Abstract
This paper makes five key points. First is that the aggregate effect of radical and sustained behavioural changes in a sufficient number of individuals potentially at risk is needed for successful reductions in HIV transmission. Second, combination prevention is essential since HIV prevention is neither simple nor simplistic. Reductions in HIV transmission need widespread and sustained efforts, and a mix of communication channels to disseminate messages to motivate people to engage in a range of options to reduce risk. Third, prevention programmes can do better. The effect of behavioural strategies could be increased by aiming for many goals (eg, delay in onset of first intercourse, reduction in number of sexual partners, increases in condom use, etc) that are achieved by use of multilevel approaches (eg, couples, families, social and sexual networks, institutions, and entire communities) with populations both uninfected and infected with HIV. Fourth, prevention science can do better. Interventions derived from behavioural science have a role in overall HIV-prevention efforts, but they are insufficient when used by themselves to produce substantial and lasting reductions in HIV transmission between individuals or in entire communities. Fifth, we need to get the simple things right. The fundamentals of HIV prevention need to be agreed upon, funded, implemented, measured, and achieved. That, presently, is not the case.
Introduction
No one thought, 25 years ago, that HIV prevention would be as difficult as it has proven to be. Despite efforts, UNAIDS now estimates that 33 million people are living with HIV, and 2·5 million new infections arise every year.1 We must do better and the question is how. We have learned that no simplistic or even simple solutions exist for HIV prevention. We need to remain humble as we approach the issue of how to keep the virus from moving from one person to another.
Key messages: Behavioural strategies.
HIV prevention is neither simple nor simplistic. We must achieve radical behavioural changes—both between individuals and across large groups of at-risk people—to reduce incidence. Once achieved, it is essential that such changes are sustained
Although cognitive-behavioural, persuasive communications, peer education, and diffusion of innovation approaches to change are beneficial within a combination prevention framework, behavioural science can and must do better. Novel theoretical and programmatic approaches are needed to inform new approaches to motivate behavioural change
Goals for behavioural strategy involve knowledge, stigma reduction, access to services, delay of onset of first intercourse, decrease in number of partners, increases in condom sales or use, and decreases in sharing of contaminated injection equipment. A multilevel approach that encompasses behavioural strategies must be taken—behavioural HIV prevention needs to be integrated with biomedical and structural approaches, and treatment for HIV infection
The fundamentals of HIV prevention need to be agreed upon, funded, implemented, measured, and achieved in a comprehensive and sustained manner. Access to HIV prevention information, messages, skills, and technologies is essential and a fundamental human right
Advances in scaling up antiretroviral treatment in resource-poor countries, the benefits of male circumcision, and the hoped for promise of pre-exposure prophylaxis and microbicides do not render behavioural strategies obsolete. If anything, behavioural strategies need to become more sophisticated, combined with advances in the biomedical field, and scaled up. But that task is not easy. Sexual behaviours and the sharing of injection equipment that cause most HIV infections worldwide occur for many motivations (eg, reproduction, desire, peer pressure, pleasure, physical or psychological dependence, self-esteem, love, access to material goods, obligation, coercion and force, habit, gender roles, custom, and culture). The varieties of sexual expression are infinitely greater than is acknowledged or sanctioned by most societies' defined legal and moral systems. Ironically, most societies—either openly or clandestinely—provide opportunities for varied sexual expression, often within the context of substance use, even if the defined legal and moral systems seem somewhat rigid. Sexual behaviour typically does not occur in public, making it difficult to motivate protection when potential transmission occurs, and making it almost impossible to verify reports of what people say they have or have not done. Substance use to the point of intoxication is not only allowed, but is central to many countries' economies, and attempts to control the distribution and sale of illegal substances—and especially drugs that are injected—have met with little success.2
Behavioural change has been responsible for the prevention successes to date. Strategies to modify risk behaviours need to remain a main priority for HIV prevention. We define behavioural strategies as those that attempt to delay onset of first intercourse, decrease the number of sexual partners, increase the number of sexual acts that are protected, provide counselling and testing for HIV, encourage adherence to biomedical strategies preventing HIV transmission, decrease sharing of needles and syringes, and decrease substance use. Behavioural strategies to accomplish these goals can focus on individuals, couples, families, peer groups or networks, institutions, and entire communities. Whereas structural strategies seek to change the context that contributes to vulnerability and risk3 and biomedical interventions block infection or decrease infectiousness,4 behavioural strategies attempt to motivate behavioural change within individuals and social units by use of a range of educational, motivational, peer-group, skills-building approaches, and community normative approaches.
This series of papers on HIV prevention in The Lancet emphasises that highly active HIV prevention5 inevitably must be combination prevention (figure 1).1 Advances in biomedical HIV prevention, as in the case of male circumcision or the potential of antiretroviral therapies for prevention, provide substantial opportunities to re-invigorate behavioural approaches to HIV prevention and challenge us to advance structural approaches so that these advances can get to those who need them the most.6 All these prevention approaches contribute to effective HIV prevention within communities, and thus behavioural strategies need to be used in combination with biomedical and structural approaches that are combined strategically to address local epidemics.3,4,7
Figure 1. Highly active HIV prevention.

This term was coined by Prof K Holmes, University of Washington School of Medicine, Seattle, WA, USA.5 STI=sexually transmitted infections.
The first successful examples of behavioural change resulting in decreases in HIV incidence emerged from communities of men who have sex with men in the USA, Canada, Europe, and Australia.8-11 Thailand and Uganda took the HIV epidemic seriously fairly early on and established measures to change transmission behaviours and reduce rates of HIV infection.12,13 Senegal averted an epidemic1,11 through behaviour change that was helped by cross-sectoral cooperation, the reach of the faith sector, and inclusion of marginalised groups with high risk of HIV. Countries that have all reported decreases in HIV transmission related to changes in sexual behaviour include Brazil, Côte d'Ivoire, Kenya, Malawi, Tanzania, Zimbabwe; rural parts of Botswana, Burkina Faso, Namibia, and Swaziland; and urban parts of Burundi, Haiti, and Rwanda.1,14,15 Approaches for harm reduction combining access to clean syringes and needles together with education, outreach, and access to drug treatment have been successful worldwide in reduction of HIV transmission acquired via sharing of injection equipment.2 Heavy alcohol use and stimulants remain major drivers of HIV transmission in many places and in many groups of people.16-19
What do some successes have in common?
To reduce major successes in HIV prevention to one or two elements (eg, reduction in the number of partners), or to one or two strategies, is always a temptation and analogous to monotherapy for treatment of HIV disease.20 We reject that simplistic analysis and instead argue that reductions in HIV transmission in entire countries or regions or in specific risk groups inevitably result from a complex combination of strategies and several risk-reduction options with strong leadership and community engagement that is sustained over a long time. The effective mix will vary by transmission dynamics and several other factors.
We use two case examples—Uganda and the Mbeya region of Tanzania—to draw attention to a number of common elements of two successful programmes (table 1). The Mbeya region reported a decrease in HIV prevalence from 20% in the mid-1990s to 13% in 2005.21 This region of Tanzania is one of the most highly affected in the country, and local leadership from parliament, district councils, and regional AIDS coordinators stimulated actions that were similar to those in Uganda. One regional plan, enhanced surveillance for planning and assessment, and improved laboratories for testing were essential to the operation (table 1).
Table 1.
Elements of two successful programmes for behavioural change
| Uganda12 | Mbeya region, Tanzania21 | |
|---|---|---|
| Political support | Political support at the highest levels of government (the President) | Political support from members of Parliament, District Councils, and the Regional Medical Officer |
| Involvement | Resistance committees, police, traditional healers, youth, midwives, performance artists, refugees | Regional AIDS Control Coordinator and Council, district medical officers, district AIDS coordinators |
| Institutional participation | Religious organisations, prisons, universities, media, women's groups, schools | Business, non-governmental organisations |
| Planning | Single national plan with input from all sectors | Single regional plan with input from all sectors |
| Surveillance | Enhanced HIV and STI surveillance | Enhanced HIV and STI surveillance |
| Laboratory | Enhanced to support surveillance and VCT | Enhanced to support surveillance and VCT |
| VCT | Came later in the effort; could be responsible for continuation of risk reduction achievements; essential for referral to care | Widespread efforts to support testing even in the absence of treatment; strong emphasis on counselling |
| Information, education, and communication | Used several channels to ensure that simple and explicit messages were widespread; local involvement in design, production, and dissemination | Used several channels to ensure that simple and explicit messages were widespread; local involvement in design, production, and dissemination |
| Behavioural options | Delayed onset of intercourse, abstinence, sticking to one partner, use of condoms | Delayed onset of intercourse, abstinence, sticking to one partner, using condoms |
| Mobilisation | Involved resistance committees and district level officials and organisations | Developed and involved district, village, and ward AIDS committees |
| Condoms | Social marketing of condoms came later in the efforts; could be essential to sustain low prevalence rates | Widespread condom distribution |
| Blood supply | Ensured safety of the blood supply | Ensured safety of the blood supply |
| Treatment of STIs | STI treatment came later in the efforts | Central to initial efforts |
| HIV-infected people | Support groups formed | Specific emphasis on addressing stigma and discrimination; reducing effect of HIV infection |
STIs=sexually transmitted infections. VCT=voluntary counselling and testing.
Namibia is a recent example of a country taking aggressive steps to reduce HIV transmission. The country has a 5-year strategic plan and has doubled its domestic spending on HIV. Life-skills based HIV prevention is now being taught in 79% of secondary schools, more than 25 million male condoms are distributed every year in the public sector, 29% of men and 18% of women have received an HIV test in the past year, knowledge levels are high (>60% of men and women aged 15-24 years got all items correct on a test of comprehensive knowledge of HIV), and sex before the age of 15 years and the percentage of people reporting multiple partners has dropped.22
Three important lessons emerge from these and other case studies. First, radical behavioural change is needed in a sufficiently large number of people who are potentially at risk to reduce HIV transmission. Uganda's 70% decrease in HIV prevalence, for example, was linked to a 60% reduction in sex with non-primary partners, a 2-year delay in onset of first intercourse, and increases in condom use. One analysis of the Uganda success surmised: “Our findings indicate that substantial HIV reductions in Uganda resulted from public-health interventions that triggered a social process of risk avoidance manifested by [radical] changes in sexual behaviours. Communications were clear and direct, and widespread involvement from various sectors of Ugandan society was achieved.”13 Modest changes in behaviour are helpful, but changes in transmission require that large numbers of people change their behaviours substantially and maintain these changes for a long time.
