Abstract
Background
Dietary lipid content effects activation of peroxisome proliferator-activated receptor-α (PPARα) and may accelerate cardiac hypertrophy and dysfunction in response to pressure overload. This study investigated the effects of a high fat diet on the development of cardiac hypertrophy.
Methods and Results
C57BL/6J mice (n=14–16/group) underwent transverse aortic constriction (TAC) or sham surgery and were fed either standard low fat diet (STD; 10% fat) or a high fat diet (HFD; 60% fat) for 16 weeks. Sham mice showed no differences between STD and HFD for heart mass or echocardiographic parameters despite greater plasma free fatty acid and leptin concentrations with HFD. TAC increased heart mass and decreased ejection fraction similarly in both groups. Left ventricular end systolic and diastolic diameters with TAC were increased compared to shams on the HFD (p < 0.05) but were not different from STD TAC mice. High fat feeding increased expression of PPARα-regulated genes. The activity of medium chain acyl-coenzyme A dehydrogenase (MCAD), a marker of fatty acid oxidation capacity, was increased in HFD TAC mice compared to STD, consistent with PPARα activation.
Conclusion
Increased fat intake prevented the fall in MCAD activity and did not exacerbate the hypertrophic response to TAC compared to a low-fat diet.
Keywords: diet, hypertrophy, mortality, metabolism, cardiac, heart, fatty acid, mitochondria
Introduction
Recommendations for dietary fat intake are aimed at preventing cardiovascular disease and are largely focused on weight management and the regulation of lipid profiles1. Commercial and clinical dietary intervention programs, however, suggest a wide spectrum for daily fat intake, from 29% in the Zone diet2 to 68% in the Atkins diet3. The effects of high fat diets on the heart are not clear. Studies in transgenic mice with overexpression of proteins involved in cardiomyocyte lipid uptake4 or in hyperlipidemic rats5 demonstrate that increased myocardial fatty acid uptake results in left ventricular chamber dilation, systolic dysfunction, and cardiac hypertrophy. Conversely, patients with hypertensive LVH present with reduced myocardial fatty acid uptake and oxidation compared to normotensive individuals6. This suggests a paradox, whereby alterations in fatty acid availability to either extreme results in LVH and systolic dysfunction. Previous studies in our laboratory have shown that Dahl salt-sensitive rats fed a high fat diet had reduced LVH and improved LV systolic function compared to rats fed a low fat diet despite similar elevations in blood pressure7;8. Taken together, the effect of a high fat diet on the development of LVH and HF needs clarification.
Though dietary lipids are the primary fuel for generation of ATP and contractile force in the normal heart, they also regulate energy metabolism through peroxisome proliferator-activated receptor α (PPARα) and its coactivators. PPARα controls the expression of proteins involved in fatty acid metabolism and is activated by long chain fatty acids and elevations in plasma fatty acids9. With advanced LVH and HF, there is a downregulation of both PPARα and key enzymes in the fatty acid oxidation pathway10. Therefore, it remains unknown whether the anti-hypertrophic effects of a high fat diet seen in our previous studies was due to increased mitochondrial fatty acid oxidation or because of the maintained expression of β-oxidation enzymes secondary to increased PPARα activation.
The purpose of this study was to determine the relationship between dietary fat intake and the development of LVH, ventricular remodeling, and molecular markers of altered cardiac metabolism in response to pressure overload. Studies were performed in the well-established mouse model of transverse aortic constriction (TAC), which causes chronic LV pressure overload, LV remodeling, and overt HF, providing a model for the human cardiac response to hypertension. Stimulation of PPARα was assessed through measurements of transcript levels of established PPARα target genes and the activity of the fatty acid β-oxidation enzyme medium chain acyl-CoA dehydrogenase (MCAD).
Methods
Animal Care
This study was conducted according to the Guidelines for the Care and Use of Laboratory Animals (National Institutes of Health publication No. 85-23) and was approved by the Institutional Animal Care and Use Committee (IACUC) of the Case Western Reserve University. Animals were maintained on a 12-hour light/dark cycle.
