Recent trials in glioma have revealed significant limitations in the end points used. This requires a critical and comprehensive review of how brain tumor trials are conducted, particularly of which end points are defined and how response and progression are defined.
LIMITATIONS OF MACDONALD'S RESPONSE AND PROGRESSION CRITERIA
In 1990, Macdonald et al1 reported criteria for response assessment in glioma. These criteria, which rely primarily on computed tomography (CT) –based two-dimensional WHO response criteria, marked the transition from a subjective interpretation of clinical and radiologic changes toward more objective radiologically based criteria. Macdonald's criteria use the enhancing tumor area as the primary measure, while considering the use of steroids and changes in the neurologic status. Although these criteria have limitations (particularly the difficulty of measuring the often irregular shape of gliomas), they have become widely accepted. However, recent observations have revealed fundamental limitations to Macdonald's criteria.2,3 At the core of Macdonald's criteria are changes in enhancement, and indeed, all too often, the enhancement of high-grade tumors is perceived as a measure of tumor. However, enhancement is nonspecific and primarily reflects a disrupted blood-brain barrier. Enhancement can be influenced by changes in corticosteroid dose and radiologic technique.4,5 Enhancement can also be induced by a variety of nontumoral processes: inflammation, seizure activity, postsurgical changes, and radiation necrosis.6–9 As a result, changes in the enhancing area cannot be equated with changes in tumor size or tumor growth/activity. Macdonald's criteria have proved to be of limited value in the following clinical situations.
Pseudoprogression and Radiation Necrosis
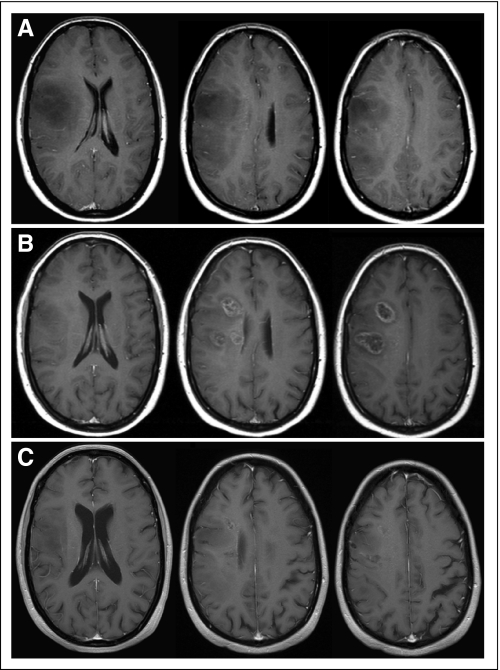
Studies of patients with glioblastoma treated with the current standard of care—chemoradiation combined with temozolomide—have shown consistently that immediately after the end of radiotherapy, 20% to 30% of patients suffer from pseudoprogression.10,11 This is defined as an increase of enhancement within the irradiated field that spontaneously subsides without new antitumor treatments (Fig 1). Pseudoprogression appears to be less frequent after fractionated external-beam radiotherapy only.12 Moreover, after combined chemoradiation, radiation necrosis also seems to occur more frequently and earlier than it does after fractionated external-beam radiotherapy only.13 This limits the validity of progression-free survival (PFS) as the primary end point in clinical trials and has significant implications for eligibility in salvage treatment trials; patients should not be eligible for such trials in the first months after the end of radiotherapy. Most trials currently use a 3-month minimum interval, which is admittedly an arbitrary period.
Fig 1.
Example of pseudoprogression after radiotherapy only. (A) Patient with biopsy-proven gemistocytic astrocytoma showing mass effect on magnetic resonance imaging (MRI) < 2 months before start of radiotherapy underwent 50.4-Gy radiotherapy in fractions of 1.8 Gy. (B) On first follow-up MRI 2 months after end of radiotherapy, new enhancing lesions were present. (C) These disappeared 7 months later without any additional treatment. The patient was asymptomatic throughout this episode and did not receive steroids.
