Abstract
Purpose
This study investigated the utility of BRAF mutation testing of thyroid fine-needle aspiration biopsy (FNAB) specimens for preoperative risk stratification in papillary thyroid cancer (PTC).
Patients and Methods
We assessed the T1799A BRAF mutation status in thyroid FNAB specimens obtained from 190 patients before thyroidectomy for PTC and its association with clinicopathologic characteristics of the tumor revealed postoperatively.
Results
We observed a significant association of BRAF mutation in preoperative FNAB specimens with poorer clinicopathologic outcomes of PTC. In comparison with the wild-type allele, BRAF mutation strongly predicted extrathyroidal extension (23% v 11%; P = .039), thyroid capsular invasion (29% v 16%; P = .045), and lymph node metastasis (38% v 18%; P = .002). During a median follow-up of 3 years (range, 0.6 to 10 years), PTC persistence/recurrence was seen in 36% of BRAF mutation–positive patients versus 12% of BRAF mutation–negative patients, with an odds ratio of 4.16 (95% CI, 1.70 to 10.17; P = .002). The positive and negative predictive values for preoperative FNAB-detected BRAF mutation to predict PTC persistence/recurrence were 36% and 88% for overall PTC and 34% and 92% for conventional PTC, respectively.
Conclusion
Preoperative BRAF mutation testing of FNAB specimens provides a novel tool to preoperatively identify PTC patients at higher risk for extensive disease (extrathyroidal extension and lymph node metastases) and those who are more likely to manifest disease persistence/recurrence. BRAF mutation, as a powerful risk prognostic marker, may therefore be useful in appropriately tailoring the initial surgical extent for patients with PTC.
INTRODUCTION
Epithelial cell–derived differentiated thyroid cancer is the most common endocrine malignancy with a rapidly increasing incidence worldwide,1–4 an estimated incidence of 37,340 cases in the United States in 2008, and prevalence of more than 300,000 cases.4 The vast majority are papillary thyroid cancer (PTC), which accounts for 80% to 90% of all thyroid malignancies.1,2,5 Although PTC is generally indolent and curable, disease persistence/recurrence is common and is associated with increased mortality.6–11 Therefore, the extent of initial surgical management of PTC should be calibrated to reduce its persistence/recurrence and mortality while minimizing potential adverse outcomes associated with extensive surgery. However, there have been unresolved controversies regarding the optimal initial surgical management of PTC, largely due to imprecise preoperative information on the aggressiveness level of the tumor.12–16
Since its initial description in thyroid cancer,17,18 the oncogenic T1799A BRAF mutation has been widely found in PTC and in some anaplastic thyroid cancers from patients of various ethnic backgrounds, with a prevalence of approximately 45% in PTC.19 Recent studies have established a strong association of BRAF mutation with aggressive clinicopathologic characteristics of primary PTC, including extrathyroidal extension, lymph node metastasis, histologic subtypes with a poorer prognosis (eg, tall cell variant PTC), and advanced disease stages, as well as disease persistence/recurrence.19–22 On the basis of these data, we hypothesized that preoperative BRAF mutation testing of fine-needle aspiration biopsy (FNAB) specimens would be an informative preoperative risk stratification strategy for predicting the extent of initial disease and subsequent clinical outcomes to assist in defining the optimal scope of initial surgical and medical managements for PTC. This study was conducted to test our hypothesis by assessing BRAF mutation status in FNAB specimens obtained before thyroidectomy, and determining its association with the surgical pathologic findings and clinical outcomes in a large series of patients with PTC.
PATIENTS AND METHODS
Patients, FNAB Specimens, and Clinicopathologic Data
With the approval of our institutional review board and patient consent, where required, we studied 190 patients with PTC who had been treated and observed at the Johns Hopkins Hospital (Baltimore, MD) from 1994 to 2007—134 patients with conventional PTC, 41 with follicular variant PTC, and 15 with tall cell variant PTC—and who were stratified on the basis of final histopathologic examination of surgical specimens. Thyroid FNAB was performed on the primary tumor in these patients, either in the outpatient clinic with ultrasound guidance or in the operating room before total thyroidectomy. FNAB specimens, either fresh or retrieved from archived samples, were processed, and genomic DNA was isolated as described previously.23,24
All patients underwent total or near-total thyroidectomy. Cervical lymphadenectomy was typically performed for treatment of lymph nodes that were suggestive of abnormality on intraoperative examination. Clinicopathologic data were collected at the Johns Hopkins Hospital as described previously.25 Briefly, electronic clinical and pathologic records were retrospectively reviewed to collect the clinicopathologic information (eg, age at initial diagnosis, patient's sex, primary tumor size, extrathyroidal extension, lymph node metastasis, and multifocality) listed in Table 1. Disease staging was performed according to the criteria of the American Joint Committee for Cancer. Patients usually received radioiodine (iodine-131 [131I]) ablation treatments within 1 to 2 months after total thyroidectomy. Patients were then routinely observed as outpatients at the Johns Hopkins Hospital, typically every 6 to 12 months, and were evaluated for cancer persistence/recurrence with serum thyroglobulin testing, 131I whole-body scan, and/or other imaging studies—most commonly, ultrasonography. FNAB was performed for masses suggestive of tumor detected radiographically. As described previously,25 disease persistence/recurrence was established by a detectable serum thyroglobulin level, positive 131I body scan, and/or a tumor mass confirmed via FNAB cytology or final surgical pathology. Patients who did not fall into this category were regarded as disease free. Cancer persistence was defined as continuous disease existence, and cancer recurrence was defined as disease reappearance after being declared disease free by the above criteria. The group with disease persistence included four patients with distant metastases to the lungs that remained detectable throughout the follow-up course. The clinical follow-up time was defined as the time from the initial treatment to the first evidence of disease persistence/recurrence and in the group without recurrence, to the most recent clinical follow-up visit. Because the first medical follow-up visit for patients with PTC at our institution was usually 6 months after initial treatments, we chose 7 months as the starting point for follow-up time.
