ABSTRACT
Objective: To present the results of the clinical study of carbon ion radiotherapy (CIRT) for skull base and paracervical spine tumors at the National Institute of Radiological Sciences in Chiba, Japan. Methods: The study is comprised of three protocols: a pilot study, a phase I/II dose escalation study, and a phase II study. All the patients were treated by 16 fractions for 4 weeks with total doses of 48.0, 52.8, 57.6, and 60.8 Gy equivalents (GyE). Results: As a result of the dose escalation study of CIRT for skull base tumors, a dose fractionation of 60.8 GyE/16 fractions for 4 weeks was decided as the recommended dose because of acceptable normal tissue reactions and good local tumor control. Conclusions: Preliminary results of the phase II clinical study of CIRT for skull base chordoma showed local control at 5 years at 100%, and normal tissues showed a mild reaction without any severe morbidity of important organs.
Keywords: Chordoma, carbon ion radiotherapy, particle radiotherapy, skull base tumor
Skull base chordomas are rare tumors originating from ectopic remnants of the embryonal notochord at the spheno-occipital synchondrosis.1 The limiting factor for x-ray radiotherapy (XRT) conventionally applied to skull base chordomas is the adjacent normal tissue because photon radiotherapy has poor local control.2 A clear dose-response relationship has been demonstrated for chordomas, in which an improved tumor control rate can be achieved for patients treated with > 75 Gy, whereas only 20% of patients receiving 40 to 60 Gy are controlled locally.3 Because the tolerance doses of surrounding normal tissues are lower than the doses needed for tumor control, the required high local doses are often difficult to apply by conventional XRT.
It is clear that the charged particle therapy is a more appropriate radiotherapy for the skull base chordoma than XRT because of excellent dose distribution.4,5 Clinical evidence of proton therapy, alone or combined with photon therapy, showed excellent tumor control and minimized normal tissue morbidity.6,7,8,9,10,11 It has been pointed out, however, that in certain patient groups it is difficult to achieve local control with proton radiotherapy, even at elevated doses. It has thus been recognized that among chordoma patients, the prognosis for paracervical chordoma patients is worse than for skull base chordoma patients, that for nonchondroid patients it is worse than for chondroid ones, and that the prognosis for females is worse than that for males.12 The high biological effectiveness of carbon ion radiotherapy (CIRT) has promising potential for these intractable skull base and paracervical chordomas. There are only a few reports from Gesellschaft für Schwerionenforschung (GSI) in Heidelberg, Germany, of CIRT for skull base chordomas.3 This article describes the National Institute of Radiological Sciences (NIRS) experience of CIRT for skull base chordomas.
MATERIALS AND METHODS
Eligibility criteria for this study were as follows: (1) age between 15 and 80 years, (2) histologically confirmed chordoma of the skull base and upper (C1 to C2) paracervical spine, (3) Karnofsky index (KI) > 50% and neurological function (NF) of grade 1 or 2, (4) anticipated life expectancy of more than 6 months, (5) no previous radiotherapy, (6) no chemotherapy within 4 weeks, (7) no multiple distant metastasis, (8) no active or uncontrollable infection in the treatment area, and (9) no serious medical or psychological conditions precluding safe administration of treatment. The histology of each patient was diagnosed by two pathologists: one from the NIRS and one from the hospital where an operation had been performed. Each patient had recovered from the effects of surgery before entry into the study. All patients signed an approved institutional review board consent form. This study was approved by the NIRS Ethical Committee on Human Clinical Research.