Second, a mix of communication channels disseminated simple and clear messages about several risk reduction and health-seeking options (eg, delay of onset of first intercourse, reduction in number of partners, condom use especially with non-primary partners, HIV testing, and treatment for sexually transmitted infections). One risk reduction strategy (eg, abstinence or partner reduction) should not be emphasised over another (eg, condom use), since people like choice and the mix of strategies is essential.
Third, local involvement in message design, production, and dissemination was essential.12 In fact, one of the most energising activities in many strategies and campaigns for HIV prevention involves using the creativity and energy of people who are most affected by the epidemic to develop messages and strategies to motivate behavioural change.
Despite these lessons, it has been difficult to develop and implement strategies and programmes that extend behaviourally-based HIV prevention to enough countries and people, and throughout a sufficient number of sectors of society to reverse or even stem the advance of HIV/AIDS. The 2007 UNAIDS report estimates that over 2 million new HIV infections occur every year.1 Countries such as Mozambique, South Africa, and Zambia show no decrease in levels of HIV infection.
Sustaining reductions in high-risk behaviour and HIV incidence once they have occurred has happened rarely, if at all.23 The number of HIV infections in men who have sex with men is now increasing in the USA and many European countries.24 Uganda has reported stable HIV prevalence in a rapidly growing population (which translates into a greater number of people living with HIV/AIDS) and increases in risky sexual behaviour.1 Despite Thailand's successes in reducing general population prevalence, HIV has remained high in injecting drug users, men who have sex with men, and informal sex workers.1
Behavioural intervention research
Experience with behavioural intervention research parallels programmatic experience. Several studies and meta-analyses have investigated individually targeted behavioural interventions to reduce HIV-related sexual risk behaviour. Historically, most approaches are based on cognitive-behavioural approaches,25 communications theory,26,27 peer education, or diffusion of innovation, and the benefits and restrictions of these approaches are now well known.25 The behavioural changes effected are statistically significant in studies that are designed to assess the efficacy of such interventions, but rarely sufficient to reduce sexually transmitted or HIV infections.28 Project EXPLORE16-18 is the only intervention study for HIV behaviour with an HIV endpoint, and it draws attention to the benefits and restrictions of behavioural interventions for individuals. It used an intensive one-to-one counselling format over ten sessions to reduce HIV incidence in 4295 men who have sex with men in six US cities.29 The counselling was highly individualised. Similar to other behavioural approaches, the counselling attempted to increase knowledge, perceived risk of acquiring HIV, motivation, and skills to change. Counsellors and clients assessed circumstances and occasions in which an individual might engage in risky behaviour, and then established risk reduction plans to assist the individual in avoiding HIV acquisition. Control participants received counselling on the basis of Project RESPECT model, in which individuals were given brief risk reduction counselling along with HIV testing twice a year.30 An earlier clinical trial showed the efficacy of the Project RESPECT model in reduction of incident sexually transmitted infections. Individuals in the intensive experimental intervention also received maintenance sessions every 4 months after the conclusion of the treatment sessions. Average follow-up was 3·25 years, which was longer than has occurred in any other intervention trial of behavioural change. The overall incidence was 2·1 per 100 person-years, and the rate of HIV acquisition in the intervention group was 18·2% lower than that in the control group, although this effect was not significant (15·7% [95% CI -8·4 to 34·4] after adjustment for baseline variables). Thus, intensive one-to-one counselling was not more effective than was twice-yearly HIV counselling, testing, and referral.
This controlled prevention trial is a good indicator of what has happened in large-scale programmes—namely, that effects are often marginal and changes are difficult to sustain. The effects of the intervention on HIV incidence seemed to be substantial in the first 12 months, with a 33% reduction in the first 6 months and a 39% reduction in the first 12 months. But the intervention and control groups did not differ significantly in HIV incidence at the end of the 3·25 years of follow-up. Had the study terminated when behavioural studies are usually stopped (ie, at 12-months' follow-up), the intervention would have been declared effective. The intervention did reduce unprotected anal intercourse with partners who were HIV-infected or whose serostatus was unknown (20·5% reduction [95% CI 10·9-29]), but this reduction was not sufficient to reduce HIV incidence. The Project EXPLORE intervention was effective in reduction of some HIV risk factors (eg, increasing safer sexual norms, communication skills, and self-efficacy to practise safer sexual behaviours).29 But important risk factors such as stimulant and other drug use, heavy alcohol use, and depressive symptoms were not affected, and these were important predictors of seroconversion in both the intervention and control groups.
Get the programmes right
Two solutions to limited efficacy and lack of sustainability in behavioural strategies for HIV prevention exist. First, we need to think differently about the goals of different levels of interventions. Behavioural strategies are necessary but not sufficient to reduce HIV transmission, but are essential in a comprehensive HIV prevention strategy. Second, behavioural strategies themselves need to be combinations of approaches at multiple levels of influence.31
Multiple behaviours collectively enhance risk, and they need to be targeted through many levels to achieve the best results. Behavioural strategy aims might involve increased knowledge about how to protect oneself from HIV infection; stigma reduction; encouraging access to services (eg, methadone maintenance, HIV counselling and testing, diagnosis and treatment of sexually transmitted infections, use of antenatal and reproductive health services); improving attitudes toward safer sexual practices; delaying onset of intercourse; decreasing number of partners; reducing use of sex workers; increasing condom sales; recognition of early symptoms of sexually transmitted infections or HIV; recognition of the benefits and limitations of male circumcision for protection against HIV; disclosure of HIV serostatus; harm reduction strategies; how to access treatment for HIV; the importance of adherence to antiretroviral drugs; and so on. The right combination of strategies, of course, depends on the profile of the populations engaging in risky activities, among whom HIV is spreading.7 Adoption of a comprehensive framework—in terms of combination HIV prevention and the use of multilevel behavioural strategies—requires that each strategy be assessed only in terms of what it is trying to achieve. Failure to show that a specific strategy reduces HIV infection does not render it useless in a comprehensive programme or a multilevel behavioural strategy for HIV prevention. The combination of strategies might be relevant to the end result.
Emphasis on some behavioural goals (eg, abstinence) to the exclusion of others has hampered prevention efforts. A so-called ABC approach to prevention of sexual transmission (abstinence, be faithful, condoms) has led to an inappropriate and ineffective focus on abstinence only, when the evidence is clear that several behavioural changes are essential for epidemic control.32,33 It would be useful if the abstinence-only controversy could be laid to rest. Although some moral systems encourage abstinence for ethical or religious reasons (and that is their right), public health in a pluralistic world needs to follow scientific findings. There have also been discussions, which are not particularly useful, as to whether condom use, partner reduction, abstinence, or delay in onset of intercourse reduced HIV prevalence in Uganda. A combination of all these approaches is essential for immediate and sustained reductions in HIV transmission.
The initial authorising legislation of the US President's Emergency Plan for AIDS Relief (PEPFAR) required that 33% of total prevention spending be spent on abstinence until marriage. The US Institute of Medicine, in its assessment of PEPFAR, concluded that: “Despite the efforts of the Office of the US Global AIDS Coordinator to administer the allocation [of the abstinence-only requirements] judiciously, it has greatly limited the ability of country teams to develop and implement comprehensive prevention programs that are well integrated with each other and with counselling and testing, care, and treatment programs and that target those populations at greatest risk”.32 The US Congress' General Accounting Office reported that the requirements that a third of PEPFAR prevention funds be spent on the “A” of the “ABCs” makes it difficult for programme planners to allocate prevention resources appropriately on the basis of the available data.34 These findings, along with the work of several advocacy groups, resulted in these provisions being removed from the 2008 PEPFAR authorising legislation.
Collins and colleagues33 summarised it well when they said: “It is time to scrap the ABCs and elevate the debate on HIV prevention beyond the incessant controversies over individual interventions. Small scale, isolated programs, however effective, will not bring the AIDS epidemic under control. To lower HIV incidence, especially in high transmission areas, policy makers, donors, and advocates need to demand national prevention efforts that are tailored to their epidemics, bring quality interventions to scale, and address environmental factors in vulnerability. That is why today's most commonly cited acronym for HIV prevention— ABC—falls severely short of what is needed to reduce HIV transmission. ABC infantilizes prevention, oversimplifying what should be an ongoing, strategic approach to reducing incidence.”
Combination behavioural prevention
Table 2 shows how the multilevel approach—a combination behavioural prevention strategy—can be used, with HIV counselling and testing as an example. HIV transmission is a dyadic event that occurs in social contexts, and thus, behavioural strategies working with social units might have greater potential than might those working with individuals in isolation. Strategies working across many levels of influence might be more likely to affect behaviour than might those working only at one level, as shown by the multilevel behavioural intervention to increase access to sterile syringes in injecting drug users in the USA (panel 1).43 This study was undertaken in Harlem, NY, USA, with the South Bronx as the comparison community. The goal was to develop a community-based participatory research programme to establish whether a multilevel intervention would increase sterile syringe access. The intervention worked to change behaviour at the level of individuals (injecting drug users and pharmacists), peer groups and networks, institutions, and the community. Positive opinion and attitudes toward pharmacy syringe sales to injecting drug users increased among pharmacists and community members in the intervention community. A significant decrease in syringe reuse and a significant increase in pharmacy use were recorded in African-American injecting drug users in the intervention communities.
Table 2.
A multilevel approach to behavioural strategies for HIV prevention with HIV counselling and testing as an example
| Examples | Applied to HIV counselling and testing | |
|---|---|---|
| Individual | Education; drug-related or sexual risk reduction counselling; skills building; prevention case management | HIV testing and counselling for individuals35 |
| Couple | Couples counselling | HIV counselling and testing for couples35-38 |
| Family | Family-based counselling programmes | Home-based family HIV counselling and testing39 |
| Peer group/network | Peer education; diffusion of innovation; network-based strategies | Voluntary counselling and testing for all network members |
| Institution (eg, school, workplace, prisons) | Institution-based programmes | Services for voluntary counselling and testing available within workplaces and other institutional settings40 |
| Community | Mass media; social marketing; community mobilisation | Community-based voluntary counselling and testing (eg, Project Accept);41,42 mobilisation and media to promote HIV counselling and testing |
Panel 1: Specific activities undertaken in a multilevel behavioural intervention to increase access to sterile syringes in injecting drug users in Harlem, NY, USA43.
