Abstract
Alcohol dependent smokers (N = 118) enrolled in an intensive outpatient substance abuse treatment program were randomized to a concurrent brief or intensive smoking cessation intervention. Brief treatment consisted of a 15-minute counseling session with 5 min follow-up. Intensive intervention consisted of three one-hour counseling sessions plus eight weeks of nicotine patch therapy. The cigarette abstinence rate, verified by breath CO, was significantly higher for intensive (27.5%) versus brief (6.6%) treatment at one month post quit date but not at six months when abstinence rates fell to 9.1% and 2.1%. Smoking treatment assignment did not significantly impact alcohol outcomes. Although intensive smoking treatment was associated with higher rates of short term tobacco abstinence, other, perhaps more intensive smoking interventions are needed to produce lasting smoking cessation in alcohol dependent smokers.
Keywords: smoking, smoking cessation, nicotine, alcoholism, alcoholism treatment
Although cigarette smoking prevalence among U.S. adults has declined to 21 percent (Centers for Disease Control and Prevention, 2005), the majority of individuals with alcohol problems remain current smokers (Hughes, 1995; Lasser et al., 2000). The Department of Health and Human Services (DHHS) Clinical Guidelines for Treating Tobacco Use and Dependence provided a consensus recommendation that smokers receiving treatment for chemical dependency should be provided smoking cessation treatments including both counseling and pharmacotherapy (Fiore, Bailey, Cohen et al., 2000). However, the Guideline panel noted that that this recommendation was made in the absence of definitive randomized clinical trials.
Most community-based treatment settings offer no smoking cessation intervention at all (Fuller et al., in press). A brief counseling intervention such as recommended by the Agency for Healthcare Policy and Research (Smoking Cessation Clinical Guideline Panel & Staff, 1996) may be the most feasible approach in these settings. However, this brief counseling approach may be inadequate in light of research that indicates a strong dose-response relationship between the amount of clinician contact time and successful treatment outcome. Additionally, the most successful of the concurrent alcohol-tobacco treatment trials included nicotine replacement as a component (Cooney, Cooney, Patten, & George, 2004). A recent review (Hughes and Kalman, 2006) found consistent evidence that comorbid alcohol problems were associated with more severe nicotine dependence, suggesting that those with alcohol problems might have a greater need for intensive smoking intervention. The present study was designed to compare brief smoking cessation counseling with a more intensive intervention that incorporated two of the DHHS practice guidelines recommendations, extended clinician contact time and pharmacotherapy for smoking cessation.
An additional concern is that concurrent alcohol and smoking cessation treatment will increase risk of alcohol relapse. With the possible exception of a study by Joseph et al. (2004), this has not seemed to occur (e.g., Hurt et al., 1994; Burling et al., 1991; Burling et al., 2001; Bobo et al., 1995, 1998). Inconsistencies among outcome studies, however, suggest a need for further evaluation of concurrent smoking cessation treatment on alcohol treatment outcome.
The primary aim of this article was to report the outcome of a randomized clinical trial that tested the hypothesis that a smoking cessation intervention consisting of intensive behavioral counseling and nicotine patch therapy will lead to greater short and long-term smoking abstinence compared with a brief smoking cessation advice condition. A second aim was to determine whether there was any effect of the intensive smoking intervention on drinking outcomes. Unlike prior studies of concurrent treatment, this study was conducted in an outpatient setting because it is often the preferred setting for alcohol treatment for patients with sufficient social resources and without serious medical or psychiatric impairment (Finney, Hahn, & Moos, 1996).