Table 1.
Association of BRAF Mutation Status Detected on Thyroid Fine-Needle Aspiration Biopsy With Poorer Clinicopathologic Characteristics of Papillary Thyroid Cancer
| Characteristic |
BRAF Positive (n = 73) |
BRAF Negative (n = 117) |
P* | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age at diagnosis, years | .28 | ||||
| Median | 43 | 46 | |||
| Range | 24-77 | 12-83 | |||
| Sex, male | 24 | 32.9 | 29 | 24.8 | .25 |
| Tumor size, cm | .35 | ||||
| Median | 1.8 | 1.5 | |||
| Range | 0.6-10.0 | 0.6-6.0 | |||
| Extrathyroidal extension | 17 | 23.3 | 13 | 11.1 | .039 |
| Capsular invasion | 21 | 28.8 | 19 | 16.2 | .045 |
| Lymph node metastasis | 28 | 38.4 | 21 | 18.0 | .002 |
| AJCC stages | .14 | ||||
| I | 54 | 74.0 | 89 | 76.1 | |
| II | 4 | 5.5 | 15 | 12.8 | |
| III | 9 | 12.3 | 9 | 7.7 | |
| IV | 6 | 8.2 | 4 | 3.4 | |
| III/IV | 15 | 20.6 | 13 | 11.1 | .093 |
| Multifocality | 34 | 46.6 | 47 | 40.2 | .45 |
Abbreviation: AJCC, American Joint Committee on Cancer.
P value from Fisher's exact test for categorical data and Wilcoxon rank sum test for continuous data.
BRAF Mutation Analysis
For the analysis of the T1799A BRAF mutation on FNAB specimens, we used a sensitive and specific colorimetric mutation detection assay (Mutector; TrimGen, Sparks, MD). The principles and reliability of this assay in detecting mutations were documented previously.26 The high sensitivity and specificity of this method for detecting the BRAF mutation have been particularly well established for thyroid FNAB specimens.23,24,27 Exon 15 of the BRAF gene containing the site where T1799A mutation occurs was first amplified by polymerase chain reaction, followed by colorimetric assay using the Mutector kit, as described previously.23,24,27 Color development in the enzymatic reaction in this assay, which defined the positive result, was quantified by a microplate reader at a wavelength of 405 nm to confirm the result. This approach previously revealed a high concordance of the BRAF mutation status between the FNAB specimens and the corresponding primary PTC tumors.23 In 38 cases of FNAB specimens in this study for which we had matched surgical tumor specimens, 37 (97.4%) of 38 patients were concordant for the BRAF status; one patient showed BRAF mutation in the surgical specimen but wild-type allele in matched FNAB specimens, possibly because of an insufficient sampling of PTC cells via FNAB.
Statistical Analysis
Categorical data were summarized with frequencies and percentages, and continuous data were summarized with medians and ranges. BRAF mutation status groups were compared using Fisher's exact test for categorical data and the nonparametric Wilcoxon rank sum test for continuous measures. Analysis of cancer persistence/recurrence was limited to those patients who had 7 or more months of follow-up. Sensitivity, specificity, and positive and negative predictive values of the FNAB-assessed BRAF mutation status for cancer persistence/recurrence were calculated using standard formulas. Univariate and multivariate logistic regression analyses were performed to estimate the odds ratios (ORs) of clinicopathologic characteristics and persistence/recurrence of disease for BRAF mutation–positive patients relative to BRAF mutation–negative patients, independent of other prognostic factors. Kaplan-Meier survival analysis and standard log-rank test were used to evaluate the effect of BRAF mutation on cancer persistence/recurrence. CIs were computed by standard methods. All reported P values are two sided. Analysis was performed using SAS version 9.1.3 software (SAS Institute Inc, Cary, NC).
