ABSTRACT
Objective: We describe a novel technique of cranioplasty using a vascularized mastoid bone flap in patients after translabyrinthine excision of vestibular schwannomas (VS). Postoperative outcomes in terms of pinna and postauricular deformity are evaluated. Study Design: Retrospective study. Setting: Tertiary referral center. Results: Seventeen patients underwent cranioplasty with bone flap after translabyrinthine VS excision. At minimum follow-up of 12 months, none had a cerebrospinal fluid (CSF) leak. The measured pinna projections between the operated and non-operated sides had a mean difference of 0.80 mm (±1.70), which is not statistically significant (p > 0.05). The mean depth of the postauricular depressions was 1.38 mm (±0.93). Over the same period, 10 patients underwent translabyrinthine VS surgery without cranioplasty. In this group, there was a significant difference of 4.71 mm (±1.53) in mean pinna underprojections on the operated sides compared with the non-operated sides. Compared with patients who have undergone cranioplasty, the retroauricular depressions were significantly deeper (p < 0.05) with a mean depth of 2.92 mm (±1.21). Conclusions: Vascularized mastoid cortical bone flap cranioplasty is a simple and effective technique to ameliorate the pinna and retroauricular deformities after translabyrinthine VS excision without increased surgical cost or the use of alloplastic materials.
Keywords: Vascularized bone flap, cranioplasty, vestibular schwannoma, mastoid reconstruction
The translabyrinthine approach is one of the most common surgical techniques performed in vestibular schwannoma (VS) and lateral skull base surgery. Since its initial description more than four decades ago, the mortality and morbidity rates have declined dramatically. Cranial nerve injuries and cerebrospinal fluid (CSF) leak continue to challenge surgeons to fine-tune this operation in an effort to reduce morbidity. Most surgeons believe a reduction in the incidence of CSF leak is possible through more meticulous techniques1,2 such as modification of the standard closure with autologous fat placed deeper into the cerebellopontine angle after meticulous wax obliteration of all visible air cells2. At the same time, newer techniques of closure surfaced, including the use of hydroxyapatite cement and titanium mesh, among others.
Part and parcel of the standard wound closure in the translabyrinthine technique is a less serious problem related to cosmesis. Frequently, a depression develops over time in the postauricular sulcus as the autologous fat atrophies and soft tissues retract, at times dramatically, into a disfiguring hollow. This is also associated with medial displacement of the pinna due to the lack of support when the volume substitute of fat is no longer there (Fig. 1). Indeed, after patients have recovered from major surgery, they begin to focus on the cosmetic results and often complain about such a deformity. Patients who wear glasses or those with short hair find these deformities distressing. This problem is often overlooked and under-reported.
Figure 1.
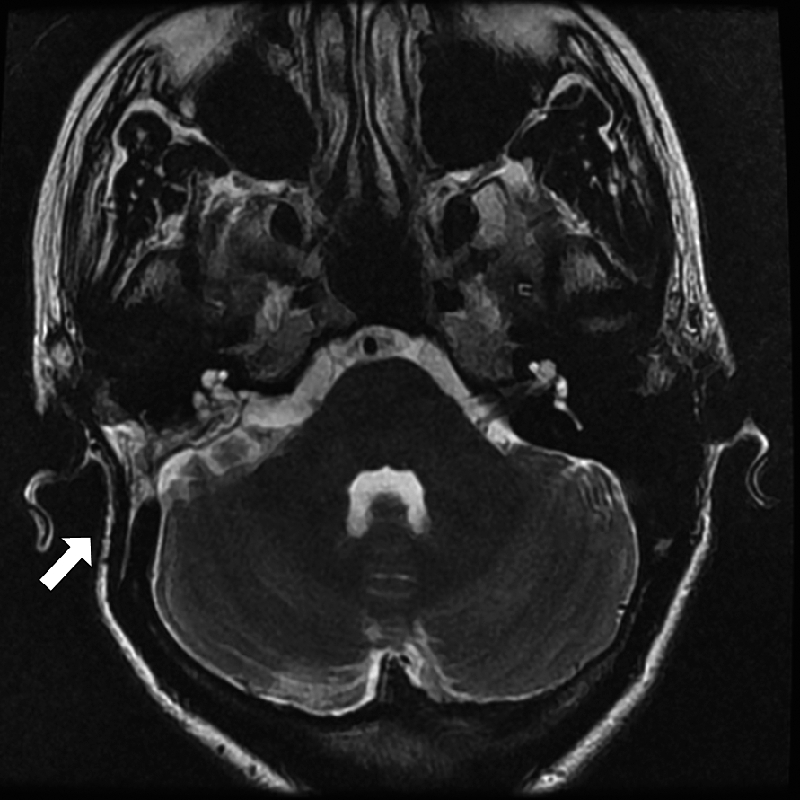
Postoperative magnetic resonance imaging scan 3 years following a translabyrinthine vestibular schwannoma excision. Note a significant retro-auricular depression and the medial displacement of the pinna (arrow) compared to the non-operated side.
