Abstract
Background and purpose
Rapid eye movement (REM) sleep behavior disorder (RBD) is a parasomnia reflecting changes in the brain, but which specific neuronal networks are involved in human RBD pathogenesis has not yet been determined. To date, only one case of idiopathic RBD has undergone autopsy, in which “incidental Lewy body disease” was found. Due to the severe neuronal loss and gliosis in the substantia nigra (SN) and locus ceruleus (LC) in this case, degeneration of brainstem monoaminergic neurons was postulated as the underlying substrate for RBD. Additional cases of idiopathic RBD with neuropathologic examination may help clarify which key brainstem structures are involved.
Patient and methods
Case report with neuropathologic analysis.
Results
A man with polysomnographically proven RBD (onset age 57 years), but no other neurologic signs or symptoms, underwent neuropathologic examination upon his death at age 72. Histopathologic analysis showed Lewy body disease, but no significant neuronal loss or gliosis was present in the SN or LC.
Conclusions
This case represents another example of Lewy body disease associated with RBD. The minimal degenerative changes in the SN and LC call into question the role of these nuclei in RBD, at least in our case. We suggest additional cases of idiopathic RBD undergo neuropathologic analyses to better delineate the neurologic substrate of this intriguing parasomnia.
Keywords: REM sleep behavior disorder, parasomnia, Lewy bodies, Lewy body disease, synuclein
Introduction
Rapid eye movement (REM) sleep behavior disorder (RBD) is characterized by loss of normal skeletal muscle atonia during REM sleep with prominent motor activity and dreaming [1,2]. There is ample data from animal and human studies that dysfunction of brainstem structures and networks are likely involved, but which specific neuronal networks are involved in RBD pathogenesis has not yet been determined [1,3]. To date, fewer than 25 cases of polysomnographically (PSG) proven RBD cases have undergone neuropathologic examination [3], and only one case of idiopathic RBD has undergone autopsy, in which “incidental Lewy body disease” was found [4]. We report herein another case of idiopathic RBD associated with Lewy body disease (LBD), but with degenerative changes topographically different from those previously reported.
Subject and Methods
The patient was followed longitudinally at Mayo Clinic Rochester, and written consent was provided for autopsy. Evaluation of antemortem and pathologic data for research purposes on patients with RBD has also been approved by the Mayo Foundation Institutional Review Board.
All available clinical, laboratory, and PSG data were analyzed and summarized. PSG included surface electromyographic (EMG) monitoring of the submental, anterior tibial and wrist extensor muscles. Neuropathologic examination was carried out in standard fashion, and sections were cut and stained with hematoxylin and eosin (H&E) and thioflavin-S and immunostained for α-synuclein and tau. The pathologic characterization was based on the National Institute on Aging – Reagan Institute criteria for Alzheimer’s disease [5], and the criteria of Kosaka et al. for Lewy body disease [6] and 3rd Report of the DLB Consortium for Lewy body dementia [7] (i.e., brainstem-, limbic-, or neocortical- predominant LBD).
Results
Antemortem Features
This right-handed retired male physician began exhibiting dream enactment behavior at the age of 57. His wife observed numerous episodes of flailing and thrashing his limbs while vocalizing. She had been struck and bitten several times. The patient would describe the dreams associated with this activity as nightmares in which he was being chased or attacked, and he bolted out of bed on several occasions while attempting to evade the attackers. The frequency and severity of these episodes increased such that by age 69, he arranged an evaluation in the Mayo Sleep Disorders Center. His PSG showed markedly increased EMG tone and aperiodic limb movements during REM sleep. His apnea/hypopnea index was 1/hour, and there were 63 periodic limb movements per hour, with 9% associated with arousals. This PSG, therefore, confirmed the clinical suspicion of RBD, and he was treated with clonazepam 0.5 mg/night, which was very effective at controlling RBD. Two years later, he attempted to wean himself off clonazepam, but dream enactment behavior quickly returned, resulting in a fractured toe after hurling himself out of bed one night.
