Abstract
Purpose
To conduct a phase II trial in chemoresistant hairy cell leukemia (HCL) with BL22, a recombinant anti-CD22 immunotoxin which showed phase I activity in HCL.
Patients and Methods
Eligible patients had relapsed/refractory HCL and needed treatment based on blood counts. Patients were stratified into three groups: response to cladribine less than 1 year, those with a response lasting 1 to 4 years, or no response and uncontrolled infection. Patients received BL22 40 μg/kg every other day for three doses on cycle 1. Those achieving hematologic remission (HR), defined as neutrophils ≥ 1,500/mm3, hemoglobin ≥ 11 g/dL, and platelets ≥ 100,000/mm3, were observed. Patients without HR were re-treated at 30 μg/kg every other day for three doses every 4 weeks beginning at least 8 weeks after cycle 1.
Results
Thirty-six patients were enrolled including 26, nine, and one in groups 1 to 3. The response after one cycle (CR, 25%; PR, 25%) improved when 56% were re-treated (CR, 47%; PR, 25%). CR rate was similar in groups 1 and 2 (P = .7). Twenty-two with baseline spleen height lower than 200 mm had higher CR (64% v 21%; P = .019) and OR rates (95% v 36%; P = .0002) compared to 14 with spleens either absent or higher than 200 mm. The only serious toxicity was reversible grade 3 hemolytic uremic syndrome, not requiring plasmapheresis, in two patients (6%). High neutralizing antibodies were observed in four patients (11%) and prevented re-treatment.
Conclusion
BL22 activity in HCL is confirmed. Best responses to BL22 after cladribine failure are achieved before the patients develop massive splenomegaly or undergo splenectomy.
INTRODUCTION
Hairy cell leukemia (HCL) is a B-cell malignancy comprising 2% of all leukemias.1 It is highly sensitive to but not curable by treatment with cladribine2–4 and pentostatin,5,6 with complete remission (CR) rates of 80% to 95%. Both lack of plateau in the disease-free survival curve,3,6 and minimal residual disease (MRD) studies,7,8 suggest the eventual need for additional treatment in many patients. The anti-CD20 monoclonal antibody (Mab) rituximab has activity in relapsed patients with HCL; of 60 patients treated in four small trials, 18 CRs (30%) were reported.9–12
In patients with relapsed or refractory HCL after prior purine analogs, activity was reported in phase I testing of recombinant immunotoxin BL22.13,14 This fusion protein, containing the variable domains of the anti-CD22 Mab RFB4,15 and a 38 kDa form of Pseudomonas exotoxin called PE38,16 is cytotoxic toward CD22+ cell lines,17 leukemic cells from patients,18 and induced regressions of human tumors in mice.17,19 In a phase I trial, of 31 patients with HCL, BL22 induced 19 CRs (61%) and five partial responses (PRs; 19%). Achievement of CR required one cycle in 11 patients and two to 14 cycles in eight patients. The CR rate was 86% in patients enrolled at the upper dose levels, 40 to 50 μg/kg every other day for three doses. The major dose-limiting toxicity (DLT) was a completely reversible hemolytic uremic syndrome (HUS) occurring in four HCL patients (13%) during cycle 2 or 3 of BL22.
While DLT on phase I was only observed during re-treatment of HCL, 63% of CRs occurred after cycle 1. Thus, retreatment may not be required in all patients. Since retreatment during the phase I trial followed cycle 1 by as little as 3 to 4 weeks, the true effect of a single cycle of BL22 was not determined. The goals of the phase II study were to confirm the high response rate of BL22 in HCL using 40 μg/kg every other day for three doses for one cycle, and to limit toxicity by retreating at a reduced dose level, and only those patients without adequate response to one cycle.