Surgery
Transverse aortic constriction (TAC) was performed using the suprasternal approach to minimize trauma and inflammation in the thorax. Male C57BL/6J mice weighing 20–25 g (Charles River, Wilmington, MA, USA) were anesthetized with 5% isoflurane. The neck and chest were shaved, and the area was cleaned with betadine and alcohol. Mice were placed supine on a heated surgical platform. Once positioned, the animal received 1.5% isoflurane by mask. A midline incision ~1.0–1.5 cm in length was made from the suprasternal notch to the third rib. The submaxillary gland was retracted, allowing for visualization of the trachea. Intubation was performed using a 20-gauge i.v. indwelling catheter. Mice were mechanically ventilated using a Harvard Apparatus Model 687 volume-controlled ventilator with tidal volume and respiratory rate adjusted for body mass. A longitudinal cut was made through the proximal portion of the sternum. The thoracic wall was retracted, the thymus bisected, and the transverse aorta located under low power magnification. A 6-0 silk suture was passed underneath the aorta between the innominate and left carotid origins. Suture was then passed around a 26-gauge bent wire and tied securely to the aorta. Following ligation, the wire was removed quickly. Chest wall and skin were closed and animals were allowed to recover in a heated chamber until fully conscious and mobile. The sham procedure was identical without aortic ligation.
Experimental Design
Initially, mice were fed standard lab chow (6% fat, 70% carbohydrates, 24% protein by calories and 0.28% sodium by mass; Harlan Teklad, Indianapolis, IN). After one week, mice underwent either TAC or sham surgery and were assigned randomly to either a standard (STD) or high fat diet (HFD). High fat and standard diet were administered concurrently; however, results on the STD-fed mice have been published previously11. Mice had access to the diets and water ad libitum for the remainder of the 16-week study with body weight and food consumption monitored weekly. Echocardiographic assessment of LV structure and function was performed at 5 and 16 weeks post-surgery. After 16 weeks of treatment, mice underwent a terminal procedure in which blood was drawn and the heart removed for biochemical analysis. Initially, all treatment groups contained 14–16 animals. Due to variable mortality in the surgical and/or dietary treatment groups, 7–14 animals were used for terminal tissue harvest and blood collection for subsequent biochemical analyses.
Diets
Diets were custom-formulated by Research Diets, Inc. (New Brunswick, NJ). Themacronutrient composition of the diets is given in Table 1 below.
Table 1.
Macronutrient composition of the diets used in this study. Diets were matched for vitamin and mineral content and were all on a low-sodium background (~0.26% by mass).
| Contribution to Total Energy Content of the Diet (% of total) | ||||||||
|---|---|---|---|---|---|---|---|---|
| FAT | CARBOHYDRATE | PROTEIN | ||||||
| Diet | Soy Oil | Cocoa Butter | Corn Starch | Fructose | Sucrose | Maltodextrin | Casein | TOTAL |
| STD | 3 | 7 | 32 | 0 | 35 | 3 | 20 | 100 |
| High Fat | 2 | 58 | 0 | 0 | 7 | 13 | 20 | 100 |
Echocardiography
LV dimensions and function were measured at 5 and 16 weeks post-surgery as described previously7;12.
Terminal Surgery
Terminal surgeries were performed on fed mice between 3 and 6 hours from the initiation of the dark phase (18:00) on the light-dark cycle. After 16 weeks of treatment, mice were weighed and anesthetized with 1.5–2.0% isoflurane. The thoracic cavity was opened by cutting from the xiphoid cartilage around the ventral portion of the ribs. The heart was removed, weighed, freeze-clamped, and stored at −80°C. Pooled blood was collected from the thoracic cavity after heart excision for analysis of plasma and serum metabolites.
Metabolic Measurements
Plasma glucose, insulin, free fatty acids, triglycerides, and total adiponectin along with serum leptin were assayed using commercially-available kits. Medium chain acyl-CoA dehydrogenase (MCAD) and citrate synthase (CS) activities were analyzed from tissue homogenates as described previously13.
