Abstract
Background
Despite the large stocks of organic nitrogen in soil, nitrogen availability limits plant growth in many terrestrial ecosystems because most plants take up only inorganic nitrogen, not organic nitrogen. Although some vascular plants can assimilate organic nitrogen directly, only recently has organic nitrogen been found to contribute significantly to the nutrient budget of any plant. Carnivorous plants grow in extremely nutrient-poor environments and carnivory has evolved in these plants as an alternative pathway for obtaining nutrients. We tested if the carnivorous pitcher plant Sarracenia purpurea could directly take up intact amino acids in the field and compared uptake of organic and inorganic forms of nitrogen across a gradient of nitrogen deposition. We hypothesized that the contribution of organic nitrogen to the nitrogen budget of the pitcher plant would decline with increasing nitrogen deposition.
Methodology and Principal Findings
At sites in Canada (low nitrogen deposition) and the United States (high nitrogen deposition), individual pitchers were fed two amino acids, glycine and phenylalanine, and inorganic nitrogen (as ammonium nitrate), individually and in mixture. Plants took up intact amino acids. Acquisition of each form of nitrogen provided in isolation exceeded uptake of the same form in mixture. At the high deposition site, uptake of organic nitrogen was higher than uptake of inorganic nitrogen. At the low deposition site, uptake of all three forms of nitrogen was similar. Completeness of the associated detritus-based food web that inhabits pitcher-plant leaves and breaks down captured prey had no effect on nitrogen uptake.
Conclusions and Significance
By taking up intact amino acids, Sarracenia purpurea can short-circuit the inorganic nitrogen cycle, thus minimizing potential bottlenecks in nitrogen availability that result from the plant's reliance for nitrogen mineralization on a seasonally reconstructed food web operating on infrequent and irregular prey capture.
Introduction
Nitrogen (N) limits plant growth in most terrestrial ecosystems [1] yet many ecosystems, including arctic tundra [2], coastal salt marshes [3], alpine meadows [4], boreal forests [5], and bogs [6] have large stocks of organic N (ON). More than 90% of soil N is bound in an organic form (humus), 20–40% of this as amino acids [7]. The availability of amino acids may drive ecosystem function in N-limited environments such as arctic tundra because of the very high turnover rates (2–24 h) of amino acids that result from microbial uptake and release [8]. Plants that can use amino acids as an N source may be able to co-exist with, or even outgrow, plants that only use inorganic N (IN), especially in environments where N mineralization rates are low and N limits plant growth [9]–[11]. In Arctic tundra, for example, the most productive species used the most abundant N forms and less productive species used less abundant forms [9].
It has been known for decades that vascular plants can assimilate ON directly when grown in culture [12], with mycorrhizae [13], or in the absence of microbial competition [14], but only in the last decade has ON been shown to be a significant N source for a wide range of plant species in different N-limited systems [15]. Standard theory of N cycling with respect to plant uptake [16] assumes that ON has to be mineralized to IN before it can be assimilated, but direct ON uptake by plants has been proposed to “short-circuit” the N cycle as plants bypass microbial mineralization of ON [17]. This short-circuit is thought to be energetically favourable to plants because ON immediately provides amino acids whereas NH4 + and NO3 − (after reduction to NH4 +) must be synthesized into amino acids [18]. Because most ecosystems are N limited and plants can potentially access multiple forms of N in the environment, more information is needed on the generality of direct acquisition of amino acids by plants to fully assess current models of N cycling for a wider range of environments.
Carnivorous plants are generally restricted to extremely N-limited habitats, such as bogs, outwash sand plains, and inselbergs [19]–[21]. In North America, carnivorous pitcher plants (Sarracenia spp. and Darlingtonia californica Torrey [both in the Sarraceniaceae]) acquire little N from root uptake; up to 80% of their N is obtained from prey captured in their pitcher-shaped leaves [19], [22]–[24]. Most North American pitcher plants secrete chitinases and proteases that directly break down the prey [25], but S. purpurea L. secretes digestive enzymes only at very low levels [26] and enzyme secretions have not been observed in D. californica. Instead, these two species rely on a food web of aquatic insect larvae, protozoa, and bacteria that inhabits the pitchers [27], [28] to break down the captured prey, mineralize the available ON to IN, and release it for absorption by the pitchers [29], [30]. In northeastern North America where N deposition rates are relatively high, Sarracenia purpurea also acquires IN directly from rainfall that collects in its pitchers [31], [32].
This “Sarracenia microecosystem” (S. purpurea plus its resident food web) has been developed as a model system in which we have examined N cycling of an entire detritus-based food web [22], [30]. In northeastern North America, S. purpurea grows in peat bogs and poor fens where plant growth is predominantly N-limited [33]. These bogs have massive stores of ON in peat that is generally assumed to be unavailable for uptake and use by vascular plants, but high nutrient flux and organic production occurs in bogs [6], and many non-carnivorous plants in these habitats have been shown to be able to take up ON (as amino acids) directly through their roots [2], [34], [35]. Carnivorous plants such as S. purpurea have weakly developed root systems (root∶shoot ratio ≈0.2) [22], and although carnivorous plants take up some nutrients from their roots, they obtain most of their nutrients from prey captured by modified leaves [36]–[38].
Recent research on the N budget of S. purpurea has focused on the relative importance of bacteria and the macroinvertebrates in its food web (larvae of the midge Metriocnemus knabi Coq., the mosquito Wyeomyia smithii (Coq.), and the sarcophagid fly Fletcherimyia fletcheri (Aldrich)) in the nutrient mineralization and excretion process [30]. This work has demonstrated that bacteria are the primary agents of N mineralization, although the mosquito and fly larvae regulate both the abundance and the diversity of the bacteria [39], [40]. Inorganic N derived from atmospheric deposition is directly assimilated by plants [22], [31]. However, neither the ability of pitcher plants to assimilate ON directly, nor the role of the food web in modulating such ON uptake has been investigated experimentally.
Here, we report the results of a 72-hour pulse-chase experiment conducted in the field in which we fed two isotopically enriched amino acids (glycine and phenylalanine) and ammonium nitrate, singly and in combination, to pitcher plants in the field. Our factorial experimental design also assessed whether the macroinvertebrate component of the pitcher-plant's associated food web altered the observed patterns of nitrogen uptake. Finally, we determined if ON uptake by S. purpurea and its associated food web differed between sites with different background levels of atmospheric nitrogen deposition.