Method
Participants
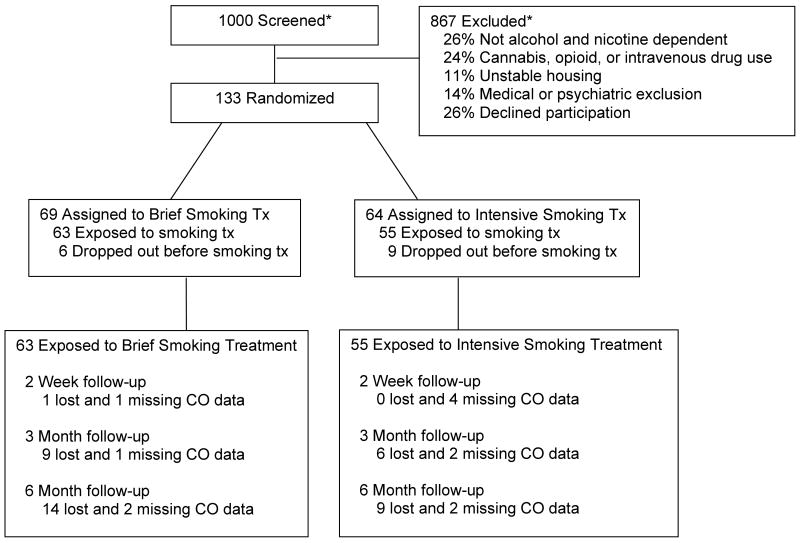
Methodological details for this study are reported in Cooney, Litt, Cooney, Pilkey, Steinberg, and Oncken (in press). Participants were recruited from two VA substance abuse intensive outpatient programs with ten non-veteran women recruited from the community for the purpose of gaining a more diverse sample. Participants had to be at least 18 years old, meet DSM-IV criteria for alcohol and nicotine dependence during the past three months, and smoke at least ten cigarettes per day. Exclusion criteria were diagnosis of current opioid dependence, current cannabis abuse or dependence, and current intravenous drug use, with additional criteria described in Cooney et al. (in press). Participant flow is described in Figure 1. This report was based on the 118 participants exposed to brief (n = 63) or intensive (n = 55) smoking cessation treatment. The study was powered to detect moderate (i.e., d = .3) differences between smoking cessation treatment conditions.
Figure 1.
Participant flow diagram.
* These numbers are estimated because screening data was available for only a subset of 198 individuals.
Design and Procedure
The substance abuse intensive outpatient program provided an initial rehabilitation treatment for substance abusers, utilizing a cognitive-behavioral coping skills approach (Monti, Kadden, Rohsenow, Cooney, & Abrams, 2002). Length of stay in the program was three weeks with program meetings five days per week. Urine and breath were monitored for drug and alcohol use at random intervals during treatment. Participants completed baseline assessment measures and were randomized to either (a) intensive smoking cessation treatment consisting of behavioral counseling and transdermal nicotine replacement, or (b) brief smoking cessation advice without nicotine replacement. During the 14 days immediately after discharge from the substance abuse intensive outpatient program, all participants were asked to participate in an Ecological Momentary Assessment protocol (reported in Cooney et al., in press).
Intensive smoking cessation treatment condition
Behavioral smoking cessation counseling was administered in three 60-minute individual sessions. The manual-guided behavioral counseling protocol followed DHHS practice guidelines (Fiore et al, 2000). The intensity of this intervention protocol was similar to that used by Joseph et al (2004). Content of counseling sessions included setting a targeted quit date, identification of antecedents for smoking urges and behavior, development of behavioral and cognitive coping skills for high risk smoking situations, and acquisition of extra-treatment support for smoking cessation. Treatment was delivered by masters or doctoral level clinicians with experience in behavioral smoking cessation treatments. Smoking quit dates were scheduled approximately one week into the three-week substance abuse treatment program. On the scheduled quit date, participants were provided a free four-week supply of 21 mg Nicoderm© transdermal patches, followed by free prescriptions for two weeks at 14 mg, and two weeks at 7 mg.
Brief smoking cessation advice condition
The brief advice intervention was based on protocols developed by the National Cancer Institute (Glynn & Manley, 1991) and recommended by the Agency for Healthcare Policy and Research (Smoking Cessation Clinical Guideline Panel & Staff, 1996). The smoking cessation therapist saw participants for one fifteen-minute session to ask about smoking status and smoking rate, advise the participant to quit smoking, assist in quitting through setting a quit date within one week and giving cessation tips, and arrange for a brief 5-minute follow-up appointment within three days of the quit date. Nicotine replacement was not offered to participants in this condition.
