Abstract
Background
It is not known how carbohydrate and fat intake impact the development of left ventricular (LV) hypertrophy and contractile dysfunction in response to pressure overload. We hypothesized that a low carbohydrate/high fat diet prevents LV hypertrophy and dysfunction compared to high carbohydrate diets.
Methods and Results
Rats were fed high carbohydrate diets comprised of either starch or sucrose, or a low carbohydrate/high fat diet, and underwent abdominal aortic banding (AAB) for two months. AAB increased LV mass with all diets. LV end diastolic and systolic volumes, and the ratio of the mRNA for myosin heavy chainβ/α were increased with both high carbohydrate diets, but not with the low carbohydrate/high fat diet. Circulating levels of insulin and leptin, both stimulants for cardiac growth, were lower, and free fatty acids higher, with the low carbohydrate/high fat diet compared to high carbohydrate diets. Among AAB animals LV volumes were positively correlated with insulin, and LV mass correlated with leptin.
Conclusion
A low carbohydrate/high fat diet attenuated pressure overload-induced LV remodeling compared to high carbohydrate diets. This effect corresponded to lower insulin and leptin concentrations, suggesting they may contribute to the development of LV hypertrophy and dysfunction under conditions of pressure overload.
Introduction
Chronic hypertension is a major cause of left ventricular hypertrophy (LVH) which, despite optimal pharmacotherapy, frequently progresses to left ventricular dysfunction and heart failure1, 2. Thus new approaches are needed to prevent hypertension-induced LVH and heart failure, specifically interventions that are independent of current therapies that address blood pressure regulation and neurohormonal activation3. Prevention of LVH and heart failure through nutritional intervention is particularly attractive because any clinical improvement would likely be additive with medicinal therapies. While nutritional guidelines generally recommend a high carbohydrate/low fat diet to prevent heart disease4, recent epidemiological studies found a twofold increase in risk of coronary heart disease in women consuming a diet with a high glycemic index (i.e. foods rich in simple sugars and rapidly digested polysaccharides5), and no benefit to reducing fat intake by 25% over an 8 year period6. Recently, we observed that a low carbohydrate/high fat diet (20% of energy from carbohydrate/60% fat) prevented development of contractile dysfunction and LV dilatation, and increased survival compared to a high carbohydrate diet (70% carbohydrate/10% fat), despite similar levels of hypertension in Dahl salt-sensitive rats7–9. The physiological mechanisms for this protective effect of a low carbohydrate/high fat diet are not well understood7–9.
In the setting of pressure overload, dietary intake of carbohydrates and fats may affect cardiomyocyte size and function via changes in circulating insulin, leptin, and free fatty acid concentrations3. A high carbohydrate diet increases both leptin and insulin, and lowers plasma free fatty acid concentration compared to a low carbohydrate/high fat diet3, 10. Insulin and leptin both stimulate cardiac growth in isolated cardiomyocytes, suggesting that diet-induced increases in their circulating concentrations could stimulate LVH independent of afterload3, 11, 12. In patients with hypertension there is a positive relationship between plasma insulin concentration and LVH that is independent of blood pressure 13–16. In addition, increased plasma leptin levels are correlated with LVH and congestive heart failure17, 18. Insulin receptor stimulation activates phosphoinositol-3 kinase (PI3K) and subsequent phosphorylation and activation of the pro-growth serine-threonine kinase Akt, resulting in increased protein synthesis and suppression of protein breakdown19, 20. Both insulin and leptin can activate AMP-activated protein kinase (AMPK)21 which could also affect cardiac protein synthesis and hypertrophy22, 23. In addition, a high fat diet increases the plasma concentration of free fatty acids, which activates peroxisome proliferators-activated receptors (PPARs), especially PPARα10, 24, and increases expression of genes encoding proteins involved in fatty acid metabolism25. The impact of dietary fat and carbohydrate intake on the activation of Akt, AMPK and PPARα under conditions of pressure overload are not known.
The present study examined the effects of dietary carbohydrate and fat on the development of LVH, LV remodeling, and contractile dysfunction in a rat model of chronic pressure overload induced by abdominal aortic banding (AAB). We hypothesized that a low carbohydrate/high fat diet would lead to a decrease in the concentrations of leptin and insulin compared to a high carbohydrate/low fat diet, and thus reduce activation of Akt and AMPK and prevent LVH and LV dysfunction. In addition, the increase in plasma free fatty acids and activation of PPARα in the heart would increase expression of genes encoding proteins involve in cardiac fatty acid metabolism, and correspond with less LVH, LV remodeling, and contractile dysfunction. Studies were performed in the well-established rat model of LVH induced by AAB. Animals were fed a high carbohydrate diet comprised of either sucrose or corn starch (70% of energy from carbohydrate/10% fat), or a low carbohydrate/high fat diet (20% carbohydrate/60% fat). LV mass, and clinically relevant echocardiographic measures of LV function were assessed after two months of pressure overload.
Methods
Experimental Design
Measurements were performed with investigators blinded to treatment. The animal protocol was conducted according to the guidelines for the care and use of laboratory animals (NIH publication No. 85–23) and was approved by the Institutional Animal Care and Use Committee of the Case Western Reserve University. Animals were maintained on a reverse 12-hour light-dark cycle. All procedures were performed in the fed state between 3 and 6 hours from initiation of the dark phase of the light/dark cycle.
Male Wistar rats (180–200g) were fed either a high fat, high starch or high sucrose chow. After one week on the assigned diet, rats underwent sham or AAB surgery (n=9–11/group), and dietary treatment was continued for 9 wks. Echocardiographic assessment of LV function was performed 8 weeks post-surgery. Nine weeks after surgery, rats were weighed and anesthetized with 1.5–2.0% isoflurane, and 3 mL of blood were drawn from the inferior vena cava for metabolic measurements. The LV was quickly removed, weighed, freeze clamped and stored at −80°C for biochemical analysis.