Individual level
Drug-related counselling sessions with injecting drug users
Fitpacks distributed (syringe-disposal containers with harm reduction information)
Risk reduction pamphlets
Peer group/network level
Harm/risk reduction group sessions for injection drug users
Institution level
Pharmacy visits
Pharmacist forums and trainings
Pharmacy guides
Posters in pharmacies
Visits to and trainings for community-based organisations serving injecting drug users
Training for community-based organisations
Community level
Health fairs
Posters, pamphlets, and stickers
Behavioural change interventions at the individual level include educational, skills-building, counselling, prevention case management, and other strategies that are delivered either one-to-one or in small groups. School-based HIV prevention falls into this category, although it is often implemented in a limited form, meaning that the number of sessions is truncated, the lessons are informational only and not skills-based, and the format addresses the biology of HIV without providing the students with practical lessons and strategies on how to avoid acquiring HIV. Although individual-level interventions might be helpful, they are not sufficiently efficacious or lasting to be used alone to reduce HIV transmission. Research and programme agendas need to move beyond intervention studies at the individual level, especially those using approaches based on cognitive theories, and explore other potentially more potent approaches to behavioural change.
Strategies for couples attempt to motivate behavioural change within a primary or secondary relationship. These strategies recognise that HIV transmission is a social event that occurs between two people, both of whom need to participate in the change. HIV testing and counselling for couples represents one very effective approach.35-37 More than 65% of new HIV infections are in sub-Saharan Africa, where most transmissions occur between heterosexual cohabiting partners. Some estimates suggest that 60-95% of new HIV infections in Rwanda and Zambia occur between married couples living together.44 Cohabiting couples in Africa represent the world's largest HIV risk group. What can be done to reverse this risk? One exemplary strategy is voluntary counselling and testing for couples, and this approach has been assessed and scaled up in Rwanda and Zambia (panel 2). This strategy has shown benefits including reduction of HIV transmission, sexually transmitted infections, and unintended pregnancies between couples. We need more experience with concordant negative couples to understand how to prevent infection outside—and thus, inside—of the relationship. Identification of concordant positive couples has the advantage of referring them for care and treatment, and encouraging outside partners or other members of the marital unit to be tested if they are in a polygamous union.
Panel 2: Addressing the social dynamics of HIV transmission within couples in Zambia and Rwanda.
Voluntary HIV counselling and testing for couples has shown efficacy in reducing risk behaviour and HIV transmission within married or cohabiting couples35-37
Voluntary counselling and testing for couples can allow them to provide mutual support for accessing treatment and for reproductive decision making45
Adverse consequences do occur, especially if the woman is infected and the man is not. Adverse consequences can be predicted from a history of alcohol abuse and violence within the relationship, and these factors should be used to advise couples about the potential negative effects of voluntary counselling and testing for HIV for couples45
Demand for voluntary counselling and testing for HIV in couples might be low because of the myth that monogamy is safe, gender inequality, concerns that individuals infected with HIV will have adverse consequences, and the inherent difficulties of a couple confronting together the possibility of one or both of them being infected with HIV46
Demand, however, is flexible and can be increased through community outreach, media, and home-based testing47
Families are clearly important in HIV risk, in addition to HIV transmission between partners, parents to children, and infections resulting from home-based care activities. A series of studies on problem behaviours in adolescents in the USA have documented the important role that families have in promotion of a variety of health-promoting and HIV-associated risk reduction strategies in adolescents.48,49 Specific strategies that focused on communication between parents and adolescents have shown efficacy in reduction of problem behaviours.50 Family-based interventions in the USA for parents with HIV infection have been efficacious in reducing emotional distress and problem behaviours in adolescents in such families.51 Enlisting families in HIV-associated risk reduction in China and other places to come to terms with their infection and reduce HIV transmission,52 and HIV prevention approaches including methadone maintenance, improve family relations and support continual risk reduction.53
One family-centric model of behavioural HIV prevention involves HIV voluntary counselling and testing, delivered in the home to the entire family. In this approach, home-based testers move from door to door, explain counselling and testing to the entire family and obtain consent, and then provide results to all family members. The perceived advantages are easier access, reduced stigma, and the possibility that counselling and disclosure for couples might be eased, especially in serodiscordant couples. Botswana, Lesotho, and Uganda among other countries, are using this strategy, and it has been assessed in cluster-randomised trials in Uganda and Zambia.39 In both cases, people randomly assigned to optional testing locations, including home testing, were four to five times more likely to agree to testing and receive test results than were those randomly assigned to testing facilities only.39
There are at least three primary approaches to use peer groups and networks as agents of change. The first involves peer education, which is especially effective when there is participation and collaboration with vulnerable groups who are often alienated from formal service providers and government structures. Peer education is especially effective in increasing condom use and reducing sexually transmitted infections in high-risk groups in sub-Saharan Africa and Asia, including female sex workers, female bar or hotel workers in truck stops, high-risk men such as transport workers, men in the military, or clients of female sex workers.54 Peer education programmes have also been successful in increasing condom use in secondary-school students (aged 13-18 years) and rural populations.54
The second approach involves diffusion of innovation and the involvement of influential leaders in the community, “…trusted trendsetters whose actions, attitudes, and views influence those of other members through interactions in existing social relationships”.55 Diffusion of innovation was first applied to HIV prevention in a series of community-level outcome trials.56,57 This approach to HIV prevention relies on nine core elements that are clustered under three main headings: developing momentum, exposure, and repetition; delivering effective, theory-based HIV prevention messages; and initiating and sustaining risk reduction conversations. Some failures to replicate this approach in other countries such as the UK have been attributed to the fact that not all the core elements of the model were incorporated. The National Institute of Mental Health Collaborative HIV/STD Prevention Trial55 adapted the community popular opinion leader model to test the efficacy of this prevention intervention with sexually transmitted infection and HIV endpoints in five international settings: China, India, Peru, Russia, and Zimbabwe. The results of this trial will be presented at the International AIDS Society meeting in Mexico City.
The third approach involves network-based interventions. Social networks are associated with HIV risk behaviours and with serostatus, especially in injecting drug users and in men who have sex with men in eastern Europe.58,59 Network-based interventions involve gaining access to social networks through key individuals; identifying members of the injection, sexual, or social networks; training network leaders as peer educators; asking leaders to disseminate HIV risk reduction messages throughout their networks; and then assessing effects. Social network interventions have been used successfully to reduce sharing of injection equipment between injecting drug users and to reduce unprotected intercourse in men who have sex with men and heterosexual men in eastern Europe.58,59
Interventions for HIV prevention have been delivered in several social institutions including workplaces,60-62 prison,63 the military, faith-based organisations, and schools. These types of institutions not only offer the opportunity to reach a large number of sometimes high-risk individuals, but might also be able to take advantage of peer networks and leaders, channels for diffusion of innovation, and media and other educational or motivational approaches. Workplace peer education programmes for prevention of HIV, for example, are quite popular but rarely assessed.60-62 The workplace is a favoured setting for reaching general populations of men and women of reproductive age and are regarded as an efficient place to deliver voluntary counselling and testing services and to promote couples and family-centred HIV services.40 Workplace programmes, however, require attention to issues of confidentiality and maintenance of quality.61 Large and multinational businesses have been able to implement these types of programmes, but they are beyond the resources of enterprises of small and medium size.64
A participatory research programme undertaken with the Thai military provides one successful example of an institution-delivered intervention.65 Entire companies were assigned to the intervention group, a diffusion group (residing in the same barracks but not receiving the intervention), and a control group. Incidence of new sexually transmitted infections was seven times lower and HIV incidence was 50% lower in the intervention group than in the diffusion and control groups. The intervention included participatory planning by the squad members, and used several strategies to reduce alcohol use and brothel patronage and increase consistent condom use, sexual negotiation, and condom skills.
Strategies at the community level involve the use of mass media, social marketing, and community mobilisation. The use of mass media and condom social marketing have been effective in increasing condom sales and distribution in a variety of populations in sub-Saharan Africa including truckers, urban and periurban adults, male miners, adolescents, and men and women seeking services for sexually transmitted infections.54
Project Accept, an example of community mobilisation, is the first international, multisite, community randomised controlled study to establish the efficacy of a multilevel structural intervention for HIV prevention, with HIV incidence and stigma reduction as study endpoints.41,42 The intervention—undertaken in South Africa, Tanzania, Thailand, and Zimbabwe—is directed at a community, and is aimed at rapidly increasing knowledge of HIV status, changing community norms about HIV risk behaviours and acceptance of people affected by HIV/AIDS, and enhancing social support for people living with HIV/AIDS. The intervention uses three major strategies: (1) community mobilisation to enhance the uptake of voluntary counselling and testing, thus increasing the rate of HIV testing, knowledge of status, and frequency of discussions about HIV; (2) community-based voluntary counselling and testing to increase access to such services beyond health-care facilities and make awareness of HIV status more normative in community settings; and (3) comprehensive post-test support services that aim to improve the psychosocial wellbeing of people infected with HIV and their social network, and assist HIV-negative people in maintaining their negative status. Outcomes are being assessed at the individual and social level, with community sampling methods and recent HIV infection as the biological endpoint.
Get the behavioural science right
Behavioural science needs to do better in supporting effective HIV prevention. Behavioural strategies need to be liberated from the strictures of present theoretical and methodological thinking. The goal is radical behavioural change, which means progressing from small focused studies of individuals on one area of HIV prevention to more comprehensive strategies that record effects on varied inputs, levels, and outcomes. We can do a better job of disseminating effective approaches and, at the same time, supporting effective strategies built from the ground up. Social and behavioural science capacity is needed to achieve this aim, especially in hyperepidemic settings.
Step 1: expand theoretical and methodological approaches
The limited benefit of behavioural strategies derives both from the present dominance of some theoretical approaches to behavioural change, and the limitations to knowledge from randomised trials testing the efficacy of interventions in individuals and small groups. The theories guiding most interventions are essentially cognitive and individualistic, and assume that people have the motivation and freedom to adopt protective actions. These theories generally do not address the fact that, whether in sexual contact or injecting networks, HIV transmission is a social event and many factors other than perceived threat, knowledge, self-efficacy, behavioural intentions, and perceived social norms affect whether or not an individual is going to share needles or have sexual intercourse and then whether or not sexual intercourse will potentially involve transmission risk.66,67 Intervention studies have focused almost exclusively on the individual or small group, and scale-up of these types of strategies to achieve an epidemic effect has never been tried and might be tenuous. Examination of the Compendium of Evidence-Based Interventions by the US Centers for Disease Control and Prevention (CDC)68,69 shows that these strategies are almost entirely delivered to individuals or small groups. Although such strategies are no doubt useful, no attempts have been made to show how they might produce region-wide or country-wide reductions in HIV incidence or prevalence. Almost all the interventions in the Compendium are based on social-cognitive theory or variations thereof.