At age 71 he began taking fluoxetine for atypical depression, which had no effect on his RBD symptoms. By age 72 he was experiencing marked dyspnea and fatigue. He was evaluated by eight different clinicians over the final year of his life (including one of us – EJO), and there were no concerns expressed by the patient, his family, or the clinicians involved in his care about any cognitive or motor dysfunction. There was also no history of orthostatic hypotension, syncope, impotence, urinary incontinence, or anosmia. He expired at age 72 from complications relating to cryptogenic organizing pneumonia. None of his relatives had been diagnosed with any neurodegenerative disorder.
Neuropathologic Features
The brain was minimally atrophic on gross examination. On microscopic examination, no senile plaques (SP) or neurofibrillary tangles (NFT) were detected in the neocortex. The Braak NFT stage was consistent with Stage II. The detailed distribution of α-synuclein pathology is shown in the Table 1, and density of Alzheimer-type pathology with thioflavin-S fluorescent microscopy is shown in Table 2. Photomicrographs of pertinent histopathologic findings are shown in the Figure. Lewy bodies (LBs), Lewy neurites (LNs), and neuronal loss were present in the dorsal motor nucleus of the vagus (Fig) and the medullary tegmentum, including the region encompassing the gigantocellular reticular formation (GCRF). There were also many LBs and LNs in the central raphe nucleus (RN) (Fig). The locus ceruleus (LC) had no significant neuronal loss and gliosis but did have many LBs and LNs (Fig). There were no other significant degenerative changes in the rostral pontine tegmentum. The substantia nigra (SN) had no significant neuronal loss, but there were LBs and LNs (Fig). The basal nucleus of Meynert had minimal neuronal loss and no NFT. On the other hand, LBs and LNs were numerous in the basal forebrain (Fig). No SP or NFT were present in the amygdala, but there were a few LBs and LNs in the cortical transition zone. The basal ganglia were unremarkable. The findings are consistent with brainstem-predominant LBD [6]. Since pathology was present in the brainstem nuclei, basal forebrain, and amygdala, the findings are also consistent with Parkinson’s disease stage 3 [8]. Given the paucity of LBs in cortical areas and the minimal Alzheimer-type pathology, the likelihood of dementia with LBs is low [7].
Table 1.
Distribution of neuronal loss and α-synuclein pathology
| Region | NL | LB | LN |
|---|---|---|---|
| Frontal cortex | 0 | 0 | 0 |
| Temporal cortex | 0 | 0 | 0 |
| Parietal cortex | 0 | 0 | 0 |
| Occipital cortex | 0 | 0 | 0 |
| Cingulate cortex | 0 | 1+ | 0 |
| Hippocampal CA2/3 | 0 | 0 | 2+ |
| Entorhinal cortex | 1+ | 1+ | 0 |
| Amygdala | 0 | 2+ | 1+ |
| Hypothalamus – anterior (infundibulum) | 0 | 1+ | 1+ |
| Hypothalamus - posterior | 1+ | 2+ | 2+ |
| Caudate/putamen | 0 | 0 | 1+ |
| Globus pallidus | 0 | 0 | 1+ |
| Basal nucleus of Meynert | 0 | 3+ | 3+ |
| Thalamus – intralaminar | 0 | 1+ | 2+ |
| Subthalamic nucleus | 0 | 1+ | 1+ |
| Laterodorsal tegmental nucleus | 0 | 2+ | 1+ |
| Pedunculopontine nucleus | 0 | 1+ | 2+ |
| Dorsal raphe (midbrain) | 0 | 2+ | 2+ |
| Substantia nigra | 0 | 3+ | 3+ |
| Locus ceruleus | 0 | 3+ | 3+ |
| Subceruleus nucleus | 0 | 3+ | 2+ |
| Central raphe (pons) | 1+ | 2+ | 2+ |
| Pontine reticular formation | 1+ | 1+ | 2+ |
| Dorsal motor nucleus | 1+ | 1+ | 2+ |
| Medullary reticular formation | 0 | 2+ | 2+ |
| Cerebellar dentate nucleus | 0 | 0 | 0 |
| Spinal cord – intermediolateral | 0 | 1+ | 2+ |
| Spinal cord – anterior horn | 0 | 0 | 1+ |
| Spinal cord – dorsal horn | 0 | 0 | 1+ |
Abbreviations: NL = neuronal loss and/or gliosis; LB = Lewy bodies; LN = Lewy neurites; 0 = none, + = mild or sparse, 2+ = moderate, 3+ = severe or frequent
Table 2.
