
In summary, EC is a rare manifestation of the EGID spectrum, which does not appear to be increasing in prevalence, in contrast to recent trends seen in esophageal disease. This dichotomy suggests a disparate pathophysiology of EC, with the possibility that EC itself is a heterogeneous entity. While the pediatric form of EC often subsides without intervention, or upon withdrawal of atopic stimuli, the adult form may relapse and require short-term steroid therapy. Importantly, EC needs to be included in the differential diagnosis of many conditions with primary or secondary involvement of the gastrointestinal tract.
Peer reviewer: Dr. Simon S Campbell, MD, Department of Gastroenterology, Manchester Royal Infirmary, Oxford Road, Manchester, M12 9WL, United Kingdom
S- Editor Li LF L- Editor Kerr C E- Editor Ma WH
References
- 1.Kaijser R. Zur Kenntnis der allergischen affektionen des verdauungskanals vom standput des chirurgen aus. Arch Klin Chir. 1937;188:36–64. [Google Scholar]
- 2.Rothenberg ME. Eosinophilic gastrointestinal disorders (EGID) J Allergy Clin Immunol. 2004;113:11–28; quiz 29. doi: 10.1016/j.jaci.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 3.Guajardo JR, Plotnick LM, Fende JM, Collins MH, Putnam PE, Rothenberg ME. Eosinophil-associated gastrointestinal disorders: a world-wide-web based registry. J Pediatr. 2002;141:576–581. doi: 10.1067/mpd.2002.127663. [DOI] [PubMed] [Google Scholar]
- 4.Arora AS, Yamazaki K. Eosinophilic esophagitis: asthma of the esophagus? Clin Gastroenterol Hepatol. 2004;2:523–530. doi: 10.1016/s1542-3565(04)00236-8. [DOI] [PubMed] [Google Scholar]
- 5.Gonsalves N. Food allergies and eosinophilic gastroi-ntestinal illness. Gastroenterol Clin North Am. 2007;36:75–91, vi. doi: 10.1016/j.gtc.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Furuta GT, Liacouras CA, Collins MH, Gupta SK, Justinich C, Putnam PE, Bonis P, Hassall E, Straumann A, Rothenberg ME. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133:1342–1363. doi: 10.1053/j.gastro.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 7.Talley NJ, Shorter RG, Phillips SF, Zinsmeister AR. Eosinophilic gastroenteritis: a clinicopathological study of patients with disease of the mucosa, muscle layer, and subserosal tissues. Gut. 1990;31:54–58. doi: 10.1136/gut.31.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan BM, Shaffer EA. Primary eosinophilic disorders of the gastrointestinal tract. Gut. 2009;58:721–732. doi: 10.1136/gut.2008.165894. [DOI] [PubMed] [Google Scholar]
- 9.Klein NC, Hargrove RL, Sleisenger MH, Jeffries GH. Eosinophilic gastroenteritis. Medicine (Baltimore) 1970;49:299–319. doi: 10.1097/00005792-197007000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Kravis LP, South MA, Rosenlund ML. Eosinophilic gastroenteritis in the pediatric patient. Clin Pediatr (Phila) 1982;21:713–717. doi: 10.1177/000992288202101202. [DOI] [PubMed] [Google Scholar]
- 11.Clouse RE, Alpers DH, Hockenbery DM, DeSchryver-Kecskemeti K. Pericrypt eosinophilic enterocolitis and chronic diarrhea. Gastroenterology. 1992;103:168–176. doi: 10.1016/0016-5085(92)91110-p. [DOI] [PubMed] [Google Scholar]
- 12.Velchuru VR, Khan MA, Hellquist HB, Studley JG. Eosinophilic colitis. J Gastrointest Surg. 2007;11:1373–1375. doi: 10.1007/s11605-006-0055-1. [DOI] [PubMed] [Google Scholar]
- 13.Shin WG, Park CH, Lee YS, Kim KO, Yoo KS, Kim JH, Park CK. Eosinophilic enteritis presenting as intussusception in adult. Korean J Intern Med. 2007;22:13–17. doi: 10.3904/kjim.2007.22.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Box JC, Tucker J, Watne AL, Lucas G. Eosinophilic colitis presenting as a left-sided colocolonic intussusception with secondary large bowel obstruction: an uncommon entity with a rare presentation. Am Surg. 1997;63:741–743. [PubMed] [Google Scholar]
- 15.Fraile G, Rodriguez-Garcia JL, Beni-Perez R, Redondo C. Localized eosinophilic gastroenteritis with necrotizing granulomas presenting as acute abdomen. Postgrad Med J. 1994;70:510–512. doi: 10.1136/pgmj.70.825.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minciu O, Wegmann D, Gebbers JO. [Eosinophilic colitis--an unusual cause of acute abdomen. Case report and literature review] Schweiz Med Wochenschr. 1992;122:1402–1408. [PubMed] [Google Scholar]
