Abstract
In pathological conditions interpretation of functional magnetic resonance imaging (fMRI) results can be difficult. This is due to a reliance on the assumed coupling between neuronal activity and changes in cerebral blood flow (CBF) and oxygenation. We wanted to investigate the coupling between blood oxygen level dependant contrast (BOLD) and CBF time courses in epilepsy patients with generalised spike wave activity (GSW) to better understand the underlying mechanisms behind the EEG-fMRI signal changes observed, especially in regions of negative BOLD response (NBR). Four patients with frequent GSW were scanned with simultaneous electroencephalographic (EEG)-fMRI with BOLD and arterial spin labeling (ASL) sequences. We examined the relationship between simultaneous CBF and BOLD measurements by looking at the correlation of the two signals in terms of percentage signal change on a voxel-by-voxel basis. This method is not reliant on coincident activation. BOLD and CBF were positively correlated in patients with epilepsy during background EEG activity and GSW. The subject average value of the ΔCBF/ΔBOLD slope lay between +19 and +36 and also showed spatial variation which could indicate areas with altered vascular response. There was not a significant difference between ΔCBF/ΔBOLD during GSW, suggesting that neurovascular coupling to BOLD signal is generally maintained between states and, in particular, within areas of NBR.
Keywords: EEG-fMRI, Generalized spike wave, BOLD, Perfusion, Correlation, Neurovascular coupling
1. Introduction
Altered brain metabolism and perfusion may confound functional imaging studies in patients with epilepsy. Generalised spike wave activity (GSW), the electroencephalographic (EEG) hallmark of absence seizures, occurs in idiopathic generalised epilepsy but can also occur in secondarily generalised patients. Some patients have GSW without any clinical manifestation and have been studied with EEG-fMRI, which characteristically shows thalamic activation and widespread cortical “deactivations” [1–3].
In pathological conditions, interpretation of functional magnetic resonance imaging (fMRI) results can be difficult. This is due to a reliance on the assumed coupling between neuronal activity and changes in cerebral blood flow (CBF) and oxygenation that are predominantly responsible for the signal changes seen in gradient echo images via blood oxygen level dependant contrast (BOLD) [4,5].
We wanted to investigate the coupling between BOLD and CBF time courses in epilepsy patients with GSW to try to better understand the underlying mechanisms behind the EEG-fMRI signal changes observed especially in regions of negative BOLD response (NBR).
In a few studies, this neurovascular coupling has been investigated with calibrated BOLD experiments (e.g., Refs. [5,6]). These allow simultaneous noninvasive estimation of perfusion and the rate of cerebral oxygen consumption. A difficulty of this method is that the investigation of these relationships relies on significant colocalised brain activation in both the BOLD and perfusion time courses and calibration with graded hypercapnia. The low signal-to-noise ratio (SNR) of both the BOLD and perfusion time courses [7] is a particular problem in epilepsy studies where responses to spontaneous activity are investigated rather than a controlled task with optimised paradigms. The different SNR of these two also means that the choice of a significant threshold for each time series is highly problematic. Even in the case where significant signal changes are seen in both BOLD and perfusion time series, there are issues to be overcome with regard to spatial localisation. Finally, even when this has been achieved, demonstrating coupling in one small brain area does not easily allow for generalisation over the rest of the brain.
In this article, we investigate in more detail the relationship between BOLD and CBF time courses using a simple method that does allow for observations to be made over the whole brain without the need for spatially consistent (de/) activations within the two different signals. In particular, we examine the relationship between CBF and BOLD changes to see if there was a difference between rest and GSW that could indicate altered cerebral oxygen status.
2. Methods
Four patients with frequent GSW were scanned with simultaneous EEG-fMRI with BOLD and arterial spin labeling (ASL) sequences. Patient and data acquisition details can be found in Hamandi et al. [8].
2.1. EEG acquisition and processing
Thirty-two channels of surface EEG were recorded in the magnetic resonance scanner using magnetic resonance imaging (MRI)-compatible hardware (BrainAmp MRplus, Brainproducts, Munich, Germany; BrainCap MR, Easycap, Herrsching-Breitbrunn, Germany). The start and stop of GSW events were visually marked on the EEG according to the fMRI time series.