Although the development of the Compendium was motivated by the need to compile and disseminate scientifically validated interventions, the major concern is that reliance on specific scientific methods—especially the randomised controlled design—determines the type of interventions that are studied rather than considering which types are needed for epidemic effect and matching the design to the research question. “…John Tukey reminds us that the public health significance of the research question should be paramount in the design of research. Important questions should not be ignored if they cannot be fitted into the framework of an RCT [randomised controlled trial]. Rather, the strongest possible design that can feasibly be implemented should be chosen, whether an RCT or an alternative design”.70
Project EXPLORE16 and other studies have shown the benefits and limitations of this theoretical approach. The main restrictions are that behavioural changes, although statistically significant, were insufficient to reduce HIV acquisition and that the interventions did not change the major contributors to infection—namely, the use of alcohol, stimulants, and other drugs.18 Clearly, enough is known about the benefits and limitations of cognitive-behavioural intervention approaches and we should move on from social-cognitive interventions that are delivered to individuals or small groups and assessed for their efficacy in randomised controlled designs. Efforts have to be made to study other approaches, especially those that can potentially lead to population-wide sustained changes in behaviour and that address the link between substances and HIV transmission. Inevitably, theoretical models and the practical implications derived from them will increase in complexity, but that might be inevitable and perhaps what is needed.
Community mobilisation efforts, which are effective in the early stages of the HIV epidemic in communities of men who have sex with men in resource-rich countries or in Uganda or Thailand, might be difficult to engineer, especially as we enter the era of antiretroviral therapy in most parts of the world. New motivational models, beyond those based on various methods of persuasive communication, are needed. One example involves the use of economic incentives, cash, or other benefits transferred to individuals or families on the completion of publicly observable behaviours that support prevention or treatment.71 Financial incentive strategies have been used quite successfully in the USA to decrease stimulant addiction72-74 and in Mexico to improve child health and education. Experiments are underway in South Africa to establish the effect of conditional cash transfers on child and family wellbeing,71 and programmatic efforts are underway in New York City (USA) to assess the benefits of cash incentives for successful completion of high school.75 Barnett and Weston71 postulate that these types of interventions, as well as microfinance and other economically-based approaches, work by increasing predictability and thus hope for the future, leading to decisions that enhance health. These types of interventions are but one example of innovative thinking in behavioural change. Clearly, the field needs many more creative approaches in view of what we know about the difficulty of preventing HIV transmission and the limits of present strategies for behavioural change.
Step 2: understand and stimulate ground-up approaches
Behavioural strategies are needed to mobilise prevention activities and programmes. Such efforts inevitably should involve building social and behavioural science capacity, particularly in resource-poor and hyperepidemic settings. We need individuals on the ground who are knowledgeable about behavioural and social science and capable of integrating that knowledge with creative thinking about prevention and appropriate assessment strategies.
An assessment of so-called reputationally strong programmes (ie, those that are perceived to be efficacious even in the absence of evaluation data) of HIV prevention in the USA by the US CDC noted that such programmes had many intervention goals and typically used multilevel intervention approaches. However, the analysis also showed that community-based programmes succeed only in the context of strong institutional support and capacity to implement and sustain the programme. Thus, stimulation of ground-up or reputationally strong programmes needs not only funding streams that allow creativity to emerge and be exercised, but also capacity building and organisational stability for community-based organisations to be able to undertake such work.
Community mobilisation is an essential component in HIV prevention, as shown by documented successful programmes (table 1). Investigations are needed to understand how such mobilisation occurs and what sustains it, especially over the long period of time that is needed for initial and maintained HIV prevention. A crucial element in successful prevention programmes is committed and sustained leadership at all levels.76
Use of data to mobilise communities, to assess successes, and to plan for the future is another key factor in several successful programmes. Agencies funding research to effect behavioural change should prioritise assessments of locally developed programmes and behavioural epidemiological and observational studies ahead of small-scale intervention studies. Assessments of existing programmes are essential, especially if we are to use all available approaches to achieve the difficult aims in behavioural HIV prevention. Behavioural epidemiological studies are very informative, particularly in elucidating focus and priorities in policies and programmes for HIV prevention. South Africa, for example, has undertaken several sero-epidemiological and behavioural-epidemiological studies of the general population and of young people (panel 3).77-80 Such studies are rare (very few countries have continual and repeated behavioural surveillance) and difficult to fund, but are essential and valuable both for establishing seroprevalence and incidence when possible, and also for describing behavioural risk patterns, such as per-partner infectivity81 and factors associated with protected and unprotected intercourse.82,85 As another example, the National HIV Behavioural Surveillance Study by the US CDC has been important in the elucidation of risk factors associated with high rates of infection with HIV in African-American men who have sex with men,86 HIV testing patterns and barriers,87 and the prevalence of stimulant use in this population.88
Panel 3: Insights from sero-epidemiological and behavioural-epidemiological studies in South Africa.
HIV prevalence is highest in black people in South Africa (12·9%), but the prevalence in white people (6·2%) and coloured people (6·1%) is very high by any standard, suggesting a generalised epidemic in all three groups in South Africa78
HIV transmission to young women is highly efficient in South Africa, with an estimated per-partnership transmission probability of 0·74-1·00. Studies in other populations have estimated this probability to be less than 0·50, suggesting that HIV prevention for young women in South Africa needs to begin at a young age and be vigorous and comprehensive if it is to be at all effective81
The best predictor of condom use at last intercourse is condom use at first intercourse, suggesting that early and comprehensive sex education is essential82
Partners who were older increased risk for HIV acquisition in young people in South Africa. But the highest risk was for partners 1-4 years older than the young woman, suggesting that strategies with slightly older men might be important in reduction of HIV risk for young women77
Living in urban areas and in townships increases risk for HIV78
Women in the work force are less likely to be infected with HIV than are men, which is the reverse of what is true in South Africa generally.83 This finding means that the young women most likely to get infected with HIV are the least likely to enter the formal workforce. Workplace programmes will not reach those at highest risk for HIV
The best predictor of whether or not a young woman will get infected is whether or not she is in school. Doing whatever we can to maintain school attendance might have health and other benefits84
The data make the point that young people in South Africa (and, by extension, much of southern Africa) are at very high risk of acquiring HIV infection. Programmatic insights are clear; action is needed.
Observational studies of change over time are essential for instigating and supporting community activism, advocacy, and change. The Australian experience is a model in this regard.11 The scientific, services, and advocacy communities established a process whereby data from yearly surveys were fed back to the community, health authorities, and AIDS service organisations to assist with prevention planning and programming, with subsequent surveys providing assessments of previous programmes and directions for the future.11
Step 3: integrate behavioural, biomedical, and structural HIV prevention strategies with HIV treatment
Cates,89 as well as others, has made the point that most forms of HIV prevention—with the exception of a prophylactic vaccine, which we do not have—need continual behaviour modification to be effective. Even male circumcision does not render a man immune from HIV but rather only reduces the risk of acquisition and requires the range of HIV prevention behaviours on his part to avert infection. Figure 2 shows graphically the prevention effect when various technologies are used perfectly and the likely effect when such approaches are used imperfectly, as is common in real life. Imperfect application was shown in the recently completed study of the diaphragm and lubricant gel to prevent HIV acquisition in women in southern Africa90 and in a study of aciclovir use to suppress herpes simplex virus 2 in Mwanza, Tanzania.91
Figure 2. Adherence to HIV prevention technologies.

Adapted from reference 89 with permission from author and publisher.
The recent report of the Institute of Medicine92 draws attention to the practical issues in ensuring adherence and the methodological challenges in measuring it in the context of prevention trials. The scientific published work for adherence suffers from the same concerns as does that for behaviour change—ie, it focuses on individuals and small groups, does not have methodological rigor, and has been undertaken primarily in high-income countries with uncertain generalisation to low-income and middle-income countries. We know that factors at the provider or clinic level, or sociocultural levels can affect adherence, and yet most strategies do not address these factors.93 Similar to prevention, adherence science needs to expand beyond individual boundaries and to think more broadly about motivational and structural strategies, especially how such strategies can be applied to large populations so that prevention technologies have a chance of working when implemented.
Step 4: prevention with positives
Treatment for HIV has extended life in resource-rich countries, and HIV prevention has yet to catch up.94 The next challenge is how to undertake effective HIV prevention in the era of more generalised access to antiretroviral therapy in resource-poor parts of the world. Prevention with positives becomes more achievable as individuals living with HIV/AIDS are encouraged to learn their serostatus and access treatment.95,96 HIV prevention typically has referred to protecting individuals from becoming infected with HIV, but substantially more efforts are being directed at helping individuals with HIV to avoid spreading it to others. Most individuals will want to remain sexually active after they learn of their positive serostatus, and this desire is even more likely as antiretroviral drugs extend not only life but also quality of life for people living with HIV/AIDS.97 People who are unaware of their serostatus are very likely to transmit a high proportion of infections, and evidence from all countries shows that individuals reduce risk and take precautions to protect their partners once they know their serostatus. Thus, one of the major tasks for HIV prevention in the developing world must involve increasing the number of people who know that they are infected with HIV. Several strategies have proven successful in this regard, including the use of community opinion leaders,98 home-based family-delivered counselling and testing,39 provider-initiated counselling and testing as in Botswana,39 and community-level counselling and testing as in Project Accept.42 Assistance with disclosure and partner testing, or the advancement of counselling and testing for couples, can help to identify partners who are infected and in need of treatment, or who are not infected and in need of protection.
Ideally, as access to treatment increases, HIV-positive individuals should enter into medical care and receive antiretroviral drugs at an early enough stage, not only for their own health, but also to reduce their infectiousness.95,96 Adherence to treatments for HIV is one of the most important factors in their success and identifies the degree of viral suppression and the potential infectiousness of people living with HIV/AIDS.99-102 Risk compensation also deserves more attention, since any advances in reduction of HIV infections could be undone by compensatory increases in risk behaviour; however, this possibility should never be an excuse for failing to implement effective HIV prevention. A frequent observation, which was noted in many places after the introduction of highly active antiretroviral therapy, was an increase in HIV risk behaviour or incidence, or the incidence of sexually transmitted infections. By contrast, risk compensation was not observed in clinical trials of the diaphragm90 or of male circumcision.103-106 Well crafted and informative observational studies are essential for understanding the extent, nature, and determinants of risk compensation, especially as innovations in HIV prevention or treatment are extended to entire populations. This approach should be a high priority for funding agencies, and repeated surveys will be essential to identify trends over time, as well as the use of strategies to address the issue.