RESULTS
Association of BRAF Mutation in FNAB Specimens With Poorer Clinicopathologic Characteristics of PTC
We examined the BRAF mutation status on preoperative FNAB specimens from 190 PTC patients and analyzed its relationship with clinicopathologic characteristics of the patients. BRAF mutation was found in 61 (45%) of 134 conventional PTC patients, five (12%) of 41 follicular variant PTC patients, and seven (47%) of 15 tall cell PTC patients, with an overall prevalence of 38%. As previously shown for BRAF mutation examined in excised surgical pathology tissues of PTC, BRAF mutation examined in FNAB specimens, in comparison with the wild-type allele, strongly predicted extrathyroidal extension (23% v 11%; P = .039), thyroid capsular invasion (29% v 16%; P = .045), and lymph node metastasis (38% v 18%; P = .002; Table 1). When the subgroup of 134 patients with conventional PTC was analyzed, BRAF mutation was still significantly associated with lymph node metastasis (39% v 19%; P = .012). The risk factors readily available before surgery (ie, patient age and sex) were not associated with the BRAF mutation status of tumors (Table 1). The patients with tall cell PTC were not separately analyzed because of their small number.
We additionally examined the relationship between BRAF mutation and PTC persistence/recurrence after the primary treatment in patients who had been observed for 7 or more months. BRAF mutation was strongly associated with PTC persistence/recurrence in these patients, who had been observed for a median of 3 years (range, 0.6 to 10 years) after the primary thyroid surgery (Table 2). Specifically, PTC persistence/recurrence was seen in 36% of BRAF mutation–positive patients versus 12% of BRAF mutation–negative patients (P = .002). This association remained highly significant in the subgroup of patients with conventional PTC alone, with tumor persistence/recurrence observed in 34% of BRAF mutation–positive patients versus 8% of BRAF mutation–negative patients (P = .004; Table 2). Postoperative 131I treatment did not explain this difference in disease persistence/recurrence, because BRAF mutation–positive patients had actually received a higher mean 131I dose postoperatively, which may have been prompted by their presentation with more advanced disease, such as lymph node metastasis and extrathyroidal extension (Table 1).
Table 2.
Association of BRAF Mutation Status Detected on Thyroid Fine-Needle Aspiration Biopsy With Persistence/Recurrence of Papillary Thyroid Cancer
| Characteristic |
BRAF Positive |
BRAF Negative |
P* | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| All types of PTC | |||||
| No. of patients | 53 | 76 | |||
| Recurrence/persistence | 19 | 35.8 | 9 | 11.8 | .002 |
| 131I dose, mCi | .01 | ||||
| Median | 100 | 79 | |||
| Range | 0-211 | 0-204 | |||
| Follow-up, months | .73 | ||||
| Median | 24 | 26 | |||
| Range | 7-114 | 7-116 | |||
| Follow-up, months† | .17 | ||||
| Median | 36 | 30 | |||
| Range | 9-114 | 7-116 | |||
| Conventional PTC | |||||
| No. of patients | 44 | 48 | |||
| Recurrence/persistence | 15 | 34.1 | 4 | 8.3 | .004 |
| 131I dose, mCi | .014 | ||||
| Median | 100 | 77 | |||
| Range | 0-209 | 0-204 | |||
| Follow-up, months | .75 | ||||
| Median | 26 | 29 | |||
| Range | 7-114 | 7-116 | |||
| Follow-up, months† | .32 | ||||
| Median | 36 | 31 | |||
| Range | 9-114 | 7-116 | |||
Abbreviations: PTC, papillary thyroid cancer; 131I, iodine-131.
P value from Fisher's exact test for categorical data and Wilcoxon rank sum test for continuous data.
Follow-up time for those who have not had recurrence.
In the overall PTC analysis based on data in Table 2, the sensitivity and specificity for FNAB-detected BRAF mutation to predict PTC persistence/recurrence were 68% (95% CI, 48% to 84%) and 66% (95% CI, 56% to 75%), respectively. The positive and negative predictive values were 36% (95% CI, 23% to 50%) and 88% (95% CI, 79% to 94%), respectively. In the analysis of conventional PTC only, the sensitivity and specificity for FNAB-detected BRAF mutation to predict disease persistence/recurrence were 79% (95% CI, 54% to 94%) and 60% (95% CI, 48% to 72%), respectively. The positive and negative predictive values for FNAB-detected BRAF mutation to predict persistence/recurrence of conventional PTC were 34% (95% CI, 20% to 50%) and 92% (95% CI, 80% to 98%), respectively.