In this study, a new technique is described as an addendum to the traditional wound closure following a translabyrinthine technique to address the postauricular cosmetic deformity.
MATERIALS AND METHODS
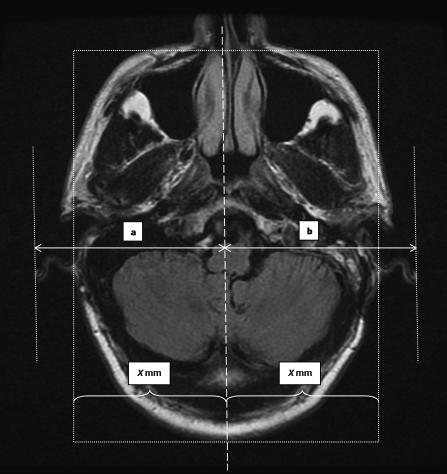
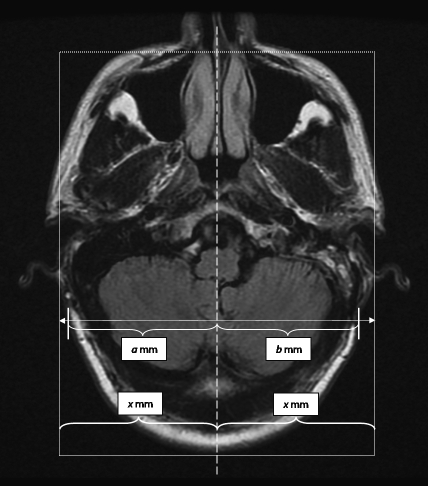
All patients who had undergone a translabyrinthine excision for a VS between April 2006 and March 2007 were identified. The hospital charts were reviewed retrospectively. Data on age, gender, side of lesion, and tumor size were noted. Postsurgical data relevant to wound complication and CSF leakage were also noted. At the 1-year follow-up, the postoperative magnetic resonance imaging (MRI) scans were analyzed to assess the presence of bony or skin deformity. Patients were also assessed clinically for the presence of retroauricular indentation or deformity. Quantification of the extent of pinna and postauricular deformity was performed in consultation with an independent radiologist. Measurements were made of the greatest distance of the outer edge of the pinna from the midline on MRI scans, on both the operated and non-operated sides, to quantify the pinna deformity (Fig. 2). At the same time, the distance of the deepest part of the retroauricular depression from the midline was measured and the difference between sides taken as the depth of the depression (Fig. 3).
Figure 2.
To quantify underprojection or overprojection of the pinna, measurements were made on 1-year postoperative magnetic resonance imaging scans. The midline of the image was determined by a line which is equidistant (X mm) from the bases of the pinnas. Measurements of the greatest distance of the outer edges of the pinna from the midline were made, on both the (a) non-operated and (b) operated sides. The difference was then recorded.
Figure 3.
To measure the retroauricular depression, the midline is first determined as in Fig. 2. The distance of the deepest part of the depression from the midline on the non-operated side (a mm) and the operated side (b mm) is then measured and the difference between sides taken as the depth of the depression.
In addition, a group of 10 consecutive patients who had had a standard closure without cranioplasty over the same period was identified to serve as controls. Identical measurements were made on their follow-up scans at 1 year.
The paired Student's t-test was used for statistical analysis and statistical significance was defined at p < 0.05.