Materials and Methods
Study species
Sarracenia purpurea grows in ombrotrophic (rain-fed) bogs [41], poor fens, and seepage swamps throughout Canada east of the Rocky Mountains and in the eastern United States from Maine to Georgia [42]. This long-lived (>50 years), perennial carnivorous plant grows as a rosette of leaves from a small rhizome crown ( Fig. 1 ). In the northeastern United States and Canada where we studied S. purpurea, it produces 6–10 new leaves each year; the leaves last 1–2 years and then senesce. These leaves are modified into pitfall traps (“pitchers”) that fill with rainwater in which captured arthropod prey drowns. The pitchers also are inhabited by an aquatic, detritus-based food web consisting of bacteria, protozoa, and invertebrates [27], [30], [39]. Prey captured by S. purpurea is shredded by aquatic larvae and mineralized by bacteria that inhabit the pitchers; the mineralized nutrients are released for uptake by the plant [29], [30], [43], [44]. Sarracenia purpurea is a somewhat inefficient predator – <3% of insect visitors are actually captured [45] – and insects and other prey account for 10–80% of their nutrient budget [23]; it obtains the remainder of its nutrients from stored reserves [22], remobilization and excretion of N and P by rotifers [46], and increasingly, atmospheric deposition [31]. As pitchers generally account for ≈80% of the total plant mass with roots and rhizome crowns accounting for the remaining ≈20% [22], S. purpurea derives <5% of its nutrients from the pore water in the peat where it grows [46], [47].
Figure 1. The pitcher plant Sarracenia purpurea.
This carnivorous plant grows as a rosette of leaves modified into pitchers that act as pitfall traps in which rainfall is collected and prey are captured.
Field sites
We measured nitrogen acquisition by S. purpurea under field conditions at Fort Albany, northern Ontario, Canada (52°15′ N, 81°35′ W) and at Tom Swamp, adjacent to Harvard Pond in Petersham, Massachusetts, U.S.A. (42°30′ N, 72°11′ W). Fort Albany is in the James Bay Lowlands of the Hudson Plains Ecoregion [48], and the dominant vegetation at the study site consists of sedges, mosses, and lichens with or without stunted black spruce (Picea mariana (Mill.) Britton, Sterns & Poggenb.) and tamarack (Larix laricina (Du Roi) K. Koch). Tom Swamp is a ≈50 ha bog at the north end of Harvard Pond, an artificial pond created in the 1800s by the construction of two dams on Riceville Creek [49]. The bog vegetation is dominated by leatherleaf (Chamaedaphne calyculata (L.) Moench.). Fort Albany is near the northern limit of S. purpurea in Ontario, but pitcher plants are abundant there as well as at Tom Swamp (densities >5/m2). Annual wet inorganic nitrogen deposition for Chapais, Quebec (the nearest data available for Fort Albany and 600 km to the southeast) was ≈2.5 kg/ha in 2002 [48] compared to ≈4.5 kg/ha for central Massachusetts [50].
Experimental design
We used a 72-hour pulse-chase experiment with isotopically enriched amino acids as our organic nitrogen (ON) source and ammonium nitrate (15NH4 15NO3) as our inorganic nitrogen (IN) source to determine if pitcher plants can acquire ON directly and to compare ON and IN uptake under different conditions. We focused on uptake of N by pitchers because our previous research showed that pitchers acquired ≈70% of added IN while roots acquired less than 2.5% of added IN [22].
At each site, 125 mature individuals were selected with at least 3 live (no sign of senescence) mature pitchers (firm and open). Five of these plants served as untreated controls and were harvested at the end of the experiment to determine baseline 15N and 13C natural abundances. The remaining 120 plants were randomly assigned to one of six treatment groups: uniformly-labeled (U-) glycine (U-Gly: 98 atom% U-13C-15N-glycine), uniformly-labeled phenylalanine (U-Phe: 98 atom% U-13C-15N-phenylalanine), I15N (98 atom% 15NH4 15NO3), U-Gly plus unlabeled phenylalanine and unlabeled NH4NO3 (hereafter U-Gly+), U-Phe plus unlabeled glycine and unlabeled NH4NO3 (hereafter U-Phe+), or I15N plus unlabeled glycine and phenylalanine (hereafter I15N+). Plants within treatment groups were assigned randomly to one of two harvests (3- or 72-hr) and one of two food web treatments (complete food webs or partial food webs, which lacked the macroinvertebrate larvae of the detritivorous midge Metriocnemus knabi and the keystone predator, the mosquito Wyeomyia smithii). Larvae of the sarcophagid fly Fletcherimyia fletcheri, which are found more commonly in pitchers in Massachusetts than in Canada, were excluded from all experimental pitchers. There were N = 5 pitchers in each treatment at each site.
Pitchers were significantly larger at Tom Swamp than at Fort Albany ( Table 1 ). Control (unfed) plants at both sites had similar concentrations of N and natural abundance levels of δ15N in leaf tissues ( Table 1 ). These control plants also had similar C concentrations and similar C∶N ratios, but background natural abundance of δ13C was slightly lower at Tom Swamp than at Fort Albany ( Table 1 ).
Table 1. Comparison of traits for pitchers of untreated plants (N = 5) harvested at Fort Albany, James Bay, Ontario, Canada (FA) and Tom Swamp, central Massachusetts, USA (TS) with significance level of unpaired t-test comparing sites.
| Site | Mean | SD | P | |
| Dry Mass (mg) | FA | 232 | 57 | 0.00003 |
| TS | 754 | 142 | ||
| Length (cm) | FA | 8.4 | 1.9 | 0.00008 |
| TS | 18.7 | 1.9 | ||
| δ15N (‰) | FA | 1.8 | 0.8 | 0.373 |
| TS | 1.1 | 1.3 | ||
| δ13C (‰) | FA | −26.8 | 0.7 | 0.0013 |
| TS | −29.4 | 1.0 | ||
| Nitrogen (%) | FA | 1.08 | 0.13 | 0.609 |
| TS | 1.02 | 0.28 | ||
| Carbon (%) | FA | 46.4 | 3.3 | 0.408 |
| TS | 47.7 | 0.3 | ||
| C∶N | FA | 43 | 4 | 0.282 |
| TS | 49 | 10 |
Any liquid in the pitchers, along with the food web, was removed from all experimental pitchers the day before the pulse-chase experiment began; the liquid removed (pitcher “liquor”) was kept for the food web manipulations. Following food web removal in the field, pitchers were rinsed with distilled water to remove as much detritus and as many microbes as possible and the pitcher opening was blocked with a fine nylon mesh to limit subsequent entry of animals and prey. In the laboratory, all living midge and mosquito larvae were removed from liquid collected from each pitcher and kept alive overnight in a solution of pitcher liquor.