High Preoperative Prognostic Value of BRAF Mutation for Poorer Clinicopathologic Outcomes of PTC
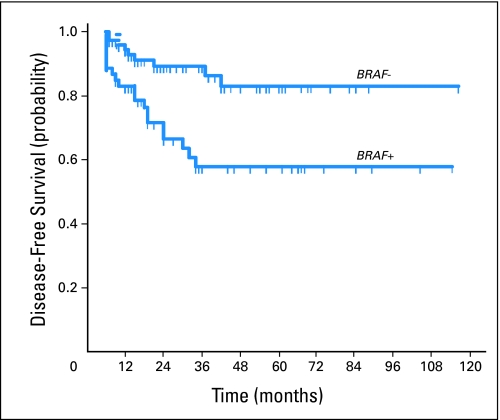
To additionally confirm the preoperative prognostic value of BRAF mutation documented in FNAB specimens of PTC, we performed a logistic regression analysis. Patients with BRAF mutation were two to three times as likely to have extrathyroidal extension, capsular invasion, and lymph node metastasis as those without (Table 3). A more than four-fold increase in the odds of PTC persistence/recurrence in patients with BRAF mutation–positive tumors was demonstrated. When adjustment was made for age and sex of the patients (the only two risk factors that can be definitely known preoperatively), the association of BRAF mutation with these clinicopathologic characteristics remained highly significant. For disease persistence/recurrence, additional adjustment was made for other potential clinicopathologic confounding factors, including extrathyroidal extension, lymph node metastasis, and other tumor characteristics as well as 131I treatment. BRAF mutation–positive patients were still significantly more likely (OR, 3.06; 95% CI, 1.10 to 8.47; P = .032) to have PTC persistence/recurrence (Table 3). Finally, the prognostic value of BRAF mutation on FNAB specimens for PTC persistence/recurrence was demonstrable as significantly reduced disease-free probability by Kaplan-Meier analysis (Fig 1).
Table 3.
Unadjusted and Adjusted Odds Ratios and 95% CIs of BRAF Mutation on Clinicopathologic Characteristics in Patients With Papillary Thyroid Cancer
| Characteristic | Unadjusted OR | 95% CI | P | Adjusted OR* | 95% CI | P |
|---|---|---|---|---|---|---|
| Extrathyroidal extension | 2.43 | 1.10 to 5.36 | 0.028 | 2.41 | 1.06 to 5.48 | .036 |
| Capsular invasion | 2.08 | 1.03 to 4.22 | 0.042 | 1.99 | 0.96 to 4.09 | .063 |
| Lymph node metastasis | 2.84 | 1.46 to 5.54 | 0.002 | 2.58 | 1.26 to 5.27 | .009 |
| AJCC stage III/IV | 2.07 | 0.92 to 4.65 | 0.078 | 1.91 | 0.83 to 4.39 | .13 |
| Persistence/recurrence | 4.16 | 1.70 to 10.17 | 0.002 | 5.16 | 1.97 to 13.48 | < .001 |
| Persistence/recurrence† | 3.06 | 1.10 to 8.47 | .032 |
Abbreviations: OR, odds ratio; AJCC, American Joint Committee on Cancer.
Tumor stage adjusted for sex; all others adjusted for age and sex.
Additional adjustment for multifocality, tumor stage, lymph node metastasis, extrathyroidal extension, tumor size, and iodine-131 dose.
Fig 1.
Kaplan-Meier estimate of papillary thyroid cancer disease-free probability in patients with or without BRAF mutation. Short vertical lines indicate censored observations (months of follow-up for those who have not had recurrence/persistence; standard log-rank test; χ2 = 9.2; P = .002).
DISCUSSION
The majority of patients with PTC are effectively treated with thyroidectomy and selective use of postoperative 131I therapy, but disease persistence/recurrence is common after initial treatments.6–11 Persistent/recurrent thyroid cancer is associated with increased morbidity and mortality. Surgery for persistent/recurrent thyroid cancer is more difficult to perform because of an increased incidence of surgical complications, including recurrent laryngeal nerve injury and hypoparathyroidism, particularly when there is a need to re-explore the central neck compartment.28–31 The extent of initial surgery for PTC can have a significant impact on disease recurrence.16 For example, total thyroidectomy, as opposed to lobectomy, can reduce disease persistence/recurrence or mortality, given that multifocality of PTC is common.32–38 BRAF mutation is often found in multiple PTC foci in the same patient,39,40 although this mutation does not seem to promote the occurrence of multifocality of PTC, as shown previously19 and in this study. Therefore, a remaining mutation-harboring PTC after subtotal thyroidectomy may potentially progress in an aggressive manner. On the other hand, total thyroidectomy is associated with an increased risk of surgical complications. Similarly, it is generally agreed that therapeutic neck dissection should be performed to remove macroscopic lymph node metastases because this reduces the chance of PTC persistence/recurrence, but whether routine prophylactic central neck dissection warrants the greater risk of complications remains debatable.29,30,33-36,38,41,42 These controversies are, to a large extent, due to the imprecision in the risk estimation of thyroid cancer aggressiveness based on the clinical and testing information available preoperatively. Pathologic characteristics of PTC shown to predict a poorer prognosis with increased disease persistence/recurrence and mortality, such as extrathyroidal extension, lymph node metastasis, advanced tumor stage, and aggressive histological subtypes (eg, tall cell PTC),6–11 are typically unknown or poorly defined preoperatively. The utility of preoperative ultrasonography to evaluate disease aggressiveness of PTC is often limited, particularly for lymph node metastasis in the central neck compartment,43–45 which is the most common source for PTC persistence/recurrence. Consequently, a novel parameter, such as the BRAF mutation, for preoperative risk estimation could be extremely valuable in the management of PTC.