After a careful evaluation of a patient's history, physical examination, and imaging, a custom-made device was used to hold the patient in the same position both during the planning computed tomography (CT) and the CIRT. In planning for the three-dimensional treatment, clinical target volume was defined with an adequate safety margin for the tumor. For this study, doses were expressed in photon-equivalent doses (Gray equivalent dose [GyE]), which were defined as the physical doses multiplied by the relative biological effectiveness (RBE) of the carbon ions. Biological flatness of the spread out Bragg peak (SOBP) was normalized by the survival fraction of the human salivary gland (HSG) tumor cells at the distal region of SOBP, where the RBE of carbon ions was assumed to be 3.0.13
The CIRT clinical study of skull base and paracervical spine tumors has been conducted since June 1995 and is comprised of three protocols: (1) between June 1995 and August 1996, a pilot study; (2) between July 1997 and July 2003, a phase I/II dose escalation study; and (3) from April 2004 till June 2007, a phase II study. All of the patients were treated with 16 fractions for 4 weeks. The total doses were 48.0 GyE in the pilot study; 48.0, 52.8, 57.6, and 60.8 GyE in the phase I/II dose escalation study; and 60.8 GyE in the phase II study.
Acute reactions were classified according to the Radiation Therapy and Oncology Group (RTOG) scoring system, with a maximum reaction within 3 months after initiation of CIRT. Late reactions were classified according to the RTOG/European Organization for Research and Treatment of Cancer (EORTC) scoring system. Acute tumor response was determined as a maximum reaction within 6 months after the onset of CIRT by serial magnetic resonance imaging (MRI) according to the World Health Organization definitions.14 Complete response (CR) was defined as the disappearance of all visible tumors, partial response (PR) was defined as > 50% reduction in the product of the perpendicular diameters of the tumor, no change (NC) was defined as < 50% reduction and < 25% increase, and progression of disease (PD) was defined as > 25% increase.
Local control probability, calculated by the Kaplan-Meier method,15 was measured from the first day of CIRT to the date of local relapse. Local control was defined as showing no evidence of tumor regrowth by MRI, CT, physical examination, or biopsy. Survival probability, also calculated by the Kaplan-Meier method, was measured from the first day of CIRT to the date of death or the date of last follow-up. A log-rank test was used to evaluate the differences between two probability curves, and a P value < 5% was considered statistically significant. The Statistical Analysis System Version 3.1 (SAS Institute, Cary, NC) was used for the entire analysis.
RESULTS
Between June 1995 and June 2007, 34 cases in 33 patients with chordoma of the skull base and the paracervical spine were treated with CIRT. One patient had been treated twice by CIRT for primary and marginal recurrence. The follow-up periods ranged from 8 to 129 months with a mean period of 53 months. The patients consisted of 14 males and 19 females, and their ages ranged from 16 to 76 years with a median age of 47 years. The KI ranged from 70 to 100% with a median of 90%. Of 34 cases, 7 (21%) involved the paracervical spine. Twenty-one of the cases (62%) required single-stage removal including biopsy (Fig. 1), nine cases (26%) required two-stage removal, two cases (6%) had three-stage removal, and two (6%) had four-stage removal.
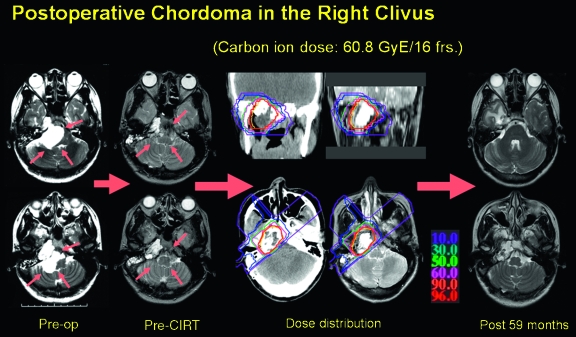
Figure 1.
A case presentation of a 25-year-old woman with skull base chordoma. After surgical removal of the tumor, the residual tumor was treated by carbon ion radiotherapy. No tumor regrowth was present 59 months after radiotherapy with limited radiation encephalopathy of the right temporal lobe. frs, fractions; op, operation; CIRT, carbon ion radiotherapy.
Four cases were treated with 48.0 GyE in the pilot study. In the phase I/II dose escalation study, one case was treated with 48.0 GyE, three were treated with 52.8 GyE, seven cases with 57.6 GyE, and five cases with 60.8 GyE. In the phase II study, 14 cases were treated with 60.8 GyE. Clinical target volume (CTV) ranged from 2 to 328 cm3, with a mean value of 51 cm3 and a median value of 32 cm3. Applied ports were two ports for 7 cases (21%), three ports for 26 cases (76%), and four ports for 1 case (3%). All patients completed their scheduled fractionation without any delay.
