Summary
In eye development the tasks of tissue specification and cell proliferation are regulated, in part, by the Pax6 and Pax6(5a) proteins respectively. In vertebrates, Pax6(5a) is generated as an alternately spliced isoform of Pax6. This stands in contrast to the fruit fly, Drosophila melanogaster, which has two Pax6(5a) homologs that are encoded by the eyegone and twin of eyegone genes. In this report we set out to determine the respective contributions that each gene makes to the development of the fly retina. Here we demonstrate that both eyg and toe encode transcriptional repressors, are expressed in identical patterns but at significantly different levels. We further show, through a molecular dissection of both proteins, that Eyg makes differential use of several domains when compared to Toe and that the number of repressor domains also differs between the two Pax6(5a) homologs. We predict that these results will have implications for elucidating the functional differences between closely related members of other Pax subclasses.
Keywords: Pax6(5a), eyegone, twin of eyegone, retina, eye, Drosophila
Introduction
Pax6 genes play an indispensable role in the development of wide range of retinal systems. Mutations within Pax6 orthologs lead to severe retinal abnormalities in humans, mice and flies (Ton et al., 1991; Hill et al., 1991; Quiring et al., 1994). In contrast forced expression of Pax6 is sufficient to rewrite the developmental program of non-retinal tissues thereby producing an ectopically situated eye (Halder et al., 1995a). Furthermore, the universal presence of Pax6 in all seeing animals examined so far has underscored its importance in eye development and sparked a rethinking of the evolutionary origins of the eye (Halder et al., 1995b; Gehring 2002; Gehring 2005). As a consequence Pax6 has turned into one of the best-studied members of the paired box (Pax) family of transcription factors (Gehring, 1996; Gehring and Ikeo, 1999; Pichaud and Desplan, 2002).
Pax6, like all other Pax proteins, contains a PAIRED DNA binding domain (PD) which itself is comprised of two functionally separable helix-turn-helix motifs, the PAI and the RED domains (Noll, 1993; Jun and Desplan, 1996). In addition Pax6 contains a third nucleic acid recognition motif, the homeodomain (HD). The composition and structure of Pax6 provides for considerable flexibility in its interactions with nucleic acids thereby allowing for the combinatorial use of three functionally independent DNA recognition domains. While vertebrates have only a single Pax6 gene, the fruit fly, Drosophila melanogaster, contains two Pax6 orthologs eyeless (ey) and twin of eyeless (toy). Both play central roles in the specification of the retina (Quiring et al., 1994; Halder et al., 1995; Cznery et al., 1999).
Alternate splicing of vertebrate Pax6 leads to the production of a second isoform, Pax6(5a). This isoform (1) lacks a functional PAI domain; (2) binds to DNA through its RED and HD; and (3) has a different PD binding specificity than canonical Pax6 (Walther and Gruss, 1991; Jaworski et al., 1997). In vertebrates Pax6 and Pax6(5a) appear to play different roles in retinal development. Pax6(5a) loss-of-function mutants have different phenotypes than those of Pax6. Likewise, overexpression of Pax6(5a) induces different developmental defects and patterns of gene expression than Pax6 (Duncan et al., 2000a,b; Chauhan et al., 2002a,b,c; Singh et al., 2002, Haubst et al., 2004).
Pax6(5a) is also found in Drosophila but, unlike vertebrates, does not result from alternate splicing of Pax6 but rather is encoded by two separate genes, eyegone (eyg) and twin of eyegone (toe). These genes arose from a relatively recent duplication event and together with vertebrate Pax6(5a) represent a novel subclass of Pax genes (Jun et al., 1998; Aldaz et al., 2003). Similar to vertebrates, Drosophila Pax6 and Pax6(5a) appear to play different roles in eye development. While ey and toy act primarily in retinal specification, the main function of eyg is to promote cell proliferation (Dominguez et al., 2004; Chao et al., 2004). Each isoform exerts its influence on development through different transcriptional mechanisms: Ey acts as an activator while Eyg has the unique property of acting as a dedicated repressor (Punzo et al., 2001, 2004; Yao and Sun, 2005).
The Pax6 genes in Drosophila do not play completely redundant roles in development. There are some differences in the expression patterns of the two genes (Quiring et al., 1994; Cznery et al., 1999; Kammermeier et al., 2001). As a result ey and toy loss and gain-of-function mutants have some significant phenotypic differences (Kammermeier et al., 2001). Even within the eye specification hierarchy toy appears to sit atop ey (Cznery et al., 1999; Kronhanm et al., 2002). Interestingly, there are also disparities between the abilities of the two genes to direct eye formation in non-retinal tissues (Halder et al., 1995a; Cznery et al., 1999; C. Salzer and J. Kumar unpublished data). Differences in the activities of individual DNA recognition domains and protein-protein interaction motifs account for these many distinctions (B. M. Weasner and J. Kumar unpublished data).
Since the Drosophila genome encodes two Pax6(5a) genes we set out to determine if there are disparities between the roles that eyg and toe play in eye development. We will show that eyg and toe are expressed in identical patterns in the eye but eyg mRNAs account for the vast majority of Pax6(5a) transcripts. A comparison of the effects that loss of each gene has on eye development demonstrates that eyg and toe are differentially required in the retina. We have gone on to show that while Toe, like Eyg, is a transcriptional repressor, the number of repressor domains is different. And finally, we demonstrate that each Pax(5a) protein makes use of a unique combination of domains during normal eye development and extra eye field induction. Together, these results suggest that although eyg and toe arose through a recent duplication event, the two Pax6(5a) proteins likely play non-redundant roles in the eye and exert their influence on retinal development through differential use of combinations of protein domains.
Materials and Methods
Fly Stocks, Reagents and Microscopy
The following stocks were used in this study: eyg[1], eyg[22-2], eyg[M3-12], wg[W11]-GAL4, eyg-GAL422-2, tub-GAL4, ey-GAL4, GMR-GAL4, dpp-GAL4, CD-Gal4, UAS-ey, UAS-toy, UAS-so, UAS-optix, UAS-eya, UAS-GFP, wg-lacZ and an additional 220 GAL4 lines from the Bloomington Drosophila Stock Center (details of these stocks are available upon request). The eyg-Gal422-2 (also referred to as EM458) carries a P[GawB] insertion 527bp upstream of the eyg transcript. It is homozygous viable and has no visible phenotype on its own (Jang et al., 2003). CD-Gal4 drives expression mimicking eyg expression (LHW and YHS, unpublished results). The following antibodies were used in this study: rat anti-ELAV, mouse anti-Eyg (gift of Ntalia Azpiazu), IgG conjugated Cy3. Adult eyes were prepared for scanning and light microscopy as essentially described in Kumar et al 1998. Developing imaginal discs, salivary glands and embryos were prepared for light and confocal microscopy as essentially described in Yao and Sun, 2005 and Jang et al., 2003.
Generation of Eyg and Toe Protein Variants
Schematic drawings of Eyg deletion, Toe deletion and Eyg/Toe chimeric proteins are diagramed in Figure 9. An alignment of the Eyg and Toe proteins, along with a demarcation of the individual domains, is provided within the Supplemental Data Section Figure S1). Our full length Eyg protein is 525 amino acids in length and represents the shortest functional isoform. Each protein domain was originally defined by Jun and Desplan, 1996. The N-terminal (NT) region consists of residues 1-13, the PD domain consists of residues 14-104, the B region contains residues 105-231, the HD domain contains residues 232-291 and the C-terminal (CT) region contains residues 292-525. The N-terminal deletion (Eyg ΔNT) contains amino acids 14–525, the paired domain deletion construct (Eyg ΔNT+PD) contains amino acids 105–525, the B domain deletion construct (Eyg ΔB) contains amino acids 1–104 fused to residues 232–525, the homeodomain deletion construct (Eyg ΔHD) contains amino acids 1–231 fused to 292–525 and the C-terminal deletion construct (Eyg ΔCT) contains amino acids 1–291. In addition we made two multiple domain deletion constructs. The combined N and C terminal deletion constructs (Eyg ΔNT+CT) contains amino acids 14–291 and the triple N terminal, B domain and C-terminal deletion construct (Eyg ΔNT+B+CT) contains amino acids 14–104 fused to residues 232-291.
Figure 9. Functional assay of domains in Eyg and Toe.
(A–C) Schematic of Eyg deletions, Toe deletions and Eyg/Toe chimeric proteins. (D) Summary of Eyg and Toe domain requirements in rescue and overexpression assays. (E) Summary of results from rescue of eyg loss-of-function mutants and extra eye field induction assays.
Our full length TOE protein is 640 amino acids in length. The N-terminal region consists of residues 1-142, the PD domain consists of residues 143-233, the B region consists of 234–383, the HD domain consists of residues 384-443 and the C-terminal region consists of residues 444-640. The N-terminal deletion (Toe ΔNT) contains amino acids 143–640, the paired domain deletion construct (Toe ΔNT+PD) contains amino acids 234–640, the B domain deletion construct (Toe ΔB) contains amino acids 1–233 fused to residues 384-640, the homeodomain deletion construct (Toe ΔHD) contains amino acids 1–383 fused to 444–640 and the C-terminal deletion construct (Toe ΔCT) contains amino acids 1–443. In addition we made two multiple domain deletion constructs. The combined N and C terminal deletion constructs (Toe ΔNT+CT) contains amino acids 143–443 and the triple N terminal, B domain and C-terminal deletion construct (Toe ΔNT+B+CT) contains amino acids 143–233 fused to residues 384-443.