PATIENTS AND METHODS
Eligibility
Patients had to have CD22+ HCL by flow cytometry after prior therapy with cladribine, with less than 2 years CR/PR after the first course or less than 4 years CR/PR to a second or later course. Eligibility required abnormal blood counts, with neutrophils fewer than 1,000/mm3, hemoglobin (Hgb) lower than 10 g/dL, platelets lower than 100,000/mm3, lymphocytes higher than 20,000/mm3, or symptomatic splenomegaly. Patients could not have high levels of neutralizing antibodies, defined as more than 75% neutralization of 1,000 ng/mL of BL22 in a cytotoxicity assay with Raji cells.14 There could be no chemotherapy within 4 weeks or monoclonal antibody within 3 months before enrollment. Eligibility required AST and ALT ≤ 2.5 times the normal upper limit (ULN), bilirubin ≤ 2.2× ULN, albumin ≥ 3 g/dL, and creatinine ≤ 1.4 mg/dL unless the creatinine clearance was ≥ 50 mL/min.
Study Design
Patients received BL22 over 30 minutes every other day for three doses. To prevent allergic reactions and fever, patients received oral hydroxyzine 25 mg and ranitidine 150 mg 1 hour before and 8 hours after each dose, acetaminophen 650 mg every 6 hours for four doses beginning 1 hour before each dose. To prevent hypovolemia and renal compromise from third spacing, patients received D5/0.45%NaCl over 2 to 4 hours before and after each dose, and during the first 8 days of each cycle received normal saline 1 L/d by portable pump when not receiving other intravenous fluid. The cycle 1 dose of BL22 was 40 μg/kg every other day for three doses. Patients with a hematologic remission (HR) by 8 weeks, defined as ANC, platelets, and Hgb at least 1.5 × 109/L, 100 ×109/L, and 11 g/dL, respectively, were not retreated and observed until relapse with blood counts low enough to meet initial eligibility criteria. Patients with high levels of neutralizing antibodies or progressive disease (PD) were not retreated. Retreatment was at 30 μg/kg every other day for three doses at ≥ 26-day intervals until two cycles after a CR without MRD, or until four cycles after a CR with MRD.
Response Criteria
CR required absence of HCL in blood and bone marrow using nonimmunologic stains, nonpalpable spleen and/or negative computed tomography (CT), and resolution of cytopenias. MRD in the bone marrow biopsy required CD20-positive cells ≥ CD3 positive cells, tartrate-resistant acid phosphatase–positive cells morphologically consistent with HCL, and more than 50% of the CD20-positive cells morphologically consistent with HCL. MRD in blood was defined as positive blood flow cytometry. PR required ≥ 50% reduction in tumor burden and improvement in cytopenias either to HR levels or ≥ 50% improvement in baseline neutrophils, platelets, and Hgb. PD was defined by ≥ 25% increase in measurable disease on CT by sum of perpendicular diameters, or a ≥ 25% increase in palpable hepatomegaly or splenomegaly, a ≥ 5 0% increase in circulating HCL cells, or a more than 25% treatment-unrelated decrease in Hgb if lower than 11 g/dL, platelets if lower than 100,000/mm3, or neutrophils if lower than 1,500/mm3. Stable disease (SD) was defined as the absence of either response or PD.
Definition of DLT
Toxicity grading was based on National Cancer Institute Common Toxicity Criteria version 3.0. BL22 was stopped for DLT, defined as at least grade 3 toxicity except for grade 3 fever, AST, ALT, gamma glutamyl transferase, grade 3 to 4 hematologic toxicity, and transient grade 3 proteinuria with grade 0 creatinine. Grade 3 HUS was defined as grade ≥ 1 creatinine increase associated with acute microangiopathic hemolytic anemia and/or thrombocytopenic puura, not requiring plasmapheresis and not associated with clinical sequelae.
RESULTS
Patient Characteristics
Thirty-six patients were prospectively stratified by response to last course of cladribine; 26 had less than 1 year CR/PR, nine had 1 to 4 year CR/PR, and one had uncontrolled infection and no response. There were 31 men and five women; their ages were 43 to 75 years (median, 56 years). The concentration of circulating HCL cells was 0.3 to 74,000 cells/mm3, and the median was 61/mm3. Patients had classic HCL which by flow cytometry was positive for CD20, CD22, CD19, CD25, CD11c, CD103, and surface immunoglobulin, except for three patients with HCL variant who were CD25 negative.
