Abstract
The ComX pheromone is a posttranslationally modified oligopeptide that stimulates natural genetic competence in Bacillus subtilis. Various ComXRO-E-2 analogs were synthesized and their biological activities were studied to investigate structure-activity-relationships. These results showed that the minimal active unit was the tripeptide, [3–5]ComXRO-E-2, and all residues except the modified tryptophan residue were replaceable by alanine without total loss of activity.
Bacteria secrete specific extracellular signaling molecules that increase in concentration with cell density. In a process known as quorum sensing, bacteria regulate their behavior in response to such molecules.1 Quorum sensing attracts attention as a promising anti-pathogenic drug target rather than anti-bacterial, notably preventing the emergence of drag-resistant bacteria.2 The ComX pheromone3 is a signaling oligopeptide that stimulates natural genetic competence controlled by quorum sensing in Bacillus subtilis.4 In the presence of the ComX pheromone, the membrane-located receptor histidine kinase ComP autophosphorylates and donates phosphate via a two-component-system to activate competence gene expression.5 Previous studies indicated that the ComX pheromone is posttranslationally modified by the addition of an isoprenoid to a tryptophan residue.6,7 Posttranslational isoprenoidal modification on the cystein residue8 is widely observed in many important proteins such as the Ras oncoproteins, which plays a crucial role in human tumor and the isoprenoidal modification is essential for the Ras functions.9 Therefore, the posttranslational isoprenoidal modification is an attractive target for cancer therapy, but on the tryptophan residue, including other amino acid residues except cystein, is unprecedented.
Recently the ComXRO-E-2 pheromone 1 from B. subtilis strain RO-E-2,6 was shown to be a hexapeptide possessing a modified tryptophan residue with a geranyl group. This novel modification resulted in the formation of a tricyclic structure (Figure 1).10
Fig 1. Chemical structure of ComXRO-E-2 pheromone.
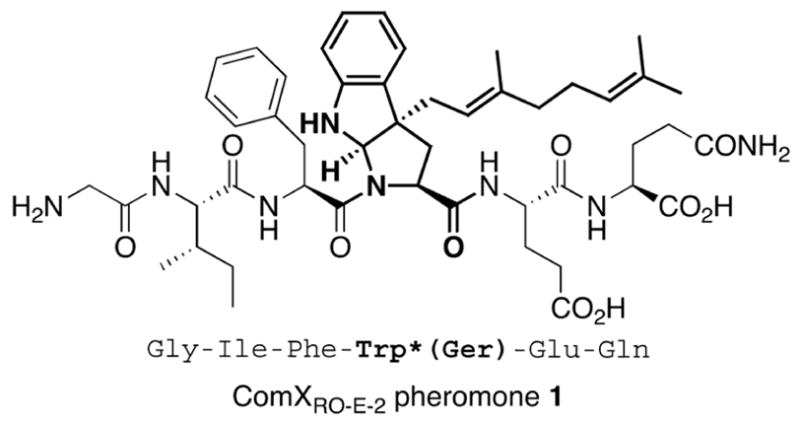
Bold Trp*(Ger) represents the modified tryptophan residue with a geranyl group in the ComXRO-E-2 pheromone (bold lines).
Since further studies are required to elucidate the details of the function through the interaction between the ComX pheromone and ComP, various ComXRO-E-2 analogs were prepared using solid phase peptide synthesis.10–13 The biological activities of these peptides as well as of ComXRO-E-2 pheromone were investigated using β-galactosidase assays.6,10–12,14 These data were used to draw the dose response curves. The biological activities of these peptides were represented by two values both normalized to the activity of the ComXRO-E-2 pheromone (Table 1). The first value presents the concentration of each peptide, needed to obtain the same β-galactosidase activity as that obtained with the ComXRO-E-2 pheromone at EC50. The second is the maximum activity as a percentage of that obtained with the ComXRO-E-2 pheromone.
Table 1.