We made a series of chimeric proteins in which single or multiple domains of Eyg were replaced with the corresponding domains of Toe. The Eyg/Toe NT chimera contains amino acids 1–142 of TOE fused to residues 14-525 of Eyg, the Eyg/Toe PD chimera was generated by replacing the PD of Eyg with amino acids 143–233 from Toe, the Eyg/Toe B chimera was generated by replacing the B domain of Eyg with amino acids 234–383 of Toe, the Eyg/Toe HD chimera was generated by replacing the HD of Eyg with amino acids 384–443 of Toe and the Eyg/Toe CT chimera contains amino acids 1–291 of Eyg fused to residues 444-640 of Toe. The Eyg/Toe NT+CT chimera was generated by replacing the NT and CT regions of Eyg with amino acids 1–142 and 444–640 of Toe while the Eyg/Toe PD + HD chimera was generated by replacing the PD and HD of Eyg with amino acids 143–233 and 384–443 of Toe.
With the exception of three constructs (Eyg ΔNT+PD, ΔHD and Eyg ΔCT) each of the remaining deletion and chimeric constructs are new and novel lines. The Eyg ΔNT+PD, Eyg ΔHD and Eyg ΔCT constructs, while independently generated, are similar to those described in Yao and Sun, 2005. The results reported here, using these lines, are in agreement with those in the earlier report.
Real-time PCR
Total RNA was isolated from 50 third instar larval eye-antennal discs. 1μg of total RNA was reverse transcribed using the First Strand cDNA Synthesis Kit (Roche). 1μl of the RT reaction was amplified using the SYBR Green PCR Master Mix (Roche Diagnostics, Germany) according to its manufactures instructions. mRNA was quantified using a LightCycler (Roche Diagnostics, Germany). Thermocycling conditions were 95°C for 10 min and 45 cycles of [95 °C/10 sec.; 60 °C/5 sec.; 72 °C/9 sec.]. Fluorescence was detected at the end of the extension phase. Melting curve analyses were performed at the end of the amplification to confirm the specificity of the amplified products and lack of primer dimers. The expected lengths of amplified products were verified in gel electrophoresis and sequenced. Quantified PCR was performed using the eyg-specific primer set (5′-AGGCAAGAGTTCAGGTGTGG-3′ and 5′-CAACGGCTGCTGAGGTG-3′) and toe-specific primer set (5′-GGCCAGGGTGCAGGTT-3′ and 5′-TTGCTGGTGCTGTACGGATA-3′), respectively.
Absolute quantification of transcripts
Two stand curves for eyg and toe, respectively, were generated using serial dilutions of a known amount of the corresponding cDNA. Plasmids containing eyg cDNA and toe cDNA were linearized. The copy number was estimated by optical density according to the molar mass derived from the plasmid size. Different dilutions were made to obtain 102, 103, 104, 105 and 106 copies in 1 μL. All standards were amplified in duplicates. Target mRNA copy numbers were calculated based on the standard curves generated by LightCycler software Version 4.05. The same cDNA samples were examined in five independent PCR. When comparing different cDNA samples, the fluorescence intensity was normalized to ribosomal gene rp49. The primers for rp49 were 5′-TACAGGCCCAAGATCGTGAA-3′ and 5′-ACGTTGTGC ACCAGGAACTT-3′.
toe miRNA generation
The 22 nucleotides (nt. 1464 to 1485) of the toe coding sequence (underlined in oligo 1 and 2) were selected as a target sequence. To generate the toemiRNAi construct, the below four long primers were synthesized. The PCR product of oligo 1 and 2 was amplified using oligos 3 and 4. The final PCR product was digested with EcoRI and NotI and then cloned into pUAST. [GGCAGCTTACTTAAACTTAATCACAGCCTTTAATGTGAAGCAGCCATATCCGTACCGCTAAGTTAATATACCATATC] – oligonucleotide 1 [AATAATGATGTTAGGCACTTTAGGTACGAAGCAGCCATATCCGTACAGCTAGATATGGTATATTAACTTAGCGG] – oligonucleotide 2 [GGCGAATTCATGTTTAAAGTCCACAACTCATCAAGGAAAATGAAAGTCAAAGTTGGCAGCTTACTTAAACTTAATCA] – oligonucleotide 3 [GGCGCGGCCGC ATCCAAAACGGCATGGTTATTCGTGTGCCAAAAAAAAAAAAAATTAAATAATGATGTTAGGCACTT] – oligonucleotide 4
in situ hybridization
Staged embryos and third instar larvae were prepared for in situ hybridization (detailed protocols are available upon request). Non-radioactive labeled antisense RNA probes were used for in situ hybridization (Tautz and Pfeifle, 1989). The pBluescript-SKII-lune plasmid was linearized and transcribed to generate eyg antisense RNA probes (Jun et al., 1998). The EST clone, pOT2a-toe was similarly transcribed to synthesize the toe antisense RNA probe. Both probes were denatured and hybridized to either embryos or imaginal discs. All hybridizations and washes were done at 65°C. Specific details of the RNA probes and hybridization conditions are available upon request.
Results
Expression of eyg and toe During Development
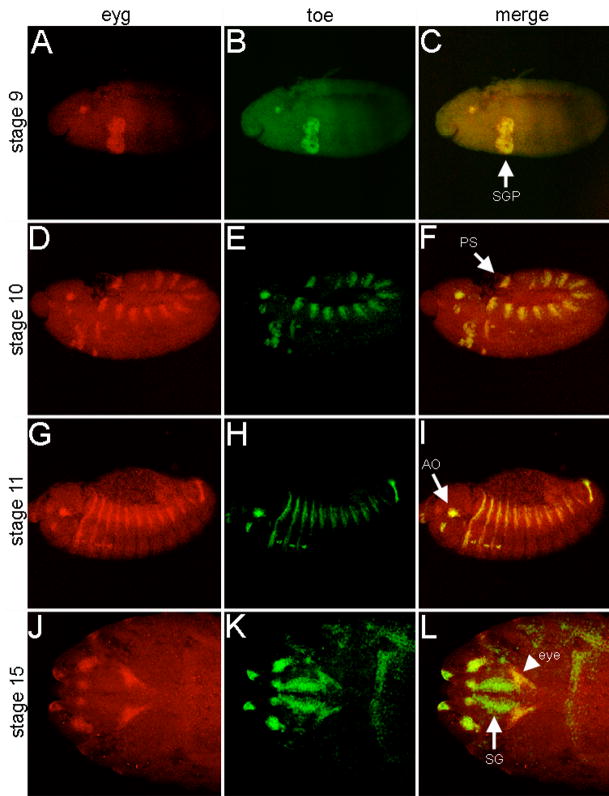
eyg transcripts are distributed within several embryonic tissues as well as the leg, wing and eye-antennal imaginal discs (Jones et al., 1998; Jun et al., 1998; Aldaz et al., 2003). Here we have characterized the expression pattern of toe and compare it to that of eyg (Figure 1). eyg and toe transcripts are first detected in stage 9 embryos within the salivary gland precursor (SGP) and a small cluster of cells within the dorsal head (Fig. 1A–C; Jones et al., 1998; Jun et al., 1998). The expression of toe transcripts in the SGP will persist through the rest of embryonic and larval development while eyg expression is terminated in late stage embryos and reinitiated later (Fig. 1D–I, Fig. 2J,K, Jones et al., 1998). By late stage 10 both transcripts are also found in identical patterns within the posterior spiracle (PS) and within a cluster of cells at the anterior edge of each thoracic and abdominal segment (Fig. 1D–F, Jones et al., 1998; Jun et al., 1998). Expression of eyg and toe expands to the larval antennal organ (AO) as well as the leg disc primordia by stage 12 (Fig. 1G–I, Jones et al., 1998; Jun et al., 1998). During the latter stages of embryogenesis both eyg and toe transcripts accumulate in the presumptive eye-antennal imaginal disc (Fig. 1J–L; Jones et al., 1998; Jun et al., 1998). Only two other members of the eye specification cascade, ey and toy, share this expression pattern (Quiring et al., 1994; Czerny et al., 1999). The remaining members are added sequentially during the larval development (Kumar et al., 2001). The only discernable difference between the expression patterns of either Pax6(5a) gene during embryogenesis is found within the SG: eyg expression is eliminated while toe transcriptional levels are maintained (Fig. 1J–L; Jones et al., 1998).
Figure 1. eyg and toe are expressed in nearly identical patterns during embryogenesis.
Transcriptional profile of eyg and toe during several stages of embryogenesis. Probes are listed at the top of each column. Embryonic stage is denoted at the right of each row. Note that the expression patterns are nearly identical except for the salivary glands in stage 15 embryos. SGP = salivary gland precursor, PS = posterior spiracle, AO = antennal organ, SG = salivary gland. Anterior is to the left.