Diet
All diets were custom made by Research Diets Inc (New Brunswick, NJ). The macronutrient compositions of the diets are given in Table 1. The high fat diet derived 58% of energy from cocoa butter (fatty acid composition of 28% palmitate, 65% stearate, 5% oleic acid, and 2% linoleic acid), as previously described10. Standard commercial rodent chows used in research facilities are low in fat (10–15% of total energy) and high in carbohydrate (65–70% of the total energy), with approximately 10–20% of the carbohydrate in the form of simple sugars (mainly sucrose) and 80–90% as starches. Thus in the present study the dietary composition of the High Starch diet (see Table 1) most resembles a standard commercial rodent chow.
Table 1.
Macronutrient composition of the diets. Diets were matched for vitamin and mineral content.
| DIET | Contribution to total energy content of the diet (% of total) | ||||||
|---|---|---|---|---|---|---|---|
| FAT | CARBOHYDRATE | PROTEIN | |||||
| Soy oil | Cocoa Butter | Corn Starch | Sucrose | Maltodextran | Casein | Total | |
| High Fat | 2 | 58 | - | 7 | 13 | 20 | 100 |
| High Starch | 2 | 8 | 53 | 10 | 7 | 20 | 100 |
| High Sucrose | 2 | 8 | 8 | 62 | - | 20 | 100 |
Abdominal Aortic Banding
Rats were anesthetized with 2.0–2.5% isoflurane by mask. The suprarenal abdominal aorta was exposed by a midline abdominal incision, and was tied with a 3–0 silk suture against a blunt needle (19G). The needle was immediately removed, leaving the aortic lumen constricted to the diameter of the needle. Sham operated animals were subjected to the same procedure but without AAB.
Echocardiography
LV function was evaluated using a Sequoia C256 system (Siemens Medical) with a 15-MHz linear array transducer as previously described 26. Briefly, rats were anesthetized with 1.5–2.0% isoflurane by mask, the chest was shaved, the animal was placed supine on a warming pad, and ECG limb electrodes were placed. 2-D guided M-mode, 2-dimensional, and Doppler echocardiographic studies of aortic flows were performed from parasternal and foreshortened apical windows. All data were analyzed offline with software resident on the ultrasound system at the end of the study as described previously 7, 26.
Metabolic and biochemical variables
Plasma free fatty acid and triglycerides concentrations were measured using enzymatic spectrophotometric assays (Wako and Sigma, respectively) 7. Blood glucose concentration was measured with an enzymatic spectrophotometric assay from perchloric acid deproteinized whole blood samples (Stannbio laboratories). Serum levels of leptin and plasma concentration of adiponectin and insulin were measured by ELISA (ALPCO Diagnostics). Myocardial activity of medium chain acyl-CoA dehydrogenase (MCAD) and citrate synthase was measured spectrophotometrically as previously described7.
mRNA measurement
RNA was isolated from frozen powdered LV tissue using RNeasy Mini Kit (Qiangen) following the manufacturer’s instructions. Quantitative RT-PCR was performed with TaqMan PCR master mix (Applied Biosystem) using an ABI 7900 Detection System as previously described27. RT-PCR was performed for each of the following genes, using TagMan Gene Expression Assay from Applied Biosystems: atrial natriuretic peptide (Nppa, Rn00561661_m1); myosin heavy chain α (Myh6, Rn00568304_m1); myosin heavy chain β(Myh7, Rn00568328_m1); PPARα (Ppara, Rn00566193_m1); MCAD (Acadm, Rn00566390_m1); carnitine palmitoyl transferase I (cpt1b, Rn00566242_m1); uncoupling protein 3 (ucp3, Rn00565874_m1); pyruvate dehydrogenase kinase 4 (Rn00585577_m1); cyclophilin A (ppia, Rn00690933_m1). mRNA values for these genes were normalize to cyclophilin A, and expressed relative to the High Starch Sham group.
Western blot analysis
Protein was extracted from frozen LV tissue 28, separated by electrophoresis in 10% SDS-PAGE gels, transferred onto a nitrocellulose membrane, and incubated with specific antibodies to either phospho-AMPK (Thr172 of α subunit) or phospho-Akt (Ser473) (all at 1:1000, from Cell Signaling Technology, Inc.) Fluorescence-conjugated secondary antibodies (IRDye 680/800, 1:5000; LI-COR Bioscience) were used for incubation before the membranes were scanned with Odyssey® infrared imaging system (LI-COR Bioscience). The digitized image was analyzed with Odyssey® software. Membranes were then stripped (Pierce Restore® stripping buffer) and re-probed for total-AMPK and Akt (1:1000; Cell Signaling Technology, Inc).
Statistical Analysis
Comparisons were made using an analysis of variance (ANOVA) with the Bonferroni test for multiple comparisons. Effect of diet on cardiac structure and function were examined with the AAB group only using one-way ANOVA and post-hoc t-test. To examine which circulating metabolic or biochemical variables were uniquely predictive of cardiac change in the AAB group, multiple regression analyses were used examining cardiac measurement as dependent measures and circulating variables as predictor variables using stepwise procedures. Values are presented as means± S.E.M and a p<0.05 was considered significant.