Distribution of Alzheimer-type pathology
| Region | SP (per 100x) | NFT (per 400x) | CAA (score) |
|---|---|---|---|
| Frontal cortex | 0 | 0 | 0 |
| Temporal cortex | 0 | 0 | 0 |
| Occipital cortex | 0 | 0 | 0 |
| Cingulate gyrus | 0 | 0 | 0 |
| Hippocampus – CA2/3 | 0 | 0–10 | na |
| Hippocampus – CA1 | 0 | 0–1 | na |
| Hippocampus – subiculum | 0 | 0 | na |
| Entorhinal cortex – layer II | 0 | 8–11 | na |
| Entorhinal cortex – layer IV | 0 | 1–5 | na |
| Amygdala – corticomedial | 0 | 0 | na |
| Amygdala – basolateral | 0 | 0 | na |
| Basal nucleus of Meynert | 0 | 0 | na |
| Basal ganglia – putamen | 0 | 0 | na |
Senile plaques (SP), neurofibrillary tangles (NFT) and amyloid angiopathy (CAA) were assessed in sections stained with thioflavin-S fluorescent microscopy. Except for the hippocampus and entorhinal cortex, there was no Alzheimer-type pathology and the Braak NFT stage was consistent with Stage II. (na = not assessed)
Figure.
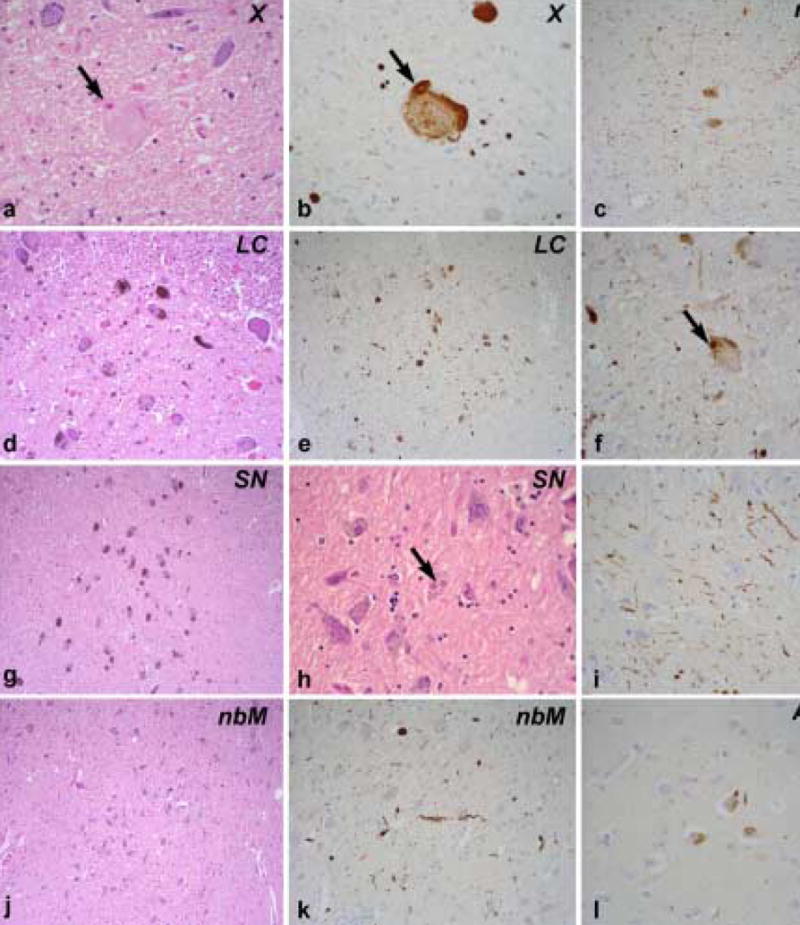
a.) The dorsal motor nucleus (X) shows mild neuronal loss and gliosis with spheroids containing LBs (arrow). b.) An adjacent section of the dorsal motor nucleus (X) immunostained for α-synuclein shows a spheroid containing a LB (arrow). c.) Low power image of the central raphe nucleus (raphe) immunostained for α-synuclein shows neuronal immunoreactivity and scattered LNs. d.) The low power H&E stained section of the locus ceruleus (LC) shows a normal neuronal population. e.) The low power section of locus ceruleus (LC) immunostained for α-synuclein shows LBs and LNs. f.) Higher magnification of the locus ceruleus (LC) shows more clearly LBs (arrow) and LNs. g.) A low power H&E stained section of the substantia nigra (SN) shows a normal neuronal population. h.) A higher power H&E stained section shows LBs (arrow) in the substantia nigra (SN). i.) High power image of α-synuclein immunostained section of the hippocampus reveals sparse LNs in the CA2 sector. j.) A low power H&E stained section of the basal nucleus of Meynert (nbM) shows a normal neuronal population. k.) At higher magnification LNs are visible in the basal nucleus of Meynert (nbM) with α-synuclein immunohistochemistry. l.) Spare LBs and LNs are detected in the amygdala (Amyg) with α-synuclein immunohistochemistry. (c, d, e, g, j – 200x; a, b, f, h, I, k, l – 400x)
Discussion
RBD has been associated with several etiologies, including medications, autoimmune/limbic encephalitis, narcolepsy, structural lesions in the brainstem, and neurodegenerative disease [1,2,9,10,11]. The few cases which have been studied with magnetic resonance imaging (MRI) have shown lesions in the dorsal pons [10,11], yet the specific neuronal networks involved in human RBD pathogenesis have not been identified with certainty. Although ubiquitin and α-synuclein immunocytochemistry were not used in the case reported by Uchiyama et al. [4], and the consensus criteria for the clinical and pathologic diagnoses of dementia with Lewy bodies (DLB) and LBD had not yet been published, the distribution of LBs suggests that their case would now be classified as brainstem-predominant LBD [6,7]. Due to the severe neuronal loss and gliosis in the SN and LC in their case, the authors postulated that degeneration of brainstem monoaminergic neurons explained RBD [4]. Additional cases of idiopathic RBD with neuropathologic examination are needed to confirm or refute this hypothesis.