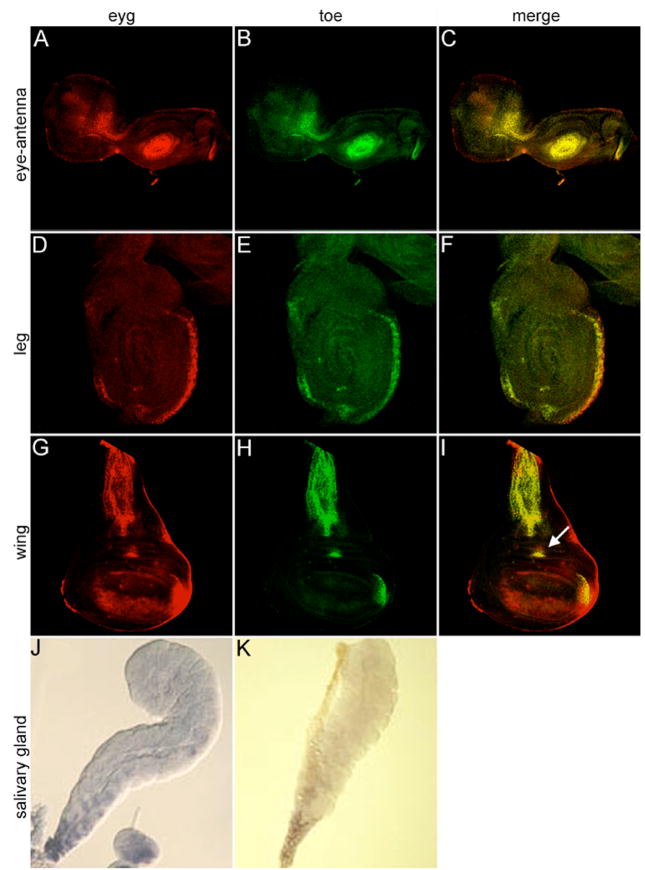
Figure 2. eyg and toe are expressed in nearly identical patterns in salivary glands and imaginal discs.
Transcriptional profile of eyg and toe during the third larval instar. Probes are listed at the top of each column. Tissue type is denoted at the right of each row. Note that the expression patterns are nearly identical. Arrow in panel I marks area of the wing disc in which ectopic eye development is supported by the expression of several retinal determination genes including ey.
Within the developing larval eye-antennal discs both eyg and toe transcripts accumulate in identical patterns. Within the antennal segment both transcripts localize to the medial and distal segments while in the eye disc expression of both genes is found anterior to the morphogenetic furrow (Fig. 2A–C, Dominguez et al., 2004). Unlike the similarities found in the embryo, eyg and toe expression is somewhat different from that of ey and toy. The Pax6 transcripts are expressed broadly ahead of the advancing furrow (Quiring et al., 1994; Cznery et al., 1999). However, eyg and toe expression is restricted to a narrow domain of cells that straddle the dorsal-ventral compartment boundary and does not extend laterally (Fig. 2A–C, Dominguez et al., 2004). This difference in expression is likely due to the requirements of eyg (and possibly toe) in Notch mediated control of cell proliferation at the organizing center versus the role of ey and toy in tissue specification. Within the developing wing primordium both transcripts are expressed broadly within the notum and in two discrete regions within the presumptive wing (Fig. 2D–F). It is interesting that one of those areas is particularly susceptible to being transformed into retinal tissue in response to forced expression of ey (Fig. 2F, arrow). Both eyg and toe transcripts are also found within identical patterns of the leg primordium (Fig. 2G–I) and the anterior duct cells of the salivary gland (Fig. 2J,K; Jones et al., 1998). The results from this and other studies of eyg and toe expression suggest at first glance that these genes may play redundant roles within several developing tissues including the compound eye. It is unlikely, however, that these genes play completely surplus roles (at least in the eye) as eyg loss-of-function mutants show near complete loss of retinal tissue and forced expression of toe is insufficient to restore eye development to these flies (see below).
Quantitative Contribution of eyg and toe mRNA Transcripts
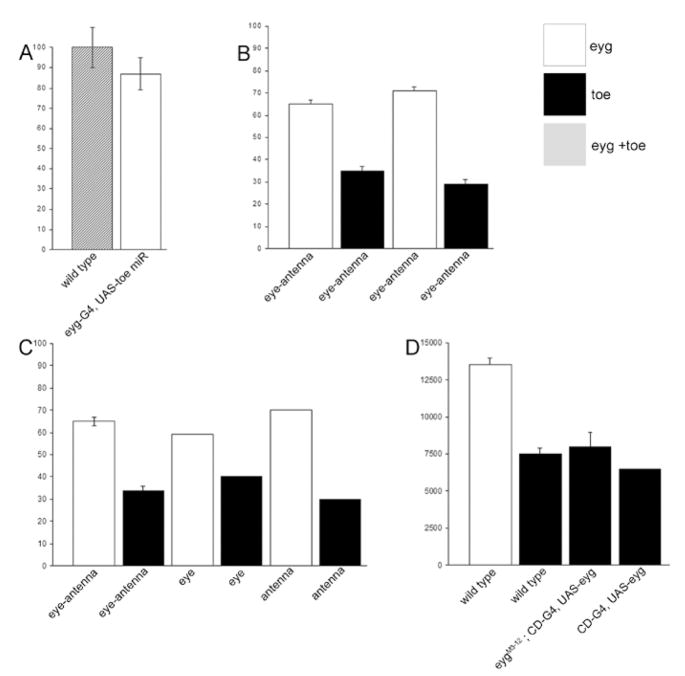
In order to further examine the contributions of eyg and toe to the development of the eye we used real-time PCR to measure the levels of each Pax6(5a) mRNA transcript in the eye-antennal disc (Figure 3). We measured the combined levels of eyg and toe in normal eye-antennal imaginal discs and compared it to discs in which we forcibly expressed a microRNA that is predicted to target and reduce the levels of toe mRNA transcripts. This method was employed because a toe specific loss-of-function mutation does not yet exist. Our results indicate that the vast majority (approximately 87%) of Pax6(5a) mRNAs are transcribed from the eyg locus (Fig. 3A). A direct comparison of eyg and toe levels in wild type eye-antennal discs confirms the unequal levels of Pax6(5a) expression (Fig. 3B). This relationship is maintained autonomously in individual eye and antennal discs (Fig. 3C). Finally, we set out to determine if the relative levels of toe are dependent upon eyg expression. In both eyg loss-of-function mutants and forced expression experiments the level of toe remained constant suggesting that toe levels are regulated independently of eyg (Fig. 3D). It appears that toe transcriptional regulation is also independent of eyg in several other tissues including the developing embryo and wing imaginal discs (Aldaz et al., 2003; Jang et al., 2003).
Figure 3. eyg is expressed at a higher level than toe in eye-antennal disc.
(A) Relative level of anti-Eyg/Toe immunostaining on third instar eye discs. The signal intensity in wild type (n=12) is taken as 100%. The signal intensity when Toe is efficiently knockdown (eyg-Gal4 driving UAS-toe miR; n=7) is reduced to about 87%. (B–D) Graphs depict the levels of eyg and toe in the developing eye and antenna as assayed by quantitative RT-PCR. White bars are eyg transcript level. Black bars are toe transcript level. (B) RT-PCR from eye-antennal disc from late third instar (left two bars) and second instar (right two bars). (C) When the eye and antenna discs were surgically separated, the eyg transcript is higher than toe in both eye and antennal discs. (D) Absolute eyg and toe transcript numbers were estimated from eye-antennal discs. toe transcript level was not significantly affected when eyg is overexpressed (CD-Gal4 driving UAS-eyg), indicate that toe is not transcriptionally regulated by eyg. eygM3-12 is a null mutation with a deletion beginning 23 bp upstream of eyg and extending 13 kb downstream of eyg transcription unit (Jang et al., 2003). Although the toe transcriptional unit is not affected, it is not clear whether toe expression is affected by the deletion. There is no eye-antennal disc in eygM3-12 to examine whether toe is affected. So we drove eyg expression by the CD-Gal4 in eygM3-12 to rescue the eye-antennal disc. In these rescued eye disc, toe transcript level is not significantly different from that in the wild type disc. This result clearly demonstrate that the eygM3-12 mutation affects only eyg.
An anti-Pax6(5a) Antibody Recognizes the PD of Eyg and Toe
An in vivo comparison of the roles played by the two Pax6(5a) genes has been hampered by the lack of available molecular markers for the distribution of Eyg and Toe proteins. To overcome this obstacle we generated a polyclonal antibody that recognizes full-length Eyg (data not shown). Since the antibody recognized a region of the eye disc in which both eyg and toe are expressed, and since both proteins share considerable sequence similarity within the DNA binding domains we wanted to determine the specificity of the anti-Eyg antibody. We expressed toe along the A/P axis of the wing disc using a dpp-GAL4 driver. Our antibody not only recognized endogenous Eyg, which is found within the hinge region, but it also recognized the exogenously added Toe protein along the A/P axis (Fig. 4A,B). Thus the antibody we recovered recognizes both Eyg and Toe and will be referred to as anti-Pax6(5a).
Figure 4. The Eyg/Toe antibody recognizes the PD of Eyg and Toe.
(A,B) wing discs. (C–I) eye-antennal discs. Arrowhead in all panels denotes endogenous Pax6(5a) expression in the wing and eye disc. Arrows in panels a and e indicate detection of exogenous Pax6(5a) protein. Genotype of each tissue is indicated in each panel. Note that wgw11 enhancer drives expression in the lateral regions of the eye disc ahead of the morphogenetic furrow. Anterior is to the right.