This study demonstrates that testing for the BRAF mutation on FNAB specimens of PTC could preoperatively predict poorer clinicopathologic outcomes. Numerous studies on primary PTC tumors from patients of various ethnic and geographic backgrounds have demonstrated the association of the BRAF mutation with aggressive clinicopathologic characteristics of PTC, such as extrathyroidal extension, lymph node metastasis, advanced tumor stage, and tumor persistence/recurrence.19 Several multivariate analyses demonstrated that the presence of BRAF mutation in PTC is an independent risk factor for disease persistence/recurrence.25,46 Strong molecular bases have been established for these associations, including BRAF mutation–associated overexpression of various tumor promoting genes and silencing of tumor suppressor genes, as well as aberrant silencing of thyroid iodide-handling genes and hence loss of 131I avidity, rendering PTC cells insensitive to 131I treatment with a consequently increased cancer persistence/recurrence rate.19 Transgenic mouse models have also demonstrated the ability of BRAF mutation to promote the aggressiveness and progression of PTC.47
BRAF mutation can be readily determined on cytologic materials from thyroid FNAB specimens. This testing has been shown to have limited diagnostic value because of the low sensitivity of BRAF mutation when used in cytologically indeterminate specimens that are mostly non-PTC and therefore do not harbor BRAF mutation.19 However, the present study demonstrates the prognostic power of preoperative testing for the BRAF mutation on FNAB specimens for PTC. It is particularly important to note that BRAF mutation in these FNAB specimens preoperatively predicted lymph node metastases, as it has been widely shown to do in studies that use archived primary PTC tumor tissue.19 In this study, prophylactic central neck dissection was not routinely performed; it was reserved mainly for grossly enlarged lymph nodes. Consequently, many metastatic lymph nodes in BRAF mutation–positive patients were likely left unresected. Therefore, the strength of association of BRAF mutation with lymph node metastasis revealed in this study was likely underestimated. This possibility was strongly supported by the result that the OR associated with BRAF mutation for PTC persistence/recurrence was much higher than that for lymph node metastasis (Table 3). Lymph node metastasis is the most important risk factor associated with PTC persistence/recurrence, and regional neck dissection to remove involved lymph nodes has been repeatedly shown to reduce disease persistence/recurrence.30,35,36,38,48 Thus, preoperative knowledge of BRAF mutation status may be particularly useful in guiding surgical decision making about prophylactic central neck dissection. Preoperative knowledge of BRAF mutation status may also help define the optimal extent of initial thyroidectomy (eg, total thyroidectomy v lobectomy) in appropriate clinical settings.
Preoperative BRAF mutation analysis can also have an impact on decision making regarding the conventional elements of postoperative medical treatments, including 131I remnant ablation and long-term thyrotropin suppression.9,34,49,50 It has long been controversial, on the basis of conventional clinicopathologic criteria, whether to treat apparently low-risk patients with 131I and how intensely to suppress their serum thyrotropin level. Given the significant association of BRAF mutation with PTC persistence/recurrence, even in conventionally low-risk patients,19 preoperative knowledge of this mutation may help physicians more appropriately plan immediate postoperative 131I ablation therapy at an early stage, perhaps with a somewhat higher 131I dose given the association of BRAF mutation with decreased 131I avidity of PTC.25,51,52 For the same reason, preoperative knowledge of BRAF mutation may facilitate early planning for appropriately aggressive thyrotropin suppression therapy and vigilant follow-up of patients with PTC. This approach may be particularly helpful and practically feasible for apparently low-stage PTC, which harbors BRAF mutation in only one third of the patients.19
Given that recurrence is the primary concern in managing differentiated PTC in most patients (given the extremely low mortality), the positive predictive value of 36% shown in this study for BRAF mutation to predict PTC recurrence is of practical importance. An OR of 4 to 5 for preoperative BRAF mutation testing on FNAB to predict PTC recurrence also represents a remarkable prognostic power, which no other known current predictors, including the conventionally used preoperative clinical factors and imaging studies, could match. Moreover, a high negative predictive value of approximately 90% for BRAF mutation to predict PTC recurrence should add to the usefulness of BRAF mutation in preoperative risk stratification of PTC. Thus, a positive BRAF mutation test should favor more aggressive surgery, such as prophylactic dissection of lymph nodes in the central neck, whereas a negative preoperative BRAF mutation test should favor less aggressive surgery, such as sparing of prophylactic central neck dissection. Taking into consideration specific conventional clinicopathologic risk factors in individual patients should enhance the accuracy of this BRAF mutation-based risk stratification and decision making for the management of PTC.
In summary, we investigated the utility of preoperative testing for the BRAF mutation on FNAB specimens as a tool to preoperatively predict more extensive disease at surgery and poorer subsequent clinical outcomes of PTC. This novel approach to preoperative risk stratification will likely have an important impact on the management of patients with PTC by better balancing the risks and benefits of aggressive initial surgical and medical treatments. Using the BRAF mutation for risk stratification may be particularly helpful in areas where controversies remain regarding the optimal type and extent of initial treatments of PTC. Efforts are needed to define specific indications and practice guidelines for such clinical use of BRAF mutation in the management of PTC.