Our patient had classic clinical and PSG features of RBD. Although he was not evaluated by a neurologist at any time after the diagnosis of RBD was made, he was seen by numerous physicians over his last year of life, and no neurologic symptoms or signs were apparent. Hence, our case is best characterized clinically as “idiopathic RBD.”
Histopathologic analysis in our case showed LBs and LNs, yet no significant neuronal loss or gliosis in the SN, LC, and RN. LBs, LNs, and degenerative changes were also present in the GCRF. Since symptomatology in LBD reflects neuronal loss more so than the presence of LBs and LNs [12], the findings in this case argue against RBD reflecting degeneration in the SN, LC, and RN.
Although our patient did not have his tissue processed in a manner so that detailed neuronal quantification could be carried out, the fact that no significant degeneration was evident in the SN and LC calls into question the role of these nuclei in RBD, at least in our case. The pedunculopontine nucleus (PPN) and laterodorsal tegmental nucleus (LDTN) could be involved, but the minimal degenerative changes in the rostral pons in our case, and the increased cholinergic mesopontine neurons in the case reported by Schenck et al. [13], argue against marked degeneration of the PPN and LDTN being central to RBD. But what other nuclei could be involved? To date, no detailed neuronal counts have been carried out on the GCRF – the most caudal nucleus that exerts inhibitory influences on the alpha-motor neurons in the spinal cord. LBs, LNs, and neuronal loss were present in the GCRF in our case, but the large size and poorly defined borders of the GCRF makes quantification of neuronal loss challenging. Studies in the cat implicate the ventral part of the oral pontine reticular nucleus (vRPO) being critical to REM sleep [14]; this nucleus has not been studied in detail in humans. Very recently, lesions to the sublaterodorsal (SLD) nucleus (analogous to the subcoeruleus/peri-coeruleus region in cats) were found to cause disinhibition of spinal motoneurons, resulting in increased EMG tone during REM sleep [15]. The SLD or homologous nucleus has not been examined rigorously in humans, but certainly this nucleus warrants further study. Clearly, further work in brainstem neuron quantification is necessary in RBD patients, yet one hindrance in deciphering RBD pathogenesis in patients with parkinsonism and/or dementia is that multiple neuronal systems degenerate, making it difficult to know which systems are truly central to the disorder. Analyses in patients with idiopathic RBD may be most enlightening, as the degenerative changes may be more mild and selective, and thus more revealing.