SURGICAL TECHNIQUE
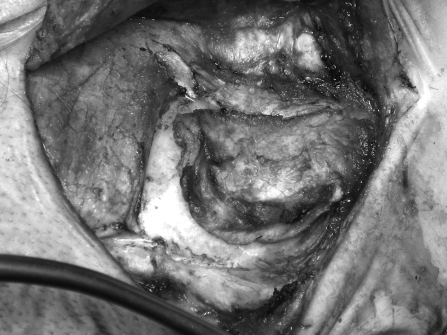
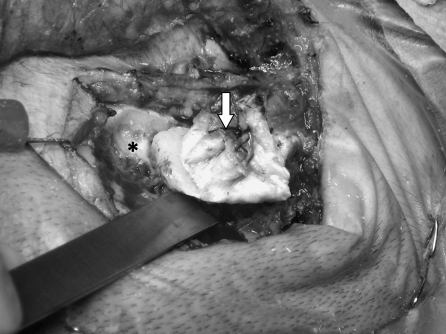
All procedures were performed by the combined neurotology and neurosurgery team. With the patient under general anesthesia, a Mayfield skull clamp is used to secure the head in the surgical position. The surgery proceeds in the standard manner using the transtemporal translabyrinthine approach through a C-shaped postauricular incision. The incision is deepened down to the periosteum. A thick musculoperiosteum is left attached to the mastoid cortex. An inferiorly based flap is designed to incorporate the lower edge of the temporalis muscle (Fig. 4). It is partially elevated from the mastoid bone to define the limits of the cranioplasty flap. The bone flap is fashioned by drilling with 6 mm and 3 mm cutting burrs circumferentially to define the underlying dura and sigmoid sinus. Once sufficient depth and safety margins are established, straight 1.0- to 1.5-cm osteotomes were used to detach the bone flap from the mastoid tip (Fig. 5). The bone flap represents the outer cortex of the mastoid bone measuring ~1.5 × 2.0 inches and is left attached to the musculoperiosteum, based inferiorly and attached to the sternocleidomastoid muscle. The bone and its musculoperiosteum are then retracted inferiorly to allow the completion of the translabyrinthine exposure (Fig. 6). Upon completion of tumor resection, all visible air cells are meticulously obliterated with bone wax, while the aditus ad antrum is plugged with temporalis fascia and wax. The dural defect is repaired with a tapered piece of abdominal fat inserted snugly into the cerebellopontine angle, also filling the surgical cavity. Subsequently, 5 cc of fibrin glue is applied. The cranioplasty flap is suture-suspended with the musculoperiosteum at the edge of the surgical cavity to help compress and medialize the fat graft (Fig. 7). A standard wound closure is performed followed by a full mastoid dressing applied for 48 hours.
Figure 4.
The vascularized mastoid bone flap is created by initially raising a musculoperiosteal flap which incorporates the inferior part of the temporalis muscle and is left attached to the mastoid cortex. It is partially elevated from the mastoid bone to define the limits of the cranioplasty flap.
Figure 5.
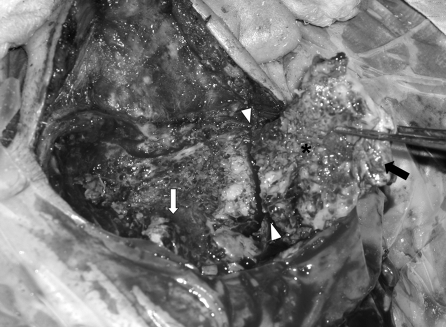
The musculoperiosteal flap (arrow) is sutured onto itself while attached to bone to avoid tearing during drilling. The bone flap is created by drilling circumferentially (asterisk) to define the underlying dura and sigmoid sinus. Once sufficient depth and safety margins are established, osteotomes are used to detach the bone flap from the mastoid tip.
Figure 6.
The vascularized mastoid bone flap (asterisk) is raised in continuity with the overlying musculoperiosteum (black arrow) and pedicled inferiorly to the nuchal and sternocleidomastoid muscles (arrowheads). The sigmoid sinus is identified early and protected (white arrow).
Figure 7.
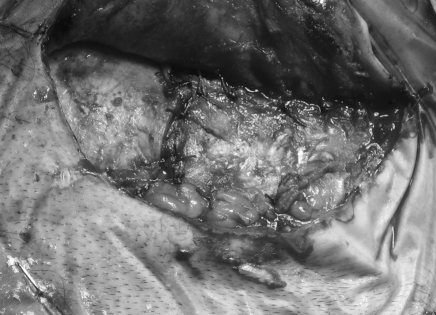
After excision of tumor, all visible air cells are obliterated with bone wax and the aditus ad antrum is plugged with temporalis fascia and wax. The dural defect is then repaired with a tapered piece of abdominal fat inserted snugly into the cerebellopontine angle, also filling the surgical cavity. The cranioplasty flap is suture-suspended with the musculoperiosteum at the edge of the surgical cavity to help compress and medialize the fat graft for a watertight closure.