Enhancement Resulting From Local Treatment
The observation of postsurgical enhancement confounding assessment of response has resulted in the exclusion from phase II studies using response as the primary end point of patients after surgery, unless an immediate postoperative scan (ie, within the first 24 to 48 hours) is obtained.7,14–16 However, local treatment–induced enhancement is not limited to surgical resection. Transient increases in enhancement (flare) not reflecting tumor progression have been reported in multiple studies of local intratumoral treatment.17–20 Therefore, PFS may not be an appropriate end point in such trials. The same holds true for interstitial brachytherapy and stereotactic radiosurgery, which may induce radiation necrosis mimicking tumor progression.21
Pseudoresponse After Treatment With Agents Affecting Angiogenesis and Blood Vessels
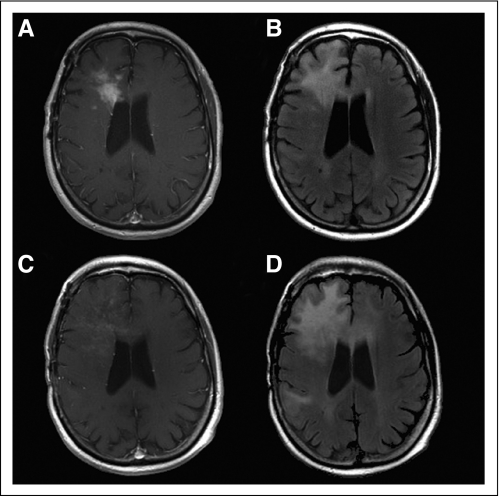
Studies of agents that modify signal transduction through the vascular endothelial growth factor (VEGF) signaling pathways (eg, bevacizumab and cediranib) have shown that initiation of therapy often produces a rapid decrease in enhancement, resulting in high response rates.22,23 However, these responses result at least partially from a rapid normalization of abnormally permeable blood vessels or regional cerebral blood volume, not from antitumor effects. Moreover, in several patients, increases in the nonenhancing portion of tumor were observed in T2- or fluid-attenuated inversion recovery (FLAIR) –weighted magnetic resonance imaging (MRI), suggestive of infiltrative tumor progression despite the continuing radiologic response of the enhancing lesions (Fig 2).24 This may explain the disappointing disparity between the unprecedented high response rates produced by these agents in recurrent glioblastoma and the modest (if any) survival benefit reported. To some extent, similar effects have been observed after treatment with platelet-derived growth factor inhibitors.25 Again, the difficulty in assessing progression limits use of 6-month PFS as the primary end point, and in response assessment, changes in T2- or FLAIR-weighted MRI sequences must also be considered.26
Fig 2.
Patient 57 years of age with secondary glioblastoma before ([A] T1-weighted magnetic resonance imaging (MRI) with contrast and [B] fluid-attenuated inversion recovery [FLAIR] –weighted MRI) and after 7 months of treatment with bevacizumab and irinotecan, showing reduction in size of initial contrast-enhancing mass but also demonstrating (C) subtle diffuse enhancement and (D) significantly increased FLAIR crossing the corpus callosum. This was associated with increased cognitive impairment. Patient was not receiving corticosteroids at time of either scan.
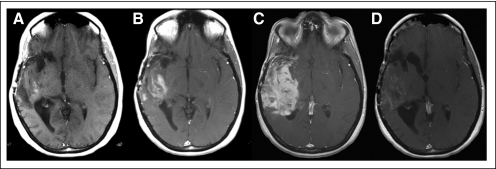
Another issue is the occurrence of rebound enhancement and edema on discontinuation of the VEGF-signaling inhibitor, which requires special attention if patients are enrolled onto clinical trials after nonresponse to VEGF-inhibiting agents. In the example illustrated by Figure 3, after a sufficiently long washout interval, a new baseline scan is needed to avoid the unjustified designation of failure of subsequent treatment because of rebound enhancement and edema.
Fig 3.
Example of rebound enhancement after discontinuation of anti–vascular endothelial growth factor (VEGF) agent. (A) Axial T1-weighted magnetic resonance imaging with contrast of patient with recurrent right temporal glioblastoma who initially responded to aflibercept (VEGF Trap; Regeneron, Tarrytown, NY); (B) at recurrence when aflibercept was discontinued; and (C) 4 weeks after, showing significant increase in contrast enhancement. (D) Patient began receiving bevacizumab with significant reduction in enhancement 4 weeks later, suggesting that worsening was in part result of aflibercept discontinuation and not just of tumor progression.
Nonenhancing Tumors: Low-Grade Glioma
Macdonald's criteria were designed primarily to evaluate high-grade gliomas and focus on changes in enhancing tumor volume. However, low-grade gliomas usually do not show contrast enhancement in CT or MRI. Untreated, these tumors often have low growth rates, on the order of 3 to 5 mm per year.27 Because it would take a considerable period of time to reach the Macdonald-specified 25% increase in area, this obscures the detection of progression. Moreover, low response rates have been observed in several trials despite significant clinical benefit (particularly seizure reduction) and prolonged disease control.28–30 One problem seems to be that residual abnormalities, which may persist after successful treatment, cannot be distinguished from active tumor in T2- or FLAIR-weighted MRI.