Results
LV Mass and Function
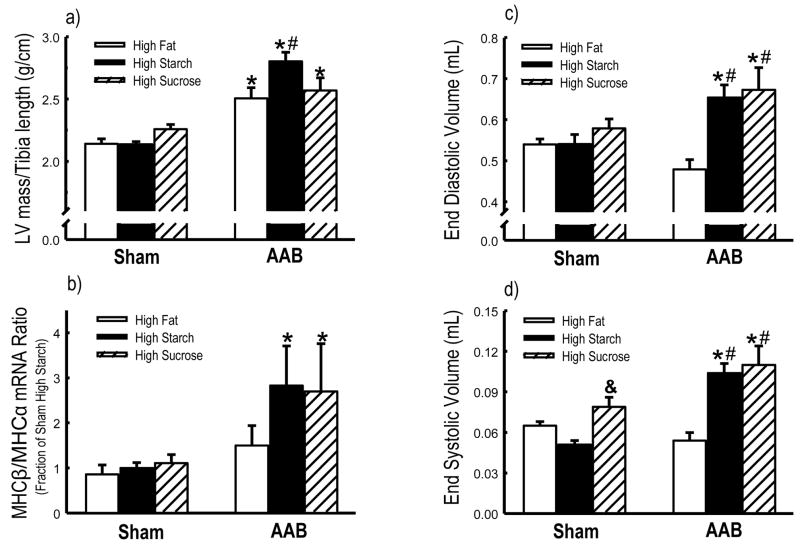
Body mass was not different among groups prior to surgery (data not shown) or at the end of the study (Table 2). Compared to shams, 9 weeks of AAB increased LV mass normalized to tibia length in all groups (Figure 1a). Additionally, the increase was greater in the high starch diet compared to the high fat diet. There were no differences among groups in tibia length or right ventricular mass (data not shown).
Table 2.
Body mass and echocardiography results.
| Sham | AAB | |||||
|---|---|---|---|---|---|---|
| High Fat n=9 | High Starch n=9 | High Sucrose n=11 | High Fat n=9 | High Starch n=10 | High Sucrose n=10 | |
| Terminal body mass (g) | 469±13 | 476±16 | 488±10 | 472±19 | 500±11 | 473±21 |
| Heart Rate (bpm) | 192±3 | 188±4 | 192±4 | 195±7 | 182±4 | 180±4 |
| Ejection fraction (%) | 88.0±1.1 | 90.6±1.1 | 86.4±1.0& | 88.6±1.1 | 83.6±1.0*# | 83.8±1.0*# |
| Anterior wall thickness (mm) | 1.78±0.04 | 1.88±0.04 | 1.92±0.04 | 2.03±0.04* | 2.24±0.04*# | 2.12±0.04* |
| Posterior wall thickness (mm) | 1.81±0.04 | 1.90±0.04 | 1.98±0.04# | 2.06±0.04* | 2.34±0.04*# | 2.13±0.04*& |
Data are the mean ± SEM;
p< 0.05 vs. respective Sham;
p<0.05 vs. High Fat diet;
p<0.05 vs. High Starch diet.
Figure 1.
LV mass/Tibia length ratio (a), and the ratio of mRNA expression for myosin heavy chain MHCα to MHCβ (b) in the left ventricle determined by qRT-PCR (Data represent as the fold change in gene expression relative to the High Starch Sham group). Echocardiographic assessments of LV end diastolic volume (c), and end systolic volume (d). *p<0.05 vs. respective Sham; #p<0.05 vs. High Fat diet; &p<0.05 vs. High Starch diet (n=9–11/group).
With both high starch and high sucrose diets, there was significant LV remodeling and systolic dysfunction with AAB compared to sham, as seen in the increase in end diastolic and systolic volumes and a reduction in ejection fraction. These effects were not observed with the high fat diet (Figure 1c–d, Table 2). Additionally, end systolic volume was significantly increased and ejection fraction was significant reduced in high sucrose sham animals compared to high starch sham (Figure 1d, Table 2). There was no different among groups in heart rate (Table 2).
Metabolic and Biochemical Variables
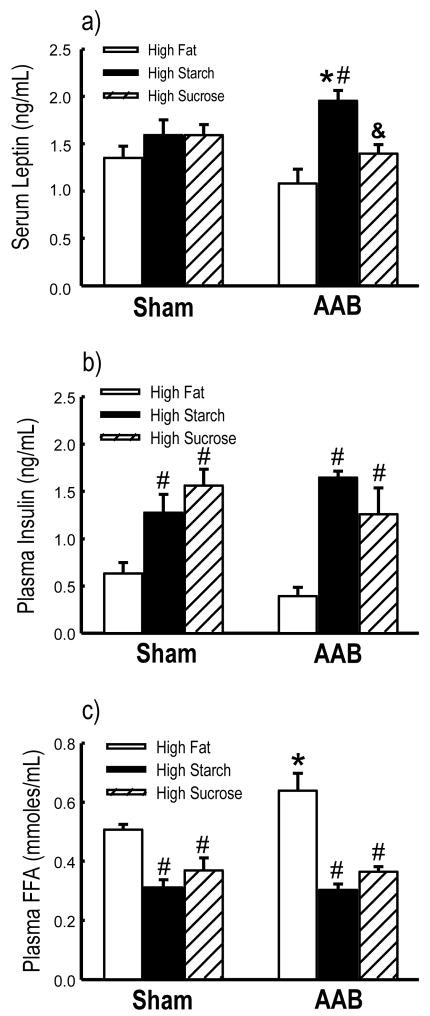
Plasma insulin levels were increased in both high starch and high sucrose diets compared to the high fat diet in both sham and AAB animals (Figure 2b). Serum leptin was unaffected by diet in the sham animals, but was significantly elevated in the ABB animals fed the high starch diet compared to both high fat and high sucrose diets (Figure 2a). Additionally, the animals fed the high fat diet had higher plasma free fatty acid concentration compared to rats on the high starch or high sugar diets, and the high fat fed AAB animals had higher plasma fatty acids than their respective sham (Figure 2c). Neither diet nor AAB affected glucose, triglycerides or adiponectin concentrations (Table 3).
Figure 2.
Circulating leptin (a), insulin (b) and plasma free fatty acids (c) concentration. *p<0.05 vs. respective Sham; #p<0.05 vs. High Fat diet; &p<0.05 vs. High Starch diet (n=9–11/group).
Table 3.