Patient Response
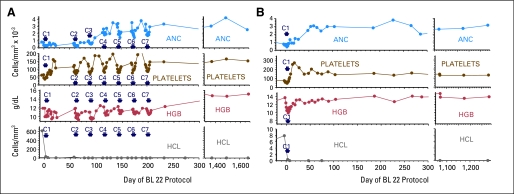
A total of 131 cycles of BL22 were administered to 36 patients. Sixteen patients (44%) received one cycle and 20 (56%) received two to 13 cycles (Fig 1). At 8 weeks, treatment was stopped for nine patients in CR (25%) and three patients in HR (8%). Two patients relapsed from HR, were re-treated, and returned to HR. Six patients (17%) were not retreated due to immunogenicity in two (6%), PD in two (6%), infection in one (3%), and HUS in one (3%). After retreatment of 56% of the patients, best response included 17 CRs (47%), five HRs (14%), and four PRs (11%) for an overall response rate (ORR) of 72%. At the time of CR, MRD was evident by marrow immunohistochemistry in three patients (18%), all of whom had received only one cycle of BL22. No patients had MRD in the blood by flow cytometry. Examples of CRs achieved after one to five cycles without MRD are shown in Figure 2.
Fig 1.
CONSORT flow diagram. Disposition of study participants. After cycle 1, patients with complete (CR) or hematologic remission (HR) were observed, and those with partial remission (PR) or stable disease (SD) were retreated if eligible. Patients ineligible for retreatment after PR or SD had dose-limiting toxicities, infection, or immunogenicity. QOD, every other day; PD, progressive disease.
Fig 2.
Patterns of response. (A) Complete response (CR) in patient BH03 requiring five cycles of BL22 before achieving negative marrow, then two consolidation cycles. (B) CR in patient BH11 after one cycle of BL22. Neither patient has relapsed after 36 to 46 months of follow-up. C, cycle; ANC, absolute neutrophil count; HGB, hemoglobin; HCL, hairy cell leukemia.
Duration of Response
Resolution of cytopenias to at least HR level was achieved by 22 patients (61%). At a median duration of follow-up of 26 months (range, 13 to 46), six HRs relapsed (Fig 3A). Decrease in counts to below HR level occurred in four of five with best response of HR compared to two of 17 with best response of CR (P = .009). As shown in Figure 3B, the median CR duration was not reached at 22+ months (range, 5 to 46+), and only four (24%) of 17 CRs relapsed. Three of 17 CRs did not clear MRD from the bone marrow biopsy, although only one of these patients relapsed from CR. As shown in Figure 3C, at a median follow-up of 18+ months (range, 4 to 46+) in CR, five additional patients became MRD+, three of whom relapsed from CR. Thus, despite withholding retreatment in patients achieving CR, most of the CRs are durable without MRD. Of eight patients requiring more than one cycle for CR, five received at least two consolidation cycles past the disappearance of MRD, and all five remain in CR, while three patients received fewer than two consolidation cycles and all three patients relapsed (P = .018). Thus patients requiring more than one cycle of BL22 for CR are probably at higher risk to relapse and need extra cycles to resolve MRD.
Fig 3.
Durability of response. Left axis show % of 36 patients remaining in (A) hematologic remission (HR), (B) complete remission (CR), and (C) CR without (W/O) MRD. Red bars indicate follow-up of nonrelapsing patients. Right axes indicate % of the 22, 17, and 14 patients originally achieving HR, CR, and CR without minimal residual disease, respectively, who remain relapse free.