Assessment
Alcohol and cigarette consumption was measured using the Form 90 (Miller & Del Boca, 1994) administered at baseline and at scheduled research follow-up interviews 14-days, 3-months, and 6-months after discharge from the substance abuse outpatient program. Note that this research follow-up schedule translates to 1 month, 3.5 months, and 6.5 months after the target smoking quit date which was two weeks before program discharge. However, unless otherwise noted, follow-up timing will be designated with reference to time since substance abuse program discharge. Participants were paid $15 for participation in the baseline and first follow-up assessments, and $50 for the second and third follow-up assessments.
Two drinking dependent measures were derived from the Form-90 data collected at each time point. The first of these, Proportion Days Heavy drinking (PDH), reflected the proportion of days of heavy drinking in the 14 days prior to the 14-day follow-up (the time since program discharge) and in the 30 days prior to the 3 and 6-month follow-ups. A heavy drinking day was defined as any day on which a man drank six or more standard drinks or a woman drank four or more standard drinks. The second measure was a dichotomous variable reflecting reported alcohol abstinence throughout the 14 or 30 days prior to a follow-up point. Two smoking dependent measures were also examined. One was repeated 7-day point prevalence smoking abstinence at each of the three scheduled follow-ups, verified by breath carbon monoxide (CO) levels of < 10 parts per million. The second measure was time to relapse, computed from the patient's smoking quit day to the day of the first cigarette in the 180 day follow-up period. These smoking outcome measures were selected because they provide biochemically verifiable measures of reported abstinence over time (Hughes, Keely, Niaura, Ossip-Klein, Richmond, & Swan, 2003).
Results
The sample (N = 118) was 89% male and 11% female. The mean age was 46.6 years (± 7.8 years). Race was 65.5% White, 30.2% Black, 1.7% Hispanic, and 2.6% other. Seventy five percent of the participants were unemployed and 25.4% were married or were living with a spouse or partner. All met DSM-IV criteria for Alcohol and Nicotine Dependence, drank on a mean of 61% of the days in the 3 months prior to seeking treatment at the VA, and consumed an average of 19.3 (± 17.1) alcoholic drinks per day. They reported a mean of 4.2 prior treatments for alcohol problems (SD = 9.1). Participants smoked a mean of 98% of days and a mean of 24.8 cigarettes (SD = 9.9) per day during the three months prior to seeking treatment. The mean Fagerström Test for Nicotine Dependence (Heatherton, Kozlowski, Frecker, & Fagerström, 1991) score was 5.5 (± 2.2). They reported smoking for a mean of 28.1 years (SD = 10.0) and attempted to quit a mean of 3.8 times (SD = 4.8). Participants met criteria for lifetime other substance abuse or dependence as follows: cocaine (32.3%), opioid (10.4%), cannabis (19.8%), stimulant (10.4%), hallucinogen (4.1%), and sedative/hypnotic/anxiolytic (6.3%). Multiple t-tests and chi-square analyses were used determine if the two treatment groups were homogeneous with respect to background variables. Variables examined were sex, age, ethnicity, education, employment status, baseline number of drinks consumed per day, number of smoking quit attempts, cigarettes smoked per day, and baseline Fagerström nicotine dependence score. Analyses indicated that the groups were equivalent on all baseline measures.
All of the study sample completed the intensive outpatient substance abuse program and were exposed to smoking cessation behavioral counseling. Among the twelve participants verified abstinent from smoking four weeks after quit date, ten were prescribed the initial four weeks of nicotine patches and nine were provided nicotine patch renewals.
Form 90 data were obtained for 99.2% of cases at the 14-day follow-up, 87.3% at the 3-month follow-up, and 80.5% of cases at the 6-month follow-up. An additional 3 to 4% of cases were lost for the smoking outcome due to missing carbon monoxide (CO) data. Attrition rates for the 3 and 6-month follow-ups were moderately higher in the Brief treatment condition than in the Intensive treatment condition (see Figure 1). A comparison of those who were lost to those who remained in the study, however, showed that their early smoking quit rates were similar [20% abstinence at 14 days among those lost, compared to 15.5% abstinence among those who remained [N=112; χ2(1)= 0.20, p > .6], suggesting that follow-up attrition was not due to poorer smoking outcomes.
