Abstract
Alcohol-tobacco interactions and relapse precipitants were examined among alcohol dependent smokers in a trial of concurrent alcohol and tobacco treatment. After discharge from treatment, participants completed 14 days of electronic diary (ED) assessments of mood, self-efficacy, urges to drink or smoke, and drinking and smoking behavior. ED data revealed an increase in frequency of alcohol urges after smoking episodes. Drinking relapse episodes were predicted by prior ED ratings of low self-efficacy to resist drinking and high urge to smoke. Smoking relapse episodes were predicted by high urge to smoke and high negative, high arousal mood. Results were seen as supportive of both a cross substance cue reactivity model of multiple substance use, and a limited-strength model, but not a cross substance coping model.
Many individuals with alcohol problems also smoke cigarettes. Hughes (1995) reviewed 11 studies that examined the prevalence of smoking in alcoholics and found that a median of 83% of alcoholics in these studies were current smokers, compared to 30% in the general population. The negative health consequences of smoking among alcohol and drug abusers are substantial. One longitudinal study has indicated that smoking killed more alcoholics than did alcohol (Hurt et al., 1996).
Studies have examined the efficacy of providing smoking cessation treatment concurrent with initial treatment for alcohol and drug problems. The impact of concurrent smoking treatment on alcohol and drug outcomes was not consistent across studies. Researchers have reported that smoking cessation treatment either did not affect alcohol and drug outcomes (Hurt et al., 1994; Burling et al., 1991; Burling et al., 2001) or served to improve these outcomes (Bobo et al., 1998). However, a mandatory smoking ban (Joseph et al., 1993) was associated with worse drug use outcomes, and a recent study comparing concurrent versus delayed smoking intervention found worse drinking outcomes in the concurrent treatment group (Joseph, Willenbring, Nugent, & Nelson, 2004). The lack of consistency among outcome studies suggests a need to focus more carefully on the processes involved in smoking and smoking cessation among treated alcohol dependent smokers.
A number of theories have been advanced to explain the association between smoking and alcohol dependence (Cooney, Cooney, Patten, & George, 2004; Kalman, 1998) including behavioral theories that focus on factors that may underlie relapse after cessation of alcohol and tobacco use. The cross-substance coping response hypothesis (Monti, Rohsenow, Colby, & Abrams, 1995), based on a social learning model, postulates that smoking may be used to cope with cravings for alcohol, or drinking may be used to cope with craving for cigarettes. Research on cross-substance coping has yielded mixed results. This theory was supported by questionnaire data from detoxified alcoholics showing that many expect that they would smoke to cope with urges to drink (Monti et al., 1995). Laboratory data contrary to this theory were reported by our research group (Cooney, Cooney, Pilkey, Kranzler, & Oncken, 2003). We examined alcohol dependent smokers enrolled in alcohol treatment and found that acute cigarette deprivation led to high levels of cigarette craving but no increase in alcohol urges. However, a similar laboratory study conducted with a hazardous drinking, non-treatment seeking sample found that cigarette deprivation was associated with an increased urge to drink (Palfai, Monti, Ostafin, & Hutchison, 2000). Cross substance coping response theory leads to the testable prediction that, among abstinent alcoholics, smoking occasions would be associated with increased alcohol urges prior to smoking and with decreased alcohol urges immediately after smoking.
An alternative theory of cross substance cue reactivity is based on classical conditioning principles. Alcohol and tobacco are often consumed together, so repeated pairings of smoking cues with drinking behavior and vice versa is thought to result in these cues acquiring conditioned stimulus properties (Istvan & Matarazzo, 1984). Thus, smoking may come to elicit urges to drink and drinking may elicit urges to smoke. Laboratory based studies of alcohol dependent smokers have supported this theory, with findings that alcohol cue exposure elicits smoking urges (Cooney et al., 2003; Drobes, 2002; Gulliver et al., 1995; Rohsenow et al., 1997). One study also demonstrated that smoking cues elicit alcohol urges (Drobes, 2002). Cross substance cue reactivity theory would predict that concurrent treatment of smoking and drinking would lead to better alcohol outcomes than would alcohol treatment alone because ex-smokers would have less exposure to cues that elicit alcohol craving compared with continuing smokers. Another prediction is that continuing smokers would experience increased alcohol craving after smoking a cigarette. Note that the cross-substance coping theory and the cross substance cue reactivity theory lead to opposite predictions for alcoholics concurrently treated for smoking. Cross-substance coping theory predicts that cessation of smoking among abstinent alcoholics would increase alcohol cravings and relapse while the cross substance cue reactivity theory predicts that smoking cessation would decrease alcohol cravings and relapse.
An additional behavioral theory, the limited strength model (Muraven & Baumeister, 2000) has relevance for treating alcohol dependent smokers. This theory postulates that self-control is a limited resource, and that exerting self-control may consume self-control strength, reducing the amount of strength available for subsequent self-control efforts. This theory has not been tested in alcohol dependent smokers, but it was supported in studies of alcohol consumption among moderate drinkers, both in the laboratory (Muraven, Collins, & Nienhaus, 2002) and in the natural environment (Muraven, Collins, Shiffman, & Paty, 2005). This theory leads to the prediction that abstinent alcohol dependent individuals who have quit smoking and are fighting cigarette cravings may be at greater risk for alcohol relapse.
The present study employed Ecological Momentary Assessment (EMA; Shiffman & Stone, 1998) methodology, which is well suited to examine alcohol-tobacco interactions as they occur in the natural environment. Data are collected in real time, avoiding bias introduced with retrospective recall (Shiffman et al., 1997). Participants carry an electronic diary (ED) throughout the day, enhancing ecological validity as data are collected in the natural environment. Problems of faked compliance (Litt, Cooney & Morse, 1998; Stone, Shiffman, Schwartz, Broderick, & Hufford, 2003) are avoided because each record is electronically date and time stamped by the ED. EMA studies of smokers have demonstrated a strong link between negative affect and smoking relapse (Shiffman, Paty, Gnys, Kassel, & Hickcox, 1996). Alcohol consumption has also been linked with smoking relapse in non-alcoholic smokers (Shiffman et al., 1996).
A cognitive behavioral model of lapse and relapse (Marlatt & Gordon, 1985) focuses on negative affect, low self-efficacy, and craving as important proximal intrapersonal determinants of first use. Negative affect is often cited as primary relapse trigger. Low self-efficacy reduces the likelihood that an individual will make an effort to cope with temptation. Craving is a complex construct with physiological, learning, and cognitive determinants (see the 2000 supplement of the journal Addiction, Volume 95, Supplement 2). Although there is much research on these predictors of relapse (Marlatt & Witkiewitz, 2005), few studies have examined them using EMA methods.
The present study used EMA methods to investigate alcohol-tobacco interaction processes in the context of a randomized clinical trial of brief versus intensive smoking cessation treatment delivered concurrently with alcohol treatment. The outcomes of this clinical trial will be presented in a subsequent report. This report will focus on the following process questions, which were formulated to test the behavioral theories of alcohol-tobacco interactions described above. What is the effect of smoking treatment intensity and smoking quit status on the frequency and intensity of alcohol urges? Among continued smokers, are alcohol urges associated with the onset of smoking occasions? Among continued smokers, what is the immediate effect of a smoking episode on alcohol urges? EMA methodology was also employed to examine proximal antecedents to the alcohol and tobacco relapses that occurred soon after treatment, comparing the predictive power of smoking and drinking urges with other potential predictors, including negative affect and self-efficacy ratings. These analyses allowed a test of cross substance relapse predictions, i.e., whether smoking urges are predictive of alcohol relapse and whether alcohol urges are predictive of smoking relapse.