Metabolic measurement and mitochondrial enzyme activities.
| Metabolic Parameter | Sham | AAB | ||||
|---|---|---|---|---|---|---|
| High Fat n=9 | High Starch n=9 | High Sucrose n=11 | High Fat n=9 | High Starch n=10 | High Sucrose n=11 | |
| (μmol/mL) | 4.33±0.15 | 4.73±0.15 | 4.69±0.26 | 3.97±0.25 | 4.29±0.27 | 4.49±0.21 |
| Plasma Triglycerydes (mg/mL) | 1.05±0.09 | 1.32±0.13 | 1.25±0.18 | 1.08±0.16 | 1.12±0.13 | 1.50±0.12 |
| Plasma Adiponectin (μg/mL) | 7.09±0.45 | 6.69±0.63 | 5.94±0.30 | 6.84±0.63 | 6.78±0.29 | 5.85±0.44 |
| Enzyme activities | ||||||
| MCAD activity (μmol min−1 g−1) | 5.96±0.26 | 5.59±0.23 | 6.44±0.42 | 5.74±0.31 | 5.59±0.28 | 4.75±0.26* |
| Citrate Synthase (μmol min−1 g−1) | 112.5±5.5 | 109.2±6.1 | 110.1±6.5 | 108.4±6.7 | 106.1±5.1 | 103.1±8.0 |
| MCAD/CS activity | 0.054±0.004 | 0.052±0.003 | 0.057±0.003 | 0.054±0.004 | 0.054±0.004 | 0.047±0.003 |
Data are the mean ± SEM;
p< 0.05 vs. respective Sham.
AAB caused a switch in the mRNA for MHC from the α isoform to β in both high starch and high sucrose, as seen in the increase ratio MHCβ/α, but not in the high fat diet group (Figure 1b and Table 4). The mRNA for ANF was increased in all animals with AAB (Table 4). The mRNA for PPARα and PPARα-regulated genes were similar among groups except for an increase in UCP3 and PDK4 mRNA in the sham high fat, and UCP3 mRNA in the sham high sucrose group (Table 4). The activities of citrate synthase and MCAD were similar among groups except for a significantly lower activity of MCAD in the AAB high sucrose group (Table 3).
Table 4.
mRNA expression in the left ventricle determined by qRT-PCR. Data represent as the fold change in gene expression relative to the High Starch Sham group.
| Gene | Sham | AAB | ||||
|---|---|---|---|---|---|---|
| High Fat n=9 | High Starch n=9 | High Sucrose n=11 | High Fat n=9 | High Starch n=10 | High Sucrose N=11 | |
| ANF | 0.59±0.08 | 1.00±0.17 | 0.83±0.11 | 2.14±0.34* | 3.55±0.80* | 2.63±0.80* |
| MHCα | 1.02±0.19 | 1.00±0.05 | 1.04±0.13 | 1.02±0.13 | 0.75±0.13 | 0.75±0.16 |
| MHCβ | 0.64±0.06 | 1.00±0.15 | 1.04±0.10 | 1.43±0.40* | 1.50±0.26 | 1.53±0.36 |
| PPARα | 1.02±016 | 1.00±0.13 | 1.10±0.08 | 1.25±0.16 | 1.03±0.11 | 1.09±0.16 |
| MCAD | 1.28±0.15 | 1.00±0.11 | 1.21±0.13 | 1.31±0.11 | 0.93±0.15 | 0.99±0.16 |
| CPT-1β | 1.02±0.17 | 1.00±0.14 | 1.13±0.10 | 1.32±0.13 | 0.98±0.15 | 0.93±0.17 |
| UCP3 | 1.74±0.31 | 1.00±0.18# | 1.79±0.21& | 1.58±0.21 | 1.03±0.16 | 1.05±0.19* |
| PDK4 | 1.87±0.32 | 1.00±0.15# | 1.51±0.28 | 1.62±0.21 | 0.89±0.17 | 0.84±0.17* |
Data are the mean ± SEM;
p<0.05 vs. respective Sham;
p<0.05 vs. High Fat diet;
p<0.05 vs. High Starch diet.
There were no differences among groups in either total or phosphorylated Akt, or the ratio of phosphorylated to total Akt, as assessed by western blot, (Table 5). Similarly, there were no differences in total or phosphorylated AMPK, or the ratio of phosphorylated to total AMPK except for an increase the ratio of phosphorylated to total AMPK in the AAB high starch group (Table 5).
Table 5.
Western blot densitometry of total and phosphorylated Akt and AMPK (in arbitrary units).