Analysis of Smoking Outcomes
Seven-day point prevalence smoking abstinence rates (CO-verified) for each follow-up point are shown in Table 1. To evaluate the influence of smoking cessation treatment Condition on abstinence from smoking over time, a generalized estimating equations (GEE) approach was used (Proc GENMOD, SAS Institute, 1999). Time was modeled as a repeated measure, and scaled as days since baseline (i.e., 14, 90, and 180 days). An autoregressive (AR1) covariance structure produced the best fit in modeling the repeated indicators of abstinence. The analysis yielded no significant effect for smoking cessation treatment Condition on smoking abstinence, and no interaction of Time X Condition. A significant effect for Time did emerge (βTime = -4.86; se = 0.74; N = 118; χ2(2) = 2.03; p < .05; RR = .007; CI = 0.002 to 0.03). Examination of abstinence rates by time period indicated that abstinence rates decreased over time. Chi-square tests at each time point did show a significantly higher cigarette abstinence rate for the Intensive treatment at the 14-day posttreatment time point. Note that this was one month after target smoking quit date.
Table 1.
7-Day Point Prevalence Abstinence (CO verified) by Smoking Cessation Treatment Condition.
| Time Period | Brief | Intensive | |||
|---|---|---|---|---|---|
| n | % Abstinent | n | % Abstinent | χ2 | |
| Baseline (N=118) | 63 | 0.0 | 55 | 0.0 | -- |
| 2 Weeks (N=112) | 61 | 6.6 | 51 | 27.5 | 8.99** |
| 3 Months (N=100) | 53 | 5.7 | 47 | 4.3 | 0.10 |
| 6 Months (N=91) | 47 | 2.1 | 44 | 9.1 | 2.12 |
p < .01
χ2 is the uncorrected result of the analysis comparing frequency of abstinence by Condition for each given time point (df = 1).
Cox regression analysis was conducted on time to relapse by smoking cessation treatment condition. Time to relapse was computed from the patient's quit day to the day of the first cigarette out to 180 days post alcohol treatment discharge. Results showed no effect for smoking treatment condition on survival. Mean days of abstinence among those who quit were 16.2 (SD = 37.6) for Brief versus 22.6 (SD = 37.0) for Intensive [F(1, 85) = 0.62, ns].
Analysis of Drinking Outcomes
Mean PDH and alcohol abstinence rates based on available data by smoking cessation treatment condition are shown in Tables 2 and 3. Analysis of PDH was conducted using a 2 (treatment condition) X 3 (Time period: 14 days; 3 months; 6 months) linear mixed model regression procedure with the baseline value of PDH used as a covariate (Proc MIXED, SAS Institute, 1999). PDH was arcsine transformed to correct for the skewness of the proportion data (Winer, 1971). The analysis yielded a significant main effect for Time [F(2, 194) = 4.94; p < .01], but no significant main effect for treatment Condition or for the interaction of Condition X Time. Examination of least squared means indicated that PDH increased slightly over time from the 14-day point to the 6-month follow-up.
Table 2.
Proportion Days Heavy Drinking (PDH) by Smoking Cessation Treatment Condition.
| Time Period | Brief | Intensive | |||||
|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | F | |
| Baselinea (N=118) | 63 | .53 | .36 | 55 | .55 | .33 | 0.16 |
| 2 Weeksb (N=117) | 62 | .02 | .06 | 55 | .04 | .18 | 0.95 |
| 3 Monthsa (N=103) | 54 | .10 | .25 | 49 | .07 | .20 | 0.45 |
| 6 Monthsa (N=95) | 49 | .01 | .03 | 46 | .02 | .07 | 0.19 |
reference time period is previous 30 days
reference time period is previous 14 days
F is the uncorrected result of the oneway analysis of variance comparing PDH by Condition for each given time point.
Table 3.