mRNA Expression Analysis
Powdered heart tissue was disrupted and homogenized by shaking in solution with 5 mm stainless-steel beads for 3 min. at 30 sec−1 (Mixermill 300; Qiagen), followed by RNA isolation (RNeasy Mini Kit, Qiagen). RNA samples were eluted in 50 μL of nuclease-free water and stored at −80°C. For cDNA synthesis, RNA (1 μg) was mixed with 2 μL Oligo(dT)16 (Applied Biosystems) and 0.5 μL random primers (Invitrogen) and brought to 15 μL total volume. Sample/primer mix was heated to 70°C for 10 min. then placed immediately on ice for 2 min., followed by the addition of the RT reaction mix containing 5x buffer (5 μL), 0.1M DTT (2.5 μL), and Superscript II RT (Invitrogen), 10 mM dNTP (1.25 μL; Invitrogen), and RNase inhibitor (0.25 μL; Applied Biosystems). Reaction mix was incubated for 2 min. at 42°C, 1 μL reverse transcriptase added, and incubation continued at 42°C for 1hr. Reaction mix was then incubated at 70° C for 10 min. and placed on ice for 2 min. The resulting cDNA samples were stored at −20°C. Quantitative RT-PCR (qRT-PCR) was performed using an ABI 7900 and the following protocol: 2 min. at 50°C, 10 min. at 95°C, 40 cycles at 95°C for 15 sec., and 1 min. at 60°C. Each reaction was 25 μL, consisting of 1.0 μL cDNA sample, 1.25 μL TaqMan Gene Expression Assay, 12.5 μL 2X TaqMan PCR master mix, and 10.25 μL nuclease-free water. The expression of murine atrial natriuretic peptide (Nppa), myosin heavy chain α (Myh6) and β (Myh7), MCAD (Acadm), CS (Cs), PPARα (Ppara), carnitine palmitoyl transferase I (cpt1b), pyruvate dehydrogenase kinase 4 (pdk4), uncoupling protein 3 (ucp3), and peptidylprolyl isomerase a (ppia, internal control) was determined by qRT-PCR using Assays On-Demand probes (Applied Biosystems). mRNA concentration was normalized to that of ppia and expressed as the fold increase relative to the STD sham group.
Western Blotting
Protein was extracted from frozen cardiac tissue, separated by electrophoresis in 10% SDS-PAGE gels, transferred onto nitrocellulose membranes, and incubated with specific antibodies to either phosphorylated-Akt (Ser473) or phosphorylated-AMPK (Thr172 of the α subunit) (all at 1:1000, from Cell Signaling Technology, Inc.). Fluorescence-conjugated secondary antibodies (IRDye 680/800, 1:5000; LI-COR Bioscience) were used for incubation before the membranes were scanned and analyzed with the Odyssey® infrared imaging system (LI-COR Bioscience). Membranes were then stripped (Pierce Restore® stripping buffer) and re-probed for total Akt and AMPK (1:1000; Cell Signaling).
Statistical Analysis
All analysis was performed with the investigator(s) blinded. Two-way analysis of variance (ANOVA) with Bonferroni post hoc adjustment was used to compare all dietary groups with and without aortic constriction. Differences in mortality were assessed by a Kaplan-Meier survival curve with Chi squared analysis (SPSS® Base 15.0, SPSS Inc., Chicago, IL). All data are presented as means ± SEM. A P < 0.05 was accepted as statistically significant.
Results
Survival Data
All sham-operated mice on the STD and HFD survived the 16-week duration of the study. TAC induced accumulated mortality of 27% in the STD- and 31% in the HFD-fed animals.
Body and Heart Mass
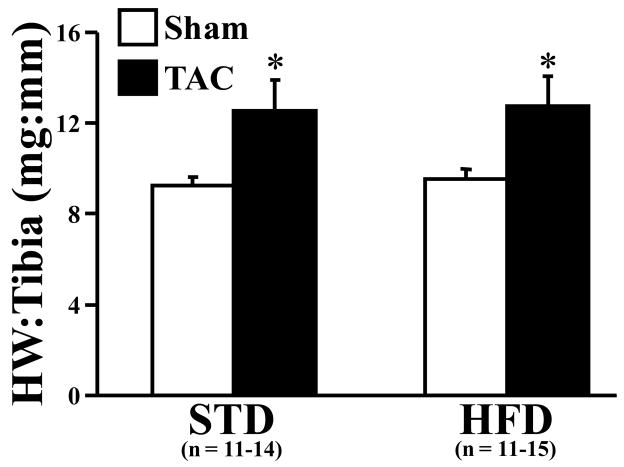
No significant differences in body mass were observed in either the sham or TAC animals fed either STD or HFD (Table 2). Average daily food consumption also was not different among all groups (data not shown). TAC increased heart weight (normalized to tibia length) by 38.7% in STD-fed mice and 33.6% in HFD-fed mice compared to their respective shams (Figure 1). Epididymal fat mass was increased in the HFD-fed sham mice compared to TAC mice on the same diet and STD-fed sham mice (Table 2).
Table 2.