Response With Respect to Clinical Factors
To determine if response was related to the degree of purine analog resistance, patients were prospectively stratified as having less than 1 or 1 to 4 years of response to last course of cladribine. As presented in Table 1, there is no significant relationship with respect to CR rate (P = .7), but there was a trend (P = .07) for higher ORR (100% v 65%) in patients with longer cladribine response. The relationship between response and spleen status was also examined. As presented in Table 2, CR rates were higher in patients with spleens smaller than 200 mm compared to patients with larger or absent spleens, either after just one cycle (41% v 0%; P = .006), or overall (64% v 21%; P = .019). Table 2 also shows that ORR, whether assessed after cycle 1 or after retreatment for best response, was significantly higher in patients with spleens smaller than 200 mm than in patients with either larger or absent spleens (P = .0002 to .017). Thus, spleen status was more important than cladribine resistance with respect to response to BL22. Nevertheless, CR occurred despite massive splenomegaly, in that patient BH01 had spleen height 250 mm before cycle 1 of BL22 and obtained CR with normal size spleen (125 mm) after six cycles.
Table 1.
Response Versus Cladribine Resistance (stratified groups)
| Group | Prior Cladribine Response | No. | % |
|||
|---|---|---|---|---|---|---|
| CR | HR | PR | ORR | |||
| 1 | < 1-year CR/PR to last cladribine | 26 | 46 | 8 | 12 | 65 |
| 2 | 1-4 year CR/PR to last cladribine | 9 | 56 | 33 | 11 | 100 |
| 3 | None, plus uncontrolled infection | 1 | 0 | 0 | 0 | 0 |
| Total | 36 | 47 | 14 | 11 | 72 | |
| P, groups 1 v 2 | .7 | .1 | 1.0 | .07 | ||
Abbreviations: CR, complete response; HR, hazard ratio; PR, partial response; ORR, overall response rate.
Table 2.
Response Related to Spleen Status
| Spleen Status (mm) | No. | Cycle 1 (%) |
P | Best Response (%) |
P | ||
|---|---|---|---|---|---|---|---|
| CR Rate | ORR | CR Rate | ORR | ||||
| CR | |||||||
| < 200 | 22 | 41 | 64 | ||||
| > 200 or absent | 14 | 0 | .006 | 21 | .019 | ||
| Total | 36 | 25 | 47 | ||||
| ORR | |||||||
| < 200 | 22 | 73 | 95 | ||||
| > 200 | 5 | 0 | .0057 | 20 | .001 | ||
| Absent | 9 | 22 | .017 | 44 | .004 | ||
| > 200 or absent | 14 | 14 | .0016 | 36 | .0002 | ||
| Total | 36 | 47 | 72 | ||||
NOTE. P values show Fisher's exact test versus spleen < 200 mm.
Abbreviations: CR, complete response; ORR, overall response rate.
Toxicity of BL22 During Phase II Resting
Adverse events were graded using the National Cancer Institute Common Toxicity Criteria Adverse events version 3.0. As shown in Figure 4, cycle 1 at 40 μg/kg every other day for three doses and retreatment at 30 μg/kg every other day for three doses gave very similar toxicity profiles, with the most common toxicities being grade 1 to 2 hypoalbuminemia, AST/ALT elevation, edema, myalgia, proteinuria, fatigue, nausea, and fever. Three (8%) of 36 patients had HUS, none of whom required plasmapheresis. Grade 3 HUS occurred in one patient after cycle 2, which was administered 11 weeks after cycle 1, and in another patient after cycle 1. Both of these patients had prior splenectomy. The third patient had grade 1 HUS along with CR during cycle 3 (30 μg/kg ×3). The patient was eligible for retreatment and HUS did not recur with cycles 4 to 7 given at 10 μg/kg for three doses. After relapse from CR the patient was treated by special exemption with 20 μg/kg for three doses and had grade 3 HUS with cycle 8, as well as another CR. In summary, BL22 was well tolerated with evidence of transient mild vascular leak syndrome in most patients, and completely reversible HUS in 8%, not requiring plasmapheresis. All grade 3-4 events shown in Figure 4 were reversible and were either nondose limiting (ie, ALT, AST, fever) or associated with HUS.
Fig 4.