Method
Participants
This study was approved by the Human Studies Subcommittee of the VA Connecticut Healthcare System. Participants were recruited from two intensive outpatient substance abuse programs at the VA Connecticut Healthcare System. Included in this group were non-veteran women recruited from the community for the purpose of gaining a more representative sample. After beginning treatment in the substance abuse program, individuals were asked if they would be interested in participating in a research study that would provide them with smoking treatment concurrent with their substance abuse treatment. To be included in the study, participants had to be at least 18 years old, meet DSM-IV criteria for alcohol and nicotine dependence during the past three months, be interested in receiving treatment for both their alcohol and cigarette use, and smoke at least 10 cigarettes per day.
Exclusion criteria were diagnosis of current opioid dependence, current cannabis abuse or dependence, current intravenous drug use, acute medical or psychiatric disorder requiring treatment, use of medications known to influence alcohol or cigarette urges (naltrexone, disulfiram, bupropion), medical problems or conditions that would contraindicate nicotine patch use, impaired vision or hearing that would interfere with using the hand-held computer, reading ability below the fifth-grade level (Slosson Oral Reading Test; Slosson, 1963), lack of reliable transportation to treatment or excessive commuting distance, unstable housing during the 14 days following treatment, and inability to provide a name of an individual who could be contacted to help locate the participant if he or she became lost to follow-up.
After obtaining written informed consent, 133 individuals enrolled and were randomized to smoking cessation treatment, but 15 dropped out of the study by leaving the substance abuse treatment program early (n = 12) or for other reasons (n = 3). Thus, 118 individuals were asked to participate in the EMA protocol in the two weeks after treatment. Of these, 102 (86%) provided EMA data for analyses with 16 individuals excluded due to failure to complete any EMA recording.
Measures and Instruments
Baseline assessment and sample characteristics
Recruited individuals were given a screening interview to identify those who were likely to meet criteria for inclusion in the study and to collect basic demographic and clinical information. The substance-related disorders and psychotic screening sections of the Structured Clinical Interview for DSM-IV Axis I Disorders, Patient edition, version 2.0 (SCID-I/P; First et al., 1996) were used to determine whether participants met inclusion/exclusion criteria for alcohol dependence, drug dependence, and psychotic disorders. The 6-item Fagerström Test for Nicotine Dependence was used to characterize the sample. This scale has an internal consistency reliability of alpha = .61, and its total score was shown to be closely related to biochemical measures of intensity of smoking (Heatherton, Kozlowski, Frecker, & Fagerström, 1991).
Retrospective measures of alcohol and tobacco use
Alcohol and cigarette consumption was measured using the Form 90 (Miller & Del Boca, 1994), a structured interview that combines the calendar prompts of the Time-Line Follow-Back method (Sobell & Sobell, 1992) and the drinking pattern estimation procedures of the Comprehensive Drinker Profile (Marlatt & Miller, 1984). The Form 90 was administered at baseline and a 14-day version (Form 14) was administered at the end of the 14-day EMA data collection period. Participants' self-report was verified by means of biochemical assessments. Breath tests were used to detect recent alcohol use at baseline and the post-EMA time points. Expired breath carbon monoxide readings ≤ 10 ppm were considered corroboration of cigarette abstinence. Participants were paid $15 for participation in the baseline and post-EMA interviews.
Design and Procedure
All participants were enrolled in a three-week intensive outpatient substance abuse treatment program located at one of two VA sites in Connecticut. Within one week of program admission, participants completed baseline assessment measures and then were randomized to either intensive smoking cessation treatment consisting of behavioral counseling and transdermal nicotine replacement, or to brief smoking cessation advice without nicotine replacement. These interventions were delivered concurrent with the three-week intensive outpatient program.
Alcohol and tobacco treatment
As a platform for the smoking cessation intervention trial, the substance abuse intensive outpatient program provided an initial rehabilitation treatment, utilizing a cognitive-behavioral coping skills approach. Length of stay in the program was three weeks with required program meetings five days/week, five hours/day. Urine and breath were monitored for drug and alcohol use throughout participation in the program.
The intensive smoking cessation intervention was administered in three 60-minute individual sessions. On the scheduled quit date, participants were provided a four-week supply of 21 mg Nicoderm® transdermal nicotine patches, followed by prescriptions for two weeks at 14 mg, and two weeks at 7 mg Nicoderm. The brief smoking cessation advice intervention was based on the recommendations of the Agency for Healthcare Policy and Research (Smoking Cessation Clinical Guideline Panel & Staff, 1996). The smoking cessation therapist saw participants for one fifteen-minute session followed by a brief 5-minute follow-up appointment within three days of the quit date. Nicotine replacement was not offered to participants in this condition.
Ecological Momentary Assessment (EMA) Protocol
During the 14 days immediately after discharge from the intensive outpatient program, all participants were asked to participate in an EMA protocol, which involved computerized self-monitoring of alcohol urges, smoking urges, smoking behavior, mood state, and alcohol abstinence self efficacy. Data were recorded on hand-held computers that were set up to sample these variables under the following three conditions: (a) immediately prior to cigarette smoking, (b) five minutes after the onset of cigarette smoking, and (c) at random time points unrelated to smoking. This EMA sampling strategy thus gathered data on background conditions that change slowly and are assessed using the random time-based strategy and also gathered data on momentary states that change rapidly and are assessed using the event-based strategy linked to before and after smoking episodes (see Shiffman (2005) for discussion of these sampling strategies). The 14-day monitoring period was selected in order to assess processes occurring outside the intensive substance abuse treatment environment, at a time when most participants were expected to be abstinent from alcohol and some would also be abstinent from cigarettes.
The ED used was a hand-held Psion® LZ-64 computer with 32K RAM, a 4-line, 20-character LCD screen, a real-time clock-calendar, an audio speaker, and data-recording capability. Signal-contingent recording occurred by programming the ED to prompt participants on a quasi-random basis four times per day; with one randomly scheduled prompt in each of four 3.5 hour recording time periods from 8:00AM to 10:00PM (i.e., 8:00 – 11:30; 11:30 – 3:00; 3:00 – 6:30; and 6:30 – 10:00PM). Participants had the option of delaying responding to the ED signals for 5 or 15 minutes when recording would be inconvenient. Participants met with research staff at the end of each week to upload ED data and to complete other assessments. As an incentive to adhere to the EMA protocol, participants were paid $5.00 for each completed ED assessment day.
Event-contingent recording occurred by instructing participants to initiate an ED recording immediately prior to smoking each cigarette. Only the first cigarette-initiated recording within each of the four recording time periods triggered the full computerized event recording questionnaire; the rest of the cigarette-initiated recordings within a recording time period caused the ED to only record the date and time of smoking and then shut off. This was done to minimize burden on the participant. The result of this programming was that participants completed the ED questionnaire on up to four cigarettes per day. Interval-contingent recording was made possible by programming the ED such that for each of those first cigarette-initiated recordings within the four time periods, the computer displayed the full event recording questionnaire, and prompted the individual to complete a second full questionnaire five minutes later. This was intended to assess self-report processes during or shortly after a period of smoking. Therefore, on up to four occasions per day, full questionnaires were recorded immediately prior to smoking and five minutes after the onset of smoking. These event- and interval-contingent recordings provided the before-cigarette and after-cigarette ratings of urge to smoke and urge to drink that were the basis of our examination of the function of tobacco smoking on alcohol urges. The protocol did not include alcohol contingent recordings because few drinking episodes were expected to occur in the two weeks immediately following intensive alcohol treatment.