Application of Macdonald's Criteria After Complete Resection of Enhancing Disease
Macdonald's criteria define disease progression as an increase in enhancing tumor area of ≥ 25% or the appearance of new enhancing lesions. Surgical techniques have evolved such that so-called gross total resection (eg, resection of all of the enhancing disease) is performed on a more regular basis.31 If there is no enhancement present at postoperative imaging study, any enhancement on subsequent scans—no matter how small or nonspecific—by definition implies tumor progression. Currently, there are no generally accepted criteria to determine progression after gross total resection.
In theory, both alternative imaging tools and alternative trial designs and end points could be used to overcome these imaging issues. Indeed, more sophisticated MRI techniques and metabolic positron emission tomography with radioactively labeled amino acid tracers may provide answers to some of these issues, but these techniques are not widely available, nor have they been validated for use in trials of glioma.32,33 Their value remains to be established in future studies.
PFS OR OVERALL SURVIVAL AS THE PRIMARY END POINT
Whether PFS can be an appropriate primary end point in phase III trials—or whether overall survival (OS) should be the primary end point in these trials as a matter of principle—is an ongoing discussion in oncology. The evaluation of the effect of a particular treatment on OS may be influenced by subsequent salvage treatments. Until recently, the lack of effective treatments in glioma made this a hypothetic consideration in glioma trials. However, the bevacizumab studies in recurrent glioblastoma have suggested that salvage treatments may indeed affect OS.23 In contrast, some recent trials of newly diagnosed glioma have shown improved PFS after initially intensified treatment without an impact on OS.34–36 None of these trials have clarified whether this increase in PFS signified clinical benefit for the patients. Prolonging PFS may be beneficial to a patient if the toxicity of initial treatment is low, good function is maintained as long as the tumor is controlled, and progression is associated with a significant deterioration in function or quality of life. Unfortunately, until recently, most studies of treatment for newly diagnosed gliomas did not gather adequate functional or quality-of-life data to assess these issues. Moreover, validated and accepted tools to assess neurologic deterioration–free survival are currently not available.
NEED FOR STUDY-SPECIFIC END POINTS
Clearly, different and study-specific end points are required depending on the type of trial, investigational treatment, and clinical setting (newly diagnosed or recurrent high-grade or low-grade glioma). If classical cytotoxic drugs that do not interfere with enhancement are tested in recurrent glioblastoma, then a classical approach with 6-month PFS as the primary end point can be used. In newly diagnosed glioblastoma, analyses have suggested that 12-month OS may be considered as a surrogate end point, although this end point would be subject to the effects of salvage treatments.37,38 PFS is not adequate in trials of local treatments, trials of newly diagnosed glioma managed with chemoradiation combined with temozolomide, or trials with antiangiogenic agents. In these cases, the diagnosis of progression as assessed by conventional MRI is too uncertain. Crossover is an issue if the investigational agent is active in recurrent disease or is likely to be used in the control arm at time of progression. If so, no OS benefit may be observed, despite clear activity of the investigational agent. In these circumstances, trial design should emphasize other parameters of clinical benefit for patients.
To address these issues and develop specific guidelines for end points in various types of neuro-oncology trials, an international working party has been formed to develop recommendations for each of these situations. It is expected that through this effort, widely accepted criteria will again become available for use in clinical trials in the coming years.