Prolonged Abstinence from Drinking by Smoking Cessation Treatment Condition.
| Time Period | Brief | Intensive | |||
|---|---|---|---|---|---|
| n | % Abstinent | n | % Abstinent | χ2 | |
| Baselinea (N=118) | 63 | 3.2 | 55 | 5.5 | 0.38 |
| 2 Weeksb (N=117) | 62 | 88.7 | 55 | 85.5 | 0.28 |
| 3 Monthsa (N=103) | 54 | 64.8 | 49 | 71.4 | 0.52 |
| 6 Monthsa (N=95) | 49 | 61.2 | 46 | 58.7 | 0.06 |
reference time period is previous 30 days
reference time period is previous 14 days
χ2 is the uncorrected result of the analysis comparing frequency of abstinence by Condition for each given time point (df = 1).
Rates of abstinence from drinking for the time period prior to each follow-up point are shown in Table 3. As in the mixed model analysis described above for smoking quit rates, a GEE approach with an autoregressive covariance structure was used. Abstinence rate at baseline was used as a covariate. The analysis yielded no significant effect for smoking cessation treatment Condition on drinking abstinence, and no interaction of Time X Condition. A significant Time effect emerged, such that longer time from baseline was associated with less abstinence (βtime = -1.47; se = 0.32; N = 118; χ2(3) = 21.27; p < .001; RR=.23; CI = 0.12 to 0.43).
Relationship between smoking and drinking outcomes
Among those assessed at the 14 day follow-up, across both treatment groups, 16% of participants reported not smoking and not drinking, with 76% of participants reporting they were smoking and not drinking. All participants who reported drinking also reported current smoking (8% of the full sample). These results suggest that maintenance of abstinence from alcohol was necessary but not sufficient for maintenance of abstinence from tobacco in the initial follow-up period. There was not enough variability in smoking outcome in the later follow-ups to examine the relationship between long-term smoking and drinking outcomes.
Discussion
This study examined treatment outcome after concurrent alcohol and smoking cessation treatment. The alcohol outcomes were reasonably good, and comparable to those reported in Project MATCH (Babor et al., 2003), with approximately 60% reporting at least 30 days continuous abstinence at the six month follow-up. The intensive smoking treatment was associated with good treatment exposure, good nicotine patch compliance, and higher short-term tobacco abstinence. However, the long-term smoking outcome results were disappointing. Six months after treatment only 9.1% were confirmed abstinent in the intensive treatment condition. This is much lower than the 17% abstinence rate reported in a review of studies of nonalcoholic smokers on nicotine patch therapy (Fiore et al, 2000), but is within the range of what is usually seen in studies of concurrent alcohol tobacco treatment (Burling, Marshall & Seidner, 1991; Bobo et al., 1995, 1998; Joseph, Nichol, & Anderson, 1993). Higher cigarette abstinence rates have been reported in studies that employed far more intensive smoking interventions (Hurt et al., 1994; Burling, Burling, & Latini, 2001). Taken together, these data suggest that alcohol dependent smokers currently in treatment for alcohol problems have more difficulty quitting than those without alcohol problems. A smoking cessation intervention more intensive than the one employed in this study may be needed for effective smoking cessation during alcohol treatment.
Compared to the brief treatment that produced minimal smoking cessation, there appears to be no negative effect of concurrent intensive smoking cessation treatment on drinking soon after treatment or six months later. However, a more effective smoking cessation intervention is required to provide a stronger test of positive or negative carryover effects from smoking cessation to drinking. The lack of any negative carryover effect found here is consistent with earlier research (Hurt et al., 1994; Burling et al., 1991; Burling et al., 2001; Bobo et al., 1995, 1998). However, the present results are not consistent with the methodologically strong study reported by Joseph et al. (2004) which found worse alcohol outcomes after concurrent alcohol tobacco treatment, in comparison to a condition in which smoking cessation was delayed six months. It is possible in that study that the process of anticipating and then participating in a four-session smoking cessation intervention six months after alcohol treatment acted as a booster alcohol treatment leading to better alcohol outcomes, even prior to the onset of the delayed smoking treatment, relative to the concurrent alcohol tobacco condition which had no further treatment during the follow-up phase.