For every recording, whether initiated by the participant or by the ED, the participant was prompted to report any occurrence of smoking or drinking behavior within the day and to rate how he or she was feeling “right now” on a series of items along an 11-point Likert-type scale ranging from 0 = “Not at all” to 10 = “Very much.” Desire to drink was assessed using the item “Alcohol Urge.” Alcohol abstinence self efficacy was assessed with the item “Can Resist Drinking.” Mood state was recorded as a potential antecedent to alcohol and tobacco relapse using 12 items derived from a semantic space analysis of mood adjectives in the circumplex model of mood experience (Larsen & Diener, 1992; Russell, 1980). Mood states were classed along two major dimensions: pleasantness (negative vs. positive) and arousal (high arousal vs. low arousal). Four quadrants of moods were thus created: positive-high arousal items (active, peppy); positive-low arousal (quiet, relaxed); negative-high arousal (nervous, angry); and negative-low arousal (bored, sad). The items were combined by quadrant to yield four reliable mood composites (internal reliability alphas exceed 0.80). Intercorrelations of Mood scores revealed that the four scores were moderately correlated with each other, but not redundant. Negative-high arousal scores and negative-low arousal scores were correlated r = .50, and positive-high arousal scores and positive-low arousal scores were correlated r = .41. As expected, positive and negative moods were modestly and negatively correlated with each other. Given these levels of correlations we regarded problems of multicollinearity in our analyses of the mood data as minimal.
Results
The sample (N = 102) was 87% male and 13% female. The mean age was 45.9 years (± 7.3 years). Race was 66.7% White, 25.5% Black, 2.0% Hispanic, and 2.9% other. Seventy two percent of the participants were unemployed and 29.3% were married or were living with a spouse or partner. All met DSM-IV criteria for Alcohol and Nicotine Dependence, drank on a mean of 64% of the days in the three months prior to seeking treatment at the VA, and consumed a mean of 17.4 (SD = 11.3) alcoholic drinks per day. They reported a mean of 3.9 prior treatments for alcohol problems (SD = 6.3). Participants smoked a mean of 99% of days and a mean of 26.6 cigarettes (SD = 10.3) per day during the three months prior to seeking treatment. The mean Fagerström Test for Nicotine Dependence score was 5.3 (SD = 2.2). They reported smoking for a mean of 28.4 years (SD = 9.6) and attempted to quit a mean of 4.0 times (SD = 5.1). Participants met lifetime criteria for other substance dependence as follows: cocaine (32.8%), opioid (9.6%), cannabis (4.0%), stimulant (8.0%), hallucinogen (1.6%), and sedative/hypnotic/anxiolytic (5.8%).
Post-treatment Abstinence Rates by Treatment Condition: Drinking and Smoking
Short-term drinking and smoking abstinence were examined using retrospective reports from the two-week post-ED assessment. Drinking data were obtained for 98% of cases, and missing cases were treated as non-abstinent. At the post-ED assessment, the 14-day point prevalence alcohol abstinence rate was 90.2% across smoking treatment conditions. Logistic regression analysis conducted with pretreatment drinking level used as a continuous covariate revealed that alcohol abstinence rates did not differ significantly by smoking treatment condition.
Fourteen-day point prevalence cigarette abstinence was also examined at the post-ED time point, with abstinence verified by CO < 10ppm. Missing cases were treated as non-abstinent. The abstinence rate for Brief treatment was 5.8% (3/52), and 24.0% for the Intensive treatment (12/50), Logistic regression analysis indicted that this was a significant treatment effect (b = 1.65, se = 0.68, OR = 5.63, CI = 1.36 to 19.74).
Influence of Smoking Treatment on Urge to Drink
Participants who provided EMA data responded to 73% of the random prompts by the ED. Those who reported cigarette smoking during the EMA assessment period initiated a mean of 15.7 (SD = 16.1) pre-cigarette assessments and 15.7 (SD = 16.0) post-cigarette assessments. Examination of the distribution of urge-to-drink ratings revealed that this variable was not normally distributed, but rather was highly skewed (skewness = 3.63), with a mode and median of 0, and scattered recordings above 0. In order to accommodate this distribution, urge to drink was recomputed as a dichotomous variable (positive urge rating) in which any recording of urge above 0 was coded as 1 and scores of 0 were coded as 0.
A random effects logistic regression, or generalized estimating equations analysis(GEE, Proc Genmod, SAS Institute) was conducted to determine if smoking cessation Treatment condition influenced occurrence of any positive alcohol urge (i.e., any recording of urge to drink greater than 0). Time was modeled as three fixed factors: Week number, Day number crossed with Week, and Recording Number crossed with Day. An autoregressive covariance structure (AR 1) was adopted for the repeated measures model. Results indicated that smoking Treatment condition did not influence likelihood of randomly prompted ED ratings of positive alcohol urges (Z = 0.46, p > .60).
Association of Smoking Status with Urge to Drink
A GEE model was conducted as described above, with the exception that smoking status during the 2-week monitoring period [smoking (n = 83) vs. abstinent (n = 19)] was substituted for the Treatment condition variable in the models. Results indicated that smoking status was not associated with occurrence of randomly prompted ED ratings of positive drinking urges (Z = -0.75, p > .40).
Influence of Cigarette Smoking Episode on Urge to Drink
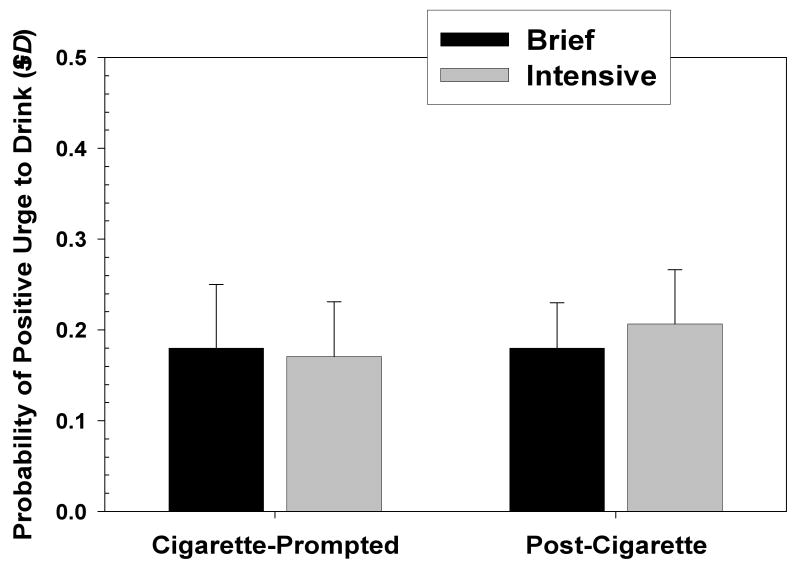
Alcohol-dependent individuals who stop drinking may use cigarettes to help control urges to drink. This hypothesis was tested by selecting those records in which a participant initiated a recording at the onset of smoking, and in which the person was subsequently prompted and responded five minutes after the onset of smoking. A total of 79 participants provided pre- and post-cigarette ED recordings. A logistic regression analysis was conducted in which occurrence of positive alcohol urge recording was examined as a function of recording type (onset of smoking versus five minutes after onset of smoking), treatment condition, and recording type X condition. Number of records completed by a participant was used as a covariate. Results indicated a significant main effect for Recording Type (B=.39; SE= .22; Wald χ2=4.18; OR = 1.64; CI: 1.02 to 2.27; p < .05), and a significant effect for number of recordings made (B=.007; SE= .001; Wald χ2=49.64; OR = 1.10; CI: 1.00 to 1.11; p < .01), but no main effect for smoking cessation Treatment condition or for Recording Type X Treatment condition. Examination of predicted probabilities indicated that probability of positive urge to drink was higher after smoking a cigarette than before smoking (see Figure 1). The absolute probability of urge to drink, however, was low both before and after smoking. The analysis was repeated selecting only those records in which participants rated the situation as one in which it was socially acceptable to drink. The results were comparable to those presented for the entire sample of records.