| Sham | AAB | |||||
|---|---|---|---|---|---|---|
| High Fat n=9 | High Starch n=9 | High Sucrose n=11 | High Fat n=9 | High Starch n=10 | High Sucrose n=11 | |
| Phospho-Akt | 9.84±0.49 | 8.70±0.32 | 9.53±0.52 | 9.28±0.63 | 8.45±0.32 | 8.77±0.33 |
| Total Akt | 18.7±2.3 | 15.1±1.44 | 18.4±1.6 | 19.5±2.5 | 19.1±2.0 | 15.7±2.0 |
| Phospho-Akt/Total Akt | 0.61±0.07 | 0.62±0.07 | 0.55±0.04 | 0.51±0.04 | 0.50±0.05 | 0.62±0.07 |
| Phospho-AMPK | 12.8±0.8 | 15.3±1.7 | 13.4±0.9 | 12.6±1.1 | 13.1±0.8 | 12.9±0.7 |
| Total AMPK | 41.0±2.3 | 41.8±5.7 | 41.1±2.4 | 36.0±2.4 | 38.9±2.3 | 42.2±2.2 |
| Phospho-AMPK/Total AMPK | 0.31±0.01 | 0.39±0.04# | 0.33±0.01& | 0.35±0.02 | 0.34±0.01 | 0.31±0.01 |
Data are the mean ± SEM;
p<0.05 vs. High Fat diet;
p<0.05 vs. High Starch diet
Association between circulating variables and cardiac measures
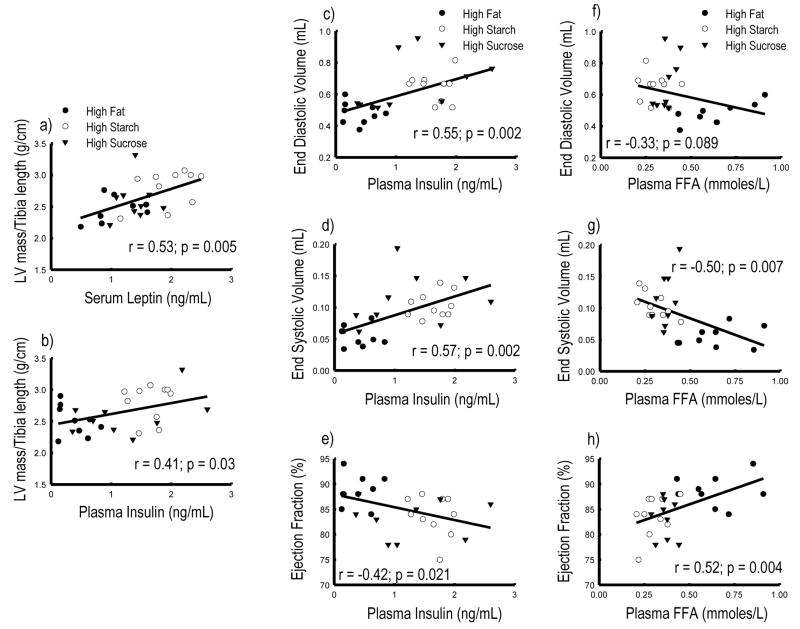
LV mass was positively correlated with serum leptin and plasma insulin concentrations (Figure 3a–b), but not other variables (data not shown). LV end diastolic and end systolic volumes were positively correlated with plasma insulin, and inversely correlated with plasma free fatty acid concentration (Figure 3c–h), but were not associated with serum leptin concentration (data not shown). Ejection fraction was positively correlated with plasma free fatty acid and inversely correlated with plasma insulin concentration (Figure 3). The mRNA expression of PPARα regulate genes were positively correlated with plasma free fatty acid concentration, with correlation coefficients of 0.55, 0.50, 0.46, and 0.54 (p<0.05) for MCAD, CPT1β, UPC3 and PDK4, respectively. There were no significant relationships between the mRNA expressions for PPARα-regulated genes and LV mass, echocardiographic measurements, or hormone levels (data not shown).
Figure 3.
LV mass/Tibia length ratio plotted as a function of leptin (a) and insulin (b) concentration. LV end diastolic volume plotted as a function of insulin (c) and free fatty acid (f). LV end systolic volume plotted as a function of insulin (d) and free fatty acid (a). Ejection fraction plotted as a function of insulin (e) and free fatty acid (h) in AAB group.
Multivariate regression analyses were used to examine the relative importance of circulating variables for cardiac outcome measures. Serum leptin levels were associated with LV mass (β = 0.53, p = 0.006), plasma insulin with end diastolic (β = 0.55, p = 0.004) and end systolic volumes (β = 0.54, p = 0.005). Plasma free fatty acid and adiponectin were associated with ejection fraction (β = 0.49, p = 0.010 and β = 0.40, p = 0.028, respectively).
Taken together, these data are consistent with the concept that under conditions of pressure overload, cardiac hypertrophy is minimized by low circulating levels of insulin and leptin, and that LV remodeling and dysfunction is reduced by elevated free fatty acids and low insulin.
Discussion
In the present study, a low carbohydrate/high fat diet attenuated pressure overload-induced LVH, and reduced LV remodeling and contractile dysfunction compared to high carbohydrate/low fat diets comprised of either starch or sugar. The lower LV mass was associated with lower circulating leptin and insulin concentrations, consistent with the concept that these hormones directly stimulate cardiac growth11, 12, 29, 30. The prevention of the increase in LV end systolic and diastolic volumes corresponded with lower circulating insulin levels and a higher free fatty acid concentration, but not leptin. These finding support the paradigm that consumption of a low carbohydrate/high fat diet attenuates the development of LVH, remodeling and contractile dysfunction with pressure overload compared to a high carbohydrate/low fat diet.
Clinical observations show that hypertensive patients with high plasma insulin have a greater occurrence of LVH13–16. Our results extend this concept to suggest that reducing plasma insulin with a low carbohydrate/high fat diet may reduce LVH and prevent ventricular dysfunction in response to chronic pressure overload (Figures 2 and 3). Multivariate regression analyses indicated that insulin was the best predictor of end diastolic and systolic volumes. Insulin stimulates cardiac growth via insulin receptor stimulation and activation of a complex network of signaling pathways, resulting in increased protein synthesis and suppression of protein breakdown19, 20. Evidence for a key role for insulin signaling in LVH comes from studies in transgenic mice, where deletion of the insulin receptor on cardiac myocytes reduces activation of Akt and its downstream targets19, 20, 31, and results in a smaller heart29. Over-expression of Akt increases insulin-stimulated protein synthesis, inhibits protein breakdown, and causes massive LVH31, 32. We found no evidence for Akt activation by either AAB or a high carbohydrate diet, illustrating that activation of Akt is not essential for the greater LVH observed in the present investigation.
As previously reported, in this non-obesity model of low carbohydrate/high fat feeding, there is a decrease in serum leptin concentration7, 10, 33 Clinical research on leptin has largely been focused on its role as a potential mediator of cardiac hypertrophy and heart failure in obese patients17, 18. Leptin receptors are expressed in the heart 34, suggesting that leptin has a direct effect on cardiomyocytes11, 12. In the present study on non-obese animals, the suppression of leptin levels with low carbohydrate/high fat diet was significantly associated with reduced LVH in response to AAB (Figure 2 and 3). While leptin was the strongest predictor of LVH, there was no relationship between leptin and activation of either AMPK or Akt, suggesting that leptin is not signaling through these kinases that are known to elevate cardiac protein synthesis and hypertrophy3, 22, 23. We previously noted that the reduction in serum leptin following treatment with the same low carbohydrate/high fat diet for 12 weeks in normal rats corresponded to a reduction in epididymal fat mass and greater thoracic fat mass. In the present study there were no differences in epididymal, thoracic, or visceral fat mass (data not shown), suggesting that the reduction in plasma leptin was not due to changes in fat distribution. Taken together, these finding suggest that lowering leptin through dietary manipulations is cardioprotective in the setting of pressure overload. In contrast, studies in rat and mouse models of obesity show that leptin may have a protective effect on the heart through suppression of appetite, increased cardiac fatty acid oxidation, and reduced accumulation of toxic lipid compounds in the heart35, 36.