Biological activities of ComXRO-E-2 analogs
| Entry | Substance | Amino acid sequence | EC50a (nM) | EDmaxb (%) |
|---|---|---|---|---|
| 1 | ComXRO-E-2 | Gly-Ile-Phe-Trp*(Ger)-Glu-Gln | 1 | 100 |
| 2 | [2–6]ComXRO-E-2 | Ile-Phe-Trp*(Ger)-Glu-Gln | 6 | 100 |
| 3 | [3–6]ComXRO-E-2 | Phe-Trp*(Ger)-Glu-Gln | 8 | 70 |
| 4 | [4–6]ComXRO-E-2 | Trp*(Ger)-Glu-Gln | >300 | 15 |
| 5 | [1–5]ComXRO-E-2 | Gly-Ile-Phe-Trp*(Ger)-Glu | 20 | 80 |
| 6 | [1–4]ComXRO-E-2 | Gly-Ile-Phe-Trp*(Ger) | 130 | 60 |
| 7 | [2–5]ComXRO-E-2 | Ile-Phe-Trp*(Ger)-Glu | 20 | 80 |
| 8 | [2–4]ComXRO-E-2 | Ile-Phe-Trp*(Ger) | 160 | 55 |
| 9 | [3–5]ComXRO-E-2 | Phe-Trp*(Ger)-Glu | 20 | 60 |
| 10 | Ala-ComXRO-E-2 | Ala-Gly-Ile-Phe-Trp*(Ger)-Glu-Gln | 1 | 100 |
| 11 | Ala-ComXRO-E-2 | Ala-Gly-Ile-Phe-Trp*(Ger)-Glu-Gln | 7 | 100 |
| 12 | [G1A]ComXRO-E-2 | Ala-Ile-Phe-Trp*(Ger)-Glu-Gln | 3 | 100 |
| 13 | [I2A]ComXRO-E-2 | Gly-Ala-Phe-Trp*(Ger)-Glu-Gln | 10 | 95 |
| 14 | [F3A]ComXRO-E-2 | Gly-Ile-Ala-Trp*(Ger)-Glu-Gln | 20 | 90 |
| 15 | [E5A]ComXRO-E-2 | Gly-Ile-Phe-Trp*(Ger)-Ala-Gln | 3 | 100 |
| 16 | [Q6A]ComXRO-E-2 | Gly-Ile-Phe-Trp*(Ger)-Glu-Ala | 20 | 95 |
Concentration of each peptide showing the same activity to the observed activity at EC50 of ComXRO-E-2 pheromone.
Ratio of maximum activity compared with ComXRO-E-2 pheromone.
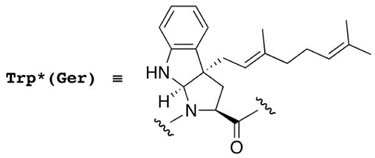
Although N-terminal truncated [2–6]ComXRO-E-2 and [3–6]ComXRO-E-2 showed significant biological activities as previously reported,12 [4–6]ComXRO-E-2 lost all activity (entry 4). The C-terminal glutamate residue was partially dispensable like the N-terminal glycine. In contrast, deletion of the glutamic acid and glutamate residues C-terminal to tryptophan dramatically decreased the activity (entry 5–8). These results indicated that for activity, either the modified tryptophan must reside at an internal position, or that contacts of the ComP receptor with the glutamic acid residue are important. A minimal active core within ComXRO-E-2 appears to consist of the tripeptide, [3–5]ComXRO-E-2 (entry 9). Elongation of ComXRO-E-2 pheromone by the addition of alanine at either the N- or C-terminus did not affect the activity (entry 10,11). The roles of individual amino acid side chains were investigated substitution of each residue except the modified tryptophan with alanine. Surprisingly, none were absolutely essential for biological activity, although alanine substitution of 3 phenylalanins and 6 glutamate decreased the activity substantially (entry 12–16).
Earlier work on the structure-activity-relationships of tremerogen A-10,15 which is the sex pheromone of basidiomycetous yeasts containing a C-terminal farnesyl modified cysteine methyl ester, revealed that removal of the C-terminus methyl ester or N-terminal residues decreased biological activity16 in contrast to the present results with the ComXRO-E-2 pheromone, which showed that only tripeptide, [3–5]ComXRO-E-2, still had considerable activity. These results indicated that the pattern of specific interaction of the ComX pheromone with its receptor are unique and different from that of the S-isoprenoidal peptides. Furthermore, the precise structure of modified tryptophan residue was essential for the biological activity, because synthetic ComXRO-E-2 peptides, containing a geranyltryptophan residue with a geranyl group replacing a tryptophanyl proton10 or with additional stereoisomers at the modified tryptophan residue,11,12 showed no biological activities. Although the farnesyl group on tremerogen A-10 was replaceable by lipophilic long chains,16 the role of the geranyl moiety in biological activity and in determining pherotype specificity is not yet known. Into investigate this question, we are synthesizing analogs of ComXRO-E-2 with other side chains replacing the geranyl moiety.
In summary, various ComXRO-E-2 analogs were synthesized and their biological activities were studied to investigate structure-activity-relationships. These results showed that the minimal active unit was the tripeptide, [3–5]ComXRO-E-2, and all residues except the modified tryptophan residue were replaceable by alanine without total loss of activity.