Figure 1.
Mean probability of positive urge to drink at smoking onset and after smoking episodes by treatment condition.
In order to ensure that any effects on drinking urges were not due to passage of time, an analysis was done examining the effects of time of day within recording day on probability of reporting positive drinking urges. Time of day had no effect on probability of positive urges (B = 0.01; se = 0.02; Z= 0.74).
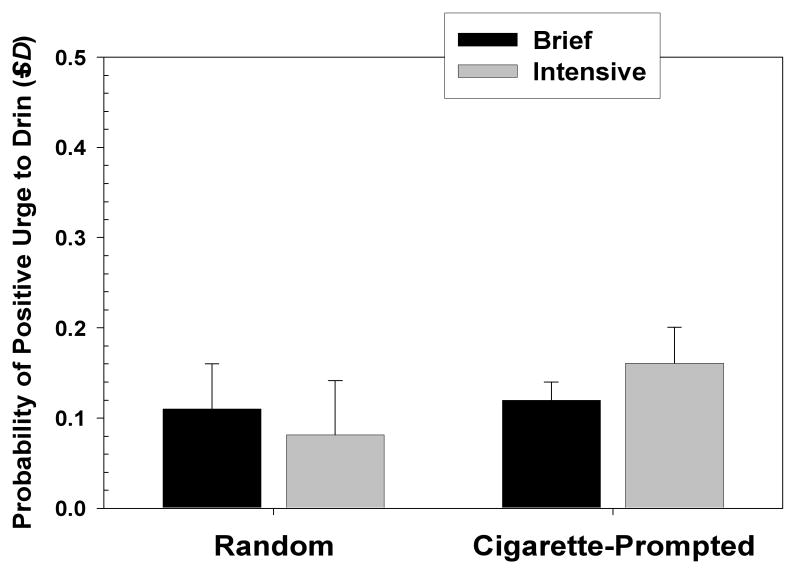
A similar analysis was used to compare probability of positive Urge to Drink during random (computer prompted) recordings versus cigarette-onset recordings. Records were selected so that the random recording and the cigarette-onset recording had to occur in the same time period on the same day (to control for variation attributable to time of day). The number of random recordings was comparable for the two treatment conditions (1975 among Brief Treatment participants and 1965 among Intensive Treatment participants). However, the number of cigarette-onset recordings differed greatly by condition (1665 for Brief Treatment participants vs. 664 for Intensive Treatment participants; χ2(1) = 274.54, p < .001). The logistic regression analysis showed no effect on the likelihood of positive Urge to Drink attributable to recording type (random v. cigarette-onset), and no effect for smoking treatment condition. There was, however, a significant interaction effect of smoking Treatment condition X recording type (B=.65; SE= .21; Wald χ2=9.23; OR = 1.91; CI: 1.26 to 1.90; p < .01). The nature of this interaction is seen in Figure 2. Analysis of simple effects via chi-square analysis indicated that for those in the Brief treatment, the likelihood of positive urge to drink was not significantly higher during the random prompts than during the cigarette-onset records. For those in the Intensive treatment, however, the likelihood of positive urge to drink was significantly higher during the cigarette-onset records than during the random ones [χ2(1) = 15.84, p < .001]. As in the previous analysis, the mean probability of positive urge to drink assessed at random or cigarette prompted recordings was fairly low, ranging no higher than 18%. As above, this analysis was repeated selecting only those records in which participants rated the situation as one in which it was socially acceptable to drink, and the results were comparable to those presented for the entire sample of situations.
Figure 2.
Mean rated urge to drink at smoking onset and at random, nonsmoking time points by treatment condition.
Momentary Predictors of First Drink
The design of the present study allowed us to examine the momentary predictors of both the first drink and the first cigarette reported by participants during the 14 day EMA monitoring period, using procedures like those described by O'Connell, Schwartz, Gerkovich, Bott, & Shiffman (2004). Of the 102 persons who received smoking treatment and provided EMA data, 13 reported their first posttreatment drink as occurring during the EMA monitoring period. These participants reported a mean of 28 days (SD = 10.4) abstinent from alcohol prior to the start of EMA data collection. A random effects logistic regression analysis was conducted for these 13 participants using the first ED record in which alcohol use was reported as the index case. Those records from the ED recording period immediately preceding the recording period in which a first drink was reported provided the momentary predictor variables. These lead recordings were completed up to 3.5 hrs prior to the recordings that reported the first drink. Comparison records were all other signal-contingent records from the same 13 participants, except those in which any drinking was reported.
Momentary predictors included urge to smoke, urge to drink, alcohol abstinence self-efficacy, and the four mood composite measures: negative-high arousal mood, negative low arousal mood, positive-high arousal mood, and positive-low arousal mood. Additionally, some situational constraints on drinking were also tested, including time of day, weekend versus weekday recording, and social appropriateness of drinking (“Is drinking socially acceptable here?”). These recordings occurred within the same day, anywhere from 3 hours to 15 minutes prior to the occurrence of drinking. Between-subjects predictors were smoking Treatment condition and baseline drinking level (percent days abstinent). Results of this analysis are shown in Table 1.
Table 1.
Analysis Of Logistic Regression Parameter Estimates. Dependent Variable = Lag of Occurrence of First Drink in EMA Monitoring Period (Nevents = 13). Reference Records = All other Non-Drinking Records From the Same Participants (Nreference = 779).