One case, which was treated with 52.8 GyE, had a grade 2 acute skin reaction. Six cases had grade 2 acute mucosal reactions; two of these cases were treated with 52.8 GyE, and four were treated with 60.8 GyE. No cases resulted in grade 3 or higher acute skin and mucosal reactions (Table 1). Two cases (52.8 GyE and 57.6 GyE) showed grade 1 late skin reactions. Two cases had grade 1 late mucosal reactions (52.8 GyE and 60.8 GyE). No cases had grade 2 or higher late skin and mucosal reactions (Table 2). There were no acute reactions of the brain and the spinal cord. One patient (60.8 GyE), who had a grade 2 late brain reaction, had radiation encephalitis that was treated with steroids for 1 year. Grade 1 late reactions of the brain were observed in five cases (one case with 48.0 GyE and four cases with 60.8 GyE). Twenty-eight patients had no reaction.
Table 1.
Acute Reactions (Skin and Mucosa)
| Total Dose (GyE) | Cases | Skin |
Mucosa |
||||
|---|---|---|---|---|---|---|---|
| G0 | G1 | G2 | G0 | G1 | G2 | ||
| GyE, Gray equivalent; G, grade. | |||||||
| 48.0 | 5 | 4 | 1 | 0 | 5 | 0 | 0 |
| 52.8 | 3 | 1 | 1 | 1 | 1 | 0 | 2 |
| 57.6 | 7 | 4 | 3 | 0 | 4 | 3 | 0 |
| 60.8 | 19 | 12 | 7 | 0 | 12 | 3 | 4 |
| Total | 34 | 21 | 12 | 1 | 22 | 6 | 6 |
Table 2.
Late Reactions (Skin, Mucosa, and Brain)
| Total Dose (GyE) | Cases | Skin |
Mucosa |
Brain |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| G0 | G1 | G2 | G0 | G1 | G2 | G0 | G1 | G2 | ||
| GyE, Gray equivalent; G, grade. | ||||||||||
| 48.0 | 5 | 5 | 0 | 0 | 5 | 0 | 0 | 4 | 1 | 0 |
| 52.8 | 3 | 3 | 1 | 0 | 2 | 1 | 0 | 3 | 0 | 0 |
| 57.6 | 7 | 6 | 1 | 0 | 7 | 0 | 0 | 7 | 0 | 0 |
| 60.8 | 19 | 19 | 9 | 0 | 18 | 1 | 0 | 14 | 4 | 1 |
| Total | 34 | 32 | 2 | 0 | 32 | 2 | 0 | 28 | 5 | 1 |
There was 1 case of PR (treated by 48.0 GyE), 32 cases of NC, and 1 case of PD (treated by 48.0 GyE).
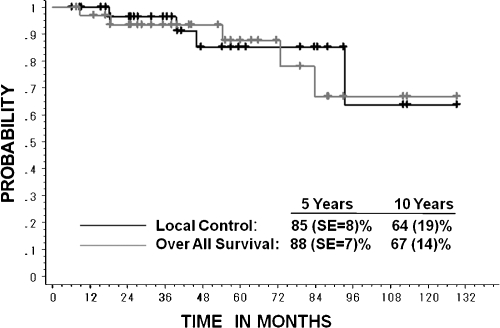
The local control rate was 85.1% (standard error [SE], 8%) at 5 years and 63.8% (SE, 19%) at 10 years (Fig. 2). Age, sex, KI, dose, and gross tumor volume (GTV) were not prognostic factors in local control. Nineteen patients who were treated with 60.8 GyE/16 fractions for 4 weeks showed no local recurrence at the time of analysis.
Figure 2.
Local control curve of 34 cases and overall survival curve of 33 patients.