The next day, the largest pitcher on each plant was fed with one of the 15N treatments. We fed each manipulated pitcher with a 0.8 mM 15N solution (2 ml for Fort Albany and 9 ml for the larger pitchers at Tom Swamp) and an equal amount of pitcher liquor, resulting in pitchers filled to approximately three-quarters of their volume. Thus, all experimental pitchers contained an enriched (15N) nutrient solution along with the microbial component of the food web (supplied in the pitcher liquor). Pitchers at Fort Albany were fed 0.022 mg N, whereas the larger pitchers at Tom Swamp were fed 0.101 mg N. The amount of N added to pitchers represented <1.0% of N content of pitcher tissue at Fort Albany and <1.9% of N content of pitcher tissues at Tom Swamp.
When we added only single forms of N (i.e., the U-Gly, U-Phe, and I15N treatments), all N added to the pitchers was enriched in 15N. When we added three forms of N (the U-Gly+, U-Phe+, and I15N+ treatments), only one-third of the N added to each pitcher was enriched in 15N; the remaining two-thirds was comprised of equal amounts of the other two forms as unlabeled N. We are confident that we minimized potential effects of excess N availability on pitcher N uptake, which could have been particularly important when only one form of N was added in a single feeding event. The total amount of N supplied and the actual concentration of N were both substantially lower than that used in other studies of Sarracenia: 1.2–3.6 mg N/plant as a mixture of amino acids to pitchers of S. flava [51]; 1–10 mM alanine fed to Nepenthes pitchers [52]; 20 ml/pitcher of 6.8–8.6 mM NH4-N (1.9–2.4 mg N/pitcher) [22], [29] or 6–8.7 mg N/pitcher as NH4Cl to S. purpurea [23], [31], which is comparable to the mass of prey-N captured by S. purpurea in a growing season [53].
Finally, for the complete food web treatments, we put invertebrate larvae into the pitchers immediately after we added the 15N solution. We added two midge and two mosquito larvae in each pitcher in the complete food web treatment at Fort Albany and nine midge and nine mosquito larvae in each pitcher in the complete food web treatment at Tom Swamp (i.e., 1 midge+1 mosquito larva per ml of pitcher liquor). Unfed (control) pitchers for which we measured natural abundance of 13C and 15N also had complete food webs (pitcher liquor+midge+mosquito larvae).
Because of the dramatic size differences between plants at the two sites ( Table 1 ), total N fed to each plant and food web manipulations (numbers of midge and mosquito larvae added to pitchers) differed at the two sites. Therefore, statistical analyses were conducted separately for each site.
Harvest
Target pitchers were removed from the rest of the plant 3 or 72 hr after feeding with a stainless steel razor blade that was rinsed in 50% ethanol between cuttings. Pitcher liquor was transferred to a sealed sterile plastic tube and the pitcher was placed in a zip-lock plastic bag. Both were stored in a cooler with cold packs and taken immediately to the laboratory for processing. Pitchers were cut open longitudinally, washed thoroughly with tap water, rinsed with 0.5 mM CaCl2 to remove any amino acids from the surface [54], and finally rinsed three times with distilled-deionized water before being transferred to paper bags. Midge and mosquito larvae were removed from the pitchers with an eye dropper, transferred through three sequential baths of distilled-deionized water and stored in new sterile vials. Because of the small mass of larvae in each pitcher, larvae from the five replicates of each harvest × treatment combination were pooled into one composite larval sample. Plant and invertebrate samples were then oven-dried at 65°C for 48 h and then weighed.
Isotopic analyses
Each pitcher and composite larval sample was ground to a fine powder in a stainless steel capsule with a stainless steel ball using a Wig-L-Bug mixer (Bratt Technologies, LLC., East Orange, New Jersey, USA). A 4-mg subsample of plant tissue or a 1-mg subsample of larvae was then placed into an 8×5 mm tin capsule (Elemental Microanalysis Mason, Ohio, USA) and combusted in a Costech ECS4010 Elemental Analyzer and DeltaPlus XP mass spectrometer at the University of New Hampshire to measure 13C/12C, %C, 15N/14N and %N concurrently. A reference standard (NIST 1515, NIST 1575a, or an internal tuna standard) was included after every five samples.
Recovery of added tracer in pitchers was calculated as:
where 15Nrec = mass of 15N tracer recovered in the labeled N pool, m pool = N mass of the total N pool, atom%15N pool = atom percent 15N in the labeled N pool, atom%15Nref = atom percent 15N in the reference N pool (non-labeled plants harvested at the end of the experiment), and atom%15Ntracer = atom percent 15N of the applied tracer [55].
Statistical analysis
First, we measured 15N and 13C in pitchers to determine if S. purpurea could acquire intact amino acids. We emphasize that observing enrichment in 13C and 15N by itself does not provide evidence for acquisition of intact glycine or phenylalanine because both 13C and 15N may be acquired in products of microbial mineralization of amino acids. Rather, a comparison of the slope of excess 13C versus 15N (per gram dry mass of plant tissue) to the slope of the 15N source provides a conservative estimate of N acquired as amino acid [56]. Ratios below the expected slope ( = 13C∶15N ratio of the amino acids) indicate loss of 13C (e.g., respiration) and/or 15N acquisition after mineralization of labeled amino acid. Such an analysis should be undertaken within a few hours of the application of dual-labeled amino acids (hence our 3-hour harvest). We used ordinary least-squares linear regression analysis to compare the slope of 13C∶15N in pitchers fed U-Gly or U-Phe as their only N source with the expected slopes of 13C∶15N if glycine (expected slope = 2) or phenylalanine (expected slope = 9) were taken up intact within the first 3 hours after feeding.