| Predictor | Estimate | Odds Ratio | SE | 95% Confidence Limits | Z | |
|---|---|---|---|---|---|---|
| Baseline Drinking | -.46 | 0.63 | 1.23 | 0.06 | 7.10 | 0.14 |
| Time of Day (1) | -0.79 | 2.28 | 1.15 | 0.23 | 22.36 | 0.49 |
| Time of Day (2) | -1.62 | -- | 0.96 | -- | -- | 0.09 |
| Time of Day (3) | -0.63 | -- | 0.91 | -- | -- | 0.49 |
| Time of Day (4) | 0.00 | -- | -- | -- | -- | -- |
| Day of Recording | 0.09 | 1.09 | 0.03 | 0.91 | 1.16 | 1.50 |
| Weekday | -0.08 | 0.92 | 0.79 | 0.20 | 4.33 | -0.10 |
| Weekend | 0.00 | -- | -- | -- | -- | -- |
| Condition 1 (Brief) | 0.71 | 2.03 | 0.79 | 0.43 | 9.59 | 0.79 |
| Condition 2 (Intensive) | 0.00 | -- | -- | -- | -- | -- |
| Urge to Drink | -1.54 | 1.22 | 1.15 | 0.23 | 2.04 | 1.79 |
| Urge to Smoke | 0.22 | 1.24 | 0.12 | 1.19 | 1.58 | 3.18* |
| Alcohol Abstinence Self Efficacy | -0.18 | 0.83 | 0.10 | 0.69 | 0.98 | 3.54* |
| Socially Acceptable to Drink (No) | -0.51 | 0.60 | 0.58 | 0.19 | 1.87 | -0.88 |
| Socially Acceptable to Drink (Yes) | 0.00 | -- | -- | -- | -- | -- |
| Negative-High Arousal Mood | 0.19 | 1.22 | 0.18 | 0.86 | 1.73 | 1.10 |
| Negative-Low Arousal Mood | -0.06 | 0.94 | 0.30 | 0.52 | 1.70 | -0.20 |
| Positive-High Arousal Mood | 0.11 | 1.12 | 0.22 | 0.72 | 1.75 | 0.50 |
| Positive-Low Arousal Mood | -0.05 | 0.95 | 0.11 | 0.77 | 1.17 | -0.51 |
p < .05;
p < .01
The results indicated that urge to smoke was predictive of occurrence of first drink, and that alcohol abstinence self-efficacy was protective. Interestingly, prior rating of positive urge to drink and negative affect were not predictive of occurrence of first drink.
Momentary Predictors of First Cigarette
In order to detect antecedents of the first cigarette smoked, it was necessary to select those participants who first had stopped smoking. Of the 102 persons who received smoking treatment and provided EMA data, 31 patients reported being abstinent from smoking for at least 2 days prior to EMA monitoring, and of those 31, 12 reported onset of smoking during the EMA monitoring period. These participants reported a mean of 5.4 days (SD =4.5) abstinent from cigarettes prior to the beginning of the EMA period. The first-cigarette records from these 12 served as the index cases. Comparison records were all other signal-contingent records from the same 12 participants, except for those records in which smoking was recorded. As with the analysis of antecedents to first drink, this analysis was a within-person comparison.
Logistic regression was used to evaluate the momentary predictors of occurrence of the first cigarette of the EMA monitoring period using assessments obtained in the recording prior to that in which the first cigarette was reported. These recordings occurred within the same day, anywhere from 3 hours to 15 minutes prior to the occurrence of smoking. Momentary predictors included urge to smoke, urge to drink, alcohol abstinence self-efficacy, and the four mood composite measures: negative-high arousal mood, negative low arousal mood, positive-high arousal mood, and positive-low arousal mood. As with the analyses of predictors of first drink, potential situational constraints on smoking were also examined, including time of day, day of recording period, weekend versus weekday, and social acceptability of drinking in that situation. Between-subjects (level 2) predictors were smoking Treatment condition and baseline drinking level (percent days abstinent).
Because of the small number of events (first cigarettes), the predictors were evaluated in sets. First, the between-subjects predictors, smoking cessation Treatment condition and baseline drinking level were tested. Second, the continuous, motivation-related variables were tested: urge to smoke, urge to drink, and alcohol abstinence self-efficacy. The third set consisted of the affective variables, negative-high arousal mood, negative-low arousal mood, positive-high arousal mood, and positive-low arousal mood. The fourth set consisted of the situational constraint variables.
As seen in the Table 2, baseline drinking level (percent days abstinent) and smoking cessation Treatment condition emerged as a predictors of first cigarette, such that less baseline drinking and assignment to the more intensive smoking treatment were protective against lapsing. Urge to smoke and negative-high arousal affect also emerged as predictors of first cigarette. A final analysis was conducted in which only baseline drinking, Treatment condition, smoking urge and negative-high arousal mood were included. In this analysis only urge to smoke emerged as a significant predictor of smoking. Treatment condition, negative-high arousal mood, and baseline drinking dropped out. Situational variables like time of day did not emerge as significant in the prediction of first cigarette.
Table 2.
Analyses of Logistic Regression Parameter Estimates. Dependent Variable = Lag of Occurrence of First Cigarette in EMA Monitoring Period (Nevents = 12). (Predictors are Variables Recorded at Time Point). Reference Records = All Other Non-Smoking Records From the Same Participants (Nreference= 1762).
| Set | Predictor | Estimate | Odds Ratio | SE | 95% Confidence Limits | Z | |
|---|---|---|---|---|---|---|---|
| 1 | Baseline Percent Days Abstinent | -0.13 | 0.88 | 0.06 | 0.78 | 0.98 | -2.26* |
| Treatment Condition 1 (Brief) | 0.13 | 0.88 | 0.02 | 0.84 | 0.92 | 5.87*** | |
| Treatment Condition 2 (Intensive) | 0.00 | -- | -- | -- | -- | -- | |
| 2 | Urge to Drink | 0.09 | 1.14 | 1.65 | 0.71 | 2.89 | 0.98 |
| Urge to Smoke | 0.17 | 1.19 | 0.05 | 1.08 | 1.30 | 3.75*** | |
| Alcohol Abstinence Self Efficacy | 0.63 | 1.88 | 0.72 | 0.89 | 3.97 | 1.65 | |
| 3 | Negative-High Arousal | 0.47 | 1.60 | 0.15 | 1.18 | 2.16 | 3.03*** |
| Negative-Low Arousal | -0.19 | 0.82 | 0.16 | 0.60 | 1.13 | -1.21 | |
| Positive-High Arousal | -0.32 | 0.72 | 0.20 | 0.49 | 1.07 | -1.64 | |
| Positive-Low Arousal | 0.09 | 1.09 | 0.11 | 0.87 | 1.37 | 0.79 | |
| 4 | Time of Day (1) | -0.74 | 0.24 | 1.15 | 0.03 | 1.79 | -0.65 |
| Time of Day (2) | 0.68 | -- | 0.78 | -- | -- | 0.87 | |
| Time of Day (3) | -0.81 | -- | 1.31 | -- | -- | -0.62 | |
| Time of Day (4) | 0.00 | -- | -- | -- | -- | -- | |
| Day Number of Period | -0.04 | 0.96 | 0.08 | 0.83 | 1.12 | -0.48 | |
| Weekday | 0.21 | 1.23 | 0.82 | 0.25 | 6.13 | 0.26 | |
| Weekend | 0.00 | -- | -- | -- | -- | -- | |
| Socially Acceptable to Drink (No) | 0.07 | 1.08 | 0.80 | 0.22 | 5.15 | 0.09 | |
| Socially Acceptable to Drink (Yes) | 0.00 | -- | -- | -- | -- | -- | |
| 5 | Percent Days Abstinent | 2.37 | 10.67 | 1.43 | 0.65 | 175.40 | 1.66 |
| Treatment Condition (Brief) | 0.51 | 1.67 | 0.79 | 0.35 | 7.92 | 0.65 | |
| Treatment Condition (Intensive) | 0.00 | -- | -- | -- | -- | -- | |
| Negative-High Arousal | 0.28 | 1.22 | 0.21 | 0.82 | 1.83 | 0.98 | |
| Urge to Smoke | 1.10 | 1.19 | 0.10 | 1.17 | 1.35 | 2.30* | |
p < .01;
p < .001
Discussion
This study examined alcohol-tobacco interaction processes immediately after concurrent alcohol and tobacco treatment. Analyses of alcohol craving across the two-week posttreatment ED recording period revealed that the frequency of alcohol craving was low with no significant differences between smokers and nonsmokers, or between those in brief or intensive smoking treatment. Others have also reported low levels of reported alcohol craving on EMA measures obtained from treated alcohol dependent individuals (Litt, Cooney & Morse, 2000; Krahn et al., 2005). Floor effects may have been operating to reduce the sensitivity of an alcohol craving measure to smoking cessation treatment or tobacco abstinence effects in a sample of intensively treated alcohol dependent individuals. However, hypotheses that smoking cessation would raise the level of alcohol craving as predicted by cross substance coping response theory or lower the level of alcohol craving as predicted by cross substance cue reactivity theory were not supported. This finding is consistent with the results of a laboratory study in this population (Cooney, et al., 2003) which looked for, but did not find, evidence that acute cigarette deprivation elicited changes in alcohol urges.