2.2. MRI acquisition
Imaging was carried out on a 3-T Siemens Allegra head scanner (Siemens, Erlangen, Germany) using a standard head transmit/receive coil.
A 30-min time series was obtained with a pulsed arterial spin labelling sequence (Q2TIPS) [9,10] with the PICORE labelling scheme. The scanning parameters were the following: TR 2.3 s, (time for acquisition of a single slice was 66 ms, TI1/TI1stop/TI2 = 600/1200/1300 ms), TE 30 ms, six axial slices (extending superiorly from the top of the corpus callosum), 4-mm slice thickness, slice gap 0.5 mm, FOV 22.4×22.4 cm, matrix 64×64 (see Ref. [10] for full sequence details).
2.3. MRI processing
In-house software written in MATLAB (www.mathworks.com) for the calculation of perfusion images. BOLD and ASL series were preprocessed separately. Standard general linear model (GLM) analyses were run in SPM2 (http://www.fil.ion.ucl.ac.uk/SPM) see ref [8].
A time series of the difference images (control-label) was calculated by subtracting adjacent control and label images; a “surround average” of preceding and following label images was used to remove effects of BOLD signal fluctuation within one TR [7] and expressed as a ratio of the control image to remove BOLD contrast present in both label and control images [11] and create a relative CBF time series. The difference images were filtered according to Garraux et al. [11]. In brief, abnormal flow values that did not fall within a physiological range (e.g., due to head motion) were removed, by retaining only (1) pairs of voxels where control signal intensity had a value greater than 80% of the global mean intensity of the control image and (2) voxels with a fractional signal change of less than ±5%.
A corresponding BOLD sensitive time series was calculated from the ASL data by summation of adjacent label and control images.
2.4. BOLD and CBF correlation analysis
Firstly, a design matrix was created using SPM2 for both the ASL and BOLD time series and used to generate a time series of the confounding variance (due to motion) that was then removed by subtraction. Then linear drift was removed from the BOLD time course (using the MATLAB detrend.m function). Both these time series were converted into units of percent change which will be referred to as ΔBOLD and ΔCBF measurements. Pixels with a very low (e.g., white matter) or very high (e.g. edges) mean ΔCBF were removed.
To investigate the coupling between the ΔCBF and ΔBOLD measurements, we performed a voxel-wise linear regression between the two using a one way analysis of covariance as implemented in the aoctool.m function in the MATLAB statistics toolbox (www.mathworks.com). An additional regressor used to assign the data at each time point to either (1) a rest epoch (images acquired during background EEG activity) or (2) a GSW epoch (images acquired during GSW). This is used to determine if there is a difference in this relationship between EEG-defined states (rest and GSW). In particular, we examined the slope of the ΔCBF and ΔBOLD on a voxelwise basis (P<.001 uncorrected), and for the significant voxels, the signal was averaged and correlation coefficients were obtained for rest and GSW periods.
3. Results
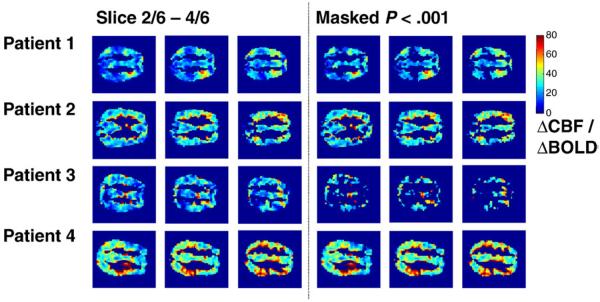
Over the whole time series, irrespective of EEG state, nearly the entire imaged brain volume showed a significant positive correlation between BOLD and CBF signal in all patients (Fig. 1). There was some considerable regional variation in the slope value. For each subject, the region of the brain demonstrating a significant correlation was used to obtain an average time course. This was then investigated to obtain an average relationship for ΔCBF/ΔBOLD (Table 1). The range of values lay between +19 and +36 and was highly significant in all subjects. It should be noted that this is despite widespread cortical NBR seen by standard GLM analysis [8]. There was not a significant difference in this relationship for the two EEG-defined states (rest and GSW) either at a voxel or brain-average level (P<.01), and the sign of the change varied.