Establishment of prevention as a standard of care, especially in medical settings, for all people living with HIV/AIDS is a major priority. It is essential to operationalise what this means for various settings and secure the resources for implementation. At the least, it will be essential that clinical-care providers undertake continual risk assessments and provide ongoing information, counselling, services for sexually transmitted infections, and referral to harm reduction and drug treatment for their patients infected with HIV. People who continue to engage in risky practices might need specialised referrals for more intensive programmes, or treatment for substance abuse or mental-health issues.
Get the simple things right
HIV prevention is hampered by unparalleled impediments. The goals of universal access should include HIV prevention technologies and devices (eg, condoms, clean needles, and drug treatment at a minimum), information, skills, and services. But this has not been the case in worldwide HIV prevention. Prevention of HIV is more controversial than is treatment for HIV/AIDS. But what should not be controversial is the imperative to use scientifically established and evidence-based strategies to save human lives. It is rare for governments to object to access to antiretroviral treatment for their populations. It is not rare for governments to object to evidence-based and proven approaches to reduce behavioural risk for HIV infection. UNAIDS reports that non-governmental informants in 63% of countries report laws, regulations, or policies that present obstacles to effective HIV prevention, treatment, care, and support for populations most at risk of HIV.22 Figure 3 shows that only 60% of sex workers, 46% of injecting drug users, and 40% of men having sex with men were reached with HIV prevention programmes (UN General Assembly special session on HIV/AIDS [UNGASS] indicator 9). The USA, a few other governments, and the International Narcotic Control Board are the only bodies to continue to oppose harm reduction principles and practices for reducing HIV infection in injecting drug users.2 The initial programmes in Uganda de-emphasised condoms because of concerns that they might encourage promiscuity,12 and similar concerns have been raised about male circumcision and widespread access to treatment;106,107 however, fear of disinhibition should never be used as an excuse not to implement effective HIV prevention strategies, and especially now that male circumcision has such potential6 and that treatment of HIV disease might reduce infectiousness.96 We can and must learn how to implement all effective HIV prevention strategies and motivate continued risk reduction.
Figure 3. Percentage of sex workers, injecting drug users, and men having sex with men who are reached by HIV prevention programmes.

*Percentage of sex workers and men having sex with men who reported knowing where they can receive an HIV test and that they were given condoms. †Percentage of injecting drug users who reported knowing where they could receive an HIV test and be provided with condoms and sterile injecting needles and syringes. Reproduced from reference 22 with permission from author and publisher.
HIV prevention is not being implemented. The aim of Millennium Development Goal 6 is to halt, and reverse, that spread of HIV/AIDS by 2015. The Special Session on HIV/AIDS of UNGASS in 2001 resulted in endorsement by 186 member states of the Declaration of Commitment on HIV/AIDS.108 UNAIDS established a multi-agency Indicator Harmonization and Registry Technical Working Group in 2006 to provide guidance about core indicators and to provide easy access to existing indicators through a web-based registry. The 40 core indicators, of which the ten behavioural indicators are listed in table 3, are designed to focus attention on key prevention components of HIV and the country-level response to the AIDS epidemic.109 The report prepared for the 2008 UNGASS includes data provided by 147 UN member states110 and additional data are provided in the most recent UNAIDS report (please see copies of the individual country reports).22 Some of the data are impressive, including increases in the total yearly resources available for HIV together with projected trends, the number and percentage of HIV-infected pregnant women receiving antiretroviral drugs,4 and the number of people receiving antiretroviral drugs in low-income and middle-income countries.
Table 3.
Ten key behavioural indicators for HIV prevention
| Number of countries reporting | Years in which countries reported | Values reported | Examples | |
|---|---|---|---|---|
| UNGASS indicator 7: percentage of women and men aged 15-49 years who received a HIV test and know their results (suggested reporting frequency every 2 years) | 85 | 2003-07 | 0-100 | Democratic Republic of the Congo (2005: 3% for men and women); Mozambique (2004: 2% for men and women); Nigeria (2005: 9% for men and 8% for women); Rwanda (2005: 11% for men and 12% for women) |
| UNGASS indicator 9: percentage of most-at-risk populations reached with HIV prevention programmes (suggested reporting frequency every 2 years) | Male sex workers: 13; female sex workers: 45; injecting drug users: 21; men having sex with men: 36 | 2007 | Male sex workers: 3-100; female sex workers: 2-100; injecting drug users: 5-82; men having sex with men: 10-100 | Figure 3 |
| UNGASS indicator 11: percentage of schools that provided school-based HIV life-skills education in the past year (suggested reporting frequency every 2 years) | 62 | 2007 | 1-100 | Angola: 1%; Central African Republic: 15%; Namibia: 79%; Nigeria: 34%; Swaziland: 51%; Zambia: 60% |
| UNGASS indicator 13: percentage of young women and men aged 15-24 years who both correctly identify ways of preventing the sexual transmission of HIV and who reject major misconceptions about HIV transmission (suggested reporting frequency every 2 years preferred; minimum every 4-5 years) | 89 | 2007 | 11-85 | Figures 4 and 5 |
| UNGASS indicator 15: percentage of young women and men aged 15-24 years who have had sexual intercourse before the age of 15 years (suggested reporting frequency every 4-5 years) | 100 | 2007 | Men: <1-43; women: <1-28 | Angola: 36% for men and 28% for women; Democratic Republic of the Congo: 31% for men and 23% for women; Kenya 28% for men and 14% for women; Mozambique 26% for men and 28% for women; Thailand: 21% for men and 5% for women |
| UNGASS indicator 16: percentage of adults aged 15-49 years who have had sexual intercourse with more than one partner in the past 12 months (suggested reporting frequency every 4-5 years) | 87 | 2003-07 | Men: <1-51; women: <1-45 | Angola (2006) 51% of men and 25% of women; Lesotho (2005) 30% of men and 11% of women; Swaziland: 23% of men and 2% of women; Tanzania 20% of men and 5% of women |
| UNGASS indicator 18: percentage of female and male sex workers reporting the use of a condom with their most recent client (suggested reporting frequency every 2 years) | Female sex workers: 57; male sex workers: 26 | 2007 | Female sex workers: 20-100; male sex workers: 4-100 | Figure 3 |
| UNGASS indicator 19: percentage of men reporting the use of a condom the last time they had anal sex with a male partner (suggested reporting frequency every 2 years) | 65 | 2007 | 24-88 | Chile: 27%; Honduras: 47%; Japan: 55%; China 64%; Thailand: 88% |
| UNGASS indicator 20: percentage of injecting drug users reporting the use of a condom the last time they had sexual intercourse (suggested reporting frequency every 2 years) | 39 | 2005-07 | 9-66 | Turkey: 13%; Morocco 21%; Russia: 31%; China: 43%; Ukraine: 56% |
| UNGASS indicator 21: percentage of injecting drug users reporting the use of sterile injecting equipment the lasttimethatthey injected (suggested reporting frequency every 2 years) | 39 | 2005-07 | 7-95 | Bangladesh: 34%; China 41%; Malaysia: 28%; Mexico: 14%; Romania: 28%; Ukraine: 84%; Vietnam: 89% |
Values taken from reference 22 and from individual country reports at http://www.unaids.org/en/KnowledgeCentre/HIVData/CountryProgress/2007/CountryProgressAIICountries.asp.
Progress on behavioural indicators is much less impressive. Table 3 provides information about the number of countries reporting on every indicator, the range of years in which those countries reported, and the averages for some indicators (when those averages have been calculated by UNAIDS)22 and the ranges for others. We use summary measures when they are provided by UNAIDS but have chosen not to provide summary statistics for others because of the range of reporting years, the low numbers of countries reporting on some indicators, and the need to weight percentages by population and estimate missing values to estimate averages.
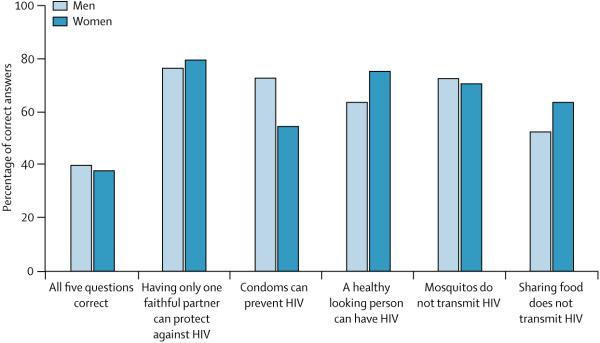
Nothing should be more important than a major focus on young people, not only in sub-Saharan Africa but in many other parts of the world as well (panel 3). The rates of comprehensive knowledge of HIV in young people aged 15-24 years (UNGASS indicator 13) are unacceptably low (figure 4),111 although the rates look better when individual items are examined (figure 5). Nonetheless, the 2005 target of 90% clearly has not been achieved and it is highly doubtful that the 2010 targets can be reached. National governments with generalised epidemics report that 67% of schools implemented skills-based HIV education (UNGASS indicator 11), but the intervention was provided only in 40% of schools on average. Further, only 42% of countries have programmes for out-of-school youth.22 The data, however, are far from complete since only 34 of the 151 countries reported on this indicator. Somewhat hopeful is a decrease in the percentage of young women and men aged 15-24 years that report sexual intercourse below the age of 15 years (UNGASS indicator 15) (figure 6). Nonetheless more progress needs to be made, especially in high prevalence areas. Adolescent girls in sub-Saharan Africa are 50% more likely than are boys to be sexually active and whereas age of first intercourse increased for boys in Mozambique, Rwanda, and Uganda, it fell in Ethiopia, Nigeria, and Tanzania (2nd, 6th, and 10th countries in the number of people living with HIV/AIDS in the world). Greater than 25% of men reportedly had sexual intercourse before 15 years of age in Angola, Democratic Republic of the Congo, and Kenya, for example. Greater than 20% of women report initiating intercourse before 15 years of age in countries such as Angola, Democratic Republic of the Congo, Mali, and Mozambique.
Figure 4. Percentage of young people aged 15-24 years who have comprehensive knowledge of HIV.

Adapted from references 22 and 110 with permission from author and publisher.
Figure 5. Comprehensive knowledge of HIV among young people, by type of question.
Reproduced from reference 22 with permission from author and publisher.
Figure 6. Percentage of young people who have first sexual intercourse before 15 years of age, by sex.

Reproduced from reference 22 with permission from author and publisher.