RESULTS
Cranioplasty Group
Thirty-two patients were identified during the study period. Only 17 had a follow-up of 1 year or longer. All patients had primary surgical treatment withour prior interventions. There were 7 men and 10 women (9 right ears and 8 left ears). Ages ranged between 38 and 88 years (mean age, 56.5 yrs). Average tumor size was 3.0 cm (range, 2.0 to 6.0 cm). Tumor size was evaluated as the maximal extrameatal (cerebellopontine angle) diameter on MRI.
Tumor removal was complete in 16 cases and planned partial in 1 case to avoid facial paralysis. The minimum follow-up period was 12 months (range, 12 to 15 mos). One patient developed necrosis of the fat graft within the cavity, which resolved with débridement and systemic antibiotics. None of the patients developed CSF leak. There was no recurrent or persistent tumor detected on follow-up scans. All patients had a minimal palpable retroauricular deformity on clinical examination and on postoperative scans. The mean difference in the largest distance of the outer edge of the pinna between the operated and non-operated side was 0.80 mm (±1.70). The mean depth of the postauricular depression was 1.38 mm (±0.93). There were no direct complications related to the creation of the bone flap, such as dural, sigmoid sinus, or ear canal laceration, or facial nerve injury.
Noncranioplasty Group
Over the same period, 10 consecutive patients underwent primary translabyrinthine excision of VS without cranioplasty reconstruction. All had a follow-up period of at least 1 year. Reasons for not performing a bone flap cranioplasty in these patients include patients' refusal, presence of a small, contracted mastoid cavity, or a sigmoid sinus that was too lateral, creating a safety concern.
There were six men and four women (seven right ears and three left ears) in this group. Ages ranged between 43 and 68 years (mean age, 51.3 yrs). Average tumor size was 2.8 cm (range, 1.5 to 4.5 cm). Identical measurements were taken from the postoperative follow-up scans. The mean difference between the operated and unoperated sides was 4.71 mm (±1.53). The mean depth of the retroauricular depression was 2.92 mm (±1.21). None of the patients in this group experienced a CSF leak. There was no recurrence or persistent tumor at 1-year follow-up in this group.
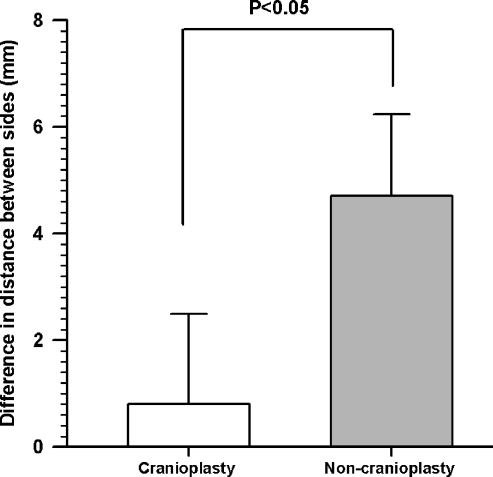
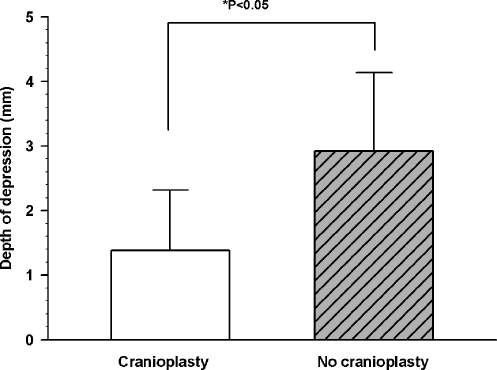
The difference in distance of the pinna from the skull on the operated and non-operated sides in the cranioplasty group compared against the group without cranioplasty was statistically significant (p < 0.05) (Fig. 8). Similarly, the depth of retroauricular depression was significantly less in the patients with cranioplasty compared with those without (Fig. 9).
Figure 8.
The difference in distance of the pinna from the skull on the operated and non-operated side in the cranioplasty group compared against the group without cranioplasty was statistically significant (p > 0.05). Values are presented as mean ± standard deviation.
Figure 9.
The depth of the postauricular depression was significantly less in the patients who underwent cranioplasty compared with those who did not (p < 0.05). Values are presented as mean ± the standard deviation.