One strength of EMA methodology is the ability to examine proximal antecedents and consequences of important events. We hypothesized that smoking a cigarette might have an immediate impact on alcohol urges. Results revealed a modest increase in the occurrence of alcohol urges from pre to post smoking. This finding was replicated when we selected only ED records that were made in situations in which it was socially acceptable to drink. This sub-analysis provided a stronger test of hypotheses than the analysis conducted across all situations because drinking was not constrained by the environment. A possible limitation of this analysis is that we were unable to determine if an increase in drinking urge occurred simply as a result of repeated recording (5 minutes apart), regardless of whether a cigarette was smoked in the interim or not. We consider this possibility unlikely however.
The finding of increased alcohol urge after smoking does not support the notion of smoking as an effective cross substance coping response in which smoking is an effective way to cope with craving for alcohol. Results are, however, consistent with findings from laboratory studies of cross substance cue reactivity in which smoking cues elicit urges to drink in alcohol dependent smokers (Drobes, 2002), or urges to use drugs in drug dependent smokers (Taylor et al., 2000). These results strengthen the rationale for recommending smoking cessation for individuals in an early phase of alcohol recovery.
EMA methodology was also employed to examine the antecedents to smoking episodes, comparing ratings obtained at the onset of multiple smoking episodes with ratings obtained at randomly sampled nonsmoking occasions. Overall, there was no significant difference between the frequency of alcohol urge reports obtained at the onset of smoking occasions compared with nonsmoking occasions, and this finding was replicated in a sub-analysis using only recordings obtained from situations that were rated as socially acceptable for drinking. This is another set of findings that failed to support the cross-substance coping hypothesis, which predicted that smoking onset would be associated with alcohol urges. A statistically significant interaction effect was found that was not predicted, indicating that those in the intensive smoking treatment reported higher alcohol urges at the onset of smoking compared to random, nonsmoking occasions. This suggests that alcohol urges were prompting smoking behavior, consistent with the cross substance coping model, but it is not clear why this process would be limited to those who received intensive smoking treatment.
Another strength of EMA methodology is the ability to examine the predictors of relapse episodes using randomly sampled assessments obtained in the hours prior to the reported first use. Note that these assessments reflect background changes in subjective states preceding drinking lapse but not the momentary state immediately proximal to the lapse. High ratings of confidence in one's ability to resist drinking predicted lower likelihood of subsequent drinking lapse and, surprisingly, high ratings of urge to smoke predicted higher likelihood of drinking lapse. High urge to smoke coupled with low confidence in ability to resist drinking can both be seen as markers of depleted self-control strength, so this finding could be seen as supporting the limited strength theory that exercising self-control over cigarette smoking consumes self-control strength, reducing the amount of strength available for subsequent efforts to exercise control over alcohol craving (Muraven & Baumeister, 2000). However, this interpretation is complicated by the fact that 10 of the 13 participants who reported drinking lapses also reported smoking prior to the drinking lapse. Future research would need to follow a larger sample of alcohol and cigarette abstinent individuals to provide a stronger test of how alcohol-tobacco interactions influence the relapse process.
Also unexpected was the finding that background changes in alcohol urge and negative affect recorded in the hours preceding a relapse did not predict drinking relapse episodes. Krahn, Bohn, Henk, Grossman, and Gosnell (2005) also found that background levels of alcohol craving and negative affect assessed via EMA did not predict alcohol relapse. One possibility is that these states do increase immediately prior to alcohol relapse but the changes are too short-lived to be detected hours before the onset of drinking. Another possibility is that automatic processes rather than conscious alcohol craving is determining initial alcohol relapse (Tiffany, 1990).
Smoking lapse episodes also were predicted by higher ED-recorded urge to smoke as well as by higher negative, high arousal mood (nervous, angry) recorded in the hours before the lapse. Studies of nonalcoholic smokers attempting smoking cessation also found that smoking relapse was predicted by smoking urge (Killen & Fortmann, 1997; Shiffman et al., 1996; West, Hajek, & Belcher, 1989) and by negative affect (Shiffman et al., 1996). The mechanism underlying these findings cannot be determined from the present study, but may involve a conditioned association of negative affect and smoking, efforts by individuals to use smoking to cope with negative affect, or affective disruption leading to a reduction in self control strength (Muraven & Baumeister, 2000).
Regarding clinical implications, there appears to be no effect of concurrent intensive smoking cessation treatment on alcohol craving in the weeks soon after treatment. Real-time in vivo assessment found a modest increase in alcohol craving immediately after smoking episodes. These process findings suggest no evidence of harmful effects of adding concurrent smoking cessation to alcohol treatment. On the other hand, smoking urges reported at randomly timed in-vivo assessments were predictive of imminent alcohol relapse. Taken together, these results suggest practitioners could recommend concurrent smoking cessation for alcohol dependent smokers, but should use intensive pharmacological and/or behavioral interventions to maximally control smoking urges in the early phase of tobacco abstinence. Relatively fast acting nicotine medications such as nicotine gum or nicotine nasal spray may be useful because they can provide a pharmacological coping strategy when alcohol dependent smokers are confronted with intense cravings, perhaps warding off both alcohol and tobacco relapse.
Study Limitations
EMA methods were only used during a two-week period after discharge from the intensive outpatient program. Thus, our findings are relevant to understanding only early craving and relapse. Future studies are needed to examine momentary process measures of later relapse. Such studies will be difficult, however, because the risk of relapse declines over time.
Although participants were instructed to complete cigarette-initiated recording at the onset of smoking, their ratings may have been affected by the smoking episode itself. Even the random EMA recordings may have been affected by the participants' reactions to being prompted by the EMA device alarm. These reactions may be positive (reminder to keep on coping) or negative (irritation at the interruption). However, short-term EMA monitoring was not found to have significant reactive effects on drinking behavior in a study using electronic diaries (Hufford, Shields, Shiffman, Paty, & Balabanis, 2002) or in a study using programmable wristwatches (Litt et al., 1998). Strengths of this methodology include the use of real-time rather than retrospective reports, the examination of proximal, momentary states surrounding smoking episodes, and the prospective examination of antecedents to both smoking and drinking relapse in a sample that recently completed concurrent alcohol and tobacco treatment.
Although EMA methodology allows one to examine large numbers of observations within subjects, the sample size for analyses of the proximal predictors of alcohol and tobacco relapse was small. These results should be considered preliminary and in need of further study. Future research on alcohol dependent smokers might need to employ a more intensive smoking cessation intervention that would generate higher cigarette abstinence rates, allowing data collection from a larger sample of patients who are abstinent from cigarettes. Theories of alcohol-tobacco interactions can be examined using both EMA studies in clinical samples and using human laboratory studies that experimentally manipulate tobacco abstinence followed by assessments of alcohol craving and/or consumption (e.g., Mckee, Krishnan-Sarin, Shi, Mase, & O'Malley, in press).