Body measurements taken at terminal procedure following 16 weeks of dietary treatment.
| STD | HFD | |||
|---|---|---|---|---|
| Measurement | Sham n = 14 | TAC n = 11 | Sham n = 15 | TAC n = 11 |
| Body Weight (g) | 28 ± 1 | 27 ± 1 | 30 ± 1 | 28 ± 1 |
| Heart Weight (mg) | 154.3 ± 5.5 | 214.0 ± 21.8* | 165.9 ± 7.3 | 221.6 ± 22.6* |
| Tibia Length (mm) | 16.77 ± 0.21 | 17.11 ± 0.13 | 17.48 ± 0.18 | 17.43 ± 0.13 |
| Epididymal Fat (mg) | 541.7 ± 40.6 | 412.7 ± 40.6 | 753.6 ± 113.9† | 490.9 ± 88.5* |
p < 0.05 vs. Sham animals on the same diet
p < 0.05 vs. Sham animals on the STD
Figure 1.
Terminal heart weight to tibia length ratios of sham (n = 11–14 per group) and TAC (n = 11–15 per group) animals. Open bars represent sham animals; closed bars represent TAC animals. Data are the mean ± SEM. *P < 0.05 compared to sham animals on the same diet.
LV Dimensions and Performance
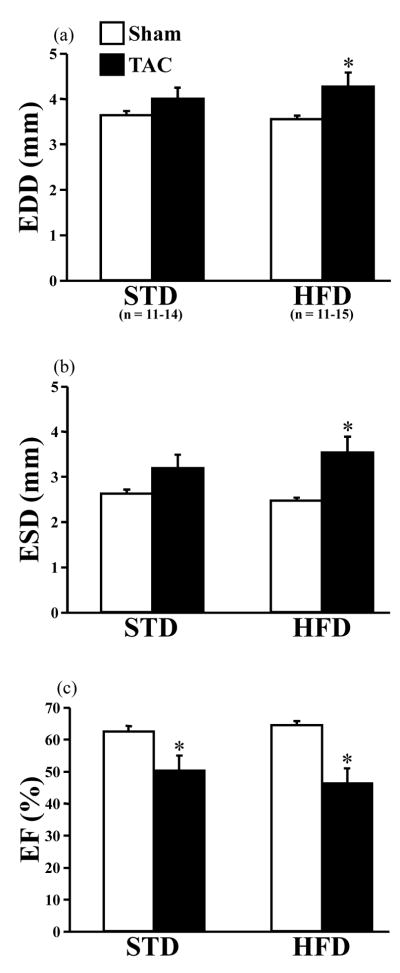
LV end diastolic diameter (EDD) and end systolic diameter (ESD) were increased significantly by TAC compared to sham in animals consuming the HFD (Figure 2a,b). No differences in LV chamber dimensions were induced by TAC in the STD-fed mice compared to their respective shams (Figure 2a,b). In general, TAC significantly decreased ejection fraction (EF%), but there was no additive dietary effect (Figure 2c).
Figure 2.
Terminal echocardiographic parameters. End diastolic diameter (EDD; a), end systolic diameter (ESD; b), and ejection fraction (EF; c) were measured after 16 weeks of treatment. Open bars represent sham animals; closed bars represent TAC animals. The number of animals used for all echocardiographic measurements is denoted in (a). Data are the mean ± SEM. *P < 0.05 compared to sham animals on the same diet.
Metabolic Parameters
Plasma glucose was unaffected by TAC or dietary treatment (Table 3). The HFD significantly elevated plasma free fatty acids in both TAC and sham groups compared to respective groups on STD (Table 3). However, neither surgical nor dietary treatment altered plasma triglyceride concentration. Plasma insulin and total adiponectin were unchanged among all groups (Table 3). Serum leptin, however, was increased by the HFD compared to STD independent of surgery.
Table 3.
Metabolite and hormone concentrations in plasma or serum in fed animals after 16 weeks of treatment.