Toxicity of BL22 on cycle 1 (left) and with retreatment (right). For each graph, grade 1 to 2 events occurring only once are not shown. LEA, lower extremity arterial; GGT, gamma glutamyl transferase; EFFU, effusion; ABD, abdominal; HEAD&N, head and neck; HUS, hemolytic uremic syndrome; ALK PHOS; alkaline phosphatase; SOB, shortness of breath.
Immunogenicity of BL22
To determine whether the bacterial toxin was recognized and neutralized by the immune system, BL22 at 1,000 ng/mL was incubated with 90% serum at 37°C, and incubated on Raji cells to determine the ability of the serum to neutralize its cytotoxicity.14 Greater than more than 75% neutralization of BL22 was observed in four patients (11%), two patients after cycle 1, and two patients after cycle 2. Patients received up to 13 cycles without neutralizing antibodies. Thus, BL22 induced immunogenicity was infrequent and appeared early during retreatment.
Pharmacokinetics of BL22
Plasma was obtained at multiple time points after the end of infusion, and assayed for BL22 levels using a cytotoxicity assay.14 The median half-life of BL22 was 157 minutes during cycle 1 (range, 5 to 228, n = 36), 153 minutes during cycle 2 (range, 93 to 241, n = 20), and 131 minutes during cycle 3 (range, 95 to 199, n = 15). To determine the relationship between tumor burden and drug exposure, area under the curve (AUC) was calculated on the first dose of the cycle for each patient from either a mono- or biexponential model based on Aikake's rule.20 AUCs for cycle 2 for patients BH05 and BH19 were calculated from the second and third doses, respectively. Data was analyzed separately in patients with and without spleens. As shown in Figures 5A and 5B, patients with normal or enlarged spleens during cycle 1 at 40 μg/kg every other day for three doses had AUCs inversely proportional to either the log of the HCL count as determined by flow cytometry (P = .0001) or the spleen size caudal-cranially (P = .007) as measured by CT. As shown in Figures 5C and 5D, patients with spleens during retreatment at 30 μg/kg every other day for three doses also had AUCs inversely proportional to either the log of the HCL count (P = .045) or spleen size (P < .0001). AUC in asplenic patients was inversely proportional to the log of the HCL count both during cycle 1 (Fig 5E; P = .045) and during retreatment (Fig 5F; P = .015). HCL counts of zero could not be shown on log plots for 28 cycles in 5C and six cycles in 5F. Thus, the AUC of cytotoxic BL22 in the plasma was inversely proportional to tumor burden, as estimated either by spleen size or by log of the concentration of circulating HCL cells. Cycles associated with HUS are labeled red, including grade 1 HUS in a patient (BH15) during cycle 3 (Figs 5C and 5D), and grade 3 HUS in two asplenic patients during either cycle 1 (BH36, Fig 5E), or cycle 2 (BH12, Fig 5F). AUCs in patients with HUS were not unusually high compared to other patients with similar tumor burden.
Fig 5.
Pharmacokinetics of BL22. Area under the curves (AUCs) are shown (A, B, E) after cycle 1 or (C, D, F) re-treatment in patients with (A-D) and without (E-F) spleens. Cycles associated with hemolytic uremic syndrome are in red. Correlations did not include BH09 in group 3 (labeled gold in E) who required blood products possibly containing antibodies during BL22 infusion. HCL, hairy cell leukemia.
DISCUSSION
We report here the results of a phase II trial with BL22 for HCL in which a single cycle of BL22 given at 40 μg/kg every other day for three doses achieved CR in nine (25%) of 36 patients, only one of whom relapsed. After retreating 20 patients (56%), a total of 17 CRs (47%) were achieved in the 36 HCL patients. Thus, the high response rate seen in HCL patients during phase I testing is confirmed in this phase II trial.