Study Limitations
This design was selected with the aim of maximizing the contrast between brief and intensive smoking cessation interventions in a way that could be readily applied during intensive outpatient alcohol treatment. This design, however, did not allow one to parse out the separate effects of intensity of behavioral counseling and nicotine patch therapy. Also, the manipulation of patch therapy was not placebo controlled and the manipulation of behavioral counseling intensity did not allow inferences regarding the impact of specific content of treatment.
Almost 90% of the individuals screened for this study were not eligible or did not enroll. This is in part because we cast a wide net, screening many who were not eligible. A relatively small number of eligible individuals (26 percent) declined participation, suggesting that there is significant interest in concurrent smoking cessation among those in alcohol treatment. Women were underrepresented in the sample, so generalization of our findings to women should be done with caution. The sample size of this trial limits our ability to detect small effects on smoking and drinking outcomes.
In conclusion, results of this trial suggest that low or moderate intensity smoking interventions delivered concurrent with alcohol treatment are unlikely to produce much long-term smoking abstinence. Without high smoking abstinence rates, one cannot provide a strong test of the impact of smoking cessation on alcohol outcomes. Future concurrent alcohol-tobacco treatment trials should consider more intensive smoking interventions such as a combination of medications, frequent behavioral counseling or contingency management.
Acknowledgments
This research was supported by Grants R01 AA11197 and P50 AA15632 from the National Institute on Alcoholism and Alcohol Abuse and by the U.S. Department of Veterans Affairs.
Contributor Information
Ned L. Cooney, Department of Psychiatry, Yale University School of Medicine and VA Connecticut Healthcare System
Mark D. Litt, Division of Behavioral Sciences and Community Health, University of Connecticut Health Center
Judith L. Cooney, Department of Psychiatry, University of Connecticut School of Medicine, University of Connecticut Health Center and VA Connecticut Healthcare System
David T. Pilkey, Department of Psychiatry, Yale University School of Medicine and VA Connecticut Healthcare System
Howard R. Steinberg, Department of Psychiatry, Yale University School of Medicine and VA Connecticut Healthcare System
Cheryl A. Oncken, Department of Medicine, University of Connecticut School of Medicine, University of Connecticut Health Center
References
- Babor TF, Steinberg K, Zweben A, Cisler R, Stout R, Tonigan JS, Anton RF, Allen JP. Treatment effects across multiple dimensions of outcome. In: Babor TF, Del Boca FK, editors. Treatment matching in alcoholism. Cambridge, UK: Cambridge University Press; 2003. pp. 150–165. [Google Scholar]
- Bobo JK, McIlvain HE, Lando HA, Walker RD, Leed-Kelly A. Effect of smoking cessation counseling on recovery from alcoholism: Findings from a randomized community intervention trial. Addiction. 1998;93:877–887. doi: 10.1046/j.1360-0443.1998.9368779.x. [DOI] [PubMed] [Google Scholar]
- Bobo JK, Walker RD, Lando HA, McIlvain HE. Enhancing alcohol control with counseling on nicotine dependence: pilot study findings and treatment implications. In: Fertig J, Allen JP, editors. Alcohol and tobacco: from basic science to clinical practice. Washington, DC: U.S. Government Printing Office; 1995. NIAAA Research Monograph no. 95-3931. [Google Scholar]
- Burling TA, Burling AS, Latini D. A controlled smoking cessation trial for substance-dependent inpatients. Journal of Consulting and Clinical Psychology. 2001;69:295–304. doi: 10.1037//0022-006x.69.2.295. [DOI] [PubMed] [Google Scholar]
- Burling TA, Marshall GD, Seidner AL. Smoking cessation for substance abuse inpatients. Journal of Substance Abuse. 1991;3:269–276. doi: 10.1016/s0899-3289(10)80011-2. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Cigarette smoking among adults--United States, 2004. Morbidity & Mortality Weekly Report. 2005;54:1121–1124. [PubMed] [Google Scholar]
