ABSTRACT
Objective: Intraosseous cavernous angioma (CA) of the petrous bone is rare and preoperative diagnosis can be challenging, especially when its epicenter is outside the internal auditory canal (IAC) or geniculate ganglion. Methods: A 45-year-old man presented to our clinic with right-sided hearing loss, tinnitus, and unsteadiness. Neuroimaging revealed a right posterior petrous mass. Aggressive subtotal resection with decompression of the IAC was achieved through a right suboccipital craniotomy. Histopathological findings were consistent with CA. Conclusion: As was the case with this patient, we believe that CA should be included in the differential diagnosis of petrous region pathology with bony involvement. Surgery is warranted due to its expansive nature and to decompress the adjacent neural structures.
Keywords: Angioma, cavernous, hemangioma, internal auditory canal, intraosseous, petrous
Cavernous angioma (CA; also called cavernous hemangioma, cavernous malformation, or cavernoma) is a common intraparenchymal lesion of the central nervous system. Extradural location constitutes 0.4 to 2% of all intracranial vascular malformations.1 Intraosseous occurrence is rare and represents 0.2% of bone tumors of the skull;2 the intraosseous skull base location, in particular, is very rare.
In this article, a case of CA located in the posterior petrous bone is presented. Unlike the majority of other reported vascular malformations in this region, the epicenter of the lesion was not in the internal auditory canal (IAC) or around the geniculate ganglion (GG).
CASE REPORT
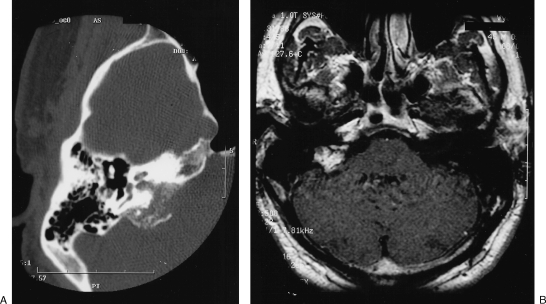
A 45-year-old man presented with a 1-year history of progressive hearing loss on the right side along with intermittent tinnitus and unsteadiness. Neurological and audiological examination confirmed the right-sided Gardner-Robertson grade 4 hearing loss. Computed tomography (CT) demonstrated an erosive and irregular calcified matrix extending from the petrous bone to the proximal jugular foramen on the right. Magnetic resonance imaging (MRI) showed a heterogeneously enhancing 2.3-cm mass in the right posterior petrous region (Fig. 1).
Figure 1.
(A) High-resolution axial computed tomography scan showing erosive and irregular calcified mass in right petrous bone expanding to jugular canal. (B) Postgadolinium, T1-weighted axial images show heterogeneously enhancing mass in right petrous region.
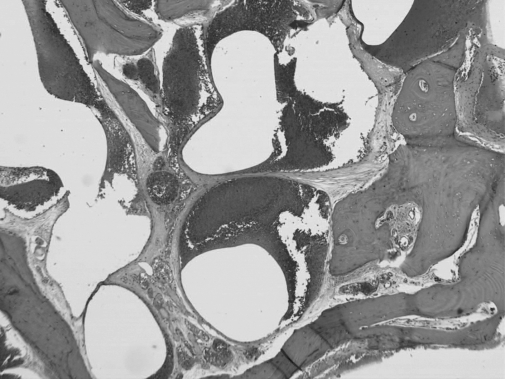
The patient underwent a right suboccipital craniectomy. Initially, no intradural lesion was identified in the cerebellopontine angle (CPA). The petrous bone, which was ventral, superior, and posterior to the IAC, was found to be very prominent. The overlying dura was very vascular. Drilling of the abnormal petrous bone was performed. The lesion, which appeared to be inside the bone, was mild to moderately vascular and trabeculated with no soft tissue component. The IAC was intimately involved with the lesion and was fully decompressed. No soft tissue was noted inside the canal. The pathological petrous bone was drilled to the limit of the jugular bulb, having achieved a subtotal resection at that point. The histopathology was marked by an intraosseous proliferation of dilated venous vessels with no involvement of arterial counterparts. The endothelial lining did not show any atypia or mitotic activity (Fig. 2).