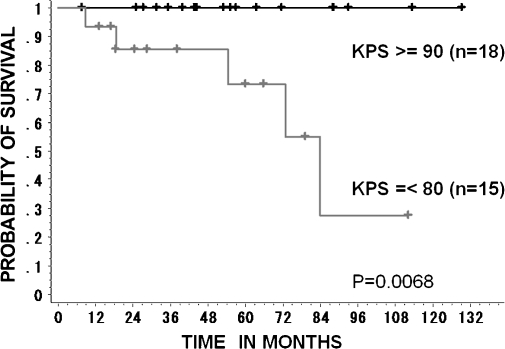
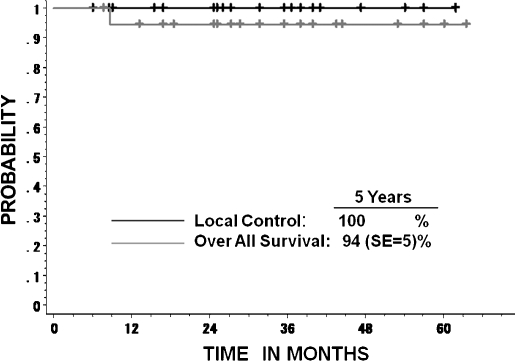
The overall survival rate of the 33 patients was 87.7% (SE, 7%) at 5 years and 67% (SE, 14%) at 10 years (Fig. 2). Five patients died: one due to local relapse (48.0 GyE), one from marginal relapse (48.0 GyE), one from lung metastasis (48.0 GyE), one because of distant cervical vertebrae metastasis (52.8 GyE), and one with interrupted liver dysfunction (60.8 GyE). The 5-year overall survival rate of 18 patients with KI ≥ 90% was 100%, and that of 15 patients with KI ≤ 80% was 73% (SE, 14%). There was statistical significance between the two groups (p = 0.0068; Fig. 3). None of the other factors, which included age, sex, dose, and GTV, were predictive of survival. Only 1 of 19 patients who were treated with 60.8 GyE/16 fractions for 4 weeks showed interrupted death from hepatic cell carcinoma at 9 months after CIRT (Fig. 4).
Figure 3.
Overall survival curves of 33 patients who were subdivided into two groups according to their Karnofsky performance score (KPS). There is statistical significance (p = .0068) between the two groups.
Figure 4.
Local control and overall survival of the patients who were treated with 60.8 GyE/16 fractions for 4 weeks.
DISCUSSION
From the results of the pilot study and phase I/II dose escalation study, the dose for the phase II study was decided as 60.8 GyE/16 fractions for 4 weeks. Preliminary results of 19 chordoma patients, who were treated by 60.8 GyE in phase I/II and II studies, showed acceptable normal tissue reactions. Maximum acute reaction of: (1) the skin was grade 1 in 7 out of 19 patients (37%), and (2) the mucosa was grade 2 in four patients (21%). In late reactions, no skin change was observed, and the maximum reaction of the mucosa was grade 1 seen only in one patient (5%) at the time of analysis. Late reactions of the brain were grade 1 in four patients (21%), which were detected by MRI and needed no medication, and grade 2 in one patient (5%), which needed short-time medication of steroid administration for his encephalitis with a headache.
The tumor control of 19 chordoma patients treated with 60.8 GyE showed excellent results, with no local recurrence and an overall survival rate of 94.4% (SE, 5.4%). Because the cause of death of one patient was interrupted liver dysfunction, the cause-specific survival rate was 100% at 5 years. Schulz-Ertner et al described GSI experience of CIRT for skull base chordomas.3 Target doses in excess of 60 GyE were associated with higher local control rates. In our study, observed normal tissue reaction and tumor control of the 60.8 GyE-treated patients were acceptable. The local control rate from our study is presently 100%; however, the follow-up periods were short. A careful and reliable follow-up study of skull base patients is needed for future determination of total dose escalation.
CONCLUSION
Skull base chordomas treated by CIRT with dose fractionation of 60.8 GyE/16 fractions for 4 weeks showed good local tumor control. With multiportal irradiation, normal tissues showed a mild reaction, and no severe morbidity of important organs occurred.
