Abstract
Background and aims
Calcium plays a central role in neuronal function and injury. Dantrolene, an inhibitor of the ryanodine receptor, inhibits intracellular calcium release from the sarcoendoplasmic reticulum and might serve as novel agent for neuroprotection and other applications in the Neurointensive Care Unit.
Methods
We reviewed the available data of dantrolene as a potential neuroprotective agent through literature searches on Ovid, Pubmed and Google Scholar.
Results
Dantrolene provides neuroprotection in multiple in vitro models and some in vivo models of neural injury. Its efficacy has an early and narrow time-window of protection. We briefly summarize its other pharmacologic effects that may have potential applications for patients in the neurointensive care unit. Areas with the need for continued research are identified.
Conclusion
Targeted use of dantrolene in selected ICU disease models of anticipated neural injury, such as impending ischemia from vasospastic syndromes, might provided neuroprotection.
INTRODUCTION
Many different forms of neuronal injury are managed in the neurointensive care unit (NICU). These include, but are not limited to, ischemic or hemorrhagic stroke, vasospasm after subarachnoid hemorrhage (SAH), elevated intracranial pressure (ICP), reperfusion injury, brain edema, and status epilepticus. Mechanisms of neuronal injury by these disease states include excitotoxicity, apoptosis, necrosis, disruption of membrane potentials and abnormal protein folding. Calcium (Ca2+) plays an integral role in regulation or activation of these pathways.
Dantrolene, a drug that has been around since 1975, has a very narrow clinical use, i.e., malignant hyperthermia. It is rarely used in the NICU for other indications such as neuroleptic malignant syndrome, heat stroke, spasticity, shivering, serotonin syndrome and ecstasy overdose 1-6. Its unique mechanism of blocking intracellular Ca2+ release might make it an attractive drug in treating or preventing neuronal injury 7.
PHARMACOLOGY OF DANTROLENE
A. MECHANISM OF ACTION AND RECEPTORS
Dantrolene inhibits ryanodine receptors (RYR) 8. RYR are intracellular Ca2+ -release channels expressed on the surface of the sarcoendoplasmic reticulum 9. RYR are regulated by the Ca2+-dependent protein, calmodulin (CaM) 10. Three different RYR isoforms have been identified in mammalian tissues. They have specific sites of expression: all three RYRs can be found in the brain, but RYR1 is found predominantly in skeletal muscle, RYR2 predominantly in cardiac muscle, and RYR3 is expressed at comparatively low levels in a variety of tissues, including the brain and smooth muscles cells 11. Dantrolene acts directly on the RYR1 and RYR3 to reduce channel activation by CaM, and thereby decreasing the Ca2+-sensitivity of the channel activation 12. RYR2 is not inhibited by dantrolene 13. This fortuitous selectivity explains why the drug has no negative inotropic effect on the heart. Binding of dantrolene to the RYRs in the brain may protect neurons from disruptions in Ca2+-homeostasis. Dantrolene has recently been used in cell models and animal models to diminish cell death resulting from a multitude of neuronal injuries, including ischemia 14, 15, epileptic seizures 16, 17, and spinal cord injury 18.
B. CHEMISTRY
Dantrolene sodium is a highly lipid soluble small molecule that is rather water insoluble, which led to the delay of the development of the intravenous (IV) formulation. Dantrolene was initially only available as an oral medication. One vial of dantrolene IV (60ml) contains 20 mg dantrolene sodium and 3000 mg mannitol and sodium hydroxide to yield a pH of 9.5, to assist with solubility. The resulting solution is irritating to veins, and should be injected preferably via a central venous catheter or large bore peripheral IV catheter.
C. PHARMACOKINETICS AND METABOLISM
Following dantrolene ingestion by mouth, about 70% of dantrolene is absorbed, with the peak plasma concentration reached in 6 hours 19. However, especially in children, plasma concentrations can have great variations 20, although it is unclear whether this leads to a difference in clinical efficacy 21. Flewellen et al. 22 investigated the clinical response after IV administration of dantrolene in relation to plasma levels. In the conscious patient, the plateau maximal depression of muscle twitch response (75% depression) and grip strength (42% depression) was accomplished after administration of an accumulative dose of 2.4 mg/kg body weight. This resulted in a dantrolene plasma concentration of 4.2 μg/ml (approximately 10 μM). Thereafter, the elimination half-life was 12 hours, although plasma concentration was maintained at a steady level within therapeutic range for about 5 hours. The residual dantrolene plasma concentration at 24 hours after such a dose was 1 μg/ml. Subjects reported a feeling of weakness at plasma levels of 1 μg/ml. This weakness persisted for up to 48 hours, by which time the residual plasma concentration of dantrolene had decreased to 0.3 μg/ml. Metabolism of dantrolene is achieved microsomally in the liver via oxidative and reductive pathways. Oxidation results in hydroxylation of the hydantoin ring to 5-hydroxydantrolene (5HD), while reduction leads to the formation of aminodantrolene, which is then acetylated to the reduced acetylated derivative (RAD) of dantrolene 20. The metabolites themselves can have some muscle relaxant properties 23. Excreted in both the urine and the bile, dantrolene is 79% excreted as 5HD, 17% as RAD, while 4% of the dose is cleared unchanged in the urine19, 20.