The DNA binding domains of Eyg and Toe (PD and HD) are likely epitopes for the anti-Pax6(5a) antibody as they share a high degree of sequence similarity between the two proteins. We set out to determine which one of these two domains the antibody recognizes. We expressed individual domains of the Pax6(5a) proteins along the margins of the eye disc using an enhancer of the wingless (wg) gene. The anti-Pax6(5a) antibody was then used to detect the expressed protein segments. In addition to the endogenous Pax6(5a) proteins, the antibody only detected the exogenously added Eyg PD (Fig. 4C–F, arrows). The antibody failed to recognize a mutant Eyg PD thus confirming the specificity of anti-Pax6(5a) to the PD (Fig. 4G). The Pax6(5a) PDs share 96% sequence similarity and the antibody fails to recognize the remaining regions of Toe (Fig. 4H; data not shown). A mutated version of the Toe PD is also not recognized by the antibody (Fig. 4H). Together, our results indicate that the anti-Pax6(5a) antibody recognizes the Eyg and Toe PDs.
As the anti-Pax6(5a) antibody recognizes both proteins we generated tagged proteins in which full-length Eyg and Toe are marked with the FLAG epitope tag in order to visualize each protein individually. When UAS-Eyg[flag] is combined with an eyg-GAL4 driver the distribution of the marked protein can be followed in the imaginal discs and salivary glands (data not shown). Unfortunately, as a toe-GAL4 line does not yet exist we are unable to specifically follow the distribution of Toe. However, combinatorial use of anti-Pax6(5a) with the Eyg[flag] is sufficient to differentiate between the distribution patterns of both proteins during normal and forced expression experiments.
Differential Requirement for eyg and toe in the Eye and Thorax
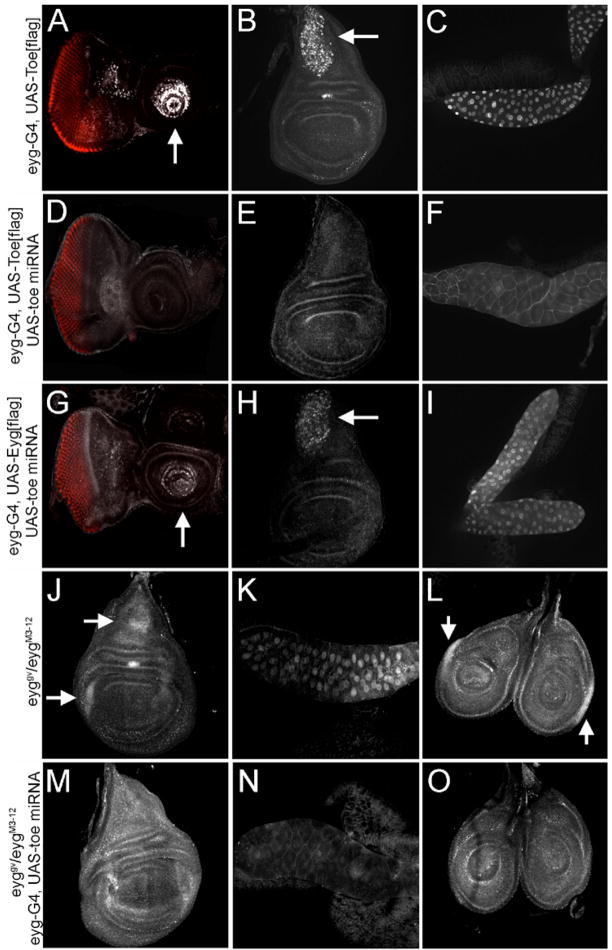
The absence of toe loss-of-function mutants has been another obstacle to clearly defining the contributions that each gene makes to retinal development. To complement the study of existing eyg loss-of-function mutant phenotypes we made use of a microRNA (miRNA) that targets toe mRNA transcript thereby reducing Toe protein levels and potentially substituting for toe loss-of-function mutants. If the miRNA is co-expressed with FLAG tagged version of either Pax6(5a) protein, only the Toe levels are eliminated (Fig. 5A–F). Additionally, only salivary gland defects that results from the overexpression of Toe-Flag, are reversed by the miRNA (Fig. 5C,F). Eyg[flag] levels remain unaffected (Fig. G–I). Furthermore, expression of the miRNA in severe eyg mutants eliminates endogenous Toe protein from both salivary glands and several imaginal discs (Fig. 5J–O). Note that the images in M-O have been overexposed to indicate that Toe protein cannot be detected in the nucleus. Together, these results indicate that the miRNA selectively targets toe transcripts.
Figure 5. An miRNA specifically knocked down Toe protein level.
(A,D,G) eye-antennal discs. (B,E,H,J,M) wing discs. (C,F,I,K,N) salivary glands. (L,O) leg discs. Genotypes of each tissue are indicated at the left of each row. Arrows in each panel indicate exogenous Toe or Eyg. Note that panels M–O have been overexposed to show that there is Toe protein is down regulated (detected by anti-Flag in A–I, by anti-Eyg/Toe in J–O). Also note that the morphology of the salivary gland is rescued when the toe miRNA is coexpressed with a full-length toe construct (panel f). The RED stain in A,D,G is Elav.
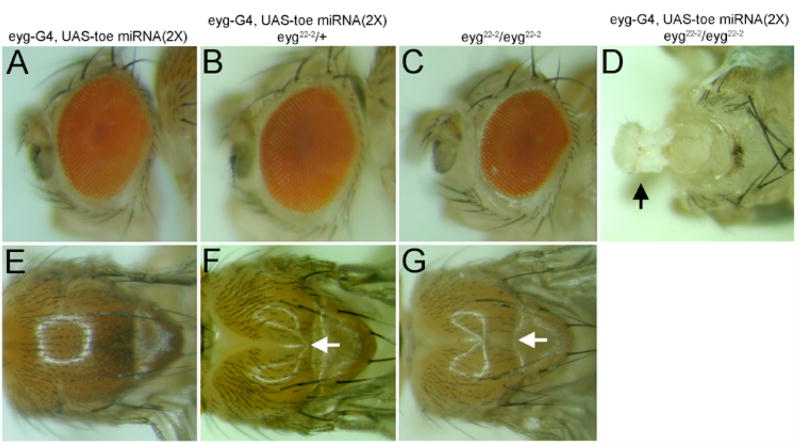
We then set out to determine what contribution, if any, to eye specification is made by toe. Using an eyg-GAL4 driver we expressed 2 copies of the toe miRNA in regions of the developing eye that normally express both Pax6(5a) proteins. Interestingly, we did not observe any discernable defects suggesting that although Toe levels are being eliminated in the retina, the endogenous levels of Eyg is sufficient to fully support eye development. The thorax, which also requires eyg, is similarly unaffected by the expression of the miRNA under control of either the eyg-GAL4 and/or tub-GAL4 (Fig. 6A,E). Since eyg and toe mRNA levels make up 87% and 13% of Pax6(5a) levels respectively we expressed the miRNA in flies heterozygous for an eyg null mutant. In this situation the eye remains unaffected but the anterior-central region of the thorax does not develop (a phenotype that is not observed in eyg heterozygotes). This is visibly manifested as a severe groove within the thorax (Fig. 6B,F). The failure to develop the anterior-central portions of the thorax is reminiscent of the effect of severely diminished levels of eyg (Fig. 6G, Aldaz et al., 2003). Interestingly, 50% reductions in Eyg protein levels are not enough to severely alter the structure of the eye (Fig. 6C). However, if eyg and toe levels are simultaneously compromised, then development of both the head and compound eyes is blocked (Fig. 6D). The thorax is much more sensitive to simultaneous reductions in Eyg and Toe levels than is the developing eye. Our results also suggest that removal of toe, on its own, has little to no effect on the development of either tissue. This is consistent with the minor contribution that the toe locus makes to the overall levels of Pax6(5a) mRNA (Fig. 3). These results suggest that Eyg and Toe proteins are differentially required in the eye and thorax.
Figure 6. toe and eyg are differentially required in the developing eye and thorax.
(A–D) adult eye and head. (E–G) adult thorax. Genotype of each tissue is indicated at the top of each column. Arrow in panel D indicates the near complete inhibition of eye and head development. Arrow in panels F and G denote defects in thorax development. Anterior is to the left.
Consistent with this hypothesis, we have noted 43 different situations in which expression of eyg and toe had differing phenotypic consequences (Supplemental Data - Table 1). For example, expression of eyg in the wing disc via a vg-GAL4 driver has no effect. However, expression of toe within the same domain leads to increased levels of cell death. Conversely, while expression of toe in the embryonic CNS and brain via the c768-GAL4 driver has no effect, expression of eyg leads to embryonic lethality. We have been able to exclude trivial explanations such as line strength and protein levels as reason for these disparities. Instead, our results further the contention that the Pax6(5a) proteins are differentially required during development. This contention is also supported by differential effects on the developing eye and wing in response to the expression of various Pax6(5a) deletion and chimeric proteins (Fig. 9; Supplemental Data - Table 2).