We used the differences in measurements instead of absolute measurements to negate any possible effects due to differences in imaging protocol, as some follow-up scans were performed in other institutions.
DISCUSSION
During the translabyrinthine approach to the cerebellopontine angle for excision of VS, a substantial amount of petrosal and mastoid bone is removed, resulting in a large bone defect. This bone defect is traditionally filled by free abdominal adipose tissue in the deeper portion. The fat is applied directly into the cerebellopontine angle leading to the closure of the dural defect, like a “champagne cork.”1,2 The periosteum and/or temporalis muscle is then closed laterally to reinforce the closure. However, as fat atrophies with time, soft tissue gradually collapses into the mastoid cavity, resulting in a permanent disfigurement.
As the incidence of major complications of translabyrinthine surgery is reduced to low levels, increasing attention has been focused on other “less serious” complications such as the development of the undesirable postoperative cosmetic deformities. Efforts to minimize this morbidity are now more critical than ever.
A variety of techniques and approaches have been employed in the reconstruction of craniectomy or craniotomy defects after neurosurgical procedures. In contrast, the literature is sparse on the reconstruction of the translabyrinthine craniectomy defect.
Issues relevant to the mastoid defect are similar to craniotomy/craniectomy reconstruction in neurosurgery and craniofacial surgery.3 An ideal cranioplasty should address both the problems of CSF leak and of cosmetic deformities. It should be relatively easy to employ with consistent and reproducible results. The materials used should emulate the tensile strength of bone. Autologous tissue should not undergo significant resorption while alloplasts need to be inert and resistant to infection and imaging artifacts. The added surgical costs relative to benefit should also be considered.
Hydroxyapatite cement (HAC) represents the most recent addition to a range of alloplastic materials used for cranioplastic reconstruction. It is a mixture of dicalcium phosphate anhydrous and tetracalcium phosphate.4,5 It could be mixed with any water-containing medium, such as blood, plasma, or saline, to form a thick paste. This can then be applied intraoperatively and can be easily contoured. The reaction is isothermic and occurs at physiological pH.5 The ability to intraoperatively mix, mold, and cure a liquid and powder composite to create the final hydroxyapatite phase offers a tremendous advantage in shaping and customizing the reconstruction. This material is highly osseoconductive and readily osseointegrated with surrounding bone.4 Both experimental and clinical data have reported infection rates of HAC from 0% to 7.7%.5,6,7,8
Kamerer et al5 described the use of HAC in four patients during translabyrinthine removal of VS. None of these patients had a CSF leak or postauricular depression.
Similarly, Kveton and colleagues8 used HAC in five patients after translabyrinthine VS surgery with promising results. Interestingly, the authors had more problems when using HAC for repair of suboccipital craniectomy defects, with resorption being a significant problem.
The high cost of HAC also limits wider application of this technique. Hydroxyapatite cement averages approximately US $80.00 per gram in the United States.9 With most cases requiring ~25 g to fill the surgical cavity to achieve the desired effect,10 the high cost is a significant consideration.
Couldwell and Fukushima11 described a method of performing a cosmetic mastoidectomy for the combined supra/infratentorial craniotomy and transtemporal exposure. The technique described the use of a free bone graft over a relatively large defect with the combined use of a microplating system. It is difficult and dangerous when attempting to preserve a large area of mastoid cortex in the context of the underlying dura and sigmoid sinus. A thin nonvascularized bone will likely suffer significant resorption and may be at risk of infection, particularly in the presence of screws and miniplates without a good vascular bed.
Tokoro and associates12 described the technique of cosmetic reconstruction after mastoidectomy using bone chips mixed with fibrin glue. Although this may be adequate in standard mastoid obliteration for chronic ear disease, it will not likely provide a rigid platform to compress and medialize the fat graft following a translabyrinthine procedure. In addition, the bone chips may be a source of infection.
Harvesting of autologous bone graft, such as a split calvarial graft, could prove time-consuming with added donor site morbidity.13 Although the rate of infection and extrusion may be low, the cosmetic results have been variable due to varying degrees of resorption of the graft. The use of titanium material to repair cranial defects is well established in neurosurgical practice.14,15 Given its nonferrous characteristic, this metal does not produce a significant degree of distortion artifacts during computed tomography and MRI that are part and parcel of postoperative monitoring. More recently, titanium mesh has been used in the reconstruction of craniectomy defects from retrosigmoid resection of VS,3 for the expressed intent of reducing postoperative headaches.