Very low urge to drink ratings obtained in this sample of treated alcohol dependent individuals made it difficult to find significant relationships with this variable. Other EMA studies with this population also found that the frequency and intensity of alcohol craving was low (Litt, et al., 2000; Lukasiewicz, Benyamina, Reynaud, & Falissard, 2005). Nevertheless, some significant effects were observed for the alcohol urge variable in the present study. Findings with urge to drink may differ in samples that have not been recently treated in intensive, abstinence-oriented programs, or for those who have high levels of alcohol dependence (Litt et al, 2000; Steinberg et al, 2006).
Acknowledgments
This research was supported by Grant R01 AA11197 from the National Institute on Alcoholism and Alcohol Abuse, by Grant P50 DA13334 from the National Institute on Drug Abuse, and by the Department of Veterans Affairs.
Contributor Information
Ned L. Cooney, Department of Psychiatry, Yale University School of Medicine and VA Connecticut Healthcare System
Mark D. Litt, Division of Behavioral Sciences and Community Health, University of Connecticut Health Center
Judith L. Cooney, Department of Psychiatry, University of Connecticut School of Medicine, University of Connecticut Health Center and VA Connecticut Healthcare System
David T. Pilkey, Department of Psychiatry, Yale University School of Medicine and VA Connecticut Healthcare System
Howard R. Steinberg, Department of Psychiatry, Yale University School of Medicine and VA Connecticut Healthcare System
Cheryl A. Oncken, Department of Medicine, University of Connecticut School of Medicine, University of Connecticut Health Center
References
- Babor TF, Steinberg K, Zweben A, Cisler R, Stout R, Tonigan JS, Anton RF, Allen JP. Treatment effects across multiple dimensions of outcome. In: Babor TF, Del Boca FK, editors. Treatment matching in alcoholism. Cambridge, UK: Cambridge University Press; 2003. pp. 150–165. [Google Scholar]
- Bobo JK, McIlvain HE, Lando HA, Walker RD, Leed-Kelly A. Effect of smoking cessation counseling on recovery from alcoholism: Findings from a randomized community intervention trial. Addiction. 1998;93:877–887. doi: 10.1046/j.1360-0443.1998.9368779.x. [DOI] [PubMed] [Google Scholar]
- Bobo JK, Walker RD, Lando HA, McIlvain HE. Enhancing alcohol control with counseling on nicotine dependence: pilot study findings and treatment implications. In: Fertig J, Allen JP, editors. Alcohol and tobacco: from basic science to clinical practice. NIAAA Research Monograph no. 95-3931. Washington, DC: U.S. Government Printing Office; 1995. [Google Scholar]
- Burling TA, Burling AS, Latini D. A controlled smoking cessation trial for substance-dependent inpatients. Journal of Consulting and Clinical Psychology. 2001;69:295–304. doi: 10.1037//0022-006x.69.2.295. [DOI] [PubMed] [Google Scholar]
- Burling TA, Marshall GD, Seidner AL. Smoking cessation for substance abuse inpatients. Journal of Substance Abuse. 1991;3:269–276. doi: 10.1016/s0899-3289(10)80011-2. [DOI] [PubMed] [Google Scholar]
- Cooney JL, Cooney NL, Patten CA, George TP. Comorbidity of nicotine dependence with affective, psychotic and substance use disorders. In: Kranzler HR, Tinsley JA, editors. Dual diagnosis and psychiatric treatment: Substance abuse and comorbid disorders. 2nd. New York: Marcel Dekker; 2004. pp. 211–259. [Google Scholar]
- Cooney JL, Cooney NL, Pilkey DT, Kranzler HK, Oncken CA. Effects of nicotine deprivation on urges to drink and smoke in alcoholic smokers. Addiction. 2003;98:913–921. doi: 10.1046/j.1360-0443.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- Drobes DJ. Cue reactivity in alcohol and tobacco dependence. Alcoholism: Clinical and Experimental Research. 2002;26:1928–1929. doi: 10.1097/01.ALC.0000040983.23182.3A. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, Gritz ER, et al. Treating tobacco use and dependence Clinical practice guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; 2000. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders – Patient Edition (SCID-I/P, Version 2.0) Biometrics Research Department; New York State Psychiatric Institute: 1996. [Google Scholar]
- Gulliver SB, Rohsenow DJ, Colby SM, Dey AN, Abrams DB, Niaura RS, Monti PM. Interrelationship of smoking and alcohol dependence, use, and urges to use. Journal of Studies on Alcohol. 1995;56:202–206. doi: 10.15288/jsa.1995.56.202. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hufford MR, Shields AL, Shiffman S, Paty J, Balabanis M. Reactivity to ecological momentary assessment: an example using undergraduate problem drinkers. Psychology of Addictive Behaviors. 2002;16:205–11. [PubMed] [Google Scholar]
- Hughes JR. Clinical implications of the association between smoking and alcoholism. In: Fertig J, Allen JP, editors. Alcohol and tobacco: from basic science to clinical practice. NIAAA Research Monograph no. 95-3931. Washington, DC: U.S. Government Printing Office; 1995. [Google Scholar]
- Hurt RD, Eberman KM, Croghan IT, Offord KP, Davis LJ, Jr, Morse RM, et al. Nicotine dependence treatment during inpatient treatment for other addictions: a prospective intervention trial. Alcoholism: Clinical and Experimental Research. 1994;18:867–872. doi: 10.1111/j.1530-0277.1994.tb00052.x. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, Morse RM, Melton LJ., 3rd Mortality following inpatient addictions treatment: role of tobacco use in a community-based cohort. JAMA. 1996;275:1097–1103. doi: 10.1001/jama.275.14.1097. [DOI] [PubMed] [Google Scholar]
- Istvan J, Matarazzo JD. Tobacco, alcohol and caffeine use: A review of their interrelationships. Psychological Bulletin. 1984;95:301–326. [PubMed] [Google Scholar]
- Joseph AM, Nichol KL, Anderson H. Effect of treatment for nicotine dependence on alcohol and drug treatment outcomes. Addictive Behaviors. 1993;18:635–644. doi: 10.1016/0306-4603(93)90017-4. [DOI] [PubMed] [Google Scholar]
- Joseph AM, Willenbring ML, Nugent SM, Nelson DB. A randomized trial of concurrent versus delayed smoking intervention for patients in alcohol dependence treatment. Journal of Studies on Alcohol. 2004;65:681–691. doi: 10.15288/jsa.2004.65.681. [DOI] [PubMed] [Google Scholar]
- Kalman D. Smoking cessation treatment for substance misusers in early recovery: A review of the literature and recommendations for practice. Substance Use & Misuse. 1998;33:2021–2047. doi: 10.3109/10826089809069815. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortman SP. Craving is associated with smoking relapse: Findings from three prospective studies. Experimental and Clinical Psychopharmacology. 1997;5:137–142. doi: 10.1037//1064-1297.5.2.137. [DOI] [PubMed] [Google Scholar]
- Krahn DD, Bohn MJ, Henk HJ, Grossman JL, Gosnell B. Patterns of urges during early abstinence in alcohol-dependent subjects. The American Journal on Addiction. 2005;14:248–255. doi: 10.1080/10550490590949424. [DOI] [PubMed] [Google Scholar]