Toe is Transcriptional Repressor
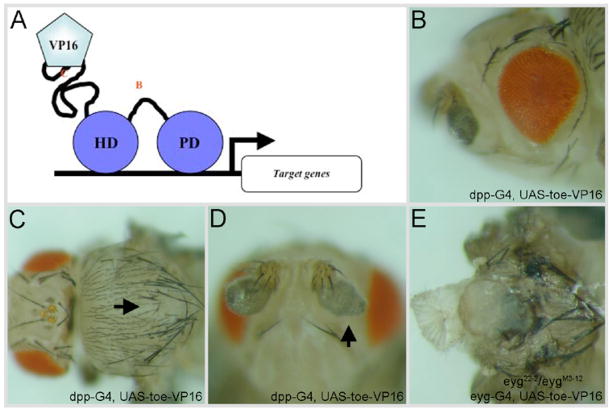
Using Eyg-VP16 (transcriptional activator) and Eyg-En (transcriptional repressor) protein fusions, eyg has been shown previously to encode a dedicated transcriptional repressor (Yao and Sun, 2005). Based on the evolutionary relationship between both Pax6(5a) genes, toe is also predicted to encode a transcriptional repressor. To test this hypothesis we created transgenic flies that expressed full-length Toe fused to the VP16 activation domain (Toe-VP16) along the A/P axis of several imaginal discs using a dpp-GAL4 driver. The activity of this transcriptional-activating form of Toe failed to mimic the activity of wild type Toe protein in several assays. In fact, in certain instances Toe-VP16 appeared to induce dominant-negative phenotypes. For instance, in contrast to Toe, which can induce extra eye fields, the expression of the transcriptional-activating form of Toe failed to promote and support eye development in a forced expression assay (compare Fig. 7A to 10B). Second, Toe-VP16 induced the formation of abnormal antennal structures and extra machrochaetes on the thorax, which are likely dominant negative effects (Fig. 7B,C). Another dominant-negative effect is seen when the expression of Toe-VP16 in eyg hypomorphic mutants leads to the production of “headless” flies (Fig. 7D,E). These phenotypes are reminiscent of the effects observed when either the toe miRNA or Eyg-VP16 is individually expressed within the same eyg mutant background (Fig. 6D, Yao and Sun, 2005). It should be noted that expression of the toe miRNA induced dominant negative phenotypes, such as the production of headless flies, only when the genetic background was compromised for eyg function. As these animals are headless and die as pharate adults we were unable to assay the effects that the toe miRNA had on macrochaetes numbers and antennal structure. The induction of dominant negative phenotypes by Toe-VP16 in an otherwise wild type background is likely due to the strong activation of putative Toe target genes via the VP16 activation domain. We also expect that Toe-VP16 would also activate some Eyg targets while the toe microRNA would only affect levels of Toe mRNA and protein. This might also contribute to the stronger Toe-VP16 phenotype. As the activity of Toe-VP16 closely mimics that of Eyg-VP16 (Yao and Sun, 2005) our results are suggestive that Toe, like Eyg, functions as a transcriptional repressor.
Figure 7. Toe is a transcriptional repressor.
(A) schematic of VP16 fusion assay. (B–E) adult eye, thorax, antenna and head. Genotypes of each tissue are indicated at the top of each column. Arrows in panels C and D mark defects in thorax and antennal development. Anterior is to the left.
Figure 10. Rescue of eyg1 loss-of-function mutants by expression of Eyg and Toe protein variants.
Scanning electron micrographs of adult compound eyes. Genotypes of each animal are listed within each panel. All UAS lines were expressed using an ey-GAL4 driver. Anterior is to the right.
Further evidence of Toe serving as a repressor comes from the partial rescue of the eyg loss-of-function retinal phenotype by expression of a chimeric protein in which Toe is fused to the Engrailed repressor (Toe-EN, data not shown). It should be noted that expression of Toe-EN failed to induce extra eye fields along the ventral surface of the head. There are two plausible explanations for these relatively mild effects. First, the Toe-EN construct may not be expressed at high enough levels to either fully rescue eyg mutants or induce extra eye fields. We think that this is unlikely as the EN domain that is fused to TOE is a strong transcriptional repressor and high levels of the fusion protein are not predicted to be required in this assay. Instead it may be that during normal development Toe functions as a repressor on some target genes and as an activator on others. Unlike Eyg, Toe may not function as a dedicated repressor. Instead it may have multiple functions with repression being one of its activities. This could account for several of the observed difference in the activities of the Pax6(5a) homologs (see below).
Mapping of the Toe Repressor Domains
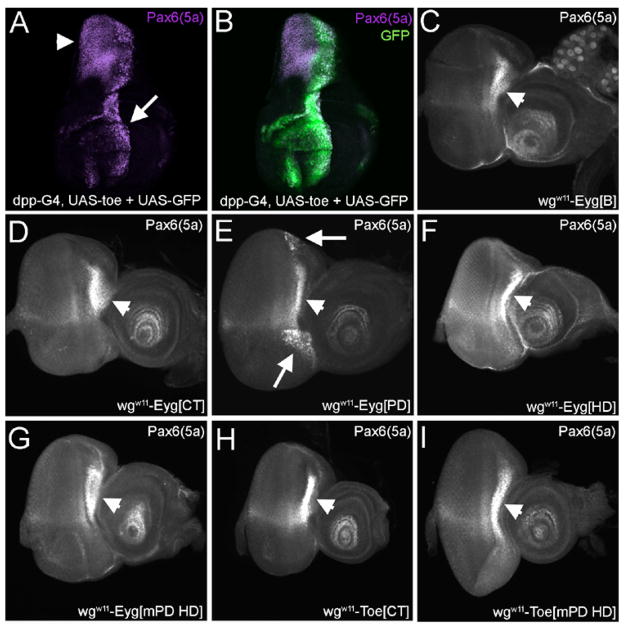
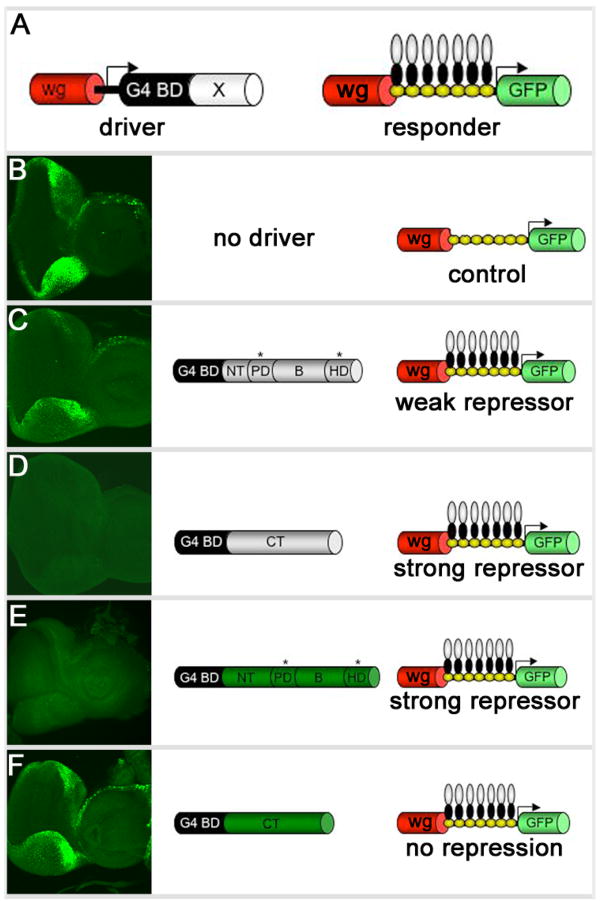
We set out to determine if the repressor domain(s) map to similar locations within Eyg and Toe. It has been previously demonstrated that Eyg contains two repressor domains: one maps to the CT region, the other is located in either the NT or the B regions of the protein (Yao and Sun, 2005; Fig. 8B–D). Expression of Eyg ΔB anterior to the furrow using an ey-GAL4 has a dominant negative effect on eye development (data not shown). Often times these dominant negative effects can be attributed to the deletion of either an activation or repressor domain. Here we show that the repressor domain within Toe is not located within the CT tail and may reside within the NT and B domains. The assay used by Yao and Sun and here is a bipartite system. In one half of the system a chimeric protein in which the GAL4 DNA binding domain is fused to either a full length Pax6(5a) protein or an individual domain is expressed along the margins of the eye under the control of a wingless (wg) enhancer element. In the second half of the system the same wg enhancer directs the expression of a GFP reporter. A cluster of UAS sites separates the enhancer element from the reporter (Fig. 8A). In flies lacking the driver construct, GFP is expressed along the margins of the eye field (Fig. 8B). When a portion of Eyg containing a strong repressor (Eyg CT) is expressed in the same pattern, expression of the reporter is completely lost (Fig. 8C). If a weak or moderate repressor domain (Eyg ΔCT mPDHD) is expressed then a reduction in reporter activity is observed (Fig. 8D). If now we express just the CT of Toe along the margins we see normal levels of the GFP reporter indicating that this domain does not contain any repressor activity (Fig. 8E). However, if the N-terminal portion of Toe containing mutated PD and HDs is expressed then strong repression of the reporter is seen. The most likely explanation is that a strong repressor domain resides within either the NT or the B domains (Fig. 8F). Thus it appears that a major functional difference between the Eyg and Toe proteins is the number and location of the repressor domains.
Figure 8. Toe repressor activity resides in its N-terminal portion.
(A) Schematic of repressor assay. (B–D) Third instar eye-antennal discs accompanied by schematic of driver/responder combinations. Note that wg-GAL4 drives expression in the lateral margins ahead of the morphogenetic furrow. Anterior is to the right.