REFERENCES
- Salisbury J R. The pathology of the human notochord. J Pathol. 1993;171:253–255. doi: 10.1002/path.1711710404. [DOI] [PubMed] [Google Scholar]
- Debus J, Hug E B, Liebsch N J, et al. Brainstem tolerance to conformal radiotherapy of skull base tumors. Int J Radiat Oncol Biol Phys. 1997;39:967–975. doi: 10.1016/s0360-3016(97)00364-7. [DOI] [PubMed] [Google Scholar]
- Schulz-Ertner D, Karger C P, Feuerhake A, et al. Effectiveness of carbon ion radiotherapy in the treatment of skull-base chordomas. Int J Radiat Oncol Biol Phys. 2007;68:449–457. doi: 10.1016/j.ijrobp.2006.12.059. [DOI] [PubMed] [Google Scholar]
- Feuvret L, Noel G, Weber D C, et al. A treatment planning comparison of combined photon-proton beams versus proton beams-only for the treatment of skull base tumors. Int J Radiat Oncol Biol Phys. 2007;69:944–954. doi: 10.1016/j.ijrobp.2007.07.2326. [DOI] [PubMed] [Google Scholar]
- Vargas C, Fryer A, Mahajan C, et al. Dose-volume comparison of proton therapy and intensity-modulated radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:744–751. doi: 10.1016/j.ijrobp.2007.07.2335. [DOI] [PubMed] [Google Scholar]
- Munzenrider J E, Liebsch N J. Proton therapy for tumors of the skull base. Strahlenther Onkol. 1999;175(Suppl. 2):57–63. doi: 10.1007/BF03038890. [DOI] [PubMed] [Google Scholar]
- Igaki H, Tokuue K, Okumura T, et al. Clinical results of proton beam therapy for skull base chordoma. Int J Radiat Oncol Biol Phys. 2004;60:1120–1126. doi: 10.1016/j.ijrobp.2004.05.064. [DOI] [PubMed] [Google Scholar]
- Noël G, Feuvret L, Calugaru V, et al. Chordomas of the base of the skull and upper cervical spine. One hundred patients irradiated by a 3D conformal technique combining photon and proton beams. Acta Oncol. 2005;44:700–708. doi: 10.1080/02841860500326257. [DOI] [PubMed] [Google Scholar]
- Weber D C, Rutz H P, Pedroni E S, et al. Results of spot-scanning proton radiation therapy for chordoma and chondrosarcoma of the skull base: the Paul Scherrer Institut experience. Int J Radiat Oncol Biol Phys. 2005;63:401–409. doi: 10.1016/j.ijrobp.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Mendenhall W M, Mendenhall C M, Lewis S B, et al. Skull base chordoma. Head Neck. 2005;27:159–165. doi: 10.1002/hed.20144. [DOI] [PubMed] [Google Scholar]
- Jereczek-Fossa B A, Krengli M, Orecchia R. Particle beam radiotherapy for head and neck tumors: radiobiological basis and clinical experience. Head Neck. 2006;28:750–760. doi: 10.1002/hed.20448. [DOI] [PubMed] [Google Scholar]
- Terahara A, Niemierko A, Goitein M, et al. Analysis of the relationship between tumor dose inhomogeneity and local control in patients with skull base chordoma. Int J Radiat Oncol Biol Phys. 1999;45:351–358. doi: 10.1016/s0360-3016(99)00146-7. [DOI] [PubMed] [Google Scholar]
- Kanai T, Endo M, Minohara S, et al. Biophysical characteristics of HIMAC clinical irradiation system for heavy-ion radiation therapy. Int J Radiat Oncol Biol Phys. 1999;44:201–210. doi: 10.1016/s0360-3016(98)00544-6. [DOI] [PubMed] [Google Scholar]
- WHO Handbook for Reporting the Results of Cancer Treatment. Definitions of Objective Response. Geneva: World Health Organization; 1979. pp. 23–25. Offset Publication No. 48.
- Kaplan E L, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]