Acknowledgment
We thank Jacolien Bromberg, MD, for providing critical comments. S.M.C. is supported in part by NIH Grant No. PO1 CA118816.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Martin J. van den Bent, Schering-Plough (C), Roche (C), Bristol-Myers Squibb (C), Ark Therapeutics (C), Siena Biotech (C), Antisense Pharma (C); Michael A. Vogelbaum, Genentech (C) Stock Ownership: None Honoraria: Martin J. van den Bent, Merck KGaA, Ark Therapeutics; Michael A. Vogelbaum, Schering-Plough, MGI Pharma; David R. Macdonald, Schering-Plough Research Funding: Martin J. van den Bent, Novartis Expert Testimony: None Other Remuneration: David R. Macdonald, Schering-Plough
AUTHOR CONTRIBUTIONS
Conception and design: Martin J. van den Bent, Michael A. Vogelbaum, Patrick Y. Wen, David R. Macdonald, Susan M. Chang
Manuscript writing: Martin J. van den Bent, Michael A. Vogelbaum, Patrick Y. Wen, David R. Macdonald, Susan M. Chang
Final approval of manuscript: Martin J. van den Bent, Michael A. Vogelbaum, Patrick Y. Wen, David R. Macdonald, Susan M. Chang
REFERENCES
- 1.Macdonald DR, Cascino TL, Schold SC, Jr, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 2.Henson JW, Ulmer S, Harris GJ. Brain tumor imaging in clinical trials. AJNR Am J Neuroradiol. 2008;29:419–424. doi: 10.3174/ajnr.A0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sorensen AG, Batchelor TT, Wen PY, et al. Response criteria for glioma. Nat Clin Pract Oncol. 2008;5:634–644. doi: 10.1038/ncponc1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cairncross JG, Macdonald DR, Pexman JH, et al. Steroid-induced CT changes in patients with recurrent glioma. Neurology. 1988;38:724–726. doi: 10.1212/wnl.38.5.724. [DOI] [PubMed] [Google Scholar]
- 5.Watling CJ, Lee DH, Macdonald DR, et al. Corticosteroid-induced magnetic resonance imaging changes in patients with recurrent malignant glioma. J Clin Oncol. 1994;12:1886–1889. doi: 10.1200/JCO.1994.12.9.1886. [DOI] [PubMed] [Google Scholar]
- 6.Finn MA, Blumenthal DT, Salzman KL, et al. Transient postictal MRI changes in patients with brain tumors may mimic disease progression. Surg Neurol. 2007;67:246–250. doi: 10.1016/j.surneu.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 7.Ulmer S, Braga TA, Barker FG, 2nd, et al. Clinical and radiographic features of peritumoral infarction following resection of glioblastoma. Neurology. 2006;67:1668–1670. doi: 10.1212/01.wnl.0000242894.21705.3c. [DOI] [PubMed] [Google Scholar]
- 8.Henegar MM, Moran CJ, Silbergeld DL. Early postoperative magnetic resonance imaging following nonneoplastic cortical resection. J Neurosurg. 1996;84:174–179. doi: 10.3171/jns.1996.84.2.0174. [DOI] [PubMed] [Google Scholar]
- 9.Kumar AJ, Leeds NE, Fuller GN, et al. Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology. 2000;217:377–384. doi: 10.1148/radiology.217.2.r00nv36377. [DOI] [PubMed] [Google Scholar]
- 10.Taal W, Brandsma D, de Bruin HG, et al. Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer. 2008;113:405–410. doi: 10.1002/cncr.23562. [DOI] [PubMed] [Google Scholar]
- 11.Brandes AA, Franceschi E, Tosoni A, et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26:2192–2197. doi: 10.1200/JCO.2007.14.8163. [DOI] [PubMed] [Google Scholar]
- 12.de Wit MC, de Bruin HG, Eijkenboom W, et al. Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology. 2004;63:535–537. doi: 10.1212/01.wnl.0000133398.11870.9a. [DOI] [PubMed] [Google Scholar]
- 13.Chamberlain MC, Glantz MJ, Chalmers L, et al. Early necrosis following concurrent Temodar and radiotherapy in patients with glioblastoma. J Neurooncol. 2007;82:81–83. doi: 10.1007/s11060-006-9241-y. [DOI] [PubMed] [Google Scholar]
- 14.Cairncross JG, Pexman JH, Rathbone MP, et al. Postoperative contrast enhancement in patients with brain tumor. Ann Neurol. 1985;17:570–572. doi: 10.1002/ana.410170607. [DOI] [PubMed] [Google Scholar]
- 15.Sato N, Bronen RA, Sze G, et al. Postoperative changes in the brain: MR imaging findings in patients without neoplasms. Radiology. 1997;204:839–846. doi: 10.1148/radiology.204.3.9280269. [DOI] [PubMed] [Google Scholar]
- 16.Smith JS, Cha S, Mayo MC, et al. Serial diffusion-weighted magnetic resonance imaging in cases of glioma: Distinguishing tumor recurrence from postresection injury. J Neurosurg. 2005;103:428–438. doi: 10.3171/jns.2005.103.3.0428. [DOI] [PubMed] [Google Scholar]
- 17.Floeth FW, Aulich A, Langen KJ, et al. MR imaging and single-photon emission CT findings after gene therapy for human glioblastoma. AJNR Am J Neuroradiol. 2001;22:1517–1527. [PMC free article] [PubMed] [Google Scholar]