Figure 2.
Photomicrograph of bone showing intraosseous cavernoma marked by proliferation of venous-type vessels (hematoxylin and eosin, original magnification ×50).
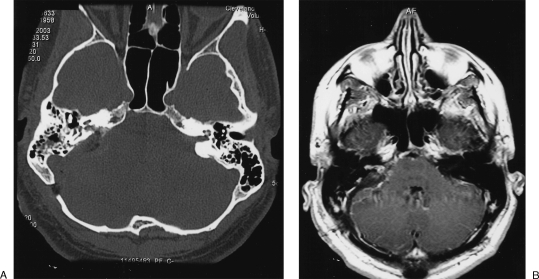
The postoperative course was uneventful. The patient completely recovered from balance problems in 10 days. At 1-year follow-up, the tinnitus persisted along with unserviceable hearing on the right. Follow-up CT and MRI showed a small residual lesion in and around the decompressed IAC (Fig. 3).
Figure 3.
Postoperative (A) high-resolution axial computed tomography scan and (B) postgadolinium, T1-weighted axial image demonstrating residual lesion.
DISCUSSION
Cavernous angioma occurs due to the failure of differentiation of the embryonic vascular network and may affect any organ system in the body. It represents the most common benign tumor of the liver, spleen, and orbit.1 Intracranially, an extra-axial location is encountered in < 2% of cases. This includes intradural, extra-axial locations such as the CPA, within the dural sinuses, and extradural locations. Among intraosseous CA locations, calvarium is relatively common, accounting for 0.2% of the skull tumors.1,2,3 However, skull base involvement, in particular, is very rare: extratemporal intraosseous locations such as the vomer,4 orbitosphenoidal region,3 clivus,5 and occipital condyle6 have been described.
Temporal bone CA deserves special attention in this context. The majority of lesions arise from the IAC intradurally or around the GG.7,8,9,10 In 9 of the 10 patients with intratemporal vascular tumors reported by Mangham et al8 and Piccirillo et al,10 the lesions were confined to these areas. This has been attributed to the dense vascular network around Scarpa's ganglion and the GG.9,10 In these locations, the endosteum surrounding these lesions may be intact,8 which may limit their bony involvement. A completely intraosseous extradural petrous bone CA with its epicenter outside the IAC or remote from GG, as in our case, is rare.
The radiological findings in intraosseous CA are nonspecific. A mottled pattern of increased T1 signal due to fatty content and increased T2 signal caused by pooling of blood, as well as early focal enhancement of the lesion forming a diffuse pattern in the late phase, have been described as characteristic MRI features.3,5 However, these may not reliably differentiate CA from other, more common lesions. Differential diagnoses include fibrous dysplasia, aneurysmal bone cyst, dermoid cyst, meningioma, facial neuroma, plasmacytoma, eosinophilic granuloma, osteoma, osteogenic sarcoma, chondrosarcoma, cholesterol granuloma, and metastatic tumor.2,3,10 Therefore, the diagnosis of an intraosseous CA is usually made at the time of surgery because it can mimic more common lesions of the skull base.11
Intraosseous CA may enlarge within time; therefore, treatment is advocated.8,11 The mechanisms underlying the growth of these lesions, unlike the intraparenchymal counterparts, are believed to be capillary budding, vascular ectasia, and vascular thrombosis and organization, rather than recurrent hemorrhage.1 However, they have been reported to have presented with epidural hematoma.12
In contrast to calvarial lesions, an en bloc resection may not always be possible for the skull base location.11 Extra-axial CA is known to cause significant intraoperative bleeding, especially when the lesion involves the dural sinuses. Intraosseous CA seems to be relatively less vascular,11 as we observed in our case. Another important intraoperative feature of these lesions is that they may infiltrate9 or even cause laceration13 of the adjacent dura. A dense perineural reaction caused by the lesion may hinder the cleavage plane between the lesion and the nerve, particularly within the IAC.9
CONCLUSION
Although rare, cavernous angioma of the petrous bone should be considered in the preoperative differential diagnosis of a petrous lesion. Surgical resection is the preferred treatment at the time of diagnosis for symptomatic lesions because these lesions may continue to grow and cause irreversible neurological deficits.