PPARα is activated by fatty acids, and thus acts as a lipid sensor in cardiomyocytes, increasing the capacity for fatty acid uptake and catabolism in response to greater exposure to lipid25. Previous studies found that a high fat diet increases plasma free fatty concentration, activates PPARα, and stimulates expression of key mitochondrial proteins involved in fatty acid oxidation in the heart10, 25. Here we show that LV end diastolic and end systolic volumes are inversely correlated with plasma fatty acid concentration among AAB animals (Figure 3). Data from the literature on the effects of activation of PPARα on cardiac hypetrophy are conflicting. There is evidence to suggest that PPARs are negative regulators of LVH, based on the decreased expression of PPARα in the heart in response to hypertrophic growth in cell culture or with LVH following aortic banding24, 25. On the other hand, PPARα −/− mice have a normal heart mass/body mass ratio37, and mice with cardiac-restricted overexpression of PPARα38 have modest cardiac hypertrophy. Treatment with a PPARα agonist or a CPT-I inhibitor39, 40 can increase the expression of PPARα-regulated genes and LV mass41, 42; however, cardiac hypertrophy is not observed with the lower degree of PPARα activation that occurs with high fat feeding10, 42.
In contrast to our findings, a previous report which showed that sucrose supplementation blunted the up-regulation in MHCβ expression and the decrease in sarcoplasmic reticulum Ca2+-stimulated ATPase with in rats with pressure overload-induced LVH43. These animals were fed regular chow and low amount of sucrose was supplied in the drinking water (0.8%, w/v). In the present study sucrose was supplied at a high amount in the chow. Most recent studies have shown adverse effects in animals fed diets high in simple sugar27, 44.
There are important limitations to the present investigation that need to be addressed. First, we did not measure arterial or LV blood pressure, thus we cannot eliminate the possibility that the effects of diet were partially mediated by changes in afterload. Previous studies indicate that long-term administration of leptin results in an increase in blood pressure45, 46 in response to sympathetic stimulation47. However, a strong positive correlation has been reported between plasma leptin and LV wall thickness that is independent of blood pressure17, suggesting leptin directly induces cardiomyocytes hypertrophy. Second, a high protein/low carbohydrate diet, which is frequently used for weight control48, was not studied in the present investigation, thus we do not know if replacing carbohydrate with protein would have an effect similar to replacing carbohydrate with fat. Future studies should incorporate high protein/high fat/low carbohydrate diets, similar to the “Atkins diet”, in nutritional studies of heart failure. Third, we did not assess histological indices of cardiac pathology (e.g. interstitial fibrosis, cardiomyocyte hypertrophy, or apoptosis) which may have provided insight into the effects of diet on LV pathology. Lastly, the present study used a relatively short duration of treatment, which is different from most clinical LVH and heart failure, which develop over several years. Future studies should assess the effects of more prolonged pressure overload and dietary treatment.
In conclusion, a low carbohydrate/high fat diet attenuated pressure overload-induced LV dysfunction compared to a high carbohydrate diet comprised of either starch or sugar. This effect corresponded to lower circulating insulin and leptin, and elevated free fatty acids. These results add further support for the concept that a diet low in carbohydrates and high in fat minimizes pathologic LV hypertrophy through multiple changes in the hormonal and metabolic milieu. These intriguing observations in rodents provide motivation to perform clinical studies to determine if this concept is valid in hypertensive patients.
Acknowledgments
This research was supported by NIH grant HL074237. The authors thank Drs. Margaret Chandler and Isidore Okere, and Cody Rutledge and Jenny Cui for assistance. The authors have no financial disclosures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996 May 22;275(20):1557–62. [PubMed] [Google Scholar]
- 2.Lloyd-Jones DM, Larson MG, Leip EP, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002 December 10;106(24):3068–72. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 3.Sharma N, Okere IC, Duda MK, Chess DJ, O’shea KM, Stanley WC. Potential impact of carbohydrate and fat intake on pathological left ventricular hypertrophy. Cardiovasc Res. 2007 January 15;73(2):257–68. doi: 10.1016/j.cardiores.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lichtenstein AH, Appel LJ, Brands M, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006 July 4;114(1):82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 5.Halton TL, Willett WC, Liu S, et al. Low-carbohydrate-diet score and the risk of coronary heart disease in women. N Engl J Med. 2006 November 9;355(19):1991–2002. doi: 10.1056/NEJMoa055317. [DOI] [PubMed] [Google Scholar]
- 6.Howard BV, Van Horn L, Hsia J, et al. Low-fat dietary pattern and risk of cardiovascular disease: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006 February 8;295(6):655–66. doi: 10.1001/jama.295.6.655. [DOI] [PubMed] [Google Scholar]