The average dose/cycle was similar for both trials (29 to 33 μg/kg ×3) but the average number of cycles/patient was lower on phase II (3.6 v 8.6; P < .0001) due to selective retreatment. Lower response rates were expected because the protocol design prevented retreatment of patients in HR after one cycle. Nevertheless, phase II versus I response rates were not significantly different (CR, 47% v 61%; P = .33, ORR, 72% v 81%, P = .8). Dose-limiting HUS was less than half as frequent during phase II, not statistically significant due to the low incidence in both trials. Immunogenicity was less than one third as frequent (11% v 35%; P = .021). Neutralizing antibodies in all cases neutralized the toxin, based on neutralization assays showing that serum neutralizing BL22 also neutralized LMB-2 and SS1P, which contain the same toxin domains. There was no evidence of antibodies exclusively against the variable fragment, probably because the mouse constant domains of the Mab are absent in recombinant immunotoxins. Immunogenicity was also probably reduced by prior therapy, disease, and limited retreatment with BL22. However, further reduction of immunogenicity is desirable and may require careful timing of immunosuppressive chemotherapy before initiating BL22, or molecular engineering, to mutate or remove immunogenic epitopes.21,22
ADAMTS-13 activity, required to cleave ultralarge multimers of von Willebrand's factor,23 was not significantly impaired in any patient with HUS to BL22, indicating the absence of circulating ultralarge multimers of von Willebrand's factor which would require removal by plasmapheresis. The biodistribution of BL22 in animals is unknown, although the anti-CD25 recombinant immunotoxin LMB-2, containing the same toxin domains as BL22, concentrated in the kidneys of mice.24 Its short half-life relative to Mabs likely avoids dose-limiting vascular leak syndrome or hepatotoxicity. The mechanism of HUS from BL22 is still unknown but its absence with other recombinant PE38-containing immunotoxins suggests the importance of CD22 or CD22-like binding as at least part of the mechanism. It is possible that one could prevent HUS in patients receiving BL22 with combinations of antiplatelet agents, and by tailoring the dose level to tumor burden.
We found no evidence of heterogeneity with respect to CD22 expression or selection for CD22-negative HCL cells, which is consistent with the high expression of CD22 in this disease.25,26 Only one patient had, before BL22, two populations of HCL cells differing in CD22 (14,000 v 52,000 sites/cell) and CD25 expression (1,400 v 14,000 sites/cell). After four cycles of BL22% and 98% reduction in total circulating HCL cells, both populations decreased, the brighter CD22 population to a greater extent. However, CD22 and CD25 sites/cell remained stable and CD22-negative HCL cells were not detected.
We found that response rates were higher in patients with normal or moderately enlarged spleens (< 200 mm) than in either patients with larger spleens or postsplenectomy (Table 2). A massive spleen may present a barrier to tumor penetration and a sink for decreasing the BL22 plasma level (Fig 5B). Patients with prior splenectomy may have tighter packing of HCL cells in the bone marrow. Alternatively, patients with either massive spleens or splenectomy may have more advanced and resistant disease. In the 22 patients with spleens smaller than 200 mm, a single cycle of BL22 was highly effective (CR, 41%; ORR, 73%), without any serious toxicity. In this group of patients, selective retreatment improved the CR rate to 64% and ORR to 95%, without dose-limiting HUS. Patients with spleens larger than 200 mm also had no HUS, probably due to low plasma BL22 levels, but also had low response rates at the doses used. Patients postsplenectomy had low response rates to one cycle and dose-limiting HUS was observed in this group on cycle 1 or 2. Nevertheless, several of these patients were able to achieve CR with retreatment. While response appears optimal when BL22 was begun before developing massive splenomegaly or undertaking splenectomy, it is possible that adjusting BL22 dose level to disease burden may lead to improved efficacy and safety regardless of HCL disease status. Meanwhile, we recommend continued study of BL22 as a potential treatment for chemoresistant HCL, since its mechanism of action is clearly different from that of purine analogs.
Acknowledgment
We thank our clinical staff: Karen Bergeron, Rita Mincemoyer, Linda Ellison, Sonya Duke, and Barbara Debrah. Margaret Rick, MD, and Jay Lozier, MD, assayed hemolytic uremic syndrome plasma for ADAMTS-13; Constance Yuan, MD, interpreted flow cytometry data; and Seth Steinberg proposed the statistical design of the trial.