Many indicators have relevance for generalised epidemics, but some are essential for concentrated epidemics. Progress on these indicators needs to improve as well. A superficial examination of the percentage of female and male sex workers using a condom with their most recent client looks promising; one difficulty in the interpretation of this finding is the lower number of countries reporting, especially for male sex workers. The percentage of injecting drug users reporting use of sterile injecting equipment (UNGASS indicator 21) is quite low with only 38 countries reporting, as well as the percentage of injecting drug users reporting the use of a condom the last time that they had sexual intercourse (with 34 countries reporting).
We do not have up to date information about how well we are doing in HIV prevention. The need for accountability is great, and all parties—donor countries, philanthropies, multinational organisations, and countries, highly affected by HIV/AIDS—need to do their part to bring down HIV transmission.112 The numbers reported are not encouraging, and neither is the fact that many countries do not report on many indicators. Those who do report do so infrequently. The consequences for failure to reach important milestones in the fight against HIV/AIDS are grave.113 In view of what we have been able to derive from the UNGASS indicators, no one should be surprised that 2·5 million new infections occur every year.1 One can improve the science, but what good will it do if the science and best practices are not implemented?
The radical behavioural change that is needed to reduce HIV transmission requires radical commitment. Prevention strategies will never work if they are not implemented completely, with appropriate resources and benchmarks, and with a view toward sustainability. The fundamentals of HIV prevention need to be agreed upon, funded, implemented, measured, and achieved. That, presently, is not the case.
Acknowledgments
Support for this work was provided primarily by The Ford Foundation; The UCLA Center for HIV Identification, Treatment, and Prevention Services (CHIPTS; Mary Jane Rotheram-Borus, Director, funded by the National Institute of Mental Health grant number 2P30MH058107); The Diana, Princess of Wales Memorial Fund; The Franklin Mint Foundation; The MAC AIDS Fund; and The UCLA AIDS Institute and the UCLA Center for AIDS Research (Jerome Zack, Principal Investigator, funded by The National Institute of Allergy and Infectious Diseases, grant number AI28697). Other supporters included: The John M Lloyd Foundation, and the Columbia Center for HIV Clinical and Behavioral Studies (Anke Ehrhrardt, Director, funded by the National Institute of Mental Health grant number P30MH43520). Support was also provided by the HIV Prevention Trials Network (HPTN) and sponsored by the National Institute of Allergy and Infectious Diseases, National Institute of Child Health and Human Development, National Institute on Drug Abuse, National Institute of Mental Health, and Office of AIDS Research, of the National Institutes of Health, US Department of Health and Human Services, through cooperative agreement U01-AI-46749 with Family Health International, U01-AI-46702 with Fred Hutchinson Cancer Research Center, U01-AI-47984 with Johns Hopkins University, and U01-AI-48014 with the University of Pennsylvania. The sponsors had no role in the preparation of this paper and none of the views expressed herein represent those of the sponsors or any employees of the sponsors. We thank Judith Auerbach and Peter Aggleton for the extensive comments on earlier drafts of this paper; Purnima Mane of UNFPA for her guidance and feedback as this paper evolved; our colleagues at UNAIDS who contributed vital data and thoughtful comments on earlier drafts of this paper including Michael Bartos, Paul De Lay, Barbara de Zalduondo, Catherine Hankins, and Matthew Warner-Smith; and Professor King Holmes for coining the term “highly active HIV prevention” in figure 1.
Footnotes
Conflict of interest statement We declare that we have no conflict of interest.
This is the third in a Series of six papers about HIV prevention
References
- 1.UNAIDS . AIDS epidemic update. Joint United Nations Programme on HIV/AIDS; Geneva: 2007. 2007. [Google Scholar]
- 2.Wodak A. The role of harm reduction in controlling HIV among injecting drug users. AIDS. doi: 10.1097/01.aids.0000327439.20914.33. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rao Gupta G, Parkhurst JO, Ogden JA, Aggleton P, Mahal A. Structural approaches to HIV prevention. Lancet. 2008 doi: 10.1016/S0140-6736(08)60887-9. published online Aug 6. DOI:10.1016/S0140-6736(08)60887-9. [DOI] [PubMed] [Google Scholar]
- 4.Padian NS, Buvé A, Balkus J, Serwadda D, Cates W., Jr Biomedical interventions to prevent HIV infection: evidence, challenges, and way forward. Lancet. 2008 doi: 10.1016/S0140-6736(08)60885-5. published online Aug 6. DOI:10.1016/S0140-6736(08)60885-5. [DOI] [PubMed] [Google Scholar]
- 5.Vandenbruaene M. King Kennard Holmes—Chair of the Department of Global Health of the University of Washington. Lancet Infect Dis. 2007;7:516–20. doi: 10.1016/S1473-3099(07)70184-6. [DOI] [PubMed] [Google Scholar]
- 6.Sawires SR, Dworkin SL, Fiamma A, Peacock D, Szekeres G, Coates TJ. Male circumcision and HIV/AIDS: challenges and opportunities. Lancet. 2007;369:708–13. doi: 10.1016/S0140-6736(07)60323-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertozzi SM, Laga M, Bautista-Arredondo S, Coutinho A. Making HIV prevention programmes work. Lancet. 2008 doi: 10.1016/S0140-6736(08)60889-2. published online Aug 6. DOI:10.1016/S0140-6736(08)60889-2. [DOI] [PubMed] [Google Scholar]
- 8.Winkelstein W, Jr, Samuel M, Padian NS, Wiley JA. Selected sexual practices of San Francisco heterosexual men and risk of infection by the human immunodeficiency virus. JAMA. 1987;257:1470–01. doi: 10.1001/jama.1987.03390110046011. [DOI] [PubMed] [Google Scholar]
- 9.Winkelstein W, Jr, Samuel M, Padian NS, et al. The San Francisco Men's Health Study: III. Reduction in human immunodeficiency virus transmission among homosexual/bisexual men, 1982-86. Am J Public Health. 1987;77:685–89. doi: 10.2105/ajph.77.6.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winkelstein W, Jr, Wiley JA, Padian NS, et al. The San Francisco Men's Health Study: continued decline in HIV seroconversion rates among homosexual/bisexual men. Am J Public Health. 1988;78:1472–74. doi: 10.2105/ajph.78.11.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kippax S, Race K. Sustaining safe practice: twenty years on. Soc Sci Med. 2003;57:1–12. doi: 10.1016/s0277-9536(02)00303-9. [DOI] [PubMed] [Google Scholar]
- 12.Slutkin G, Okware S, Naamara W, et al. How Uganda reversed its HIV epidemic. AIDS Behav. 2006;10:351–60. doi: 10.1007/s10461-006-9118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stoneburner RL, Low-Beer D. Population-level HIV declines and behavioral risk avoidance in Uganda. Science. 2004;304:714–18. doi: 10.1126/science.1093166. [DOI] [PubMed] [Google Scholar]
- 14.UNAIDS . HIV prevention needs and successes: a tale of three countries--an update on HIV prevention success in Senegal, Thailand, and Uganda. Joint United Nations Programme on HIV/AIDS; Geneva: 2001. [Google Scholar]
- 15.UNAIDS . Report on the global AIDS epidemic: a UNAIDS 10th anniversary special edition. Joint United Nations Programme on HIV/AIDS; Geneva: 2006. [Google Scholar]
- 16.Koblin B, Chesney M, Coates TJ, for the EXPLORE Study Team Effects of a behavioural intervention to reduce acquisition of HIV infection among men who have sex with men: the EXPLORE randomised controlled study. Lancet. 2004;364:41–50. doi: 10.1016/S0140-6736(04)16588-4. [DOI] [PubMed] [Google Scholar]
- 17.Koblin BA, Chesney MA, Husnik MJ, et al. High-risk behaviors among men who have sex with men in 6 US cities: baseline data from the EXPLORE Study. Am J Public Health. 2003;93:926–32. doi: 10.2105/ajph.93.6.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koblin BA, Husnik MJ, Colfax G, et al. Risk factors for HIV infection among men who have sex with men. AIDS. 2006;20:731–39. doi: 10.1097/01.aids.0000216374.61442.55. [DOI] [PubMed] [Google Scholar]
- 19.Kalichman SC, Simbayi LC, Jooste S, Cain D. Frequency, quantity, and contextual use of alcohol among sexually transmitted infection clinic patients in Cape Town, South Africa. Am J Drug Alcohol Abuse. 2007;33:687–98. doi: 10.1080/00952990701522716. [DOI] [PubMed] [Google Scholar]
- 20.Potts M, Halperin DT, Kirby D, et al. Public health. Reassessing HIV prevention. Science. 2008;320:749–50. doi: 10.1126/science.1153843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vogel UF. UNAIDS Best Practices Collection. Joint United Nations Programme on HIV/AIDS; Geneva: 2007. Towards universal acces to prevention, treatment and care: experiences and challenges from the Mbeya region in Tanzania—a case study. [Google Scholar]
- 22.UNAIDS . Report for the global AIDS epidemic. UNAIDS; Geneva: 2008. [Google Scholar]
- 23.Stall RD, Ekstrand ML, Pollack A, McKusick L, Coates TJ. Relapse from safer sex: the next challenge for AIDS prevention efforts. J Acquir Immune Defic Syndr. 1990;3:1181–87. [PubMed] [Google Scholar]
- 24.Jaffe HW, Valdiserri RO, De Cock KM. The reemerging HIV/AIDS epidemic in men who have sex with men. JAMA. 2007;298:2412–14. doi: 10.1001/jama.298.20.2412. [DOI] [PubMed] [Google Scholar]
- 25.Noar SM, Chabot M, Zimmerman RS. Applying health behavior theory to multiple behavior change: Considerations and approaches. Prev Med. 2008;46:275–80. doi: 10.1016/j.ypmed.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Bertrand JT, Anhang R. The effectiveness of mass media in changing HIV/AIDS-related behaviour among young people in developing countries. World Health Organ Tech Rep Ser. 2006;938:205–41. discussion 317-41. [PubMed] [Google Scholar]