For all other analyses, we analyzed nitrogen uptake by pitchers as μg 15N per gram dry mass. Data were arcsin-square-root transformed to reduce heteroscedasticity. Although 15N enrichment was measured in midge and mosquito larvae ( Fig. 2 ), analysis of variance with a main effect for the invertebrate food web manipulation revealed no significant differences in 15N uptake at each site for pitchers with and without the invertebrate food web (Fort Albany F1,96 = 0.137, P = 0.71; Tom Swamp F1,94 = 1.68, P = 0.20). Therefore, 15N uptake by pitchers was analyzed for each site separately using a fixed-effect two-way analysis of variance to test for differences in 15N uptake only as a function of the form of N fed to pitchers and harvest time. In these analyses, pitchers in the two food-web treatments were pooled within each N addition treatment. A priori contrasts of the N-form treatment at the 72-h harvest were used to compare plant N uptake across treatments and between sites at the end of the experiment.
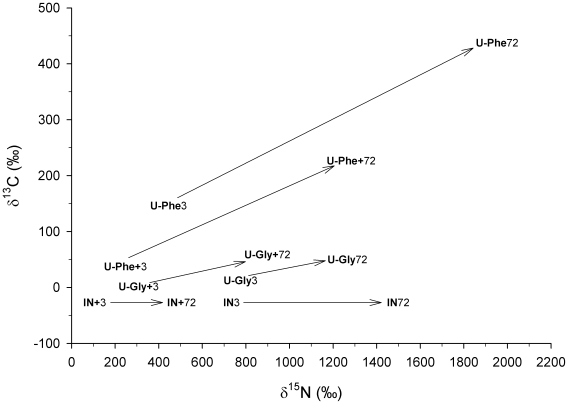
Figure 2. Enrichment of mosquito and midge larvae collected from Sarracenia purpurea pitchers.
The arrows connect points showing enrichment after 3 and 72 hours of plants at Tom Swamp in Massachusetts that were fed 15N as ammonium nitrate (15NH4 15NO3), uniformly-labeled (U-) 13C-15N-glycine or U-13C-15N-phenylalanine alone (IN, U-Gly, and U-Phe, respectively) or in combination (IN+, Gly+, and Phe+, respectively). Larvae in pitchers fed Phe or Phe+ had the highest 13C and 15N enrichment after 72 hours.
Results
Uptake of intact amino acids
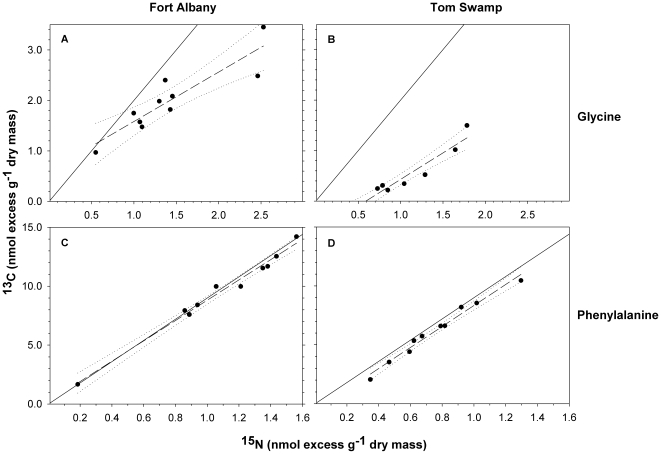
Pitcher plants rapidly assimilated the two amino acids we fed to them. There was a significant positive relationship between tissue 13C and 15N for pitchers harvested 3-h after receiving only U-Gly at Fort Albany (P = 3.83×10−4; Fig. 3A ) and Tom Swamp (P = 6.84×10−6; Fig. 3B ). The slope of the 13C∶15N line at both sites was significantly less than 2 – the value expected if intact glycine was taken up directly – at both Fort Albany (slope = 0.98, 95% CI = 0.6–1.4) and at Tom Swamp (slope = 1.1, 95% CI = 0.8–1.3).
Figure 3. Concentration of 13C and 15N recovered in Sarracenia purpurea pitchers 3 h after feeding.
Nitrogen solutions were applied into the pitcher leaves at Fort Albany in Ontario, Canada (left, panels A, C) and at Tom Swamp in Massachusetts, U S A (right, panels B, D) as uniformly-labeled (U-) 13C-15N-glycine (top row, panels A, B) or as U-13C-15N-phenylalanine (bottom row, panels C, D). Solid lines represent the 13C∶15N ratio of glycine (2∶1) or phenylalanine (9∶1); dashed-lines represent ordinary least squares regression (with 95% confidence intervals as dotted lines) for plant uptake.
There was a highly significant positive relationship between tissue 13C and 15N for pitchers harvested 3-h after receiving U-Phe at both Fort Albany (P = 1.28×10−8; Fig. 3C ) and Tom Swamp (P = 7.17×10−8; Fig. 3D ). The slope of the 13C∶15N relationship for pitcher tissue at both sites did not differ from 9 – the value expected if intact phenylalanine was taken up directly – at both Fort Albany (slope = 8.6 with a 95% CI = 7.7–9.5; Fig. 3C ) and at Tom Swamp (slope = 8.9 with a 95% CI = 7.8–10.0; Fig. 3D ).