- 17.Ong GY, Hsu CC, Changchien CS, Lu SN, Huang SC. Eosinophilic gastroenteritis involving the distal small intestine and proximal colon. Chang Gung Med J. 2002;25:56–61. [PubMed] [Google Scholar]
- 18.Rubio CA. A method for the detection of eosinophilic granulocytes in colonoscopic biopsies from IBD patients. Pathol Res Pract. 2003;199:145–150. doi: 10.1078/0344-0338-00367. [DOI] [PubMed] [Google Scholar]
- 19.Cacopardo B, Onorante A, Nigro L, Patamia I, Tosto S, Romano F, Zappala C, Bruno S, Nunnari A. Eosinophilic ileocolitis by Enterobius vermicularis: a description of two rare cases. Ital J Gastroenterol Hepatol. 1997;29:51–53. [PubMed] [Google Scholar]
- 20.Macedo T, MacCarty RL. Eosinophilic ileocolitis secondary to Enterobius vermicularis: case report. Abdom Imaging. 2000;25:530–532. doi: 10.1007/s002610000042. [DOI] [PubMed] [Google Scholar]
- 21.Al Samman M, Haque S, Long JD. Strongyloidiasis colitis: a case report and review of the literature. J Clin Gastroenterol. 1999;28:77–80. doi: 10.1097/00004836-199901000-00021. [DOI] [PubMed] [Google Scholar]
- 22.Corsetti M, Basilisco G, Pometta R, Allocca M, Conte D. Mistaken diagnosis of eosinophilic colitis. Ital J Gastroenterol Hepatol. 1999;31:607–609. [PubMed] [Google Scholar]
- 23.Chandrasekhara V, Arslanlar S, Sreenarasimhaiah J. Whipworm infection resulting in eosinophilic colitis with occult intestinal bleeding. Gastrointest Endosc. 2007;65:709–710. doi: 10.1016/j.gie.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Friedberg JW, Frankenburg FR, Burk J, Johnson W. Clozapine-caused eosinophilic colitis. Ann Clin Psychiatry. 1995;7:97–98. doi: 10.3109/10401239509149034. [DOI] [PubMed] [Google Scholar]
- 25.Anttila VJ, Valtonen M. Carbamazepine-induced eosinophilic colitis. Epilepsia. 1992;33:119–121. doi: 10.1111/j.1528-1157.1992.tb02293.x. [DOI] [PubMed] [Google Scholar]
- 26.Lange P, Oun H, Fuller S, Turney JH. Eosinophilic colitis due to rifampicin. Lancet. 1994;344:1296–1297. doi: 10.1016/s0140-6736(94)90782-x. [DOI] [PubMed] [Google Scholar]
- 27.Bridges AJ, Marshall JB, Diaz-Arias AA. Acute eosinophilic colitis and hypersensitivity reaction associated with naproxen therapy. Am J Med. 1990;89:526–527. doi: 10.1016/0002-9343(90)90386-r. [DOI] [PubMed] [Google Scholar]
- 28.Jimenez-Saenz M, Gonzalez-Campora R, Linares-Santiago E, Herrerias-Gutierrez JM. Bleeding colonic ulcer and eosinophilic colitis: a rare complication of nonsteroidal anti-inflammatory drugs. J Clin Gastroenterol. 2006;40:84–85. doi: 10.1097/01.mcg.0000190776.65526.da. [DOI] [PubMed] [Google Scholar]
- 29.Saeed SA, Integlia MJ, Pleskow RG, Calenda KA, Rohrer RJ, Dayal Y, Grand RJ. Tacrolimus-associated eosinophilic gastroenterocolitis in pediatric liver transplant recipients: role of potential food allergies in pathogenesis. Pediatr Transplant. 2006;10:730–735. doi: 10.1111/j.1399-3046.2006.00538.x. [DOI] [PubMed] [Google Scholar]
- 30.Martin DM, Goldman JA, Gilliam J, Nasrallah SM. Gold-induced eosinophilic enterocolitis: response to oral cromolyn sodium. Gastroenterology. 1981;80:1567–1570. [PubMed] [Google Scholar]
- 31.Barbie DA, Mangi AA, Lauwers GY. Eosinophilic gastroenteritis associated with systemic lupus erythematosus. J Clin Gastroenterol. 2004;38:883–886. doi: 10.1097/00004836-200411000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Ahmad M, Soetikno RM, Ahmed A. The differential diagnosis of eosinophilic esophagitis. J Clin Gastroenterol. 2000;30:242–244. doi: 10.1097/00004836-200004000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Ashida T, Shimada T, Kawanishi K, Miyatake J, Kanamaru A. Eosinophilic colitis in a patient with acute myeloid leukemia after allogeneic bone marrow transplantation. Int J Hematol. 2003;78:76–78. doi: 10.1007/BF02983245. [DOI] [PubMed] [Google Scholar]