Recently, Fayad et al16 described the use of titanium mesh after translabyrinthine acoustic neuroma surgery in 389 patients. The titanium mesh was fixed with four microscrews to the cranium over strips of fat filling the mastoid cavity. The titanium plate, in addition to reconstructing the surface of the cranium, also applies pressure over the fat and keeps it in position. In addition, there was no case of titanium implant extrusion or infection in the patients.
Like HAC, titanium cranioplasty suffers from the disadvantage of increased surgical cost, which has been estimated by Fayad and colleagues16 to be approximately US $400.00 per use.
Vascularized osteofascial calvarial bone grafts and flaps have been used for craniofacial reconstruction.17,18 The superficial temporal, postauricular, and occipital vessels provide a rich vascular network to the fascia and periosteum of the ear and lateral skull.19 By preserving the overlying musculoperiosteum, the technique of vascularized cranioplasty described in the current study protects the vascularity and viability of the bone flap; it reduces the risk of resorption, and allows rapid access to the cerebellopontine angle if necessary, while at the same time avoiding the introduction of foreign materials into the surgical field. It is relatively straightforward, consists of the best biological material available (autologous bone), and could be employed during the same operation adding perhaps an extra 10 minutes to the total operative time, without any additional costs. This reconstruction is much more rigid compared with previous methods of direct skin closure or periosteal closure over fat and it retains the skull contour without the use of miniplates and screws (Figs. 10, 11).
Figure 10.
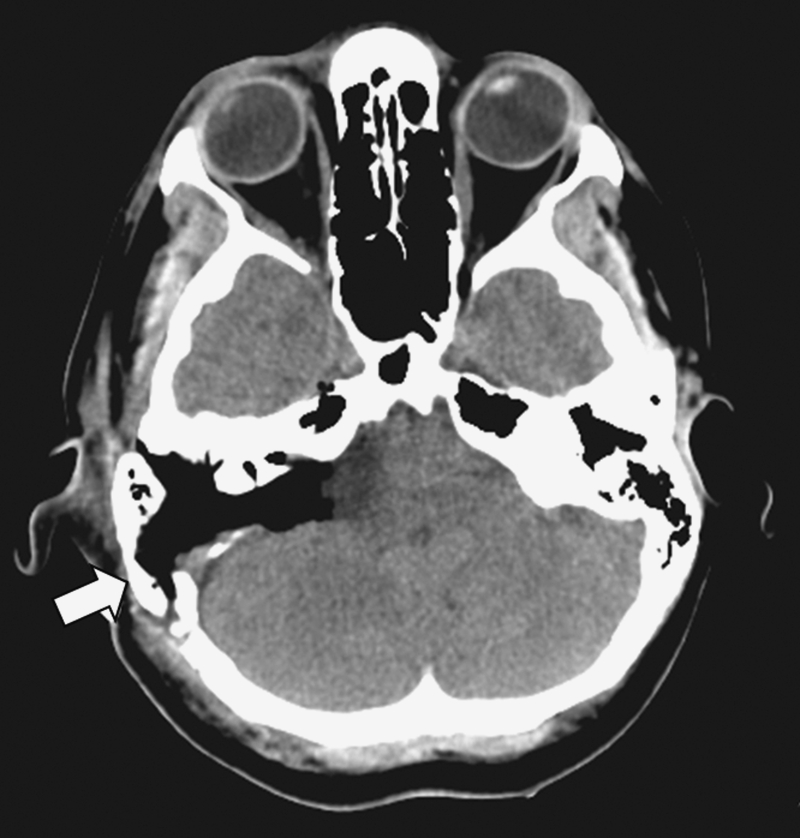
Postoperative computed tomography scan image of a patient with a vascularized bone flap cranioplasty (arrow) showing the tamponade effect of the bone flap against the underlying abdominal fat graft, and at the same time reestablishing the bony contour of the mastoid bone.
Figure 11.