Uptake of different forms of nitrogen
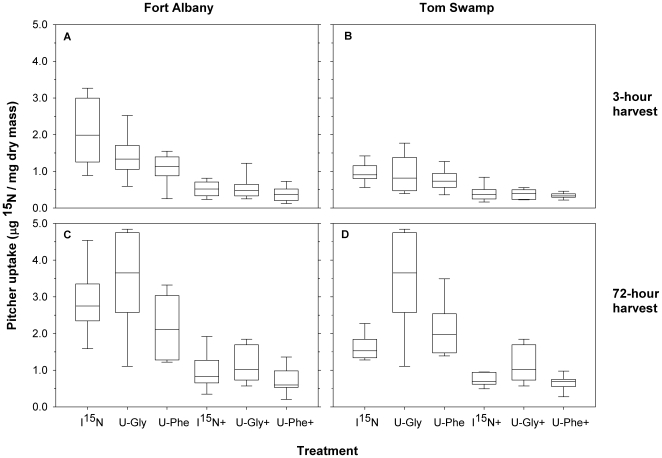
At each site, there were highly significant differences among treatments (Fort Albany: F5,108 = 41.8, P<0.0001; Tom Swamp: F5,106 = 47.0, P<0.0001) and harvest times (Fort Albany: F1,108 = 62.3, P<0.0001; Tom Swamp: F1,106 = 158.3, P<0.0001), and significant treatment × harvest time interactions (Fort Albany: F5,108 = 2.3, P = 0.05; Tom Swamp: F5,106 = 4.5, P = 0.0009; Fig. 4 ). At Fort Albany, pitchers acquired significantly more (P = 0.004) 15N from glycine than from phenylalanine when those forms were provided in isolation ( Fig. 4A ), and showed a similar trend when all three forms of N were available (P = 0.07; Fig. 4C ). At Tom Swamp pitchers acquired similar amounts (P = 0.56) of N from glycine and phenylalanine when ON was provided in isolation ( Fig. 4B ) but tended to favour glycine when all forms of N were available (P = 0.08; Fig. 4D ).
Figure 4. 15N acquisition by Sarracenia purpurea pitchers supplied with multiple forms of nitrogen.
Nitrogen was provided to pitcher leaves as ammonium nitrate (15NH4 15NO3), uniformly-labeled (U-) 13C-15N-glycine or U-13C-15N-phenylalanine (IN, U-Gly, and U-Phe, respectively) or in combination (IN+, U-Gly+, U-Phe+, respectively) at Fort Albany, Ontario, Canada (left, panels A, C) and at Tom Swamp, Massachusetts, U S A (right, panels B, D). Uptake was measured after 3 hours (top, panels A, B) and 72 hours (bottom, panels C, D) in a pulse-chase experiment. The total amount of N provided to pitchers at Fort Albany was 0.022 mg, and was 0.101 mg N at Tom Swamp. Pitchers with and without the invertebrate food webs were pooled for these analyses because there were no significant differences in 15N uptake in the two food-web treatments (Fort Albany F1,96 = 0.137, P = 0.71; Tom Swamp F1,94 = 1.68, P = 0.20).
At Tom Swamp, pitchers took up significantly more N from either glycine or phenylalanine than from ammonium nitrate (P = 0.034 and P = 0.009, respectively) when only one form of 15N was provided ( Fig. 4D ). In contrast, at Fort Albany, pitchers took up similar amounts of 15N either the amino acids or the ammonium nitrate when only one form of 15N was available (P = 0.22 and P = 0.09, respective contrasts; Fig. 4C ). At both sites, pitchers acquired similar amounts of 15N from the amino acids and ammonium nitrate when all three forms of N were provided simultaneously (P≥0.29). Finally, highly significant differences of 15N uptake were found for each form of N when it was provided in isolation relative to when it was provided in mixture ( Figs. 4C, 4D ).
Discussion
Uptake of intact amino acids has been demonstrated for species in ecosystems ranging from alpine and arctic tundra to a subtropical rainforest and in ephemeral pools in the Namibian desert [15]. The use of a variety of forms of different nutrients could provide selective advantages to plants inhabiting nutrient-limited environments. Carnivorous plants, whose growth and reproduction are strongly limited by nutrient availability and that grow in extremely nutrient-poor habitats [19], [21] acquire organic nutrients as prey, but whether or not they can directly take up intact amino acids had not been studied previously.
Our data are consistent with the hypothesis that S. purpurea takes up amino acids directly. However, while this was clearly demonstrated for phenylalanine, S. purpurea either did not assimilate glycine directly or else it assimilated glycine but metabolized it more rapidly than our 3-hour sample could detect ( Fig. 3 ). All pitchers fed U-Gly and U-Phe were highly enriched in 13C and 15N. Rapid enrichment of pitcher tissue with both 13C and 15N at a 13C∶15N ratio similar to that of the amino acid fed to the plant would suggest uptake of intact amino acids. Whereas pitchers fed U-Phe had 13C∶15N ratios similar to the expected value of 9, pitchers fed U-Gly were highly enriched in both 13C and 15N but their 13C∶15N ratio was significantly less than the expected value of 2. The results for acquisition of intact glycine, and comparison to phenylalanine, however, must be interpreted with caution [57], [58]. First, carbon respiration following acquisition of phenylalanine will have less effect on the 13C∶15N relationship than it would for glycine because of the greater amount of 13C acquired per unit phenylalanine (C∶N = 9) compared to glycine (C∶N = 2). Carbon acquired from glycine is rapidly catabolised [59], which can lead to slopes of 13C∶15N substantially below the expected value of 2 ( Figs. 3A, 3B ). This may have been exacerbated by the relatively small amounts of 13C- and 15N-enriched amino acids provided to pitchers; in order to avoid potential effects of excess N availability on the plant, added N represented <2% of the total N in the pitchers.
Furthermore, the substantial enrichment of pitchers ( Fig. 4 ) and the differences in 13C and 15N enrichment of invertebrate larvae ( Fig. 2 ) between pitchers fed U-Gly and U-Phe provides some additional support for intact glycine acquisition by S. purpurea pitchers under field conditions. As expected if plants preferentially acquire amino acids with low C∶N [60], the 15N enrichment of S. purpurea pitchers was generally greater for plants fed U-Gly than U-Phe ( Fig. 4 ).
More detailed comparisons of enrichment of 15N in plant tissues of the different treatment groups suggests that Sarracenia should show preferential uptake of amino acids with lower C∶N ratios (such as glycine) than those with higher C∶N ratios (such as phenylalanine). After 72-h, 15N concentration in plants fed glycine alone was significantly greater than 15N concentration in plants fed phenylalanine alone at Fort Albany but not at Tom Swamp ( Fig. 4 ). At both sites, uptake of 15N from glycine tended to be higher than uptake of 15N from phenylalanine when all three forms of N were available to pitchers ( Fig. 4 ). Because Fort Albany has cooler air temperatures, a shorter growing season, and lower atmospheric N deposition than Tom Swamp, we interpret these results to suggest that pitchers at the Canadian site can maximize N uptake by taking up relative more amino acids with low C∶N ratios (e.g., glycine) and avoid the energetically costly synthesis of new amino acids from IN plus a carbon skeleton [18]. In contrast, at Tom Swamp in Massachusetts, the climate is warmer, the growing season is longer, and IN is more readily available because of higher atmospheric deposition rates. Thus, direct uptake of low C∶N amino acids such as glycine may not be as important at Tom Swamp because suitable environmental conditions exist to synthesize amino acids from readily available IN plus available carbon.