- Cooney JL, Cooney NL, Patten CA, George TP. Comorbidity of nicotine dependence with affective, psychotic and substance use disorders. In: Kranzler HR, Tinsley JA, editors. Dual diagnosis and psychiatric treatment: Substance abuse and comorbid disorders. 2. New York: Marcel Dekker; 2004. pp. 211–259. [Google Scholar]
- Cooney NL, Litt MD, Cooney JL, Pilkey DT, Steinberg HR, Oncken CA. Alcohol and tobacco cessation in alcohol dependent smokers: Analysis of real-time reports. Psychology of Addictive Behaviors. doi: 10.1037/0893-164X.21.3.277. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobes DJ. Cue reactivity in alcohol and tobacco dependence. Alcoholism: Clinical and Experimental Research. 2002;26:1928–1929. doi: 10.1097/01.ALC.0000040983.23182.3A. [DOI] [PubMed] [Google Scholar]
- Finney JW, Hahn AC, Moos RH. The effectiveness of inpatient and outpatient treatment for alcohol abuse: The need to focus on mediators and moderators of setting effects. Addiction. 1996;9:1773–1796. [PubMed] [Google Scholar]
- Fiore MC, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, Gritz ER, et al. Treating tobacco use and dependence: Clinical practice guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2000. [Google Scholar]
- Fuller BE, Guydish J, Tsoh J, Ried MS, Resnick M, Zammarelli L, et al. Attitudes toward the integration of smoking cessation treatment into drug abuse clinics. Journal of Substance Abuse Treatment. doi: 10.1016/j.jsat.2006.06.011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn TJ, Manley MW. How to help your patient stop smoking: A National Cancer Institute manual for physicians. National Cancer Institute; Washington, DC: 1991. DHHS Publication No. 89-3064. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Clinical implications of the association between smoking and alcoholism. In: Fertig J, Allen JP, editors. Alcohol and tobacco: from basic science to clinical practice. Washington, DC: U.S. Government Printing Office; 1995. NIAAA Research Monograph no. 95-3931. [Google Scholar]
- Hughes JR, Kalman D. Do smokers with alcohol problems have more difficulty quitting? Drug and Alcohol Dependence. 2006;82:91–102. doi: 10.1016/j.drugalcdep.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine & Tobacco Research. 2003;5:13–25. [PubMed] [Google Scholar]
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine and Tobacco Research. 2003;5:13–25. [PubMed] [Google Scholar]
- Hurt RD, Eberman KM, Croghan IT, Offord KP, Davis LJ, Jr, Morse RM, et al. Nicotine dependence treatment during inpatient treatment for other addictions: a prospective intervention trial. Alcoholism: Clinical and Experimental Research. 1994;18:867–872. doi: 10.1111/j.1530-0277.1994.tb00052.x. [DOI] [PubMed] [Google Scholar]
- Joseph AM, Nichol KL, Anderson H. Effect of treatment for nicotine dependence on alcohol and drug treatment outcomes. Addictive Behaviors. 1993;18:635–644. doi: 10.1016/0306-4603(93)90017-4. [DOI] [PubMed] [Google Scholar]
- Joseph AM, Willenbring ML, Nugent SM, Nelson DB. A randomized trial of concurrent versus delayed smoking intervention for patients in alcohol dependence treatment. Journal of Studies on Alcohol. 2004;65:681–691. doi: 10.15288/jsa.2004.65.681. [DOI] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study. JAMA. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Miller WR, Del Boca FK. Measurement of drinking behavior using the Form-90 family of instruments. Journal of Studies of Alcohol. 1994;12:112–118. doi: 10.15288/jsas.1994.s12.112. [DOI] [PubMed] [Google Scholar]
- Monti PM, Kadden RM, Rohsenow D, Cooney NL, Abrams DB. Treating alcohol dependence: A coping skills training guide. 2nd. New York: Guilford; 2002. [Google Scholar]
- SAS Institute. SAS/STAT software: changes and enhancements through V7 and V8. Cary, NC: SAS Institute; 1999. [Google Scholar]
- Smoking Cessation Clinical Guideline Panel & Staff. The Agency for Health Care Policy and Research smoking cessation clinical practice guideline. Journal of the American Medical Association. 1996;275:1270–1280. [PubMed] [Google Scholar]
- Winer BJ. Statistical principles in experimental design. New York: McGraw-Hill; 1971. [Google Scholar]