Footnotes
Supported by an R0-1 grant from the National Institutes of Health.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Mingzhao Xing
Financial support: Mingzhao Xing
Administrative support: Mingzhao Xing
Provision of study materials or patients: Mingzhao Xing, Douglas Clark, Alan Dackiw, Matthew Kim, Anthony Tufaro, Paul Ladenson, Martha Zeiger, Ralph Tufano
Collection and assembly of data: Mingzhao Xing, Haixia Guan, Meiju Ji
Data analysis and interpretation: Mingzhao Xing, Haixia Guan, Meiju Ji, Kathryn A. Carson
Manuscript writing: Mingzhao Xing, Douglas Clark, Kathryn A. Carson, Paul Ladenson, Martha Zeiger, Ralph Tufano
Final approval of manuscript: Mingzhao Xing, Douglas Clark, Haixia Guan, Meiju Ji, Alan Dackiw, Kathryn A. Carson, Matthew Kim, Anthony Tufaro, Paul Ladenson, Martha Zeiger, Ralph Tufano
REFERENCES
- 1.Leenhardt L, Grosclaude P, Cherie-Challine L. Increased incidence of thyroid carcinoma in France: A true epidemic or thyroid nodule management effects? Report from the French Thyroid Cancer Committee. Thyroid. 2004;14:1056–1060. doi: 10.1089/thy.2004.14.1056. [DOI] [PubMed] [Google Scholar]
- 2.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 3.Mazzaferri EL. An overview of the management of thyroid cancer. In: Mazzaferri EL, Harmer C, Mallick UK, et al., editors. Practical Management of Thyroid Cancer: A Multidisciplinary Approach. London, United Kingdom: Springer-Verlag; 2006. pp. 1–28. [Google Scholar]
- 4.Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2005. Bethesda, MD: National Cancer Institute; 2007. http://seer.cancer.gov/csr/1975_2005/ [Google Scholar]
- 5.Hundahl SA, Fleming ID, Fremgen AM, et al. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985-1995. Cancer. 1998;83:2638–2648. doi: 10.1002/(sici)1097-0142(19981215)83:12<2638::aid-cncr31>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 6.Fonseca E, Soares P, Rossi S, et al. Prognostic factors in thyroid carcinomas. Verh Dtsch Ges Pathol. 1997;81:82–96. [PubMed] [Google Scholar]
- 7.Gilliland FD, Hunt WC, Morris DM, et al. Prognostic factors for thyroid carcinoma: A population-based study of 15,698 cases from the Surveillance, Epidemiology and End Results (SEER) program 1973-1991. Cancer. 1997;79:564–573. doi: 10.1002/(sici)1097-0142(19970201)79:3<564::aid-cncr20>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 8.Hay ID, Bergstralh EJ, Goellner JR, et al. Predicting outcome in papillary thyroid carcinoma: Development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993;114:1050–1057. [PubMed] [Google Scholar]
- 9.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–428. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 10.Sherman SI, Brierley JD, Sperling M, et al. Prospective multicenter study of thyroid carcinoma treatment: Initial analysis of staging and outcome: National Thyroid Cancer Treatment Cooperative Study Registry Group. Cancer. 1998;83:1012–1021. doi: 10.1002/(sici)1097-0142(19980901)83:5<1012::aid-cncr28>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 11.LiVolsi VA, Fadda G, Baloch ZW. Prognostic factors in well-differentiated thyroid cancer. Rays. 2000;25:163–175. [PubMed] [Google Scholar]
- 12.Cooper DS, Doherty GM, Haugen BR, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006;16:109–142. doi: 10.1089/thy.2006.16.109. [DOI] [PubMed] [Google Scholar]
- 13.Doherty GM. Routine central neck lymph node dissection for thyroid carcinoma. Surgery. 2006;140:1007–1008. doi: 10.1016/j.surg.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Kebebew E, Duh QY, Clark OH. Total thyroidectomy or thyroid lobectomy in patients with low-risk differentiated thyroid cancer: Surgical decision analysis of a controversy using a mathematical model. World J Surg. 2000;24:1295–1302. doi: 10.1007/s002680010215. [DOI] [PubMed] [Google Scholar]
- 15.Sherman SI, Angelos P, Ball DW, et al. Thyroid carcinoma. J Natl Compr Canc Netw. 2007;5:568–621. doi: 10.6004/jnccn.2007.0052. [DOI] [PubMed] [Google Scholar]
- 16.Witt RL. Initial surgical management of thyroid cancer. Surg Oncol Clin N Am. 2008;17:71–91. doi: 10.1016/j.soc.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Cohen Y, Xing M, Mambo E, et al. BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst. 2003;95:625–627. doi: 10.1093/jnci/95.8.625. [DOI] [PubMed] [Google Scholar]