D. SIDE EFFECTS
Most commonly reported side effects are dizziness, lightheadedness and drowsiness, but no other CNS effects with IV or oral administration have been reported 19, 20. Oral administration can result in diarrhea. The most serious reported adverse effect, hepatotoxicity, has accompanied its chronic administration by mouth 24, 25. However, there have been several reports indicating the contrary, so that it remains unclear whether dantrolene is truly hepatotoxic. Flewellen et al. 22 were unable to show any changes in liver function tests after prolonged oral exposure. No hepatotoxicity was achieved in mice exposed to dantrolene, even after enhancement of any hepatotoxic potential by the inhibition of acetylation, depletion of glutathione, induction of biotransformation and the promotion of reductive metabolism 26. In fact, dantrolene is hepato-protective in an in vivo rat model of liver injury 27. In a hepatocyte culture model, dantrolene did not produce any hepatotoxicity 28.
Other noteworthy adverse effects are rare cases of dantrolene-induced eosinophilic pleural and pericardial effusion 29, 30, which rapidly resolve after short steroid therapy 31. Prolonged oral exposure to dantrolene has been associated with an acneiform rash 19.
E. MAJOR DRUG INTERACTIONS
While dantrolene itself has no myocardial effects, marked myocardial depression was reported in swine and dogs after co-administration of verapamil 32. It is important to note, however, that this observation cannot be extrapolated to other calcium-channel-blocking agents, which are widely used in the NICU and might raise concern regarding the use of dantrolene. No adverse cardiac effect was observed in swine treated with dantrolene and amlodipine 33, and our personal observations in over 30 patients have not witnessed bradycardia or hypotension in the presence of nimodipine or nicardipine.
MOLECULAR MECHANISMS OF NEUROPROTECTION
A. CALCIUM'S LINK TO THE PATHOPHYSIOLOGY OF NEURONAL INJURY
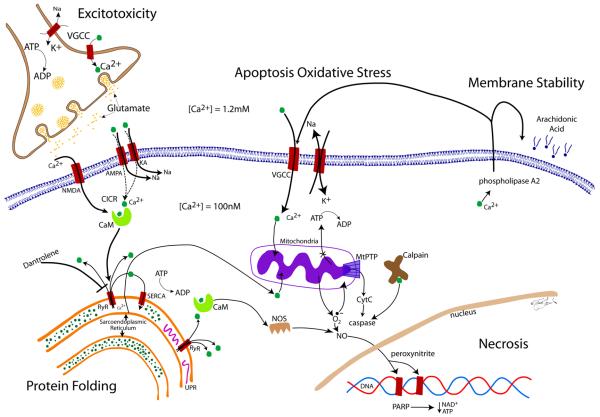
Fundamental for the survival of neurons is the fine balance between metabolic demand and energy supply. Any imbalance may lead to neuronal damage and potential cell demise. Calcium, in particular intracellular Ca2+, plays a pivotal role in neuronal injury. The link between calcium and the following forms of neuronal injury will be introduced in order to understand the role of dantrolene later: excitotoxicity induced by excitatory amino acids (i.e. glutamate), apoptosis, membrane destabilization, protein misfolding and necrosis (Figure).
FIGURE. The role of calcium in cellular injury.