Fig. 1.
A comparison of the percentage change in CBF to the percentage change in BOLD signal for 4 patients (each row). The ratio of ΔCBF/ΔBOLD (i.e., the slope of the linear fit between the two signal changes) is shown for each voxel on the left hand side. On the right hand side voxels are thresholded at a level of P<.001 (uncorrected) the average time signal from these voxels was obtained and tested (see Table 1).
Table 1.
The average slope of ΔCBF/ΔBOLD signal changes taken over all significant voxels for each patient assessed over the whole time series and then for differences between rest and GSW
| Patient | ΔCBF/ ΔBOLD all |
P | ΔCBF/ ΔBOLD rest |
ΔCBF/ΔBOLD GSW |
P |
|---|---|---|---|---|---|
| 1 | 19.3 | < .001 | 18.1 | 20.5 | .772 |
| 2 | 22.3 | < .001 | 11.3 | 33.3 | .042 |
| 3 | 29.1 | < .001 | 30.7 | 27.5 | .805 |
| 4 | 36.5 | < .001 | 44.2 | 28.8 | .035 |
4. Discussion
BOLD and CBF were positively correlated in patients with epilepsy during background EEG activity and GSW. Interestingly, the value of the ΔCBF/ΔBOLD slope did seem to show spatial variation which could indicate areas with altered vascular response as has been seen in hypoxia [12] or regions with different ranges of oxygen consumption. Significant differences in ΔCBF/ΔBOLD were not found due to GSW compared to background changes in spontaneous activity.
Preprocessing of the data followed by the analysis presented could introduce some level of correlation into the data and/or alter their statistical significance by reducing the apparent degrees of freedom. However, the analysis was run without removing motion confounds and similar results were obtained. We assumed a linear relationship between ΔCBF and ΔBOLD which is a reasonable approximation over the likely range of ΔCBF [5]. Additionally, we chose a relatively high level of significance (P<.001 uncorrected) as a threshold for ΔCBF/ΔBOLD. Significant differences in ΔCBF/ΔBOLD between rest and GSW were not found. The values of the ΔCBF/ΔBOLD slope are in broad agreement with those obtained in cognitive paradigms in healthy subjects [5,6] where changes in oxygen consumption make the ΔCBF/ΔBOLD gradient higher than would be expected for changes in CBF alone (as for hypercapnia). This indicates that neurovascular coupling and cerebral energy consumption is not altered between states or in regions exhibiting deactivations. While we do not have a direct measure of neural activity or oxygen consumption, because we know GSW is associated with cortical inhibition, we infer that this inhibition lowers metabolic demands reflected in a reduction in perfusion that leads to a reduction in the BOLD signal in a proportion suggesting that neurovascular coupling to the BOLD signal is maintained as in the experimental studies of Shmuel et al. [13]. However, we cannot exclude the possibility that differences were below a detectable level.
In previous work, the examination of BOLD and perfusion time courses have relied on analysing spatially overlapping (de/)activations in both BOLD and perfusion time courses [6]. This is problematic due to the differing sensitivity of the two methods [7] and presents a particular difficulty when the task is not controlled — as for spontaneous epileptic discharges. In brain areas that do show coincident BOLD and perfusion positive or negative responses, it is highly likely that normal neurovascular coupling will be present. We have adopted a simple approach of looking at the correlation of the two signals on a voxel-by-voxel basis to examine this relationship in areas where BOLD and perfusion do not necessarily both show coincident activation.
For regional assessment we note that constructing a full model containing both confounds and the perfusion time course for comparison to the BOLD signal is preferred for the most accurate statistical determination of the strength of this relationship. However, for voxel-by-voxel analysis, this would require a different “design matrix” to be used for each voxel.