- 18.Smith MM, Thompson JE, Castillo M, et al. MR of recurrent high-grade astrocytomas after intralesional immunotherapy. AJNR Am J Neuroradiol. 1996;17:1065–1071. [PMC free article] [PubMed] [Google Scholar]
- 19.Matheus MG, Castillo M, Ewend M, et al. CT and MR imaging after placement of the GliaSite radiation therapy system to treat brain tumor: Initial experience. AJNR Am J Neuroradiol. 2004;25:1211–1217. [PMC free article] [PubMed] [Google Scholar]
- 20.Parney IF, Kunwar S, McDermott M, et al. Neuroradiographic changes following convection-enhanced delivery of the recombinant cytotoxin interleukin 13-PE38QQR for recurrent malignant glioma. J Neurosurg. 2005;102:267–275. doi: 10.3171/jns.2005.102.2.0267. [DOI] [PubMed] [Google Scholar]
- 21.Bernstein M, Laperriere N, Glen J, et al. Brachytherapy for recurrent malignant astrocytoma. Int J Radiat Oncol Biol Phys. 1994;30:1213–1217. doi: 10.1016/0360-3016(94)90331-x. [DOI] [PubMed] [Google Scholar]
- 22.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 24.Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: Efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70:779–787. doi: 10.1212/01.wnl.0000304121.57857.38. [DOI] [PubMed] [Google Scholar]
- 25.Raymond E, Brandes AA, Dittrich C, et al. Open label phase II study of imatinib in patients with recurrent gliomas of various histologies: A European Organisation for Research and Treatment of Cancer Brain Tumor Group Study. J Clin Oncol. 2008;26:4659–4665. doi: 10.1200/JCO.2008.16.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 27.Mandonnet E, Delattre JY, Tanguy ML, et al. Continuous growth of mean tumor diameter in a subset of grade II gliomas. Ann Neurol. 2003;53:524–528. doi: 10.1002/ana.10528. [DOI] [PubMed] [Google Scholar]
- 28.Stege EM, Kros JM, de Bruin HG, et al. Successful treatment of low-grade oligodendroglial tumors with a chemotherapy regimen of procarbazine, lomustine, and vincristine. Cancer. 2005;103:802–809. doi: 10.1002/cncr.20828. [DOI] [PubMed] [Google Scholar]
- 29.Brada M, Viviers L, Abson C, et al. Phase II study of primary temozolomide chemotherapy in patients with WHO grade II gliomas. Ann Oncol. 2003;14:1715–1721. doi: 10.1093/annonc/mdg371. [DOI] [PubMed] [Google Scholar]
- 30.Hoang-Xuan K, Capelle L, Kujas M, et al. Temozolomide as initial treatment for adults with low-grade oligodendrogliomas or oligoastrocytomas and correlation with chromosome 1p deletions. J Clin Oncol. 2004;22:3133–3138. doi: 10.1200/JCO.2004.10.169. [DOI] [PubMed] [Google Scholar]
- 31.Stummer W, Pichlmeier U, Meinel T, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 32.Chen W, Delaloye S, Silverman DH, et al. Predicting treatment response of malignant gliomas to bevacizumab and irinotecan by imaging proliferation with [18F] fluorothymidine positron emission tomography: A pilot study. J Clin Oncol. 2007;25:4714–4721. doi: 10.1200/JCO.2006.10.5825. [DOI] [PubMed] [Google Scholar]
- 33.Chen W, Silverman DH, Delaloye S, et al. 18F-FDOPA PET imaging of brain tumors: Comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. J Nucl Med. 2006;47:904–911. [PubMed] [Google Scholar]
- 34.van den Bent MJ, Afra D, De Witte O, et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: The EORTC 22845 randomised trial. Lancet. 2005;366:985–990. doi: 10.1016/S0140-6736(05)67070-5. [DOI] [PubMed] [Google Scholar]
- 35.van den Bent MJ, Carpentier AF, Brandes AA, et al. Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: A randomized European Organisation for Research and Treatment of Cancer phase III trial. J Clin Oncol. 2006;24:2715–2722. doi: 10.1200/JCO.2005.04.6078. [DOI] [PubMed] [Google Scholar]
- 36.Cairncross G, Berkey B, Shaw E, et al. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J Clin Oncol. 2006;24:2707–2714. doi: 10.1200/JCO.2005.04.3414. [DOI] [PubMed] [Google Scholar]
- 37.Ballman KV, Buckner JC, Brown PD, et al. The relationship between six-month progression-free survival and 12-month overall survival end points for phase II trials in patients with glioblastoma multiforme. Neuro Oncol. 2007;9:29–38. doi: 10.1215/15228517-2006-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lamborn KR, Yung WK, Chang SM, et al. Progression-free survival: An important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol. 2008;10:162–170. doi: 10.1215/15228517-2007-062. [DOI] [PMC free article] [PubMed] [Google Scholar]