These inferences are supported by our results showing greater 15N uptake from ON than from IN (energetic benefit) when each form was provided in isolation at Tom Swamp ( Fig. 4 ). At the more N-limited Fort Albany site, however, 15N uptake by S. purpurea pitchers was similar for all three forms of 15N when each was provided in isolation. Sarracenia purpurea pitchers are open to the atmosphere, collecting rainwater as well as prey, and the difference in ON uptake between sites may represent a response to the higher atmospheric IN deposition at Tom Swamp. Similar preferential acquisition of the predominant forms of N in the local environment has been observed for plants in boreal forests [61], arctic tundra [62], [63], alpine meadows [35], and cold-temperate forests [64].
Finally, our results illustrate that the acquisition of any one form of N provided in isolation will exceed uptake of this form when multiple forms of N are made available to the plant simultaneously. At both sites and for each form of 15N supplied, uptake of 15N was significantly greater when only one form was made available compared to that same form of 15N provided in mixture. However, there were no significant differences in 15N uptake among the three forms when all forms of 15N were made available simultaneously ( Fig. 4 ). This result highlights the versatility of N acquisition by S. purpurea because N uptake of any one particular form decreases when all three forms are available.
Sarracenia purpurea is one of only two species of North American pitcher plants (the other being Darlingtonia californica) in which a food web mineralizes captured prey and simultaneously competes with S. purpurea for N. Our results revealed no differences in 15N uptake between pitchers with and without higher trophic levels in their associated food webs. This result is consistent with previous results showing that the upper trophic levels in the S. purpurea microecosystem actively process detritus but that the activity of the microbial component of the food web ultimately determines N availability for Sarracenia [30].
Taken as a whole, our field experiments indicate versatility of N acquisition by this carnivorous plant and variability in N acquisition across a gradient of atmospheric deposition. These results are consistent with data reported from other N-limited environments [54], [65]. Similarly, there is pronounced spatiotemporal variation in the availability, form, quantity, and proportions of each form of N in bogs in general and in the Sarracenia microecosystem in particular. The growth of plants in eastern North American bogs is predominantly N-limited [33] but bogs have massive stores of ON in peat with high nutrient flux and organic production [6] and receive variable inputs of atmospheric IN deposition throughout the growing season. Additionally, pitcher plants collect varying amounts of organic and inorganic N via trapped prey. We suggest that the energetic benefits of direct and rapid (<3 h) acquisition of ON as intact amino acids allow Sarracenia to short-circuit the inorganic N cycle and to minimize potential bottlenecks in N availability because of the plant's reliance for N mineralization on a seasonally reconstructed food web [66] operating on irregular and infrequent seasonal pulses of prey capture [45], [67]. Experiments employing a greater range of N concentrations for a longer duration would improve our ability to determine the upper limit of N acquisition by Sarracenia and characterize the importance of the acquisition of intact amino acids to the N budget of this carnivorous plant.
Acknowledgments
We thank Erik Hobbie and Andew Ouimette at the University of New Hampshire Stable Isotopes Laboratory for analyzing our samples. L. Tsuji, E. Liberda, C. Hart, X. Wen, D. Kozlovic and J. Liedtke assisted in the field. Paul Grogan and Steve Heard provided helpful comments on an earlier draft.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by an NSERC of Canada Postdoctoral Fellowship to JDK and by NSF grants 02-35128 and 05-46180 to AME. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Vitousek PM, Aber J, Howarth RW, Likens GE, Matson PA, et al. Human alteration of the global nitrogen cycle: sources and consequences. Ecol App. 1997;7:737–750. [Google Scholar]
- 2.Kielland K. Amino acid absorption by arctic plants: implications for plant nutrition and nitrogen cycling. Ecology. 1994;75:2373–2383. [Google Scholar]
- 3.Henry HAL, Jefferies RL. Free amino acid, ammonium and nitrate concentrations in soil solutions of a grazed coastal marsh in relation to plant growth. Plant Cell Env. 2002;25:665–675. [Google Scholar]
- 4.Miller AE, Bowman WD. Alpine plants show species-level differences in the uptake of organic and inorganic nitrogen. Plant Soil. 2003;250:283–292. [Google Scholar]
- 5.Persson J, Nasholm T. Amino acid uptake: a widespread ability among boreal forest plants. Ecol Lett. 2001;4:434–438. [Google Scholar]
- 6.Bridgham SD, Pastor J, Janssens JA, Chapin CT, Malterer TJ. Multiple limiting gradients in peatlands: a call for a new paradigm. Wetlands. 1996;16:45–65. [Google Scholar]
- 7.Senwo ZN, Tabatabai MA. Amino acid composition of soil organic matter. Biol Fert Soil. 1998;26:235–242. [Google Scholar]
- 8.Jones DL, Farrar JF, Newsham KK. Rapid amino acid cycling in Arctic and Antarctic soils. Water Air Soil Pollut, Focus. 2004;4:169–175. [Google Scholar]