| STD | HFD | ||||
|---|---|---|---|---|---|
| Metabolite or Hormone | Sham n = 9 | TAC n = 8 | Sham n = 10 | TAC n = 9 | STD vs. HFD |
| Plasma Glucose (mM) | 8.80 ± 0.61 | 8.16 ± 0.32 | 9.59 ± 0.70 | 7.89 ± 0.36 | NS |
| Plasma Free Fatty Acids (mM) | 0.38 ± 0.05 | 0.48 ± 0.04 | 0.58 ± 0.08 | 0.64 ± 0.07 | p = 0.004 |
| Plasma Triglycerides (mg/mL) | 0.36 ± 0.04 | 0.33 ± 0.04 | 0.45 ± 0.03 | 0.41 ± 0.06 | NS |
| Plasma Insulin (nM) | 0.59 ± 0.07 | 0.70 ± 0.15 | 0.89 ± 0.26 | 0.66 ± 0.24 | NS |
| Plasma Adiponectin (μg/mL) | 2.09 ± 0.26 | 2.48 ± 0.24 | 2.04 ± 0.26 | 1.86 ± 0.22 | NS |
| Serum Leptin (ng/mL) | 2.13 ± 0.30 | 2.34 ± 0.27 | 4.71 ± 0.62 | 5.29 ± 0.22 | p < 0.001 |
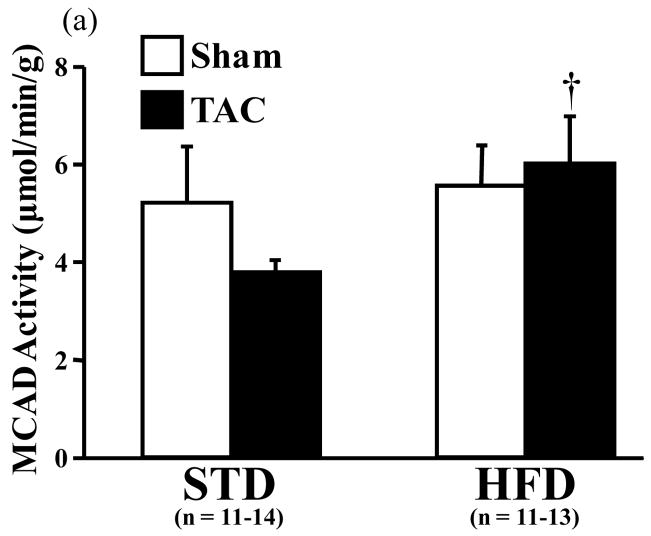
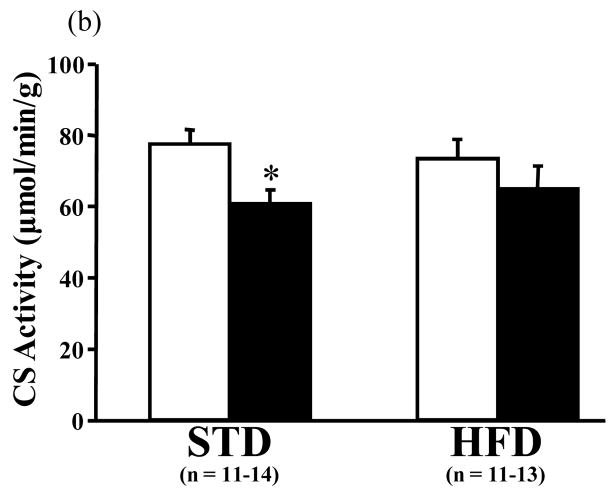
The activity of MCAD was not different between the STD- and HFD-fed sham groups. In TAC mice, however, MCAD activity was significantly increased by the HFD compared to STD diet (Figure 3a). CS activity was significantly decreased by TAC in mice consuming the STD (Figure 3b). The ratio of MCAD to CS activity was trending towards a greater value with the HFD compared to STD (p = 0.057, data not shown).
Figure 3.
Activities of (a) medium chain acyl-coenzyme A dehydrogenase (MCAD) and (b) citrate synthase (CS) in cardiac tissue measured spectrophotometrically. Open bars represent sham animals; closed bars represent TAC animals. Data are the mean ± SEM. †P < 0.05 compared to TAC animals on the STD. *P < 0.05 compared to sham animals on the same diet.
mRNA Expression
The transcript levels for ANF, MHCα, and CS were unchanged by surgical or dietary treatment. Expression of MHCβ was increased by TAC compared to sham but was unaffected by diet (Figure 4a). PPARα mRNA was significantly reduced by TAC in the HFD-fed animals compared to their respective sham. While no change was observed in MCAD and CPT-Iβ expression, there was significant upregulation of UCP3 and PDK4 in both sham and TAC groups fed the HFD compared to both surgical groups consuming the STD (Figure 4b).
Figure 4.