Molecular Dissection of Eyg and Toe During Normal Development
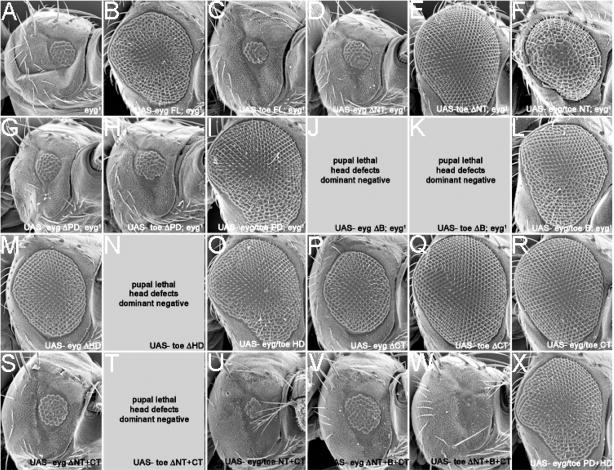
As Eyg and Toe are derived from the same ancestral gene, are expressed in identical patterns and function as transcriptional repressors, yet have differential effects on eye and thorax development, we conducted a molecular dissection of both proteins in an effort to understand the biochemical basis that underlies these unique functions. These experiments which are aimed at elucidating the differences between two Pax6(5a) proteins (Eyg and Toe) extend those of Yao and Sun, 2005 which focused on the functional differences between the activities of Eyg and Pax6 (Ey). An alignment of the Eyg and Toe proteins, along with a demarcation of the individual domains, is provided within the Supplemental Data Section. The reagents that we generated for our studies include a series of protein deletions in which individual or multiple domains of either Eyg or Toe were removed. These Pax6(5a) variants were used to test the functional requirements for each domain. We also generated a series of chimeric Pax6(5a) proteins in which single or multiple domains of Eyg were deleted and replaced with the corresponding regions of Toe. These chimeric proteins were used to test the degree to which each domain has been functionally conserved. Diagrams of the Pax6(5a) deletions and chimeras are depicted in Figure 9A–C. Each variant was assayed for the ability to rescue an eyg[1] loss-of-function mutant (Figs. 9,10) and to induce extra eye fields within ventral head segments (Figs. 9,11). It should be noted that for each deletion and chimera we tested multiple UAS insertion lines and conducted our experiments at several temperatures. We did this in an attempt to eliminate the possibility that our results are affected by expression levels or insert integrity. We also crossed each construct to several GAL4 lines to ensure that each deletion or chimeric protein was functional.
Figure 11. Induction of extra eye fields by the expression of Eyg and Toe variants.
Scanning electron micrographs of adult compound eyes and extra eye fields. Genotypes of each animal are listed within each panel. All UAS lines were expressed using a dpp-GAL4 driver. Yellow arrow in panel A indicates position of extra eye field. Anterior is to the right.
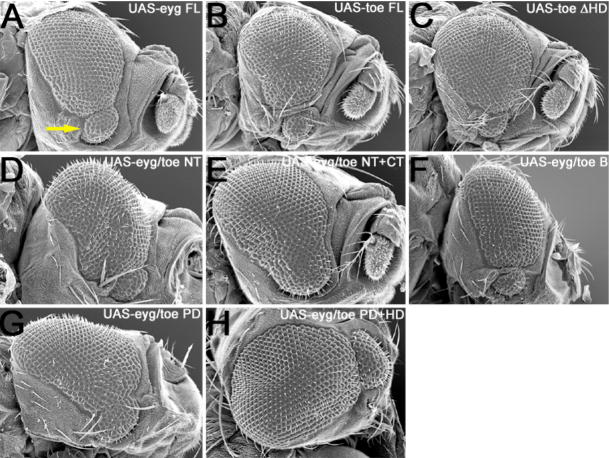
Both wild type and all variants of Eyg and Toe were expressed in eyg[1] homozygous mutant retinas, which contain between 40–50 ommatidia (Fig. 10A). Flies that are homozygous for eyg null alleles die during embryogenesis and are therefore not appropriate for this particular assay. Expression of wild type Eyg but not Toe is sufficient to return eyg[1] mutant retinas to near wild type structure suggesting that these genes have functionally diverged since the duplication (Fig. 9D, 10B,C). These genes are thus unlikely to play redundant roles in eye development.
Requirements for the non DNA-Binding Domains in Normal Eye Development
We first proceeded to test the functional requirements of the non DNA-binding domain. We started with deleting the sequences that lie upstream of the RED DNA binding domain in both proteins (Eyg ΔNT, Toe ΔNT). Toe ΔNT, but not Eyg ΔNT, restored eye development to eyg[1] mutant retinas (Fig. 9D, 10D,E) suggesting that not only is there a functional requirement for the NT region of both proteins but also that this region may functional distinguish the two Pax6(5a) proteins from each other. Surprisingly, expression of the Eyg/Toe NT chimera is also capable of rescuing eyg[1] (Fig. 9D, 10F). This result indicates that while the NT domain may functionally distinguish one Pax6(5a) from another, their function is still context dependent. Eyg contains two repressor domains, of which one lies within the first 443 amino acids of the protein (Yao and Sun, 2005). This region includes the NT, PD, B and HD as shown in Fig. 9A. Mutational analysis excluded the PD and HD regions thus leaving either the NT or B regions as possible sites for the repressor activity of Eyg. Our results raise the possibility that the repressor activity of Eyg resides within the NT region.
We then looked at the requirements for the B domain, a stretch of amino acids that lies between the PD and HD DNA binding motifs but is yet to be assigned a role in Pax protein function. Forced expression of constructs in which the B regions from Eyg and Toe were deleted individually or in combination with the NT and CT regions (Eyg ΔB, Toe ΔB, Eyg ΔNT+B+CT, Toe ΔNT+B+CT) had a dominant negative effect on eyg[1] flies; the heads were severely deformed, the retinas were not restored to wild type and the flies died in their pupal cases (Fig. 9D, 10J–K,V,X). In contrast, eyg[1] mutant retinas were restored to near wild type levels when region B of Eyg was replaced with homologous region from Toe (Eyg/Toe B; Fig. 9D, 10L). These results suggest that the B domain is functionally essential for Pax6(5a) activity and has been functionally conserved between the two transcription factors. This conclusion is supported by the observation, from a related set of experiments in which the Pax6 proteins Ey and Toy do not function normally in the absence of the B domain. Proteins lacking this domain produce ectopic eyes that are less frequently observed and are significantly smaller in size than those produced by the full-length proteins (B.M. Weasner and J.P. Kumar, unpublished data). There is the possibility, however, that region B simply acts as a linker or spacer for the two DNA binding motifs and that deleting this region from any Pax protein may disrupt the normal structural configuration as the RED and HD motifs are brought together. One could interpret the rescue of eyg[1] by Eyg/Toe B as simply the result of restoring the spacing between the DNA binding motifs. We think that is rather unlikely as a similar chimera in which the B domains of EY and TOY have been interchanged appear to have acquired new activities and do not simply function as the parental Pax6 protein (B.M. Weasner and J.P. Kumar, unpublished data).
The C-terminal tail (residues 3′ of the HD) of Eyg but not Toe contains a transcriptional repressor domain (Yao and Sun, 2005; this report). We deleted the CT region in an attempt to determine if this domain serves to functionally distinguish one Pax6(5a) protein from the other. Expression of Eyg ΔCT and Toe ΔCT fully restored eye development to eyg[1] mutant retinas (Yao and Sun, 2005; Figs. 9D, 10P,Q). While these results suggest that the CT is dispensable for Eyg function, it appears that the CT region is required for Toe activity. In fact, the results also suggest that there is a combinatorial interaction between the Toe NT and CT regions as the presence of both domains prevents Toe from rescuing the eyg mutants. Removal of either the NT or the CT is sufficient to then allow for rescue. These results are also intriguing as they suggest that the repressor domain within the CT of Eyg is not essential for its normal activity. Rather, it seems that the second repressor site, which is located in either the NT or B regions of the protein, is more essential to Eyg function. There appears, however, to be a genetic interaction between the NT and CT regions of both proteins. Expression of either Eyg ΔNT+CT or Toe ΔNT+CT was insufficient to support eye development in eyg[1] mutants (Figs, 9D, 10S,T). This is in contrast to the near full rescue of eyg[1] retinas that is observed when either NT or CT regions of Toe are individually removed (Fig. 9D, 10E,Q). Such interactions are also evidenced by the inability of Eyg/Toe NT+CT to rescue eyg[1] flies when expression of chimeras involving individual domains (Eyg/Toe NT, Eyg/Toe CT) is sufficient to restore eye development (Figs. 9D, 10F,R,U).