- Larsen RJ, Diener E. Promises and problems with the circumplex model of emotion. In: Clark MS, editor. Emotion (Review of personality and social psychology. Vol. 13. Newbury Park, CA: Sage Publications; 1992. [Google Scholar]
- Litt MD, Cooney NL, Morse PM. Ecological momentary assessment (EMA) with alcoholics: methodological problems and potential solutions. Health Psychology. 1998;17:48–52. doi: 10.1037//0278-6133.17.1.48. [DOI] [PubMed] [Google Scholar]
- Litt MD, Cooney NL, Morse P. Reactivity to alcohol-related stimuli in the laboratory and in the field: Predictors of craving in treated alcoholics. Addiction. 2000;95:889–900. doi: 10.1046/j.1360-0443.2000.9568896.x. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz M, Benyamina A, Reynaud M, Falissard B. An in vivo study of the relationship between craving and reaction time during alcohol detoxification using the ecological momentary assessment. Alcoholism: Clinical and Experimental Research. 2005;29:2135–2143. doi: 10.1097/01.alc.0000191760.42980.50. [DOI] [PubMed] [Google Scholar]
- Mckee SA, Krishnan-Sarin S, Shi J, Mase T, O'Malley SS. Modeling the effect of alcohol on smoking lapse behavior. Psychopharmacology. doi: 10.1007/s00213-006-0551-8. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt GA, Gordon JR, editors. Relapse prevention: Maintenance strategies in the treatment of addictive behaviors. 1st. New York: Guilford Press; 1985. [Google Scholar]
- Marlatt GA, Miller WR. Comprehensive drinker profile. Odessa, FL: Psychological Assessment Resources; 1984. [Google Scholar]
- Marlatt GA, Witkiewitz K. Relapse prevention for alcohol and drug problems. In: Marlatt GA, Donovan DM, editors. Relapse prevention: Maintenance strategies in the treatment of addictive behaviors. 2nd. New York: Guilford Press; 2005. pp. 1–44. [Google Scholar]
- Miller WR, Del Boca FK. Measurement of drinking behavior using the Form-90 family of instruments. Journal of Studies of Alcohol. 1994;12:112–118. doi: 10.15288/jsas.1994.s12.112. [DOI] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Colby SM, Abrams DB. Smoking among alcoholics during and after treatment: Implications for models, treatment strategies, and policy. In: Fertig JB, Allen JP, editors. Alcohol and tobacco: From basic science to clinical practice (NIAAA Research Monograph 30) Washington, DC: U.S. Government Printing Office; 1995. pp. 187–206. NIH Publication No 95-3931. [Google Scholar]
- Muraven M, Baumeister RF. Self-regulation and depletion of limited resources: Does self-control resemble a muscle? Psychological Bulletin. 2000;126:247–259. doi: 10.1037/0033-2909.126.2.247. [DOI] [PubMed] [Google Scholar]
- Muraven M, Collins RL, Nienhaus K. Self-control and alcohol restraint: An initial application of the self-control strength model. Psychology of Addictive Behaviors. 2002;16:113–120. doi: 10.1037//0893-164x.16.2.113. [DOI] [PubMed] [Google Scholar]
- Muraven M, Collins RL, Shiffman S, Paty JA. Daily fluctuations on self-control demands and alcohol intake. Psychology of Addictive Behaviors. 2005;19:140–147. doi: 10.1037/0893-164X.19.2.140. [DOI] [PubMed] [Google Scholar]
- O'Connell K, Schwartz J, Gerkovich M, Bott M, Shiffman S. Playful and rebellious states vs. negative affect in explaining the occurrence of temptations and lapses during smoking cessation. Nicotine & Tobacco Research. 2004;6:661–74. doi: 10.1080/14622200410001734049. [DOI] [PubMed] [Google Scholar]
- Palfai TP, Monti PM, Ostafin B, Hutchison K. Effects of nicotine deprivation on alcohol-related information processing and drinking behavior. Journal of Abnormal Psychology. 2000;109:96–106. doi: 10.1037//0021-843x.109.1.96. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Colby SM, Gulliver SB, Sirota AD, Niaura RS, Abrams DB. Effects of alcohol cues on smoking urges and topography among alcoholic men. Alcoholism Clinical & Experimental Research. 1997;21:101–107. [PubMed] [Google Scholar]
- Russell JA. A circumplex model of affect. Journal of Personality and Social Psychology. 1980;39:1161–1178. doi: 10.1037//0022-3514.79.2.286. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Dynamic influences on smoking relapse process. Journal of Personality. 2005;73:1715–1748. doi: 10.1111/j.0022-3506.2005.00364.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Hufford M, Hickcox M, Paty JA, Gnys M, Kassel JD. Remember that? A comparison of real-time versus retrospective recall of smoking lapses. Journal of Consulting and Clinical Psychology. 1997;65:292–300. doi: 10.1037/0022-006x.65.2.292.a. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Gwaltney CJ, Balabanis MH, Liu KS, Paty JA, Kassel JD, Hickcox M, Gnys M. Immediate antecedents of cigarette smoking: An analysis from ecological momentary assessment. Journal of Abnormal Psychology. 2002;111:531–545. doi: 10.1037//0021-843x.111.4.531. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking; Within-subjects analysis of real-time reports. Journal of Consulting and Clinical Psychology. 1996;64:366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Stone AA. Ecological momentary assessment: A new tool for behavioral medicine research. In: Krantz D, Baum A, editors. Technology and methods in behavioral medicine. Mahwah, NJ: Erlbaum; 1998. pp. 117–131. [Google Scholar]
- Slosson RL. Slosson Oral Reading Test manual. East Aurora, NY: Slosson Educational Publications; 1963. [Google Scholar]
- Smoking Cessation Clinical Guideline Panel & Staff. The Agency for Health Care Policy and Research smoking cessation clinical practice guideline. Journal of the American Medical Association. 1996;275:1270–1280. [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, editors. Measuring alcohol consumption. Humana Press; 1992. pp. 41–71. [Google Scholar]
- Steinberg HR, Cooney NL, Pilkey DT, Litt MD, Cooney JL, Oncken CA. Predictors of momentary smoking and drinking urges in alcohol dependent smokers. Poster presented at the annual meeting of the Society for Research on Nicotine and Tobacco; Orlando, FL. 2006. Feb, [Google Scholar]
- Stone AA, Shiffman S, Schwartz JE, Broderick JE, Hufford MR. Patient compliance with paper and electronic diaries. Controlled Clinical Trials. 2003;24:182–199. doi: 10.1016/s0197-2456(02)00320-3. [DOI] [PubMed] [Google Scholar]
- Taylor RC, Harris NA, Singleton EG, Moolchan ET, Heishman SJ. Tobacco craving: Intensity-related effects of imagery scripts in drug abusers. Experimental and Clinical Psychopharmacology. 2000;8:75–87. doi: 10.1037//1064-1297.8.1.75. [DOI] [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: Role of automatic and nonautomatic processes. Psychological Review. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- West RJ, Hajek P, Belcher M. Severity of withdrawal symptoms as a predictor of outcome of an attempt to quit smoking. Psychological Medicine. 1989;19:981–985. doi: 10.1017/s0033291700005705. [DOI] [PubMed] [Google Scholar]