Acknowledgments
This work was funded by the Wellcome Trust, grant number 067176 (KH, LL); the Deutsche Forschungsgemeinschaft, grant number LA 1452/3-1 (HL) and the MRC, grant number G0301067. The National Society for Epilepsy supports the MRI Unit. We thank Professor Ray Dolan (Institute of Neurology, UCL) for his support of this work, Ulrike Nöth (Institute of Neurology, UCL) for developing and assisting with data acquisition and Oliver Josephs (Institute of Neurology, UCL), Dr Laura Parkes (MARIAC, University of Liverpool), Professor Robert Turner (Institute of Neurology, UCL) and Dr Torben Lund for helpful discussions on the perfusion analysis.
References
- 1.Salek-Haddadi A, Lemieux L, Merschhemke M, Friston KJ, Duncan JS, Fish DR. Functional magnetic resonance imaging of human absence seizures. Ann Neurol. 2003;53:663–7. doi: 10.1002/ana.10586. [DOI] [PubMed] [Google Scholar]
- 2.Hamandi K, Salek-Haddadi A, Laufs H, Liston A, Friston K, Fish DR, et al. EEG-fMRI of idiopathic and secondarily generalized epilepsies. NeuroImage. 2006;31:1700–10. doi: 10.1016/j.neuroimage.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 3.Gotman J, Grova C, Bagshaw A, Kobayashi E, Aghakhani Y, Dubeau F. Generalized epileptic discharges show thalamocortical activation and suspension of the default state of the brain. Proc Natl Acad Sci. 2005;102:15236–40. doi: 10.1073/pnas.0504935102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci. 1990;87:9868–72. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB. Investigation of BOLD signal dependence on cerebral blood flow and oxygen consumption: the deoxyhemoglobin dilution model. Magn Reson Med. 1999;42(5):849–63. doi: 10.1002/(sici)1522-2594(199911)42:5<849::aid-mrm4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 6.Stefanovic B, Warnking JM, Kobayashi E, Bagshaw AP, Hawco C, Dubeau F, et al. Hemodynamic and metabolic responses to activation, deactivation and epileptic discharges. NeuroImage. 2005;28:205–15. doi: 10.1016/j.neuroimage.2005.05.038. [DOI] [PubMed] [Google Scholar]
- 7.Aguirre GK, Detre JA, Zarahn E, Alsop DC. Experimental design and the relative sensitivity of BOLD and perfusion fMRI. NeuroImage. 2002;15:488–500. doi: 10.1006/nimg.2001.0990. [DOI] [PubMed] [Google Scholar]
- 8.Hamandi K, Laufs H, Nöth U, Carmichael DW, Duncan JS, Lemieux L. BOLD and perfusion changes during epileptic generalised spike wave activity. NeuroImage. 2008;39(2):608–18. doi: 10.1016/j.neuroimage.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Luh WM, Wong EC, Bandettini PA, Hyde JS. QUIPSS II with thin-slice TI1 periodic saturation: a method for improving accuracy of quantitative perfusion imaging using pulsed arterial spin labeling. Magn Reson Med. 1999;41:1246–54. doi: 10.1002/(sici)1522-2594(199906)41:6<1246::aid-mrm22>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 10.Nöth U, Meadows GE, Kotagima R, Deichmann R, Corfield DR, Turner R. Cerebral vascular response to hypercapnia: determination with perfusion MRI at 1.5 and 3.0 Tesla using a pulsed arterial spin labelling technique. J Magn Reson Imaging. 2006;24(6):1229–35. doi: 10.1002/jmri.20761. [DOI] [PubMed] [Google Scholar]
- 11.Garraux G, Hallett M, Talagala SL. CASL fMRI of subcortico-cortical perfusion changes during memory-guided finger sequences. NeuroImage. 2005;25:122–32. doi: 10.1016/j.neuroimage.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Nöth U, Kotajima F, Deichmann R, Turner R, Corfield DR. Mapping of the cerebral vascular response to hypoxia and hypercapnia using quantitative perfusion MRI at 3 T. NMR Biomed. 2007 doi: 10.1002/nbm.1210. [DOI] [PubMed] [Google Scholar]
- 13.Shmuel A, Augath M, Oeltermann A, Logothetis NK. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci. 2006;9:569–77. doi: 10.1038/nn1675. [DOI] [PubMed] [Google Scholar]