REFERENCES
- Voelker J L, Stewart D H, Schochet S S., Jr Giant intracranial and extracranial cavernous malformation. Case report. J Neurosurg. 1998;89:465–469. doi: 10.3171/jns.1998.89.3.0465. [DOI] [PubMed] [Google Scholar]
- Heckl S, Aschoff A, Kunze S. Cavernomas of the skull: review of the literature 1975–2000. Neurosurg Rev. 2002;25:56–62. doi: 10.1007/s101430100180. [DOI] [PubMed] [Google Scholar]
- Slaba S G, Karam R H, Nehme J I, Nohra G K, Hachem K S, Salloum J W. Intraosseous orbitosphenoidal cavernous angioma. J Neurosurg. 1999;91:1034–1036. doi: 10.3171/jns.1999.91.6.1034. [DOI] [PubMed] [Google Scholar]
- Nakahira M, Kishimoto S, Miura T, Saito H. Intraosseous hemangioma of the vomer: a case report. Am J Rhinol. 1997;11:473–477. doi: 10.2500/105065897780914956. [DOI] [PubMed] [Google Scholar]
- Tashiro T, Inoue Y, Nemoto Y, et al. Cavernous hemangioma of the clivus: case report and review of the literature. AJNR Am J Neuroradiol. 1991;12:1193–1194. [PMC free article] [PubMed] [Google Scholar]
- Garcia-Marin V, Ravina J, Trujillo E, Gonzales-Feria L. Symptomatic cavernous hemangioma of the occipital condyle treated with methacrylate embolization. Surg Neurol. 2001;56:301–303. doi: 10.1016/s0090-3019(01)00613-9. [DOI] [PubMed] [Google Scholar]
- Dufour J J, Michaud L A, Mohr G, Pouliot D, Picard C. Intratemporal vascular malformations (angiomas): particular clinical features. J Otolaryngol. 1994;23:250–253. [PubMed] [Google Scholar]
- Mangham C A, Carberry J N, Brackmann D E. Management of intratemporal vascular tumors. Laryngoscope. 1981;91:867–876. doi: 10.1288/00005537-198106000-00002. [DOI] [PubMed] [Google Scholar]
- Martin N, Sterkers O, Nahum H. Haemangioma of the petrous bone: MRI. Neuroradiology. 1992;34:420–422. doi: 10.1007/BF00596506. [DOI] [PubMed] [Google Scholar]
- Piccirillo E, Agarwal M, Rohit , Khrais T, Sanna M. Management of temporal bone hemangiomas. Ann Otol Rhinol Laryngol. 2004;113:431–437. doi: 10.1177/000348940411300603. [DOI] [PubMed] [Google Scholar]
- Glasscock M E, III, Smith P G, Schwaber M K, Nissen A J. Clinical aspects of osseous hemangioma of the skull base. Laryngoscope. 1984;94:869–873. doi: 10.1288/00005537-198407000-00001. [DOI] [PubMed] [Google Scholar]
- Kessler L A, Lubic L G, Koskoff Y D. Epidural hemorrhage secondary to cavernous hemangioma of the petrous portion of the temporal bone. J Neurosurg. 1957;14:329–331. doi: 10.3171/jns.1957.14.3.0329. [DOI] [PubMed] [Google Scholar]
- Honda M, Toda K, Baba H, Yonekura M. Congenital cavernous angioma of the temporal bone: case report. Surg Neurol. 2003;59:120–123. doi: 10.1016/s0090-3019(02)00985-0. [DOI] [PubMed] [Google Scholar]