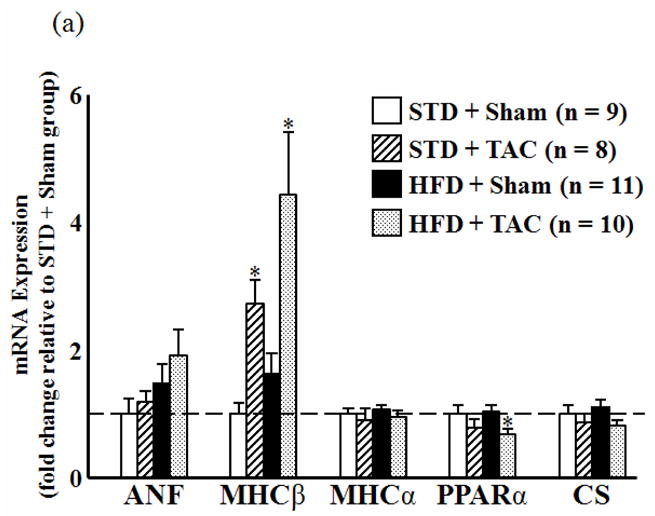
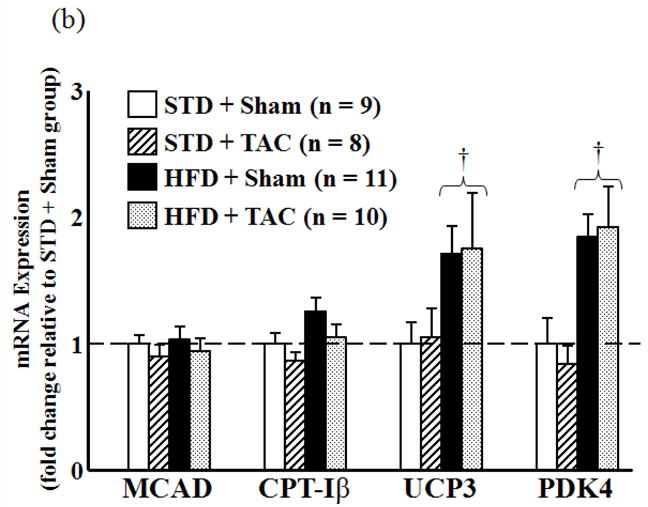
mRNA expression of cardiac genes as determined by qRT-PCR. (a) Expression of atrial natriuretic factor (ANF), myosin heavy chain β (MHCβ) and MHCα, peroxisome proliferator-activated receptor α (PPARα), and citrate synthase CS relative to STD sham group (dashed line). (b) Expression of the PPARα-regulated genes MCAD, carnitine palmitoyl transferase Iβ (CPT-Iβ), uncoupling protein 3 (UCP3), and pyruvate dehydrogenase kinase 4 (PDK4) relative to STD sham group (dashed line). *P < 0.05 compared to sham animals on the same diet. †P < 0.05 compared to TAC animals on the STD.
Western Blot Analysis
There were no significant differences in total Akt protein levels or the ratio of phosphorylated-Akt (Ser473)/total Akt between any of the treatment groups (Table 4). Similarly, there were no significant differences in total AMPK protein levels or the ratio of phosphorylated-AMPK (Thr172 of the α subunit)/total AMPK (Table 4).
Table 4.
Western blot densitometry of Akt and phosphor-Akt (Ser473) and AMPK and phosphor-AMPK (Thr172) in the heart (measured in arbitrary units). Data are presented as the fold change in protein expression relative to the STD Sham group.
| STD | HFD | |||
|---|---|---|---|---|
| Protein | Sham n = 7 | TAC n = 8 | Sham n = 10 | TAC n = 9 |
| Phospho-Akt | 1.00 ± 0.08 | 0.83 ± 0.06 | 0.90 ± 0.05 | 0.83 ± 0.02 |
| Total Akt | 1.00 ± 0.06 | 0.99 ± 0.06 | 0.94 ± 0.05 | 1.02 ± 0.02 |
| Phospho-Akt/Total Akt | 1.00 ± 0.07 | 0.86 ± 0.08 | 0.98 ± 0.09 | 0.84 ± 0.02 |
| Phospho-AMPK | 1.00 ± 0.07 | 0.87 ± 0.06 | 0.92 ± 0.06 | 0.87 ± 0.02 |
| Total AMPK | 1.00 ± 0.06 | 0.99 ± 0.09 | 1.02 ± 0.03 | 1.25 ± 0.03 |
| Phospho-AMPK/Total AMPK | 1.00 ± 0.08 | 0.89 ± 0.06 | 0.88 ± 0.07 | 0.73 ± 0.02 |
Discussion
This study demonstrated that a high fat diet did not adversely affect the development of LVH and contractile dysfunction in response to pressure overload compared to a low fat diet. High fat feeding increased plasma free fatty acids and preserved the activity of medium chain acyl-CoA dehydrogenase (MCAD) and citrate synthase (a marker enzyme for citric acid cycle flux) compared to the low fat diet (Figures 3a,b). These findings suggest that increased saturated fat intake does not exacerbate pathological cardiac hypertrophy that develops in response to pressure overload.