- 7.Okere IC, Young ME, McElfresh TA, et al. Low carbohydrate/high-fat diet attenuates cardiac hypertrophy, remodeling, and altered gene expression in hypertension. Hypertension. 2006 December;48(6):1116–23. doi: 10.1161/01.HYP.0000248430.26229.0f. [DOI] [PubMed] [Google Scholar]
- 8.Sharma N, Okere IC, Duda MK, et al. High fructose diet increases mortality in hypertensive rats compared to a complex carbohydrate or high fat diet. Am J Hypertens. 2007 April;20(4):403–9. doi: 10.1016/j.amjhyper.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 9.Okere IC, Chess DJ, McElfresh TA, et al. High-fat diet prevents cardiac hypertrophy and improves contractile function in the hypertensive dahl salt-sensitive rat. Clin Exp Pharmacol Physiol. 2005 October;32(10):825–31. doi: 10.1111/j.1440-1681.2005.04272.x. [DOI] [PubMed] [Google Scholar]
- 10.Okere IC, Chandler MP, McElfresh TA, et al. Differential effects of saturated and unsaturated fatty acid diets on cardiomyocyte apoptosis, adipose distribution, and serum leptin. Am J Physiol Heart Circ Physiol. 2006 July;291(1):H38–H44. doi: 10.1152/ajpheart.01295.2005. [DOI] [PubMed] [Google Scholar]
- 11.Rajapurohitam V, Gan XT, Kirshenbaum LA, Karmazyn M. The obesity-associated peptide leptin induces hypertrophy in neonatal rat ventricular myocytes. Circ Res. 2003 August 22;93(4):277–9. doi: 10.1161/01.RES.0000089255.37804.72. [DOI] [PubMed] [Google Scholar]
- 12.Rajapurohitam V, Javadov S, Purdham DM, Kirshenbaum LA, Karmazyn M. An autocrine role for leptin in mediating the cardiomyocyte hypertrophic effects of angiotensin II and endothelin-1. J Mol Cell Cardiol. 2006 August;41(2):265–74. doi: 10.1016/j.yjmcc.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Ilercil A, Devereux RB, Roman MJ, et al. Associations of insulin levels with left ventricular structure and function in American Indians: the strong heart study. Diabetes. 2002 May;51(5):1543–7. doi: 10.2337/diabetes.51.5.1543. [DOI] [PubMed] [Google Scholar]
- 14.Karason K, Sjostrom L, Wallentin I, Peltonen M. Impact of blood pressure and insulin on the relationship between body fat and left ventricular structure. Eur Heart J. 2003 August;24(16):1500–5. doi: 10.1016/s0195-668x(03)00312-9. [DOI] [PubMed] [Google Scholar]
- 15.Stiefel P, Miranda ML, Rodriguez-Puras MJ, et al. Glucose effectiveness is strongly related to left ventricular mass in subjects with stage I hypertension or high-normal blood pressure. Am J Hypertens. 2004 February;17(2):146–53. doi: 10.1016/j.amjhyper.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Rutter MK, Parise H, Benjamin EJ, et al. Impact of glucose intolerance and insulin resistance on cardiac structure and function: sex-related differences in the Framingham Heart Study. Circulation. 2003 January 28;107(3):448–54. doi: 10.1161/01.cir.0000045671.62860.98. [DOI] [PubMed] [Google Scholar]
- 17.Paolisso G, Tagliamonte MR, Galderisi M, et al. Plasma Leptin Level Is Associated With Myocardial Wall Thickness in Hypertensive Insulin-Resistant Men. Hypertension. 1999 November 1;34(5):1047–52. doi: 10.1161/01.hyp.34.5.1047. [DOI] [PubMed] [Google Scholar]
- 18.Perego L, Pizzocri P, Corradi D, et al. Circulating leptin correlates with left ventricular mass in morbid (grade III) obesity before and after weight loss induced by bariatric surgery: a potential role for leptin in mediating human left ventricular hypertrophy. J Clin Endocrinol Metab. 2005 July;90(7):4087–93. doi: 10.1210/jc.2004-1963. [DOI] [PubMed] [Google Scholar]
- 19.Abel ED. Myocardial insulin resistance and cardiac complications of diabetes. Curr Drug Targets Immune Endocr Metabol Disord. 2005 June;5(2):219–26. doi: 10.2174/1568008054064869. [DOI] [PubMed] [Google Scholar]
- 20.Matsui T, Rosenzweig A. Convergent signal transduction pathways controlling cardiomyocyte survival and function: the role of PI 3-kinase and Akt. J Mol Cell Cardiol. 2005 January;38(1):63–71. doi: 10.1016/j.yjmcc.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Fryer LG, Carling D. AMP-activated protein kinase and the metabolic syndrome. Biochem Soc Trans. 2005 April;33(Pt 2):362–6. doi: 10.1042/BST0330362. [DOI] [PubMed] [Google Scholar]
- 22.Chan AY, Soltys CL, Young ME, Proud CG, Dyck JR. Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. J Biol Chem. 2004 July 30;279(31):32771–9. doi: 10.1074/jbc.M403528200. [DOI] [PubMed] [Google Scholar]
- 23.Kovacic S, Soltys CL, Barr AJ, Shiojima I, Walsh K, Dyck JR. Akt activity negatively regulates phosphorylation of AMP-activated protein kinase in the heart. J Biol Chem. 2003 October 10;278(41):39422–7. doi: 10.1074/jbc.M305371200. [DOI] [PubMed] [Google Scholar]
- 24.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005 July;85(3):1093–129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 25.Huss JM, Kelly DP. Nuclear receptor signaling and cardiac energetics. Circ Res. 2004 September 17;95(6):568–78. doi: 10.1161/01.RES.0000141774.29937.e3. [DOI] [PubMed] [Google Scholar]
- 26.Morgan EE, Faulx MD, McElfresh TA, et al. Validation of echocardiographic methods for assessing left ventricular dysfunction in rats with myocardial infarction. Am J Physiol Heart Circ Physiol. 2004 November;287(5):H2049–H2053. doi: 10.1152/ajpheart.00393.2004. [DOI] [PubMed] [Google Scholar]
- 27.Chess DJ, Lei B, Hoit BD, Azimzadeh AM, Stanley WC. Deleterious effects of sugar and protective effects of starch on cardiac remodeling, contractile dysfunction, and mortality in response to pressure overload. Am J Physiol Heart Circ Physiol. 2007 September;293(3):H1853–H1860. doi: 10.1152/ajpheart.00544.2007. [DOI] [PubMed] [Google Scholar]