Under normal conditions, there is a 10,000-fold Ca2+-gradient between the intra- and extracellular space. When cellular oxygen levels reach below-threshold levels, the Na+-K+-pump, which consumes 70% of neuronal ATP, fails, leading to depolarization of the membrane and influx of Ca2+ through VGCC and glutamate release into the synaptic cleft. Glutamate binds to NMDA, AMPA and KA receptors, leading to direct and indirect influx of Ca2+ into the cell. Elevated levels of intracellular Ca2+ lead to further calcium-induced calcium release (CICR) via binding of Ca2+ to CaM. CICR is mediated by RyR binding and calcium release from the sarcoendoplasmatic reticulum, the largest store of intracellular Ca2+. Increases in intracellular Ca2+ cannot be compensated for by the energy-dependent SERCA pump, located in the membrane of the sarcoendoplasmatic reticulum. A reduction in endoplasmic Ca2+ results in misfolding of proteins, leading to UFR as a cellular stress response. Influx of Ca2+ into mitochondria leads to formation of oxygen radicals and energy failure through decreased production of ATP. Binding of Ca2+ to CaM also leads to activation of NOS and formation of NO. Oxygen radicals react with NO to form peroxinitrite, a highly reactive molecule that damages DNA and proteins. DNA cleavage activates the DNA-repair enzyme PARP, which requires ATP for its action. PARP-related energy depletion adds further to cellular stress causing necrosis. The increased (toxic) levels of intracellular Ca2+ lead to increased mitochondrial permeability via MtPTP. This causes mitochondria to become permeable to molecules smaller than 1.5 kDa, which increases the osmolar load inside the mitochondria, causing mitochondrial swelling and outer membrane rupture, releasing CyC. CyC activates pro-apoptotic factors (caspases) by reacting with Ca2+-activated calpain. Elevated intracellular Ca2+ activates phospholipase A2 which cleaves cell membrane phospholipids, releasing arachidonic acid, which in turn can be further activated to proinflammatory molecules, such as prostaglandins, leukotrienes, and thromboxanes (not shown). Dantrolene is neuroprotective by blocking ryanodine receptors at the sarcoendoplasmatic reticulum, and thereby inhibiting further increase in intracellular Ca2+-levels.
In case of metabolic failure, depolarization of the neuronal membrane ensues. This in turn results in elevated levels of glutamate, an excitotoxic amino acid released from the presynaptic vesicles 34, which binds to postsynaptic glutamate receptors 35. Many of the glutamate receptors are ligand-gated, ionotropic Ca2+-channels (N-methyl-D-aspartic acid [NMDA], alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid [AMPA], and kainate [KA] receptors). The opening of glutamate-gated channels leads to Ca2+-influx from the extracellular space into the cytosol, which in turn activates Ca2+-efflux from intracellular stores 7. The Ca2+-overload in the cytosol activates various Ca2+-dependent enzymes that can initiate irreparable cell injury and mitochondrial dysfunction leading to cell death 36. In neurons, the endoplasmatic reticulum (ER) is the primary intracellular storage site of Ca2+-ions 37, 38. As shown in animal models, metabolic failure results in massive Ca2+-release from the ER 39. By inhibiting Ca2+-discharge from the sarcoendoplasmic reticulum, dantrolene might have implications in neuroprotection.
Apoptosis is a genetically controlled, programmed and highly regulated cell death. It can be brought on by a loss of Ca2+-homeostasis. It can trigger, as well as modulate, apoptosis by promoting mitochondria-mediated caspase activation 40.
Cell membranes, when disturbed by external toxins or other triggers, can lead to a rapid Ca2+-influx, which in turn can activate cell death by mechanisms explained above.
The ER is not only the major intracellular Ca2+-store, but it is also responsible for proper protein folding. Significant Ca2+-efflux from the ER induces stress molecules and protein misfolding, which can trigger apoptosis 41, 42.
Necrosis on the other hand is characterized by cytoplasmatic swelling, plasma membrane rupture and inflammation of the surrounding tissues 40. It is now known that specific pathways exist for necrosis 43. Glutamate is released, which results in excitatory cell death as described above.
B. DANTROLENE AND NEUROPROTECTION
1. CYTOTOXICITY BY EXCITATORY AMINO ACIDS
In an in vitro model of mouse cerebral cortical neurons, dantrolene reduced the glutamate-induced increases in intracellular Ca2+-concentrations by 70% under physiologic conditions, and protected against glutamate-induced neurotoxicity 7. Complete block of glutamate toxicity by dantrolene was seen in the absence of extracellular Ca2+. This neuroprotective effect is dose-dependent 44. Dantrolene does not appear to affect the kinetics of glutamate binding to membranes or voltage-gated Ca2+-channels 45, which indicates that release of Ca2+ from intracellular stores is essential for the propagation of glutamate-induced neuronal damage 7, 45-47. Dantrolene also protects against excitotoxicity induced by quisqualate (QA) and N-methyl-D-aspartate (NMDA), whereas no protection is observed on cell damage mediated by kainate (KA) or 2-amino-3-(3-hydroxy-5-methylisoxazol-4- yl)propionate (AMPA) receptor stimulation 48. The discrepancy in glutamate receptor protection is likely explained by the fact that the increase in intracellular Ca2+ stimulated by glutamate, NMDA and QA is predominantly through release of Ca2+ from intracellular stores, whereas KA and AMPA increase intracellular Ca2+ largely from extracellular influx 49, 50. An in vivo ischemia model using microdialysis in rats showed several interesting findings: first, dantrolene and vehicle (DMSO) groups had the same level of extracellular glutamate, suggesting that dantrolene does not prevent the release of glutamate. Compared to vehicle and control, dantrolene had a significantly higher number of preserved hippocampal neurons when given 15 minutes prior to ischemia, suggesting that dantrolene is neuroprotective due to the downstream blockade in the cytoplasmic increase of Ca2+ 51..