In contrast, numerous studies have shown that exposure of the heart to excess lipids can worsen remodeling and contractile dysfunction. Obese Zucker rats exhibit hypertriglyceridemia and diabetes, resulting in cardiomyocyte fat deposition and apoptosis and decreased contractile function5. In mice, cardiomyocyte-specific overexpression of lipoprotein lipase, an enzyme responsible for triglyceride hydrolysis, results in increased uptake of very low density lipoprotein, cardiac hypertrophy, and mortality4. Furthermore, cardiac overexpression of PPARα leads to upregulation of genes involved in lipid uptake and causes ventricular hypertrophy and contractile dysfunction in response to high fat feeding14. Thus, further investigation is needed to clarify the effect of increased lipid exposure on ventricular hypertrophy and remodeling.
A potential protective role of fat is the regulation of β-oxidation enzymes and PPARα activity. In the current study, there was a significant increase in MCAD activity in TAC animals on high fat compared to those fed a low fat diet (Figure 3a) despite no change in MCAD mRNA (Figure 4b). This finding differs from our previous observation that MCAD activity and protein expression were unchanged despite reductions in MCAD gene expression15 and disputes evidence of HF-induced downregulation of fatty acid oxidation genes16;17. Thus, high fat feeding may sustain mitochondrial function during pressure overload either by providing sufficient substrate for fatty acid oxidation or through PPARα signaling and increased expression of nuclear-derived mitochondrial proteins.
Another potential mediator of LVH that is increased by a high fat diet is leptin, an adipokine known to trigger satiety and reduce food intake18. It has been shown that leptin can be a direct stimulant for increased protein synthesis and cell size in cultured neonatal rat cardiomyocytes19;20. Previous studies in our lab showed that feeding rats a high saturated fat diet for 12 weeks reduced serum leptin concentrations by 50% compared to low fat chow. This decrease in leptin, however, had no effects on body mass, blood pressure, LV mass, or cardiac function12. In the current study, we found an increase in serum leptin concentration but no effects on body weight or heart weight (Figure 1) or ejection fraction (Figures 2c). Furthermore, AMP-activated protein kinase (AMPK), a known leptin target in skeletal muscle that increases fatty acid oxidation21, and its potential effector molecule, the serine/threonine kinase Akt22, were unchanged by high fat feeding (Table 4). These results suggest that elevated leptin does not activate pro-hypertrophic signaling pathways and is not deleterious to the pressure-overloaded heart.
In summary, the findings of the current study demonstrate that in response to chronic pressure overload, increased fat intake prevented the fall in MCAD activity and did not exacerbate the hypertrophic response compared to a low fat diet. Though current dietary recommendations suggest a low fat diet1, there is a lack of evidence demonstrating a detrimental effect of a high fat diet in the development of LVH. Thus, additional studies are needed to elucidate the effects of high fat on the progression from LVH to heart failure.