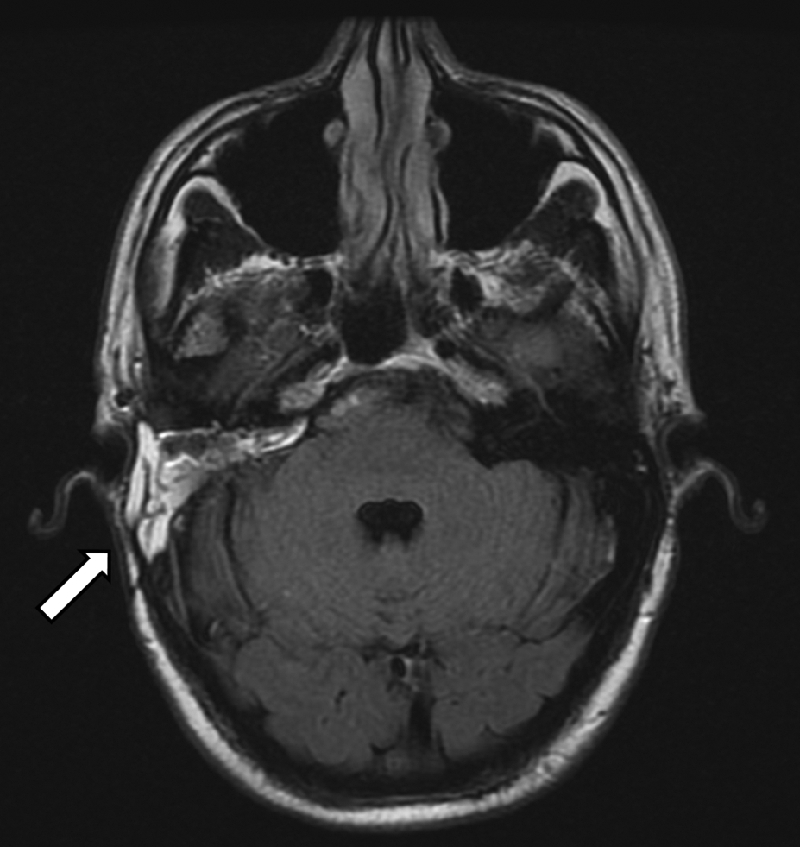
One-year postoperative magnetic resonance imaging scan of a patient who underwent vascularized bone flap cranioplasty (arrow). There is minimal pinna and postauricular deformity.
The measured pinna projections between the operated and non-operated sides in the cranioplasty group was not statistically significant. This is in contrast to the patients in the noncranioplasty group in whom there was a significant difference in mean pinna underprojections on the operated sides compared with the non-operated sides with a mean of 4.71 mm. In addition, the mean depth of the postauricular depressions was 1.38 mm in the cranioplasty patients compared with 2.92 mm in the noncranioplasty group of patients. On the surface, these differences, measured in millimeters, seem unlikely to translate into clinical significance even though they are statistically significant. However, clinical experience and observations tell us that the differences are sufficiently perceived by patients to make a real difference and thus must be part of our ongoing study on the subjective aspects of cranioplasty.
This cranioplasty technique is not suitable for patients with small, contracted mastoid cavities, or those with laterally placed sigmoid sinus, as these conditions render fashioning of the bone flap too dangerous. In addition, this technique does not avoid the potential morbidity at the abdominal graft donor site.
We acknowledge the inherent weakness in the retrospective nature and limited numbers in this study, but we believe the technique described offers a rapid, safe, and effective method of cranioplasty in patients who have undergone translabyrinthine VS surgery.
CONCLUSION
With major complications of VS surgery at an all-time low, efforts to minimize other morbidities like postoperative cosmetic deformities are becoming critical. Cranioplasty with a vascularized mastoid cortical bone flap after translabyrinthine excision of VS is a technically simple and effective technique. It is successful in alleviating the disfiguring retroauricular deformity without complications or the use of alloplastic materials.