Requirements of the RED and Homeobox DNA Binding Domains in Normal Eye Development
We were also interested in determining if functional distinctions between Eyg and Toe could be accounted for by differences in the use and requirements of the RED and HD motifs. Eye development could be restored to eyg[1] mutants through expression of Pax6(5a) variants that in which the RED domain was interchanged but not deleted (Yao and Sun, 2005; Fig. 9D, 10G–I). These results suggest that both Eyg and Toe exert their influence on transcription through the RED domain and that these domains have been functionally conserved. We similarly deleted and substituted the HDs and observed that expression of EYG ΔHD and Eyg/Toe HD rescued the small eye phenotype of eyg[1] mutants while Toe ΔHD failed in this respect (Yao and Sun, 2005; Fig. 9D, 10M–O). These results indicate that in contrast to absolute requirement for the RED domain it appears that the Eyg HD is completely dispensable for eye development. As a consequence Eyg primarily uses its RED domain to interact with DNA. There are several precedents for this observation. Several Pax genes including Drosophila pox meso and mammalian Pax1 and Pax9 completely lack the HD (Noll, 1993; Mansouri et al., 1999). Second, during eye development the HD of EY/Pax6 is also dispensable as an EY protein lacking the HD is sufficient to rescue loss-of-function ey mutants (Punzo et al., 2004). These results do not speak to the requirements of the Toe HD since the Toe full-length protein also failed to rescue. However, data presented below on the generation of extra eye fields indicates that Toe also does not make use of the HD (see below).
A large body of evidence indicates that a considerable degree of flexibility exists for the combinatorial use of DNA binding motifs by Pax proteins. We attempted to test the extreme limits of this feature by simultaneously replacing both the RED and HD regions of Eyg with the corresponding domains of Toe. Surprisingly expression of the Eyg Toe PD+HD chimera rescued the structural defects of eyg[1] mutants (Figs. 9D, 10X). This result provides further evidence that the contextual framework motifs provided by the remaining non-DNA binding regions can influence how certain combinations of DNA binding domains are used during development.
Domain Requirements for Extra Eye Field Induction
We set out to determine if Toe, like Eyg, is only capable of inducing extra eye fields adjacent to the developing endogenous retinal epithelium (as opposed to ectopic eye formation in other non-retinal tissues). We expressed each Pax6(5a) gene within 219 different developmental patterns and looked for the presence of retinal tissue. In the case of the full-length Eyg and Toe proteins, we were only able to induce extra eye fields adjacent to the normal compound eye (Fig. 11A,B; Jang et al., 2003). We were interested in determining if the domain requirements for the generation of extra eye fields are the same as those needed for the promotion of normal eye development. There is a precedent for the two processes requiring different protein domains. The activity of the CT regions of the SIX family proteins Sine Oculis and Optix (results of an ancient duplication) is an example. These regions are not interchangeable during normal eye development and in fact are thought to confer, in part, functional specificity upon SIX proteins. However, this is not the case for ectopic eye generation. The CT domains are in fact interchangeable under these conditions (Weasner et al., 2007). This result suggests that there are different molecular and biochemical requirements for normal and ectopic eye formation.
Of all the deletion constructs only the Toe ΔHD, in which the HD has been deleted, is capable of promoting the formation of an extra eye field (Fig. 11C). This implies that each domain of Eyg and all but the HD of Toe are required. This stands in contrast to our rescue assays in which the HD and CT regions of Eyg are dispensable for normal eye formation (Fig. 10M,P). Other differences were observed when the chimeric proteins were used to induce extra eye fields. In these cases the NT, B and PD domains can be individually substituted. Certain domain combinations (Eyg/Toe NT+CT and the Eyg/Toe PD+HD) could also be substituted successfully (Fig. 11D–H). Again, these requirements are dissimilar from those needed for Eyg and Toe to function properly during normal eye development. Our rescue assays concluded that all individual domains and only the PD+HD combination could be exchanged and still rescue eyg[1] mutants (Fig. 10F,I,L,O,R,X). As mentioned earlier, similar differences in domain requirements during normal eye development and ectopic eye formation (or extra eye field generation) are observed with other eye specification genes. These apparent disparities may reflect actual differences in the protein-protein interactions that occur between eye specification proteins and their cofactors. Such a model might imply that there is some flexibility in the path to producing an eye.
Discussion
In eye development the tasks of tissue specification and cell proliferation are regulated, in part, by the Pax6 and Pax6(5a) proteins respectively. In vertebrates, Pax6(5a) is generated as an alternately spliced isoform of Pax6. However, in Drosophila Pax6(5a) homologs are encoded by the eyegone and twin of eyegone genes. In this report we sought to determine the respective contributions that each gene makes to the specification of the fly. An initial analysis of transcriptional patterns indicates that both Pax6(5a) genes are expressed in identical patterns within the retina. However, eyg is expressed at a much higher level than toe. Not surprisingly, while mutations in eyg nearly delete the eye, a reduction in toe via miRNA treatments has no effects on its own. Simultaneous reductions in both genes, in contrast, result in a “headless” phenotype. Using a set of mini genetic screens and activator/repressor fusion assays we also demonstrated that both proteins function as transcriptional repressors. In total, these characteristics suggest that eyg and toe might play redundant roles in during development.
However, the high level of sequence divergence within the non-DNA binding domains hints that their functions may only be partially redundant. We set out to molecularly dissect both Pax6(5a) proteins and determine what, if any, differences exist between the activities of each protein. In two experimental contexts we were able to demonstrate that such differences between eyg and toe exist. First, a comparison of eyg and toe loss-of-function phenotypes indicated that toe played a greater role in the development of the thorax than the eye. Second, forced expression of both full-length proteins throughout the developing fly identified 43 different instances in which expression of one Pax6(5a) gene induced a different phenotype than the other. Taken together, these results hint that the roles of eyg and toe may be not be completely redundant.
We then set to determine which domain(s) might account for the differences seen in loss-of-function mutants and forced expression assays. We generated a set of deletion and chimeric proteins to dissect the requirement for each domain as well as the level of functional conservation. We attempted to rescue eyg1 mutants as well as generate extra eye fields with these protein variants. Our results indicate that Eyg and Toe make differential use of several domains. Many of these differences map to the non-DNA binding domains. We have also demonstrated that one possible mechanism for this is that Toe has only one repressor domain, while Eyg has two. Our prediction is that the differences in the non-DNA binding domains are the primary determinants of how each Pax6(5a) protein will influence development. It is less likely that the two DNA binding domains functionally distinguish one protein from another as there is an extremely high level of sequence conservation within these motifs. Thus our model for how Eyg and Toe Function is that both transcription factors bind to similar target genes but can differentially influence transcription through differing levels of repressor activity and/or interactions with disparate binding partners (Fig. 12A,B).
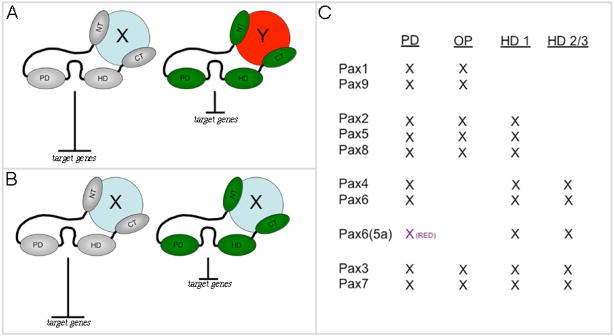
Figure 12. Putative models for Pax6(5a) activity and structure of Pax subclasses.
(A,B) putative models. In model A the difference in the strength of repression is due to the interaction of Eyg and Toe with different binding partners. In model B these strength differences are simply due to different protein levels (binding partner is the same in this model). Note that the relative strength of repression can vary between the eye and thorax. (C) Table of mammalian Pax genes with a list of putative domains. PD = paired domain, HD = homeodomain, OP = octapeptide, HD1-3 refers to the three alpha helices.
These results may have broad implications for the activities of other Pax genes in both Drosophila and vertebrates. The fly genome contains two Pax6 genes, eyeless (ey) and twin of eyeless (toy), both of which also arose through a relatively recent duplication. Both share high degrees of homology within the DNA binding domains while having significantly lower levels of sequence conservation in the non-DNA binding regions (R. Datta and J.P. Kumar, unpublished data). Functionally, Ey and Toy have differing abilities to induce eye formation when expressed in non-retinal tissues (Halder et al., 1995; Cznery et al., 1999). Some of these differences have been attributed to the C-terminal tail section of each protein (Punzo et al., 2004).
Mammalian Pax genes are grouped, in part, according to their structure (Fig. 12C). Individual classes are defined by the presence or absence of the octapeptide and the two DNA recognition (Paired and Homeobox) motifs. Like the fly genes, members within each Pax subclass share a very high degree of sequence conservation within the DNA binding domains thus they are likely to bind to very similar targets. Our results, if extended to these other Pax genes, would suggest that their activity could be distinguished by examining the localization of activation and repressor domains as well as the use of different binding partners.