- 18.Kimura ET, Nikiforova MN, Zhu Z, et al. High prevalence of BRAF mutations in thyroid cancer: Genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003;63:1454–1457. [PubMed] [Google Scholar]
- 19.Xing M. BRAF mutation in papillary thyroid cancer: Pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007;28:742–762. doi: 10.1210/er.2007-0007. [DOI] [PubMed] [Google Scholar]
- 20.Abubaker J, Jehan Z, Bavi P, et al. Clinicopathological analysis of papillary thyroid cancer with PIK3CA alterations in a Middle Eastern population. J Clin Endocrinol Metab. 2008;93:611–618. doi: 10.1210/jc.2007-1717. [DOI] [PubMed] [Google Scholar]
- 21.Frasca F, Nucera C, Pellegriti G, et al. BRAF(V600E) mutation and the biology of papillary thyroid cancer. Endocr Relat Cancer. 2008;15:191–205. doi: 10.1677/ERC-07-0212. [DOI] [PubMed] [Google Scholar]
- 22.Nakayama H, Yoshida A, Nakamura Y, et al. Clinical significance of BRAF (V600E) mutation and Ki-67 labeling index in papillary thyroid carcinomas. Anticancer Res. 2007;27:3645–3649. [PubMed] [Google Scholar]
- 23.Cohen Y, Rosenbaum E, Clark DP, et al. Mutational analysis of BRAF in fine needle aspiration biopsies of the thyroid: A potential application for the preoperative assessment of thyroid nodules. Clin Cancer Res. 2004;10:2761–2765. doi: 10.1158/1078-0432.ccr-03-0273. [DOI] [PubMed] [Google Scholar]
- 24.Xing M, Tufano RP, Tufaro AP, et al. Detection of BRAF mutation on fine needle aspiration biopsy specimens: A new diagnostic tool for papillary thyroid cancer. J Clin Endocrinol Metab. 2004;89:2867–2872. doi: 10.1210/jc.2003-032050. [DOI] [PubMed] [Google Scholar]
- 25.Xing M, Westra WH, Tufano RP, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005;90:6373–6379. doi: 10.1210/jc.2005-0987. [DOI] [PubMed] [Google Scholar]
- 26.Benoit NE, Goldenberg D, Deng SX, et al. A colorimetric approach to high-throughput mutation analysis. Biotechniques. 2005;38:635–639. doi: 10.2144/05384PF01. [DOI] [PubMed] [Google Scholar]
- 27.Jin L, Sebo TJ, Nakamura N, et al. BRAF mutation analysis in fine needle aspiration (FNA) cytology of the thyroid. Diagn Mol Pathol. 2006;15:136–143. doi: 10.1097/01.pdm.0000213461.53021.84. [DOI] [PubMed] [Google Scholar]
- 28.Wilson DB, Staren ED, Prinz RA. Thyroid reoperations: Indications and risks. Am Surg. 1998;64:674–678. [PubMed] [Google Scholar]
- 29.White ML, Gauger PG, Doherty GM. Central lymph node dissection in differentiated thyroid cancer. World J Surg. 2007;31:895–904. doi: 10.1007/s00268-006-0907-6. [DOI] [PubMed] [Google Scholar]
- 30.White ML, Doherty GM. Level VI lymph node dissection for papillary thyroid cancer. Minerva Chir. 2007;62:383–393. [PubMed] [Google Scholar]
- 31.Wingert DJ, Friesen SR, Ilinopoulos JI, et al. Post-thyroidectomy hypocalcemia: Incidence and risk factors. Am J Surg. 1986;152:606–610. doi: 10.1016/0002-9610(86)90435-6. [DOI] [PubMed] [Google Scholar]
- 32.Hay ID, Grant CS, Taylor WF, et al. Ipsilateral lobectomy versus bilateral lobar resection in papillary thyroid carcinoma: A retrospective analysis of surgical outcome using a novel prognostic scoring system. Surgery. 1987;102:1088–1095. [PubMed] [Google Scholar]
- 33.Kouvaraki MA, Lee JE, Shapiro SE, et al. Preventable reoperations for persistent and recurrent papillary thyroid carcinoma. Surgery. 2004;136:1183–1191. doi: 10.1016/j.surg.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 34.Jonklaas J, Sarlis NJ, Litofsky D, et al. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid. 2006;16:1229–1242. doi: 10.1089/thy.2006.16.1229. [DOI] [PubMed] [Google Scholar]
- 35.Ito Y, Higashiyama T, Takamura Y, et al. Risk factors for recurrence to the lymph node in papillary thyroid carcinoma patients without preoperatively detectable lateral node metastasis: Validity of prophylactic modified radical neck dissection. World J Surg. 2007;31:2085–2091. doi: 10.1007/s00268-007-9224-y. [DOI] [PubMed] [Google Scholar]
- 36.Farrag TY, Agrawal N, Sheth S, et al. Algorithm for safe and effective reoperative thyroid bed surgery for recurrent/persistent papillary thyroid carcinoma. Head Neck. 2007;29:1069–1074. doi: 10.1002/hed.20634. [DOI] [PubMed] [Google Scholar]