- 34.Kosugi S, Date K, Minagawa M, Ishikawa H, Hatakeyama K, Endo K, Kimura Y. Eosinophilic colitis accompanied by Tolosa-Hunt syndrome: report of a case. J Gastroenterol. 2003;38:613–614. [PubMed] [Google Scholar]
- 35.Roufosse FE, Goldman M, Cogan E. Hypereosinophilic syndromes. Orphanet J Rare Dis. 2007;2:37. doi: 10.1186/1750-1172-2-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inamura H, Kashiwase Y, Morioka J, Suzuki K, Igarashi Y, Kurosawa M. Accumulation of mast cells in the interstitium of eosinophilic colitis. Allergol Immunopathol (Madr) 2006;34:228–230. doi: 10.1157/13094031. [DOI] [PubMed] [Google Scholar]
- 37.Kweon MN, Yamamoto M, Kajiki M, Takahashi I, Kiyono H. Systemically derived large intestinal CD4(+) Th2 cells play a central role in STAT6-mediated allergic diarrhea. J Clin Invest. 2000;106:199–206. doi: 10.1172/JCI8490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lamouse-Smith ES, Furuta GT. Eosinophils in the gastrointestinal tract. Curr Gastroenterol Rep. 2006;8:390–395. doi: 10.1007/s11894-006-0024-6. [DOI] [PubMed] [Google Scholar]
- 39.Hogan SP, Mishra A, Brandt EB, Royalty MP, Pope SM, Zimmermann N, Foster PS, Rothenberg ME. A pathological function for eotaxin and eosinophils in eosinophilic gastrointestinal inflammation. Nat Immunol. 2001;2:353–360. doi: 10.1038/86365. [DOI] [PubMed] [Google Scholar]
- 40.Chen MJ, Chu CH, Lin SC, Shih SC, Wang TE. Eosinophilic gastroenteritis: clinical experience with 15 patients. World J Gastroenterol. 2003;9:2813–2816. doi: 10.3748/wjg.v9.i12.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khan S. Eosinophilic gastroenteritis. Best Pract Res Clin Gastroenterol. 2005;19:177–198. doi: 10.1016/j.bpg.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 42.Tan AC, Kruimel JW, Naber TH. Eosinophilic gastroenteritis treated with non-enteric-coated budesonide tablets. Eur J Gastroenterol Hepatol. 2001;13:425–427. doi: 10.1097/00042737-200104000-00021. [DOI] [PubMed] [Google Scholar]
- 43.Hill SM, Milla PJ. Colitis caused by food allergy in infants. Arch Dis Child. 1990;65:132–133. doi: 10.1136/adc.65.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suzuki J, Kawasaki Y, Nozawa R, Isome M, Suzuki S, Takahashi A, Suzuki H. Oral disodium cromoglycate and ketotifen for a patient with eosinophilic gastroenteritis, food allergy and protein-losing enteropathy. Asian Pac J Allergy Immunol. 2003;21:193–197. [PubMed] [Google Scholar]
- 45.Melamed I, Feanny SJ, Sherman PM, Roifman CM. Benefit of ketotifen in patients with eosinophilic gastroenteritis. Am J Med. 1991;90:310–314. [PubMed] [Google Scholar]
- 46.Neustrom MR, Friesen C. Treatment of eosinophilic gastroenteritis with montelukast. J Allergy Clin Immunol. 1999;104:506. doi: 10.1016/s0091-6749(99)70404-5. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz DA, Pardi DS, Murray JA. Use of montelukast as steroid-sparing agent for recurrent eosinophilic gastroenteritis. Dig Dis Sci. 2001;46:1787–1790. doi: 10.1023/a:1010682310928. [DOI] [PubMed] [Google Scholar]
- 48.Perez-Millan A, Martin-Lorente JL, Lopez-Morante A, Yuguero L, Saez-Royuela F. Subserosal eosinophilic gastroenteritis treated efficaciously with sodium cromoglycate. Dig Dis Sci. 1997;42:342–344. doi: 10.1023/a:1018818003002. [DOI] [PubMed] [Google Scholar]
- 49.Stein ML, Collins MH, Villanueva JM, Kushner JP, Putnam PE, Buckmeier BK, Filipovich AH, Assa’ad AH, Rothenberg ME. Anti-IL-5 (mepolizumab) therapy for eosinophilic esophagitis. J Allergy Clin Immunol. 2006;118:1312–1319. doi: 10.1016/j.jaci.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 50.Foroughi S, Foster B, Kim N, Bernardino LB, Scott LM, Hamilton RG, Metcalfe DD, Mannon PJ, Prussin C. Anti-IgE treatment of eosinophil-associated gastrointestinal disorders. J Allergy Clin Immunol. 2007;120:594–601. doi: 10.1016/j.jaci.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shah AM, Joglekar M. Eosinophilic colitis as a complication of the hypereosinophilic syndrome. Postgrad Med J. 1987;63:485–487. doi: 10.1136/pgmj.63.740.485. [DOI] [PMC free article] [PubMed] [Google Scholar]