- 9.McKane RB, Johnson LC, Shaver GR, Nadelhoffer KJ, Rastetter EB, et al. Resource-based niches provide a basis for plant species diversity and dominance in arctic tundra. Nature. 2002;415:68–71. doi: 10.1038/415068a. [DOI] [PubMed] [Google Scholar]
- 10.Miller AE, Bowman WD, Nash Suding K. Plant uptake of inorganic and organic nitrogen: neighbor identity matters. Ecology. 2007;88:1832–1840. doi: 10.1890/06-0946.1. [DOI] [PubMed] [Google Scholar]
- 11.Wiegelt A, Bol R, Bardgett RD. Preferential uptake of soil nitrogen forms by grassland plant species. Oecologia. 2005;142:627–635. doi: 10.1007/s00442-004-1765-2. [DOI] [PubMed] [Google Scholar]
- 12.Virtanen AI, Linkola H. Organic nitrogen compounds as nitrogen nutrition for higher plants. Nature. 1946;158:515. doi: 10.1038/158515a0. [DOI] [PubMed] [Google Scholar]
- 13.Melin E, Nilsson H. Transfer of labelled nitrogen from glutamic acid to pine seedlings through the mycelium of Boletus variegatus (Sw.) Fr. Nature. 1953;171:134. doi: 10.1038/171134a0. [DOI] [PubMed] [Google Scholar]
- 14.Miller RH, Schmidt EL. Uptake and assimilation of amino acids supplied to the sterile soil∶root environment of the bean plant (Phaseolus vulgaris). Soil Sci. 1965;100:323–330. [Google Scholar]
- 15.Lipson D, Nasholm T. The unexpected versatility of plants: organic nitrogen use and availability in terrestrial ecosystems. Oecologia. 2001;128:305–316. doi: 10.1007/s004420100693. [DOI] [PubMed] [Google Scholar]
- 16.Stark JM. Nutrient transformations. In: Sala OE, Jackson RB, Mooney HA, Howarth RW, editors. Methods in ecosystem science. New York: Springer-Verlag; 2000. pp. 215–234. [Google Scholar]
- 17.Neff JC, Chapin FS, Vitousek PM. Breaks in the cycle: dissolved organic nitrogen in terrestrial ecosystems. Front Ecol Env. 2003;1:205–211. [Google Scholar]
- 18.Clarkson DT. Factors affecting mineral nutrient acquisition by plants. Ann Rev Plant Physiol. 1985;36:77–115. [Google Scholar]
- 19.Ellison AM, Gotelli NJ. Evolutionary ecology of carnivorous plants. Trends Ecol Evol. 2001;16:623–629. [Google Scholar]
- 20.Givnish TJ. Ecology and evolution of carnivorous plants. In: Abrahamson WG, editor. Plant-Animal Interactions. New York: McGraw-Hill; 1989. pp. 242–290. [Google Scholar]
- 21.Porembski S, Barthlott W. Inselbergs: biotic diversity of isolated rock outcrops in tropical and temperate regions. Berlin: Springer-Verlag; 2000. p. 524. [Google Scholar]
- 22.Butler JL, Ellison AM. Nitrogen cycling dynamics in the carnivorous northern pitcher plant, Sarracenia purpurea. Func Ecol. 2007;21:835–843. [Google Scholar]
- 23.Chapin CT, Pastor J. Nutrient limitations in the northern pitcher plant Sarracenia purpurea. Can J Bot. 1995;73:728–734. [Google Scholar]
- 24.Schulze W, Schulze E-D, Pate JS, Gillison AN. The nitrogen supply from soils and insects during growth of the pitcher plants Nepenthes mirabilis, Cephalotus follicularis and Darlingtonia californica. Oecologia. 1997;112:464–471. doi: 10.1007/s004420050333. [DOI] [PubMed] [Google Scholar]
- 25.Hepburn JS, Jones FM, St. John EQ. The biochemistry of the American pitcher plants. Trans Wagner Free Inst Sci. 1927;11:1–95. [Google Scholar]
- 26.Gallie DR, Chang SC. Signal transduction in the carnivorous plant Sarracenia purpurea. Plant Physiol. 1997;115:1461–1471. doi: 10.1104/pp.115.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Addicott JF. Predation and prey community structure: an experimental study of the effect of mosquito larvae on the protozoan communities of pitcher plants. Ecology. 1974;55:475–492. [Google Scholar]
- 28.Naeem S. Resource heterogeneity fosters coexistence of a mite and a midge in pitcher plants. Ecol Mono. 1988;58:215–227. [Google Scholar]
- 29.Bradshaw WE, Creelman RA. Mutualism between the carnivorous purple pitcher plant Sarracenia purpurea and its inhabitants. Am Midl Nat. 1984;112:294–304. [Google Scholar]
- 30.Butler JL, Gotelli NJ, Ellison AM. Linking the brown and green: nutrient transformation and fate in the Sarracenia microecosystem. Ecology. 2008;89:898–904. doi: 10.1890/07-1314.1. [DOI] [PubMed] [Google Scholar]
- 31.Ellison AM, Gotelli NJ. Nitrogen availability alters the expression of carnivory in the northern pitcher plant Sarracenia purpurea. Proc Natl Acad Sci U S A. 2002;99:4409–4412. doi: 10.1073/pnas.022057199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gotelli N J, Ellison AM. Nitrogen deposition and extinction risk in the northern pitcher plant Sarracenia purpurea. Ecology. 2002;83:2758–2765. [Google Scholar]
- 33.Bedford BL, Walbridge MR, Aldous A. Patterns in nutrient availability and plant diversity of temperate North American wetlands. Ecology. 1999;80:2151–2169. [Google Scholar]
- 34.Clemmensen KE, Sorensen PL, Michelsen A, Jonasson S, Ström L. Site-dependent N uptake from N-form mixtures by arctic plants, soil microbes and ectomycorrhizal fungi. Oecologia. 2008;155:771–738. doi: 10.1007/s00442-008-0962-9. [DOI] [PubMed] [Google Scholar]
- 35.Raab TK, Lipson DA, Monson RK. Soil amino acid utilization among species of the Cyperaceae: plant and soil processes. Ecology. 1999;80:2408–2419. [Google Scholar]
- 36.Darwin C. Insectivorous plants. New York: Appleton and Company; 1875. p. 462. [Google Scholar]
- 37.Ellison AM, Gotelli NJ. Energetics and the evolution of carnivorous plants - Darwin's “most wonderful plants in the world”. J Exp Bot. 2009;60:19–42. doi: 10.1093/jxb/ern179. [DOI] [PubMed] [Google Scholar]