- 37.Bilimoria KY, Bentrem DJ, Ko CY, et al. Extent of surgery affects survival for papillary thyroid cancer. Ann Surg. 2007;246:375–381. doi: 10.1097/SLA.0b013e31814697d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sosa JA, Udelsman R. Total thyroidectomy for differentiated thyroid cancer. J Surg Oncol. 2006;94:701–707. doi: 10.1002/jso.20695. [DOI] [PubMed] [Google Scholar]
- 39.Giannini R, Ugolini C, Lupi C, et al. The heterogeneous distribution of BRAF mutation supports the independent clonal origin of distinct tumor foci in multifocal papillary thyroid carcinoma. J Clin Endocrinol Metab. 2007;92:3511–3516. doi: 10.1210/jc.2007-0594. [DOI] [PubMed] [Google Scholar]
- 40.Jovanovic L, Delahunt B, McIver B, et al. Most multifocal papillary thyroid carcinomas acquire genetic and morphotype diversity through subclonal evolution following the intra-glandular spread of the initial neoplastic clone. J Pathol. 2008;215:145–154. doi: 10.1002/path.2342. [DOI] [PubMed] [Google Scholar]
- 41.Cheah WK, Arici C, Ituarte PH, et al. Complications of neck dissection for thyroid cancer. World J Surg. 2002;26:1013–1016. doi: 10.1007/s00268-002-6670-4. [DOI] [PubMed] [Google Scholar]
- 42.Henry JF, Gramatica L, Denizot A, et al. Morbidity of prophylactic lymph node dissection in the central neck area in patients with papillary thyroid carcinoma. Langenbecks Arch Surg. 1998;383:167–169. doi: 10.1007/s004230050111. [DOI] [PubMed] [Google Scholar]
- 43.Kouvaraki MA, Shapiro SE, Fornage BD, et al. Role of preoperative ultrasonography in the surgical management of patients with thyroid cancer. Surgery. 2003;134:946–954. doi: 10.1016/s0039-6060(03)00424-0. [DOI] [PubMed] [Google Scholar]
- 44.Stulak JM, Grant CS, Farley DR, et al. Value of preoperative ultrasonography in the surgical management of initial and reoperative papillary thyroid cancer. Arch Surg. 2006;141:489–494. doi: 10.1001/archsurg.141.5.489. [DOI] [PubMed] [Google Scholar]
- 45.Ito Y, Tomoda C, Uruno T, et al. Clinical significance of metastasis to the central compartment from papillary microcarcinoma of the thyroid. World J Surg. 2006;30:91–99. doi: 10.1007/s00268-005-0113-y. [DOI] [PubMed] [Google Scholar]
- 46.Kebebew E, Weng J, Bauer J, et al. The prevalence and prognostic value of BRAF mutation in thyroid cancer. Ann Surg. 2007;246:466–470. doi: 10.1097/SLA.0b013e318148563d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knauf JA, Ma X, Smith EP, et al. Targeted expression of BRAFV600E in thyroid cells of transgenic mice results in papillary thyroid cancers that undergo dedifferentiation. Cancer Res. 2005;65:4238–4245. doi: 10.1158/0008-5472.CAN-05-0047. [DOI] [PubMed] [Google Scholar]
- 48.Hughes CJ, Shaha AR, Shah JP, et al. Impact of lymph node metastasis in differentiated carcinoma of the thyroid: A matched-pair analysis. Head Neck. 1996;18:127–132. doi: 10.1002/(SICI)1097-0347(199603/04)18:2<127::AID-HED3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 49.Cooper DS, Specker B, Ho M, et al. Thyrotropin suppression and disease progression in patients with differentiated thyroid cancer: Results from the National Thyroid Cancer Treatment Cooperative Registry. Thyroid. 1998;8:737–744. doi: 10.1089/thy.1998.8.737. [DOI] [PubMed] [Google Scholar]
- 50.Sawka AM, Thephamongkhol K, Brouwers M, et al. Clinical review 170: A systematic review and metaanalysis of the effectiveness of radioactive iodine remnant ablation for well-differentiated thyroid cancer. J Clin Endocrinol Metab. 2004;89:3668–3676. doi: 10.1210/jc.2003-031167. [DOI] [PubMed] [Google Scholar]
- 51.Riesco-Eizaguirre G, Gutiérrez-Martínez P, García-Cabezas MA, et al. The oncogene BRAF V600E is associated with a high risk of recurrence and less differentiated papillary thyroid carcinoma due to the impairment of Na+/I-targeting to the membrane. Endocr Relat Cancer. 2006;13:257–269. doi: 10.1677/erc.1.01119. [DOI] [PubMed] [Google Scholar]
- 52.Mian C, Barollo S, Pennelli G, et al. Molecular characteristics in papillary thyroid cancers (PTCs) with no 131I uptake. Clin Endocrinol (Oxf) 2008;68:108–116. doi: 10.1111/j.1365-2265.2007.03008.x. [DOI] [PubMed] [Google Scholar]