Footnotes
Supported by the Intramural Research Program, National Institutes of Health, National Cancer Institute; and MedImmune Inc.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical Trials repository link available on JCO.org.
Clinical trial information can be found for the following: NCT00074048.
Published by the American Society of Clinical Oncology
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Pierre Noel, Novartis (C) Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: Robert J. Kreitman, coinventor on NIH patent for BL22 (C); David J.P. FitzGerald, coinventor on the NIH patent for BL22 (C); Ira Pastan, coinventor on the NIH patent for BL22 (C)
AUTHOR CONTRIBUTIONS
Conception and design: Robert J. Kreitman, Ira Pastan
Collection and assembly of data: Robert J. Kreitman, Inger Margulies
Data analysis and interpretation: Robert J. Kreitman, Maryalice Stetler-Stevenson, Pierre Noel, David J.P. FitzGerald, Wyndham H. Wilson, Ira Pastan
Manuscript writing: Robert J. Kreitman, Ira Pastan
Final approval of manuscript: Robert J. Kreitman, Ira Pastan
REFERENCES
- 1.Bouroncle BA, Wiseman BK, Doan CA. Leukemic reticuloendotheliosis. Blood. 1958;13:609–630. doi: 10.1182/blood-2016-01-696179. [DOI] [PubMed] [Google Scholar]
- 2.Tallman MS, Hakimian D, Rademaker AW, et al. Relapse of hairy cell leukemia after 2-chlorodeoxyadenosine: Long-term follow-up of the Northwestern University experience. Blood. 1996;88:1954–1959. [PubMed] [Google Scholar]
- 3.Saven A, Burian C, Koziol JA, et al. Long-term follow-up of patients with hairy cell leukemia after cladribine treatment. Blood. 1998;92:1918–1926. [PubMed] [Google Scholar]
- 4.Goodman GR, Burian C, Koziol JA, et al. Extended follow-up of patients with hairy cell leukemia after treatment with cladribine. J Clin Oncol. 2003;21:891–896. doi: 10.1200/JCO.2003.05.093. [DOI] [PubMed] [Google Scholar]
- 5.Fanta PT, Saven A. Hairy cell leukemia. Cancer Treat Res. 2008;142:193–209. doi: 10.1007/978-0-387-73744-7_8. [DOI] [PubMed] [Google Scholar]
- 6.Grever MR. Pentostatin: Impact on outcome in hairy cell leukemia. Hematol Oncol Clin N Amer. 2006;20:1099–1108. doi: 10.1016/j.hoc.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Filleul B, Delannoy A, Ferrant A, et al. A single course of 2-chloro-deoxyadenosine does not eradicate leukemic cells in hairy cell leukemia patients in complete remission. Leukemia. 1994;8:1153–1156. [PubMed] [Google Scholar]
- 8.Carbone A, Reato G, Di Celle PF, et al. Disease eradication in hairy cell leukemia patients treated with 2- chlorodeoxyadenosine. Leukemia. 1994;8:2019–2020. [PubMed] [Google Scholar]
- 9.Hagberg H, Lundholm L. Rituximab, a chimaeric anti-CD20 monoclonal antibody, in the treatment of hairy cell leukaemia. Br J Haematol. 2001;115:609–611. doi: 10.1046/j.1365-2141.2001.03143.x. [DOI] [PubMed] [Google Scholar]
- 10.Lauria F, Lenoci M, Annino L, et al. Efficacy of anti-CD20 monoclonal antibodies (Mabthera) in patients with progressed hairy cell leukemia. Haematologica. 2001;86:1046–1050. [PubMed] [Google Scholar]
- 11.Nieva J, Bethel K, Saven A. Phase 2 study of rituximab in the treatment of cladribine-failed patients with hairy cell leukemia. Blood. 2003;102:810–813. doi: 10.1182/blood-2003-01-0014. [DOI] [PubMed] [Google Scholar]