- 27.Bertrand JT, O'Reilly K, Denison J, Anhang R, Sweat M. Systematic review of the effectiveness of mass communication programs to change HIV/AIDS-related behaviors in developing countries. Health Educ Res. 2006;21:567–97. doi: 10.1093/her/cyl036. [DOI] [PubMed] [Google Scholar]
- 28.Noar SM. Behavioral interventions to reduce HIV-related sexual risk Behavior: review and synthesis of meta-analytic evidence. AIDS Behav. 2008;12:335–53. doi: 10.1007/s10461-007-9313-9. [DOI] [PubMed] [Google Scholar]
- 29.Chesney MA, Koblin BA, Barresi PJ, et al. An individually tailored intervention for HIV prevention: baseline data from the EXPLORE Study. Am J Public Health. 2003;93:933–38. doi: 10.2105/ajph.93.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamb ML, Fishbein M, Douglas JM, Jr, et al. Efficacy of risk-reduction counseling to prevent human immunodeficiency virus and sexually transmitted diseases: a randomized controlled trial. Project RESPECT Study Group. JAMA. 1998;280:1161–67. doi: 10.1001/jama.280.13.1161. [DOI] [PubMed] [Google Scholar]
- 31.Auerbach JD, Coates TJ. HIV prevention research: accomplishments and challenges for the third decade of AIDS. Am J Public Health. 2000;90:1029–32. doi: 10.2105/ajph.90.7.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sepulveda J, Carpenter C, Curran J, et al. PEPFAR implementation: progress and promise. National Academies Press; Washington, DC: 2007. [Google Scholar]
- 33.Collins C, Coates TJ, Curran J. Moving beyond the alphabet soup of HIV prevention. AIDS. doi: 10.1097/01.aids.0000327431.82795.49. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.United States Government Accountability Office . Global health: spending requirements presents challenges for allocating prevention funding under the President's Emergency Plan for AIDS Relief. Government Accountability Office; Washington DC: 2006. [Google Scholar]
- 35.The Voluntary HIV-1 Counseling and Testing Efficacy Study Group Efficacy of voluntary HIV-1 counselling and testing in individuals and couples in Kenya, Tanzania, and Trinidad: a randomised trial. Lancet. 2000;356:103–12. [PubMed] [Google Scholar]
- 36.Allen S. Effect of serotesting with counseling on condom use and seroconversion among HIV-serodiscordant couples in Africa. BMJ. 1992;304:1605–09. doi: 10.1136/bmj.304.6842.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allen SA. Confidential HIV testing and condom promotion in Africa: impact on HIV and gonorrhea rates. JAMA. 1992;268:3338–43. [PubMed] [Google Scholar]
- 38.Allen S, Meinzen-Derr J, Kautzman M, et al. Sexual behavior of HIV discordant couples after HIV counseling and testing. AIDS. 2003;17:733–40. doi: 10.1097/00002030-200303280-00012. [DOI] [PubMed] [Google Scholar]
- 39.Bateganya MH, Abdulwadud OA, Kiene SM. Home-based HIV voluntary counseling and testing in developing countries. Cochrane Database Syst Rev. 2007;4:CD006493. doi: 10.1002/14651858.CD006493.pub2. [DOI] [PubMed] [Google Scholar]
- 40.Corbett EL, Dauya E, Matambo R, et al. Uptake of workplace HIV counselling and testing: a cluster-randomised trial in Zimbabwe. PLoS Med. 2006;3:e238. doi: 10.1371/journal.pmed.0030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawichai S, Celentano DD, Chariyalertsak S, et al. Community-based voluntary counseling and testing services in rural communities of Chiang Mai Province, northern Thailand. AIDS Behav. 2007;11:770–77. doi: 10.1007/s10461-007-9242-7. [DOI] [PubMed] [Google Scholar]
- 42.Morin SF, Khumalo-Sakutukwa G, Charlebois ED, et al. Removing barriers to knowing HIV status: same-day mobile HIV testing in Zimbabwe. J Acquir Immune Defic Syndr. 2006;41:218–24. doi: 10.1097/01.qai.0000179455.01068.ab. [DOI] [PubMed] [Google Scholar]
- 43.Fuller CM, Galea S, Caceres W, Blaney S, Sisco S, Vlahov D. Multilevel community-based intervention to increase access to sterile syringes among injection drug users through pharmacy sales in New York City. Am J Public Health. 2007;97:117–24. doi: 10.2105/AJPH.2005.069591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dunkle KL, Stephenson R, Karita E, et al. New heterosexually transmitted HIV infections in married or cohabiting couples in urban Zambia and Rwanda: an analysis of survey and clinical data. Lancet. 2008;371:2183–91. doi: 10.1016/S0140-6736(08)60953-8. [DOI] [PubMed] [Google Scholar]
- 45.Grinstead OA, Gregorich SE, Choi KH, Coates T. Voluntary HIV-1 Counselling and Testing Efficacy Study Group. Positive and negative life events after counselling and testing: the Voluntary HIV-1 Counselling and Testing Efficacy Study. AIDS. 2001;15:1045–52. doi: 10.1097/00002030-200105250-00013. [DOI] [PubMed] [Google Scholar]
- 46.Chomba E, Allen S, Kanweka W, et al. Evolution of couples' voluntary counseling and testing for HIV in Lusaka, Zambia. J Acquir Immune Defic Syndr. 2008;47:108–15. doi: 10.1097/QAI.0b013e31815b2d67. [DOI] [PubMed] [Google Scholar]
- 47.Kempf MC, Allen S, Zulu I, et al. Enrollment and retention of HIV discordant couples in Lusaka, Zambia. J Acquir Immune Defic Syndr. 2008;47:116–25. doi: 10.1097/QAI.0b013e31815d2f3f. [DOI] [PubMed] [Google Scholar]
- 48.Youngblade LM, Theokas C, Schulenberg J, Curry L, Huang IC, Novak M. Risk and promotive factors in families, schools, and communities: a contextual model of positive youth development in adolescence. Pediatrics. 2007;119(suppl 1):S47–53. doi: 10.1542/peds.2006-2089H. [DOI] [PubMed] [Google Scholar]
- 49.Pantin H, Coatsworth JD, Feaster DJ, et al. Familias Unidas: the efficacy of an intervention to promote parental investment in Hispanic immigrant families. Prev Sci. 2003;4:189–201. doi: 10.1023/a:1024601906942. [DOI] [PubMed] [Google Scholar]
- 50.Prado G, Pantin H, Briones E, et al. A randomized controlled trial of a parent-centered intervention in preventing substance use and HIV risk behaviors in Hispanic adolescents. J Consult Clin Psychol. 2007;75:914–26. doi: 10.1037/0022-006X.75.6.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rotheram-Borus MJ, Lee MB, Gwadz M, Draimin B. An intervention for parents with AIDS and their adolescent children. Am J Public Health. 2001;91:1294–302. doi: 10.2105/ajph.91.8.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou YR. “If you get AIDS… you have to endure it alone”: understanding the social constructions of HIV/AIDS in China. Soc Sci Med. 2007;65:284–95. doi: 10.1016/j.socscimed.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 53.Pang L, Hao Y, Mi G, et al. Effectiveness of first eight methadone maintenance treatment clinics in China. AIDS. 2007;21(suppl 8):S103–07. doi: 10.1097/01.aids.0000304704.71917.64. [DOI] [PubMed] [Google Scholar]
- 54.Foss AM, Hossain M, Vickerman PT, Watts CH. A systematic review of published evidence on intervention impact on condom use in sub-Saharan Africa and Asia. Sex Transm Infect. 2007;83:510–16. doi: 10.1136/sti.2007.027144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.NIMH International Multisite HIV/STI Prevention Group Methodological overview of a five-country community-level HIV/sexually transmitted disease prevention trial. AIDS. 2007;21(suppl):S3–S18. doi: 10.1097/01.aids.0000266453.18644.27. [DOI] [PubMed] [Google Scholar]
- 56.Kelly JA, Murphy DA, Sikkema KJ. Randomised, controlled, community-level HIV-prevention intervention for sexual-risk behaviour among homosexual men in US cities. Community HIV Prevention Research Collaborative. Lancet. 1997;350:1500–05. doi: 10.1016/s0140-6736(97)07439-4. [DOI] [PubMed] [Google Scholar]
- 57.Sikkema KJ, Kelly JA, Winett RA, et al. Outcomes of a randomized community-level HIV prevention intervention for women living in 18 low-income housing developments. Am J Public Health. 2000;90:57–63. doi: 10.2105/ajph.90.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kelly JA, Amirkhanian YA, Kabakchieva E, et al. Prevention of HIV and sexually transmitted diseases in high risk social networks of young Roma (Gypsy) men in Bulgaria: randomised controlled trial. BMJ. 2006;333:1098. doi: 10.1136/bmj.38992.478299.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kelly JA, Somlai AM, Benotsch EG, et al. Programmes, resources, and needs of HIV-prevention nongovernmental organizations (NGOs) in Africa, central/eastern Europe and central Asia, Latin America and the Caribbean. AIDS Care. 2006;18:12–21. doi: 10.1080/09540120500101757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brink B, Pienaar J. Business and HIV/AIDS: the case of Anglo American. AIDS. 2007;21(suppl 3):S79–84. doi: 10.1097/01.aids.0000279697.40568.fd. [DOI] [PubMed] [Google Scholar]
- 61.Mahajan AP, Colvin M, Rudatsikira JB, Ettl D. An overview of HIV/AIDS workplace policies and programmes in southern Africa. AIDS. 2007;21(suppl 3):S31–39. doi: 10.1097/01.aids.0000279692.54029.a1. [DOI] [PubMed] [Google Scholar]
- 62.Oppenheimer J. Business and AIDS in South Africa. AIDS. 2007;21(suppl 3):S11–12. doi: 10.1097/01.aids.0000279689.31158.66. [DOI] [PubMed] [Google Scholar]
- 63.Grinstead O, Seal DW, Wolitski R, et al. HIV and STD testing in prisons: perspectives of in-prison service providers. AIDS Educ Prev. 2003;15:547–60. doi: 10.1521/aeap.15.7.547.24045. [DOI] [PubMed] [Google Scholar]
- 64.Coates TJ, Fiamma A, Szekeres G, et al. Business' role in exercising leadership, promoting equity, embracing accountability, and developing partnerships. AIDS. 2007;21(suppl 3):S3–9. doi: 10.1097/01.aids.0000279688.23535.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Celentano DD, Bond KC, Lyles CM, et al. Preventive intervention to reduce sexually transmitted infections: a field trial in the Royal Thai Army. Arch Intern Med. 2000;160:535–40. doi: 10.1001/archinte.160.4.535. [DOI] [PubMed] [Google Scholar]
- 66.Noar SM. A 10-year retrospective of research in health mass media campaigns: where do we go from here? J Health Commun. 2006;11:21–42. doi: 10.1080/10810730500461059. [DOI] [PubMed] [Google Scholar]