- 38.Lloyd FE. The carnivorous plants. New York: Ronald Press; 1942. p. 352. [Google Scholar]
- 39.Gotelli NJ, Ellison AM. Food-web models predict species abundance in response to habitat change. PLoS Biol. 2006;44:e324. doi: 10.1371/journal.pbio.0040324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peterson CN, Day S, Wolfe BE, Ellison AM, Kolter R, et al. A keystone predator controls bacterial diversity in the pitcher plant (Sarracenia purpurea) microecosystem. Env Microbiol. 2008;10:2257–2266. doi: 10.1111/j.1462-2920.2008.01648.x. [DOI] [PubMed] [Google Scholar]
- 41.Damman AWH. Nutrient status of ombrotrophic peat bogs. Aquilo Ser Bot. 1990;28:5–14. [Google Scholar]
- 42.Schnell DE. Carnivorous plants of the United States and Canada, 2nd edition. Portland: Timber Press; 2002. p. 468. [Google Scholar]
- 43.Cochran-Stafira DL, von Ende CN. Integrating bacteria into food webs: studies with Sarracenia purpurea inquilines. Ecology. 1998;79:880–898. [Google Scholar]
- 44.Heard SB. Pitcher plant midges and mosquitoes: a processing chain commensalism. Ecology. 1994;75:1647–1660. [Google Scholar]
- 45.Newell SJ, Nastase, AJ Efficiency of insect capture by Sarracenia purpurea (Sarraceniaceae), the northern pitcher plant. Am J Bot. 1998;85:88–91. [PubMed] [Google Scholar]
- 46.Błędzki LA, Ellison AM. Population growth and production of Habrotrocha rosa Donner (Rotifera: Bdelloidea) and its contribution to the nutrient supply of its host, the northern pitcher plant, Sarracenia purpurea L. (Sarraceniaceae). Hydrobiologia. 1998;385:193–200. [Google Scholar]
- 47.Błędzki LA, Ellison AM. Nutrient regeneration by rotifers in New England (USA) bogs. Ver Int Ver Limnol. 2002;28:1328–1331. [Google Scholar]
- 48.Environment Canada. Canadian acid deposition science assessment. Toronto: Meteorological Service of Canada; 2004. p. 479. [Google Scholar]
- 49.Swan JM, Gill AM. The origins, spread, and consolidation of a floating bog in Harvard Pond, Petersham, Massachusetts. Ecology. 1970;51:829–840. [Google Scholar]
- 50.National Atmospheric Deposition Program (NRSP-3) 2009. Champaign, Illinois: NADP Program Office, Illinois State Water Survey.
- 51.Plummer GL, Kethley JB. Foliar absorption of amino acids, peptides, and other nutrients by the pitcher plant, Sarracenia flava. Bot Gaz. 1964;125:245–260. [Google Scholar]
- 52.Lüttge U. Untersuchungen zur Physiologie der Carnivoren-Drusen. II. Mitteilung. Über die Resorption verschiedener Substanzen. Planta. 1965;66:331–334. [Google Scholar]
- 53.Heard S. Capture rates of invertebrate prey by the pitcher plant, Sarracenia purpurea L. Am Midl Nat. 1998;139:79–89. [Google Scholar]
- 54.Persson J, Hogberg P, Ekblad A, Hogberg M, Nordgren A, et al. Nitrogen acquisition from inorganic and organic sources by boreal forest plants in the field. Oecologia. 2003;137:252–257. doi: 10.1007/s00442-003-1334-0. [DOI] [PubMed] [Google Scholar]
- 55.Nadelhoffer K, Fry B. Nitrogen isotope studies in forested ecosystems. In: Michener RH, Lajtha K, editors. Methods in ecology: stable isotopes in ecology and environmental science. London: Blackwell Scientific Publications; 1994. pp. 22–44. [Google Scholar]
- 56.Nasholm T, Ekblad A, Nordin A, Giesler R, Hogberg M, et al. Boreal forest plants take up organic nitrogen. Nature. 1998;392:914–916. [Google Scholar]
- 57.Harrison KA, Bol R, Bardgett RD. Preferences for different nitrogen forms by coexisting plant species and soil microbes. Ecology. 2007;88:989–999. doi: 10.1890/06-1018. [DOI] [PubMed] [Google Scholar]
- 58.von Felton S, Buchmann N, Scherer-Lorenzen M. Preferences for different nitrogen forms by coexisting plant species and soil microbes: comment. Ecology. 2008;89:878–879. doi: 10.1890/07-1015.1. [DOI] [PubMed] [Google Scholar]
- 59.Hodge A, Robinson D, Fitter A. Are microorganisms more effective than plants at competing for nitrogen? Trends Plant Sci. 2000;5:304–307. doi: 10.1016/s1360-1385(00)01656-3. [DOI] [PubMed] [Google Scholar]
- 60.Lipson DA, Raab TK, Schmidt SK, Monson RK. Variation in competitive abilities of plants and microbes for specific amino acids. Biol Fert Soil. 1999;29:257–261. [Google Scholar]
- 61.Nordin A, Hogberg P, Nasholm T. Soil nitrogen form and plant nitrogen uptake along a boreal forest productivity gradient. Oecologia. 2001;129:125–132. doi: 10.1007/s004420100698. [DOI] [PubMed] [Google Scholar]
- 62.Chapin FS, Moilanen L, Kielland K. Preferential use of organic nitrogen for growth by a non-mycorrhizal arctic sedge. Nature. 1993;361:150–153. [Google Scholar]
- 63.Nordin A, Schmidt I, Shaver G. Nitrogen uptake by arctic soil microbes and plants in relation to soil nitrogen supply. Ecology. 2004;85:955–962. [Google Scholar]
- 64.Finzi AD, Berthrong ST. The uptake of amino acids by microbes and trees in three cold-temperate forests. Ecology. 2005;86:3345–3353. [Google Scholar]
- 65.Atkin OK. Reassessing the nitrogen relations of Arctic plants: a mini-review. Plant Cell Env. 1996;19:695–704. [Google Scholar]
- 66.Ellison AM, Gotelli NJ, Brewer JS, Cochran-Stafira DL, Kneitel J, et al. The evolutionary ecology of carnivorous plants. Adv Ecol Res. 2003;33:1–74. [Google Scholar]
- 67.Fish D, Hall DW. Succession and stratification of aquatic insects inhabiting the leaves of the insectivorous pitcher plant Sarracenia purpurea. Am Midl Nat. 1978;99:172–183. [Google Scholar]