Supplementary Material
Acknowledgments
We thank Cheng-ting Chien, Gwo-Jen Liaw, Peter Cherbas and Thom Kaufman for valuable suggestions. We thank Chun-Lan Hsu and Yu-Chi Yang for preparing fly food and maintaining fly stocks. This work was supported by grants to Y.H.S from the National Science Council (NSC93-2321-B-001-010 and 94-2321-B-001 -011) of the Republic of China and a grant from the National Institutes of Health (R01 EY014863) to J.P.K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aldaz S, Morata G, Azpiazu N. The Pax-homeobox gene eyegone is involved in the subdivision of the thorax of Drosophila. Development. 2003;130:4473–82. doi: 10.1242/dev.00643. [DOI] [PubMed] [Google Scholar]
- Chao JL, Tsai YC, Chiu SJ, Sun YH. Localized Notch signal acts through eyg and upd to promote global growth in Drosophila eye. Development. 2004;131:3839–47. doi: 10.1242/dev.01258. [DOI] [PubMed] [Google Scholar]
- Chauhan BK, Reed NA, Yang Y, Cermak L, Reneker L, Duncan MK, Cvekl A. A comparative cDNA microarray analysis reveals a spectrum of genes regulated by Pax6 in mouse lens. Genes Cells. 2002a;7:1267–83. doi: 10.1046/j.1365-2443.2002.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan BK, Reed NA, Zhang W, Duncan MK, Kilimann MW, Cvekl A. Identification of genes downstream of Pax6 in the mouse lens using cDNA microarrays. J Biol Chem. 2002b;277:11539–48. doi: 10.1074/jbc.M110531200. [DOI] [PubMed] [Google Scholar]
- Chauhan BK, Zhang W, Cveklova K, Kantorow M, Cvekl A. Identification of differentially expressed genes in mouse Pax6 heterozygous lenses. Invest Ophthalmol Vis Sci. 2002c;43:1884–90. [PMC free article] [PubMed] [Google Scholar]
- Czerny T, Halder G, Kloter U, Souabni A, Gehring WJ, Busslinger M. twin of eyeless, a second Pax-6 gene of Drosophila, acts upstream of eyeless in the control of eye development. Mol Cell. 1999;3:297–307. doi: 10.1016/s1097-2765(00)80457-8. [DOI] [PubMed] [Google Scholar]
- Dominguez M, Ferres-Marco D, Gutierrez-Avino FJ, Speicher SA, Beneyto M. Growth and specification of the eye are controlled independently by Eyegone and Eyeless in Drosophila melanogaster. Nat Genet. 2004;36:31–9. doi: 10.1038/ng1281. [DOI] [PubMed] [Google Scholar]
- Gehring W. The genetic control of eye development and its implications for the evolution of the various eye-types. Int J Dev Biol. 2002;46:65–73. [PubMed] [Google Scholar]
- Gehring WJ. The master control gene for morphogenesis and evolution of the eye. Genes Cells. 1996;1:11–5. doi: 10.1046/j.1365-2443.1996.11011.x. [DOI] [PubMed] [Google Scholar]
- Gehring WJ. New perspectives on eye development and the evolution of eyes and photoreceptors. J Hered. 2005;96:171–84. doi: 10.1093/jhered/esi027. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Ikeo K. Pax 6: mastering eye morphogenesis and eye evolution. Trends Genet. 1999;15:371–7. doi: 10.1016/s0168-9525(99)01776-x. [DOI] [PubMed] [Google Scholar]
- Halder G, Callaerts P, Gehring WJ. Induction of ectopic eyes by target expression of the eyeless gene in Drosophila. Science. 1995a;267:1788–1792. doi: 10.1126/science.7892602. [DOI] [PubMed] [Google Scholar]
- Halder G, Callaerts P, Gehring WJ. New perspectives on eye evolution. Curr Opin Genet Dev. 1995b;5:602–9. doi: 10.1016/0959-437x(95)80029-8. [DOI] [PubMed] [Google Scholar]
- Haubst N, Berger J, Radjendirane V, Graw J, Favor J, Saunders GF, Stoykova A, Gotz M. Molecular dissection of Pax6 function: the specific roles of the paired domain and homeodomain in brain development. Development. 2004;131:6131–40. doi: 10.1242/dev.01524. [DOI] [PubMed] [Google Scholar]
- Hill RE, Favor J, Hogan BL, Ton CC, Saunders GF, Hanson IM, Prosser J, Jordan T, Hastie ND, van Heyningen V. Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature. 1991;354:522–5. doi: 10.1038/354522a0. [DOI] [PubMed] [Google Scholar]
- Jang CC, Chao JL, Jones N, Yao LC, Bessarab DA, Kuo YM, Jun S, Desplan C, Beckendorf SK, Sun YH. Two Pax genes, eye gone and eyeless, act cooperatively in promoting Drosophila eye development. Development. 2003;130:2939–51. doi: 10.1242/dev.00522. [DOI] [PubMed] [Google Scholar]
- Jaworski C, Sperbeck S, Graham C, Wistow G. Alternative splicing of Pax6 in bovine eye and evolutionary conservation of intron sequences. Biochem Biophys Res Commun. 1997;240:196–202. doi: 10.1006/bbrc.1997.7623. [DOI] [PubMed] [Google Scholar]
- Jones NA, Kuo YM, Sun YH, Beckendorf SK. The Drosophila Pax gene eye gone is required for embryonic salivary duct development. Development. 1998;125:4163–74. doi: 10.1242/dev.125.21.4163. [DOI] [PubMed] [Google Scholar]
- Jun S, Wallen RV, Goriely A, Kalionis B, Desplan C. Lune/eye gone, a Pax-like protein, uses a partial paired domain and a homeodomain for DNA recognition. Proc Natl Acad Sci U S A. 1998;95:13720–5. doi: 10.1073/pnas.95.23.13720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammermeier L, Leemans R, Hirth F, Flister S, Wenger U, Walldorf U, Gehring WJ, Reichert H. Differential expression and function of the Drosophila Pax6 genes eyeless and twin of eyeless in embryonic central nervous system development. Mech Dev. 2001;103:71–8. doi: 10.1016/s0925-4773(01)00328-8. [DOI] [PubMed] [Google Scholar]
- Kronhamn J, Frei E, Daube M, Jiao R, Shi Y, Noll M, Rasmuson-Lestander A. Headless flies produced by mutations in the paralogous Pax6 genes eyeless and twin of eyeless. Development. 2002;129:1015–26. doi: 10.1242/dev.129.4.1015. [DOI] [PubMed] [Google Scholar]
- Kumar JP, Moses K. EGF receptor and Notch signaling act upstream of Eyeless/Pax6 to control eye specification. Cell. 2001;104:687–97. doi: 10.1016/s0092-8674(01)00265-3. [DOI] [PubMed] [Google Scholar]
- Kumar JP, Tio M, Hsiung F, Akopyan S, Gabay L, Seger R, Shilo BZ, Moses K. Dissecting the roles of the Drosophila EGF receptor in eye development and MAP kinase activation. Development. 1998;125:3875–3885. doi: 10.1242/dev.125.19.3875. [DOI] [PubMed] [Google Scholar]
- Mansouri A, Goudreau G, Gruss P. Pax genes and their role in organogenesis. Cancer Res. 1999;59:1707s–1709s. discussion 1709s–1710s. [PubMed] [Google Scholar]
- Noll M. Evolution and role of Pax genes. Curr Opin Genet Dev. 1993;3:595–605. doi: 10.1016/0959-437x(93)90095-7. [DOI] [PubMed] [Google Scholar]
- Pichaud F, Desplan C. Pax genes and eye organogenesis. Curr Opin Genet Dev. 2002;12:430–4. doi: 10.1016/s0959-437x(02)00321-0. [DOI] [PubMed] [Google Scholar]
- Punzo C, Kurata S, Gehring WJ. The eyeless homeodomain is dispensable for eye development in Drosophila. Genes Dev. 2001;15:1716–23. doi: 10.1101/gad.196401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punzo C, Plaza S, Seimiya M, Schnupf P, Kurata S, Jaeger J, Gehring WJ. Functional divergence between eyeless and twin of eyeless in Drosophila melanogaster. Development. 2004;131:3943–53. doi: 10.1242/dev.01278. [DOI] [PubMed] [Google Scholar]
- Quiring R, Walldorf U, Kloter U, Gehring WJ. Homology of the eyeless gene of Drosophila to the Small eye gene in mice and Aniridia in humans [see comments] Science. 1994;265:785–9. doi: 10.1126/science.7914031. [DOI] [PubMed] [Google Scholar]
- Singh S, Mishra R, Arango NA, Deng JM, Behringer RR, Saunders GF. Iris hypoplasia in mice that lack the alternatively spliced Pax6(5a) isoform. Proc Natl Acad Sci U S A. 2002;99:6812–5. doi: 10.1073/pnas.102691299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Tang HK, Lee JY, Saunders GF. Truncation mutations in the transactivation region of PAX6 result in dominant-negative mutants. J Biol Chem. 1998;273:21531–41. doi: 10.1074/jbc.273.34.21531. [DOI] [PubMed] [Google Scholar]
- Ton CC, Hirvonen H, Miwa H, Weil MM, Monaghan P, Jordan T, van Heyningen V, Hastie ND, Meijers-Heijboer H, Drechsler M, et al. Positional cloning and characterization of a paired box- and homeobox- containing gene from the aniridia region. Cell. 1991;67:1059–74. doi: 10.1016/0092-8674(91)90284-6. [DOI] [PubMed] [Google Scholar]
- Walther C, Guenet JL, Simon D, Deutsch U, Jostes B, Goulding MD, Plachov D, Balling R, Gruss P. Pax: a murine multigene family of paired box-containing genes. Genomics. 1991;11:424–34. doi: 10.1016/0888-7543(91)90151-4. [DOI] [PubMed] [Google Scholar]
- Weasner B, Salzer C, Kumar JP. Sine oculis, a member of the SIX family of transcription factors, directs eye formation. Dev Biol. 2007;303:756–71. doi: 10.1016/j.ydbio.2006.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao JG, Sun YH. Eyg and Ey Pax proteins act by distinct transcriptional mechanisms in Drosophila development. Embo J. 2005;24:2602–12. doi: 10.1038/sj.emboj.7600725. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.