Acknowledgments
This study was supported by grants P50 NS40256, P50 AG16574, and RO1 AG15866.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schenck C, Mahowald M. REM sleep behavior disorder: Clinical, developmental, and neuroscience perspectives 16 years after its formal identification in SLEEP. Sleep. 2002;25:120–38. doi: 10.1093/sleep/25.2.120. [DOI] [PubMed] [Google Scholar]
- 2.Olson E, Boeve B, Silber M. Rapid eye movement sleep behavior disorder: demographic, clinical, and laboratory findings in 93 cases. Brain. 2000;123:331–9. doi: 10.1093/brain/123.2.331. [DOI] [PubMed] [Google Scholar]
- 3.Boeve B, Silber M, Parisi J, et al. Synucleinopathy pathology and REM sleep behavior disorder plus dementia or parkinsonism. Neurology. 2003;61:40–5. doi: 10.1212/01.wnl.0000073619.94467.b0. [DOI] [PubMed] [Google Scholar]
- 4.Uchiyama M, Isse K, Tanaka K, et al. Incidental Lewy body disease in a patient with REM sleep behavior disorder. Neurology. 1995;45:709–12. doi: 10.1212/wnl.45.4.709. [DOI] [PubMed] [Google Scholar]
- 5.Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Neurobiol Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- 6.Kosaka K, Yoshimura M, Ikeda K, Budka H. Diffuse type of Lewy body disease: progressive dementia with abundant cortical Lewy bodies and senile changes of varying degree—A new disease? Clin Neuropathol. 1984;3:185–192. [PubMed] [Google Scholar]
- 7.McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, et al. Dementia with Lewy bodies: Diagnosis and management: Third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 8.Braak H, Del Tredici K, Rub U, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 9.Iranzo A, Graus F, Clover L, et al. Rapid eye movement sleep behavior disorder and potassium channel antibody-associated limbic encephalitis. Ann Neurol. 2006;59:178–181. doi: 10.1002/ana.20693. [DOI] [PubMed] [Google Scholar]
- 10.Kimura K, Tachibana N, Kohyama J, Otsuka Y, Fukazawa S, Waki R. A discrete pontine ischemic lesion could cause REM sleep behavior disorder. Neurology. 2000;55:894–895. doi: 10.1212/wnl.55.6.894. [DOI] [PubMed] [Google Scholar]
- 11.Tippmann-Peikert M, Boeve BF, Keegan BM. REM sleep behavior disorder initiated by acute brainstem multiple sclerosis. Neurology. 2006;66:1277–1279. doi: 10.1212/01.wnl.0000208518.72660.ff. [DOI] [PubMed] [Google Scholar]
- 12.Benarroch E, Schmeichel A, Low P, et al. Involvement of medullary regions controlling sympathetic output in Lewy body disease. Brain. 2005;128:338–44. doi: 10.1093/brain/awh376. [DOI] [PubMed] [Google Scholar]
- 13.Schenck CH, Garcia-Rill E, Skinner RD, et al. A case of REM sleep behavior disorder with autopsy-confirmed Alzheimer’s disease: postmortem brain stem histochemical analyses. Biol Psychiatry. 1996;40:422–5. doi: 10.1016/0006-3223(96)00070-4. [DOI] [PubMed] [Google Scholar]
- 14.Rodrigo-Angulo M, Rodriguez-Veiga E, Reinoso-Suarez F. A quantitative study of the brainstem cholinergic projections to the ventral part of the oral pontine reticular nucleus (REM sleep induction site) in the cat. Exp Brain Res. 2005;160:334–43. doi: 10.1007/s00221-004-2015-x. [DOI] [PubMed] [Google Scholar]
- 15.Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006 May 10; doi: 10.1038/nature04767. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