Acknowledgments
This study was supported by National Institutes of Health grant HL-074237. We would like to thank Dr. Ken Walsh and Dr. Kaori Sato at Boston University for teaching us the TAC procedure. We would also like to acknowledge Karen O’Shea, Kristen King, and Celvie Yuan for their help with the terminal surgeries, Dr. Willem Kop for his assistance in the survival analyses, and Dr. Brian Barrows for his technical support with the qRT-PCR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Lichtenstein AH, Appel LJ, Brands M, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 2.St Jeor ST, Howard BV, Prewitt TE, et al. Dietary protein and weight reduction: a statement for healthcare professionals from the Nutrition Committee of the Council on Nutrition, Physical Activity, and Metabolism of the American Heart Association. Circulation. 2001;104:1869–1874. doi: 10.1161/hc4001.096152. [DOI] [PubMed] [Google Scholar]
- 3.Atkins RC, Sears B, Eaton B, et al. Dissecting the diets. Newsweek. 2003;141:55. [PubMed] [Google Scholar]
- 4.Yagyu H, Chen G, Yokoyama M, et al. Lipoprotein lipase (LpL) on the surface of cardiomyocytes increases lipid uptake and produces a cardiomyopathy. J Clin Invest. 2003;111:419–426. doi: 10.1172/JCI16751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou YT, Grayburn P, Karim A, et al. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci U S A. 2000;97:1784–1789. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de las Fuentes L, Herrero P, Peterson LR, et al. Myocardial fatty acid metabolism: independent predictor of left ventricular mass in hypertensive heart disease. Hypertension. 2003;41:83–87. doi: 10.1161/01.hyp.0000047668.48494.39. [DOI] [PubMed] [Google Scholar]
- 7.Okere IC, Chess DJ, McElfresh TA, et al. High-fat diet prevents cardiac hypertrophy and improves contractile function in the hypertensive dahl salt-sensitive rat. Clin Exp Pharmacol Physiol. 2005;32:825–831. doi: 10.1111/j.1440-1681.2005.04272.x. [DOI] [PubMed] [Google Scholar]
- 8.Okere IC, Young ME, McElfresh TA, et al. Low carbohydrate/high-fat diet attenuates cardiac hypertrophy, remodeling, and altered gene expression in hypertension. Hypertension. 2006;48:1116–1123. doi: 10.1161/01.HYP.0000248430.26229.0f. [DOI] [PubMed] [Google Scholar]
- 9.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 10.Huss JM, Kelly DP. Nuclear receptor signaling and cardiac energetics. Circ Res. 2004;95:568–578. doi: 10.1161/01.RES.0000141774.29937.e3. [DOI] [PubMed] [Google Scholar]
- 11.Chess DJ, Lei B, Hoit BD, et al. Deleterious Effects of Sugar and Protective Effects of Starch on Cardiac Remodeling, Contractile Dysfunction, and Mortality in Response to Pressure Overload. Am J Physiol Heart Circ Physiol. 2007 doi: 10.1152/ajpheart.00544.2007. [DOI] [PubMed] [Google Scholar]
- 12.Okere IC, Chandler MP, McElfresh TA, et al. Differential effects of saturated and unsaturated fatty acid diets on cardiomyocyte apoptosis, adipose distribution, and serum leptin. Am J Physiol Heart Circ Physiol. 2006;291:H38–H44. doi: 10.1152/ajpheart.01295.2005. [DOI] [PubMed] [Google Scholar]
- 13.Panchal AR, Stanley WC, Kerner J, et al. Beta-receptor blockade decreases carnitine palmitoyl transferase I activity in dogs with heart failure. J Card Fail. 1998;4:121–126. doi: 10.1016/s1071-9164(98)90252-4. [DOI] [PubMed] [Google Scholar]
- 14.Finck BN, Lehman JJ, Leone TC, et al. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest. 2002;109:121–130. doi: 10.1172/JCI14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgan EE, Chandler MP, Young ME, et al. Dissociation between gene and protein expression of metabolic enzymes in a rodent model of heart failure. Eur J Heart Fail. 2006;8:687–693. doi: 10.1016/j.ejheart.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 16.de Brouwer KF, Degens H, Aartsen WM, et al. Specific and sustained down-regulation of genes involved in fatty acid metabolism is not a hallmark of progression to cardiac failure in mice. J Mol Cell Cardiol. 2006;40:838–845. doi: 10.1016/j.yjmcc.2006.03.429. [DOI] [PubMed] [Google Scholar]
- 17.Pellieux C, Aasum E, Larsen TS, et al. Overexpression of angiotensinogen in the myocardium induces downregulation of the fatty acid oxidation pathway. J Mol Cell Cardiol. 2006;41:459–466. doi: 10.1016/j.yjmcc.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Halaas JL, Gajiwala KS, Maffei M, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 19.Rajapurohitam V, Javadov S, Purdham DM, et al. An autocrine role for leptin in mediating the cardiomyocyte hypertrophic effects of angiotensin II and endothelin-1. J Mol Cell Cardiol. 2006;41:265–274. doi: 10.1016/j.yjmcc.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Rajapurohitam V, Gan XT, Kirshenbaum LA, et al. The obesity-associated peptide leptin induces hypertrophy in neonatal rat ventricular myocytes. Circ Res. 2003;93:277–279. doi: 10.1161/01.RES.0000089255.37804.72. [DOI] [PubMed] [Google Scholar]
- 21.Minokoshi Y, Kim YB, Peroni OD, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 22.Sharma N, Okere IC, Duda MK, et al. Potential impact of carbohydrate and fat intake on pathological left ventricular hypertrophy. Cardiovasc Res. 2007;73:257–268. doi: 10.1016/j.cardiores.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]