- 28.Lei B, Lionetti V, Young ME, et al. Paradoxical downregulation of the glucose oxidation pathway despite enhanced flux in severe heart failure. J Mol Cell Cardiol. 2004 April;36(4):567–76. doi: 10.1016/j.yjmcc.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Belke DD, Betuing S, Tuttle MJ, et al. Insulin signaling coordinately regulates cardiac size, metabolism, and contractile protein isoform expression. J Clin Invest. 2002 March;109(5):629–39. doi: 10.1172/JCI13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma N, Okere IC, Duda MK, Chess DJ, O’Shea KM, Stanley WC. Potential impact of carbohydrate and fat intake on pathological left ventricular hypertrophy. Cardiovasc Res. 2007 January 15;73(2):257–68. doi: 10.1016/j.cardiores.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shiojima I, Yefremashvili M, Luo Z, et al. Akt signaling mediates postnatal heart growth in response to insulin and nutritional status. J Biol Chem. 2002 October 4;277(40):37670–7. doi: 10.1074/jbc.M204572200. [DOI] [PubMed] [Google Scholar]
- 32.Matsui T, Li L, Wu JC, et al. Phenotypic spectrum caused by transgenic overexpression of activated Akt in the heart. J Biol Chem. 2002 June 21;277(25):22896–901. doi: 10.1074/jbc.M200347200. [DOI] [PubMed] [Google Scholar]
- 33.Okere IC, Chandler MP, McElfresh TA, et al. Carnitine palmitoyl transferase-I inhibition is not associated with cardiac hypertrophy in rats fed a high-fat diet. Clin Exp Pharmacol Physiol. 2007 January;34(1–2):113–9. doi: 10.1111/j.1440-1681.2007.04545.x. [DOI] [PubMed] [Google Scholar]
- 34.Purdham DM, Zou MX, Rajapurohitam V, Karmazyn M. Rat heart is a site of leptin production and action. Am J Physiol Heart Circ Physiol. 2004 December;287(6):H2877–H2884. doi: 10.1152/ajpheart.00499.2004. [DOI] [PubMed] [Google Scholar]
- 35.Unger RH. Hyperleptinemia: protecting the heart from lipid overload. Hypertension. 2005 June;45(6):1031–4. doi: 10.1161/01.HYP.0000165683.09053.02. [DOI] [PubMed] [Google Scholar]
- 36.Zhou YT, Grayburn P, Karim A, et al. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci U S A. 2000 February 15;97(4):1784–9. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finck BN, Han X, Courtois M, et al. A critical role for PPARalpha-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: modulation by dietary fat content. Proc Natl Acad Sci U S A. 2003 February 4;100(3):1226–31. doi: 10.1073/pnas.0336724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Finck BN, Lehman JJ, Leone TC, et al. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest. 2002 January;109(1):121–30. doi: 10.1172/JCI14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rupp H, Rupp TP, Maisch B. Fatty acid oxidation inhibition with PPARalpha activation (FOXIB/PPARalpha) for normalizing gene expression in heart failure? Cardiovasc Res. 2005 June 1;66(3):423–6. doi: 10.1016/j.cardiores.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 40.Lionetti V, Linke A, Chandler MP, et al. Carnitine palmitoyl transferase-I inhibition prevents ventricular remodeling and delays decompensation in pacing-induced heart failure. Cardiovasc Res. 2005 June 1;66(3):454–61. doi: 10.1016/j.cardiores.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 41.Hamano T, Kobayashi K, Sakairi T, Hayashi M, Mutai M. Peroxisome proliferator-activated receptor alpha (PPAR alpha) agonist, WY-14,643, increased transcription of myosin light chain-2 in cardiomyocytes. J Toxicol Sci. 2001 December;26(5):275–84. doi: 10.2131/jts.26.275. [DOI] [PubMed] [Google Scholar]
- 42.Morgan EE, Rennison JH, Young ME, et al. Effects of chronic activation of peroxisome proliferator-activated receptor-alpha or high-fat feeding in a rat infarct model of heart failure. Am J Physiol Heart Circ Physiol. 2006 May;290(5):H1899–H1904. doi: 10.1152/ajpheart.01014.2005. [DOI] [PubMed] [Google Scholar]
- 43.Rupp H, Elimban V, Dhalla NS. Sucrose feeding prevents changes in myosin isoenzymes and sarcoplasmic reticulum Ca2+-pump ATPase in pressure-loaded rat heart. Biochem Biophys Res Commun. 1988 October 31;156(2):917–23. doi: 10.1016/s0006-291x(88)80931-8. [DOI] [PubMed] [Google Scholar]
- 44.Sharma N, Okere IC, Barrows BR, et al. High sugar diets increase cardiac dysfunction and mortality in hypertension compared to low carbohydrate or high starch diets. J Hypertens. 2007 doi: 10.1097/HJH.0b013e3283007dda. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Correia ML, Morgan DA, Sivitz WI, Mark AL, Haynes WG. Leptin acts in the central nervous system to produce dose-dependent changes in arterial pressure. Hypertension. 2001 March;37(3):936–42. doi: 10.1161/01.hyp.37.3.936. [DOI] [PubMed] [Google Scholar]
- 46.Shek EW, Brands MW, Hall JE. Chronic leptin infusion increases arterial pressure. Hypertension. 1998 January;31(1 Pt 2):409–14. doi: 10.1161/01.hyp.31.1.409. [DOI] [PubMed] [Google Scholar]
- 47.Aizawa-Abe M, Ogawa Y, Masuzaki H, et al. Pathophysiological role of leptin in obesity-related hypertension. J Clin Invest. 2000 May;105(9):1243–52. doi: 10.1172/JCI8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Astrup A, Meinert LT, Harper A. Atkins and other low-carbohydrate diets: hoax or an effective tool for weight loss? Lancet. 2004 September 4;364(9437):897–9. doi: 10.1016/S0140-6736(04)16986-9. [DOI] [PubMed] [Google Scholar]