REFERENCES
- Sanna M, Rohit M S, Skinner L J, Jain Y. Technique to prevent post-operative CSF leak in the translabyrinthine excision of vestibular schwannoma. J Laryngol Otol. 2003;117:965–968. doi: 10.1258/002221503322683849. [DOI] [PubMed] [Google Scholar]
- House J L, Hitselberger W E, House W F. Wound closure and cerebrospinal fluid leak after translabyrinthne surgery. Am J Otol. 1982;4:126–128. [PubMed] [Google Scholar]
- Wazen J J, Sisti M, Lam S M. Cranioplasty in acoustic neuroma surgery. Laryngoscope. 2000;110:1294–1297. doi: 10.1097/00005537-200008000-00013. [DOI] [PubMed] [Google Scholar]
- Costantino P D, Friedman C D, Jones K, Chow L C, Pelzer H J, Sisson G A., Sr Hydroxyapatite cement. I. Basic chemistry and histologic properties. Arch Otolaryngol Head Neck Surg. 1991;117:379–384. doi: 10.1001/archotol.1991.01870160033004. [DOI] [PubMed] [Google Scholar]
- Kamerer D B, Hirsch B E, Snyderman C H. Hydroxyapatite cement: a new method of achieving watertight closure in transtemporal surgery. Am J Otol. 1994;15:47–49. [PubMed] [Google Scholar]
- Costantino P D, Chaplin J M, Wolpoe M E, et al. Applications of fast-setting hydroxyapatite cement: cranioplasty. Otolaryngol Head Neck Surg. 2000;123:409–412. doi: 10.1067/mhn.2000.107679. [DOI] [PubMed] [Google Scholar]
- Poetker D M, Pytynia K B, Meyer G A, Wackym P A. Complication rate of transtemporal hydroxyapatite cement cranioplasties: a case series review of 76 cranioplasties. Otol Neurotol. 2004;25:604–609. doi: 10.1097/00129492-200407000-00031. [DOI] [PubMed] [Google Scholar]
- Kveton J F, Friedman C D, Constantino P D. Indications for hydroxyapatite cement reconstruction in lateral skull base surgery. Am J Otol. 1995;16:465–469. [PubMed] [Google Scholar]
- Arriaga M A, Chen D A, Burke E L. Hydroxyapatite cement cranioplasty in translabyrinthine acoustic neuroma surgery—update. Otol Neurotol. 2007;28:538–540. doi: 10.1097/mao.0b013e3180423ad9. [DOI] [PubMed] [Google Scholar]
- Arriaga M A, Chen D A. Hydroxyapatite cement cranioplasty in translabyrinthine acoustic neuroma surgery. Otolaryngol Head Neck Surg. 2002;126:512–517. doi: 10.1067/mhn.2002.124436. [DOI] [PubMed] [Google Scholar]
- Couldwell W T, Fukushima T. Cosmetic mastoidectomy for the combined supra/infratentorial transtemporal approach. J Neurosurg. 1993;79:460–461. doi: 10.3171/jns.1993.79.3.0460. [DOI] [PubMed] [Google Scholar]
- Tokoro K, Chiba Y, Murai M, et al. Cosmetic reconstruction after mastoidectomy for the transpetrosal-presigmoid approach: Technical note. Neurosurgery. 1996;39:186–188. doi: 10.1097/00006123-199607000-00044. [DOI] [PubMed] [Google Scholar]
- McCarthy J G, Zide B M. The spectrum of calvarial bone grafting: introduction of the vascularized calvarial bone flap. Plast Reconstr Surg. 1984;74:10–18. doi: 10.1097/00006534-198407000-00002. [DOI] [PubMed] [Google Scholar]
- Simpson D. Titanium in cranioplasty. J Neurosurg. 1965;22:292–293. doi: 10.3171/jns.1965.22.3.0292. [DOI] [PubMed] [Google Scholar]
- Gordon D S, Blair G A. Titanium cranioplasty. BMJ. 1974;2:478–481. doi: 10.1136/bmj.2.5917.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayad J N, Schwartz M S, Slattery W H, Brackmann D E. Prevention and treatment of cerebrospinal fluid leak after translabyrinthine acoustic tumor removal. Otol Neurotol. 2007;28:387–390. doi: 10.1097/01.mao.0000265188.22345.d4. [DOI] [PubMed] [Google Scholar]
- Choung P H, Nam I W, Kim K S. Vascularized cranial bone grafts for mandibular and maxillary reconstruction. The parietal osteofascial flap. J Craniomaxillofac Surg. 1991;19:235–242. doi: 10.1016/s1010-5182(05)80063-0. [DOI] [PubMed] [Google Scholar]
- Choung P H. The auriculomastoid fasciocutaneous island flap: a new flap for orofacial reconstruction. J Oral Maxillofac Surg. 1996;54:559–567. doi: 10.1016/s0278-2391(96)90632-1. [DOI] [PubMed] [Google Scholar]
- Marty F, Montandon D, Gumener R, Zbrodowski A. Subcutaneous tissue in the scalp: anatomical, physiological and clinical study. Ann Plast Surg. 1986;16:368–376. doi: 10.1097/00000637-198605000-00004. [DOI] [PubMed] [Google Scholar]