- 12.Thomas DA, O'Brien S, Bueso-Ramos C, et al. Rituximab in relapsed or refractory hairy cell leukemia. Blood. 2003;102:3906–3911. doi: 10.1182/blood-2003-02-0630. [DOI] [PubMed] [Google Scholar]
- 13.Kreitman RJ, Wilson WH, Bergeron K, et al. Efficacy of the Anti-CD22 recombinant immunotoxin BL22 in chemotherapy-resistant hairy-cell leukemia. N Engl J Med. 2001;345:241–247. doi: 10.1056/NEJM200107263450402. [DOI] [PubMed] [Google Scholar]
- 14.Kreitman RJ, Squires DR, Stetler-Stevenson M, et al. Phase I trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with B-cell malignancies. J Clin Oncol. 2005;23:6719–6729. doi: 10.1200/JCO.2005.11.437. [DOI] [PubMed] [Google Scholar]
- 15.Li JL, Shen GL, Ghetie MA, et al. The epitope specificity and tissue reactivity of four murine monoclonal anti-CD22 antibodies. Cell Immunol. 1989;118:85–99. doi: 10.1016/0008-8749(89)90359-6. [DOI] [PubMed] [Google Scholar]
- 16.Pastan I, Hassan R, Fitzgerald DJ, et al. Immunotoxin treatment of cancer. Annu Rev Med. 2007;58:221. doi: 10.1146/annurev.med.58.070605.115320. [DOI] [PubMed] [Google Scholar]
- 17.Mansfield E, Amlot P, Pastan I, et al. Recombinant RFB4 immunotoxins exhibit potent cytotoxic activity for CD22-bearing cells and tumors. Blood. 1997;90:2020–2026. [PubMed] [Google Scholar]
- 18.Kreitman RJ, Margulies I, Stetler-Stevenson M, et al. Cytotoxic activity of disulfide-stabilized recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) towards fresh malignant cells from patients with B-cell leukemias. Clin Cancer Res. 2000;6:1476–1487. [PubMed] [Google Scholar]
- 19.Kreitman RJ, Wang QC, FitzGerald DJP, et al. Complete regression of human B-cell lymphoma xenografts in mice treated with recombinant anti-CD22 immunotoxin RFB4(dsFv)-PE38 at doses tolerated by Cynomolgus monkeys. Int J Cancer. 1999;81:148–155. doi: 10.1002/(sici)1097-0215(19990331)81:1<148::aid-ijc24>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 20.Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–723. [Google Scholar]
- 21.Onda M, Beers R, Xiang L, et al. An immunotoxin with greatly reduced immunogenicity by identification and removal of B cell epitopes. Proc Natl Acad Sci U S A. 2008;105:11311–11316. doi: 10.1073/pnas.0804851105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weldon JE, Xiang L, Chertov O, et al. A protease-resistant immunotoxin against CD22 with greatly increased activity against CLL and diminished animal toxicity. Blood. doi: 10.1182/blood-2008-08-173195. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moake JL. Thrombotic microangiopathies. N Engl J Med. 2002;347:589–600. doi: 10.1056/NEJMra020528. [DOI] [PubMed] [Google Scholar]
- 24.Kreitman RJ, Pastan I. Accumulation of a recombinant immunotoxin in a tumor in vivo: Fewer than 1000 molecules per cell are sufficient for complete responses. Cancer Res. 1998;58:968–975. [PubMed] [Google Scholar]
- 25.Cordone I, Annino L, Masi S, et al. Diagnostic relevance of peripheral blood immunocytochemistry in hairy cell leukaemia. J Clin Pathol. 1995;48:955–960. doi: 10.1136/jcp.48.10.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robbins BA, Ellison DJ, Spinosa JC, et al. Diagnostic application of two-color flow cytometry in 161 cases of hairy cell leukemia. Blood. 1993;82:1277–1287. [PubMed] [Google Scholar]