Acknowledgments
The work done at Nagoya Univ. was supported by Grant-in-Aid for COE (14COEA02) and Scientific Research (No. 18101009). The work done at PHRI was supported by US National Institutes of Health grant GM57720.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.Recent reviews: Lyon GJ, Muir TW. Chem Biol. 2003;10:1007. doi: 10.1016/j.chembiol.2003.11.003.Waters CM, Bassler BL. Annu Rev Cell Dev Biol. 2005;21:319. doi: 10.1146/annurev.cellbio.21.012704.131001.Camilli A, Bassler BL. Science. 2006;311:1113. doi: 10.1126/science.1121357.
- 2.Dong Y-H, Wang L-H, Xu J-L, Zhang H-B, Zhang X-F, Zhang L-H. Nature. 2001;411:813. doi: 10.1038/35081101. [DOI] [PubMed] [Google Scholar]
- 3.Magnuson R, Solomon J, Grossman AD. Cell. 1994;77:207. doi: 10.1016/0092-8674(94)90313-1. [DOI] [PubMed] [Google Scholar]
- 4.(a) Weinrauch Y, Penchev R, Dubnau E, Smith I, Dubnau D. Genes Dev. 1990;4:860. doi: 10.1101/gad.4.5.860. [DOI] [PubMed] [Google Scholar]; (b) Piazza F, Tortosa P, Dubnau D. J Bacteriol. 2001;181:4540. doi: 10.1128/jb.181.15.4540-4548.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Recent reviews: Tortosa P, Dubnau D. Curr Opin Microbiol. 1999;2:588. doi: 10.1016/s1369-5274(99)00026-0.Hamoen LW, Venema G, Kuipers OP. Microbiology. 2003;149:9. doi: 10.1099/mic.0.26003-0.
- 6.Ansaldi M, Dubnau D. Mol Microbiol. 2002;44:1561. doi: 10.1046/j.1365-2958.2002.02977.x. [DOI] [PubMed] [Google Scholar]
- 7.Bacon Schneider K, Palmer TM, Grossman AD. J Bacteriol. 2002;184:410. doi: 10.1128/JB.184.2.410-419.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke S. Ann Rev Biochem. 1992;61:355. doi: 10.1146/annurev.bi.61.070192.002035. [DOI] [PubMed] [Google Scholar]
- 9.Mazieres J, Pradines A, Favre G. Cancer Lett. 2004;206:159. doi: 10.1016/j.canlet.2003.08.033. [DOI] [PubMed] [Google Scholar]
- 10.Okada M, Sato I, Cho SJ, Iwata H, Nishio T, Dubnau D, Sakagami Y. Nat Chem Biol. 2005;1:23. doi: 10.1038/nchembio709. [DOI] [PubMed] [Google Scholar]
- 11.Okada M, Sato I, Cho SJ, Suzuki Y, Ojika M, Dubnau D, Sakagami Y. Biosci Biotech Biochem. 2004;68:2374. doi: 10.1271/bbb.68.2374. [DOI] [PubMed] [Google Scholar]
- 12.Okada M, Yamaguchi H, Sato I, Dubnau D, Sakagami Y. Tetrahedron. 2006;62:8907. [Google Scholar]
- 13.Fmoc-protected modified tryptophan residue with a geranyl group was synthesized as previously reported,10–12 and other residues were purchased from commercial sources (Watanabe chemical, Nova biochem). Peptide bond formation was accomplished with a peptide synthesizer except for the modified tryptophan coupling. After each crude peptide was purified by HPLC, the resulting peptides were confirmed by HRMS and MS/MS analyses. Each ComXRO-E-2 peptide (about 1 mg) was obtained in 1–10% yield based on the each resin.
- 14.Using the B. subtilis tester strain employed the expression of an srfA-lacZ fusion, which responds to add ComXRO-E-2 pheromone,6 biological activity was investigated as previously reported.6,10–12 Briefly, the strain was grown overnight, and then diluted 100–fold. The culture was added to a sample solution, and incubated at 37 °C for 5 h at 150 rpm, then β-galactosidase activity was measured at 420 nm with a standard method using o-nitrophenyl-β-D-galactopyranoside at 30 °C. Each analog was tested at concentrations up to 300 nM, because the response to the ComXRO-E-2 pheromone and to analogs possessing the modified tryptophan residue achieved saturation at this concentration.
- 15.Sakagami Y, Yoshida M, Isogai A, Suzuki A. Science. 1981;212:1525. doi: 10.1126/science.212.4502.1525. [DOI] [PubMed] [Google Scholar]
- 16.Fujino M, Kitada C, Sakagami Y, Isogai A, Tamura S, Suzuki A. Naturwissenschaften. 1980;67:406. [Google Scholar]