- 67.Noar SM. An interventionist's guide to AIDS behavioral theories. AIDS Care. 2007;19:392–402. doi: 10.1080/09540120600708469. [DOI] [PubMed] [Google Scholar]
- 68.Centers for Disease Control and Prevention . Compendium of HIV Prevention Interventions. 2001. http://www.cdc.gov/hiv/pubs.hivcompendium/section2.htm (accessed May 15, 2008) [Google Scholar]
- 69.Centers for Disease Control and Prevention . Updated Compendium of Evidence-Based Interventions in HIV Prevention Research Synthesis Project. CDC; Atlanta: 2007. [Google Scholar]
- 70.West SG, Duan H, Pequegnat W, et al. Alternatives to the randomized controlled design. Am J Public Health. doi: 10.2105/AJPH.2007.124446. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barnett A, Weston M. Wealth, health, and HIV. AIDS. doi: 10.1097/01.aids.0000327434.28538.51. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rawson RA, McCann MJ, Flammino F, et al. A comparison of contingency management and cognitive-behavioral approaches for stimulant-dependent individuals. Addiction. 2006;101:267–74. doi: 10.1111/j.1360-0443.2006.01312.x. [DOI] [PubMed] [Google Scholar]
- 73.Roll JM, Shoptaw S. Contingency management: schedule effects. Psychiatry Res. 2006;144:91–93. doi: 10.1016/j.psychres.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 74.Strona FV, McCright J, Hjord H, et al. The acceptability and feasibility of the Positive Reinforcement Opportunity Project, a community-based contingency management methamphetamine treatment program for gay and bisexual men in San Francisco. J Psychoactive Drugs. 2006;(suppl 3):377–83. doi: 10.1080/02791072.2006.10400601. [DOI] [PubMed] [Google Scholar]
- 75. http://www.rockfound.org/about_us/press_releases/2007/032907cct_nyc.pdf. (accessed June 5, 2008)
- 76.Szekeres G, Coates TJ, Ehrhardt A. Leadership development and HIV/AIDS. AIDS. doi: 10.1097/01.aids.0000327433.90419.61. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pettifor AE, Rees HV, Kleinschmidt I, et al. Young people's sexual health in South Africa: HIV prevalence and sexual behaviors from a nationally representative household survey. AIDS. 2005;19:1525–34. doi: 10.1097/01.aids.0000183129.16830.06. [DOI] [PubMed] [Google Scholar]
- 78.Shisana O, Rehle T, Simbayi LC, et al. South African national HIV prevalence, HIV incidence, behaviour and communication survey. HSRC Press; Cape Town: 2005. [Google Scholar]
- 79.Shisana O, Stoker D, Simbayi LC, et al. South African national household survey of HIV/AIDS prevalence, behavioural risks and mass media impact—detailed methodology and response rate results. S Afr Med J. 2004;94:283–88. [PubMed] [Google Scholar]
- 80.Simbayi LC, Chauveau J, Shisana O. Behavioural responses of South African youth to the HIV/AIDS epidemic: a nationwide survey. AIDS Care. 2004;16:605–18. doi: 10.1080/09540120410001716405. [DOI] [PubMed] [Google Scholar]
- 81.Pettifor AE, Hudgens MG, Levandowski BA, Rees HV, Cohen MS. Highly efficient HIV transmission to young women in South Africa. AIDS. 2007;21:861–65. doi: 10.1097/QAD.0b013e3280f00fb3. [DOI] [PubMed] [Google Scholar]
- 82.Hendriksen ES, Pettifor A, Lee SJ, et al. Predictors of condom use among young adults in South Africa: the Reproductive Health and HIV Research Unit National Youth Survey. Am J Public Health. 2007;97:1241–48. doi: 10.2105/AJPH.2006.086009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Colvin M, Connolly C, Madurai L. The epidemiology of HIV in South African workplaces. AIDS. 2007;21(suppl 3):S13–19. doi: 10.1097/01.aids.0000279690.69276.c9. [DOI] [PubMed] [Google Scholar]
- 84.Moyo W, Levandowski BA, Macphail C, Rees H, Pettifor A. Consistent condom use in South African youth's most recent sexual relationships. AIDS Behav. 2008;12:431–40. doi: 10.1007/s10461-007-9343-3. [DOI] [PubMed] [Google Scholar]
- 85.Sayles JN, Pettifor A, Wong MD, et al. Factors associated with self-efficacy for condom use and sexual negotiation among South African youth. J Acquir Immune Defic Syndr. 2006;43:226–33. doi: 10.1097/01.qai.0000230527.17459.5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Centers for Disease Control and Prevention (CDC) HIV prevalence, unrecognized infection, and HIV testing among men who have sex with men-five U.S. cities, June 2004-April 2005. MMWR Morb Mortal Wkly Rep. 2005;54:597–601. [PubMed] [Google Scholar]
- 87.Centers for Disease Control and Prevention (CDC) Number of persons tested for HIV-United States, 2002. MMWR Morb Mortal Wkly Rep. 2004;53:1110–13. [PubMed] [Google Scholar]
- 88.Koblin BA, Murrill C, Camacho M, et al. Amphetamine use and sexual risk among men who have sex with men: results from the National HIV Behavioral Surveillance study—New York City. Subst Use Misuse. 2007;42:1613–28. doi: 10.1080/10826080701212519. [DOI] [PubMed] [Google Scholar]
- 89.Cates W., Jr . Technical advances in HIV prevention, in HIV/AIDS Annual Update 2006. In: Phair JP, editor. Clinical Care Options. 2006. http://clinicaloptions.com/ccohiv2006 (accessed June 10, 2008) [Google Scholar]
- 90.Padian NS, van der Straten A, Ramjee G, et al. Diaphragm and lubricant gel for prevention of HIV acquisition in southern African women: a randomised controlled trial. Lancet. 2007;370:251–61. doi: 10.1016/S0140-6736(07)60950-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Watson-Jones D, Weiss HA, Rusizoka M, et al. Effect of herpes simplex suppression on incidence of HIV among women in Tanzania. N Engl J Med. 2008;358:1560–71. doi: 10.1056/NEJMoa0800260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lagakos SW, Gable AR, editors. Methodological challenges in biomedical HIV prevention trials. The National Academies Press; Washington DC: 2008. [Google Scholar]
- 93.Gordon CM. Commentary on meta-analysis of randomized controlled trials for HIV treatment adherence interventions. Research directions and implications for practice. J Acquir Immune Syndr. 2006;43(suppl 1):S36–40. doi: 10.1097/01.qai.0000248347.87512.ba. [DOI] [PubMed] [Google Scholar]
- 94.Coates TJ. Commentary: what is to be done? AIDS. 2008;22:1079–80. doi: 10.1097/QAD.0b013e3282f8afb0. [DOI] [PubMed] [Google Scholar]
- 95.Cohen MS. Preventing sexual transmission of HIV. Clin Infect Dis. 2007;45(suppl 4):S287–92. doi: 10.1086/522552. [DOI] [PubMed] [Google Scholar]
- 96.Cohen MS, Gay C, Kashuba AD, Blower S, Paxton L. Narrative review: antiretroviral therapy to prevent the sexual transmission of HIV-1. Ann Intern Med. 2007;146:591–601. doi: 10.7326/0003-4819-146-8-200704170-00010. [DOI] [PubMed] [Google Scholar]
- 97.Remien RH, Mellins CH. Long-term psychosocial challenges for people living with HIV: let's not forget the individual in our global response to the pandemic. AIDS. 2007;21(suppl 5):S55–63. doi: 10.1097/01.aids.0000298104.02356.b3. [DOI] [PubMed] [Google Scholar]
- 98.Allen S, Karita E, Chomba E, et al. Promotion of couples' voluntary counseling and testing for HIV through influential networks in two African capital cities. BMC Public Health. 2007;7:349. doi: 10.1186/1471-2458-7-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bangsberg DR. Less than 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clin Infect Dis. 2006;43:939–41. doi: 10.1086/507526. [DOI] [PubMed] [Google Scholar]
- 100.Bangsberg DR, Kroetz DL, Deeks SG. Adherence-resistance relationships to combination HIV antiretroviral therapy. Curr HIV/AIDS Rep. 2007;4:65–72. doi: 10.1007/s11904-007-0010-0. [DOI] [PubMed] [Google Scholar]
- 101.Bangsberg DR, Perry S, Charlebois ED, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001;15:1181–83. doi: 10.1097/00002030-200106150-00015. [DOI] [PubMed] [Google Scholar]
- 102.Bangsberg DR, Ware N, Simoni JM. Adherence without access to antiretroviral therapy in sub-Saharan Africa? AIDS. 2006;20:140–41. doi: 10.1097/01.aids.0000196168.50303.31. [DOI] [PubMed] [Google Scholar]
- 103.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005;2:e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bailey RC, Moses S, Parker CB, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369:643–56. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 105.Gray RH, Kigozi G, Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369:657–66. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 106.Gray RH, Li X, Kigozi G, et al. The impact of male circumcision on HIV incidence and cost per infection prevented: a stochastic simulation model from Rakai, Uganda. AIDS. 2007;21:845–50. doi: 10.1097/QAD.0b013e3280187544. [DOI] [PubMed] [Google Scholar]
- 107.The Lancet Newer approaches to HIV prevention. Lancet. 2007;369:615. doi: 10.1016/S0140-6736(07)60285-2. [DOI] [PubMed] [Google Scholar]
- 108.United Nations Declaration of Commitment on HIV/AIDS Can HIV AIDS Policy Law Rev. 2002;6:42–43. [PubMed] [Google Scholar]
- 109.UNAIDS . Monitoring the Declaration of Commitment on HIV/AIDS: guidelines on construction of core indicators, 2008 reporting. Joint United Nations Programme on HIV/AIDS; Geneva: 2007. [Google Scholar]
- 110.Secretary General of the United Nations . Implementation of the Declaration of Commitment on HIV/AIDS and the Political Declaration on HIV/AIDS: midway to the millennium development goals. UN; New York: 2008. [Google Scholar]
- 111.Allen S, Meinzen-Derr J, Kautzman M, et al. Sexual behavior of HIV discordant couples after HIV counseling and testing. AIDS. 2003;17:733–40. doi: 10.1097/00002030-200303280-00012. [DOI] [PubMed] [Google Scholar]
- 112.Collins C, Coates TJ, Szekeres G. Accountabililty in the global response to HIV: measuring progress, driving change. AIDS. doi: 10.1097/01.aids.0000327442.66656.01. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stemple L. Health and human rights in today's fight against HIV/AIDS. AIDS. doi: 10.1097/01.aids.0000327443.43785.a1. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]