2. APOPTOSIS
Several apoptosis models demonstrate dantrolene neuroprotection via different mechanisms. One mechanism is the protection from bioenergetic failure during DNA repair. Calmodulin-dependent nitric oxide (NO) synthase, an important Ca2+-activated enzyme, forms NO from L-arginine 35. During ischemia and reperfusion, superoxide anion is produced, which can react with NO to form peroxynitrite. Both superoxide anion and peroxynitrite can cause DNA breaks. DNA cleavage activates the nuclear repair enzyme poly(ADP-ribose) polymerase (PARP). This enzyme utilizes NAD+ to transfer ADP-ribose during DNA repair. Cell death may ensue due to consumption of NAD+, which may cause energy failure as ATP is depleted in the efforts to resynthesize NAD+ 52, 53. Dantrolene, when applied early (0-40 minutes), but not late (40-120 minutes) after ischemia ameliorated PARP-related bioenergetic failure and led to a significant 25% increase of high energy phosphates in an in vitro neonatal rat brain model 54. The exact mechanism of blocking PARP-related bioenergetic failure is unknown. Whether this is due to blocking activation of PARP via prevention of DNA fragmentation or blocking other downstream mechanism of PARP, such as activation of calpains (Ca2+-dependent proteases) needs to be determined 55.
A different mechanism by which dantrolene protects against apoptosis was found in experiments using thapsigargin, an inducer of apoptosis. This chemical is a specific inhibitor of the sarcoendoplasmic reticulum Ca2+-ATPase. In an in vitro neuronal cell model, dantrolene partially abolished apoptosis induced by thapsigargin 56. It is likely that the initial Ca2+ rise may be the signaling event in the apoptotic pathway, which is partially inhibited by dantrolene.
In an in vitro Alzheimer's disease model using cells expressing the proapoptotic presenilin-1 (PS-1) mutation, dantrolene significantly prevented amyloid-β induced apoptosis and resulted in lower cellular peroxide levels, thereby protecting against oxidative stress. This protection is likely due to an altered calcium release in these cells 57.
Dantrolene has been reported to upregulate the anti-apoptotic protein Bcl-2. In two different cell lines pretreatment with dantrolene robustly suppressed 3-hydroxykynurenineinduced apoptosis 58. Upregulation of the anti-apoptotic protein Bcl-2 was associated with this protection 58, 59.
In an in vitro and in vivo apoptosis model with kainic acid-induced status epilepticus, dantrolene significantly reduced apoptosis in both models 24 hours after induction of status epilepticus 60. Using a different in vivo apoptosis model with hypoxic-ischemic injury in neonatal rats, intraventricular dantrolene significantly reduced the lipid/creatine ratio as assessed by 1H magnetic resonance spectroscopy in the alive rat pups, as well as the brain infarct area and morphologic appearance of the infarct 61.
The role of the mitochondria in apoptosis is well established 62. Elevated levels of intracellular Ca2+ released from the ER can rapidly be taken up by mitochondria 63, causing collapse of the mitochondrial membrane potential and triggering the apoptotic cascade 64. In a cell model of mitochondrial-induced apoptosis dantrolene has been shown to prevent cell death 65.
3. MEMBRANE STABILIZATION
A single paper 66 has investigated the properties of dantrolene as a possible plasma membrane stabilizer in a cell culture model of neuroblastoma cells. The authors exposed the cells to compound 48/80 (C48/80), a membrane stimulator, leading to rapid, dose-dependent Ca2+-influx. Dantrolene inhibited the induced increase in Ca2+-permeability of plasma membrane in a concentration-dependent manner. In addition, dantrolene (10μM) itself did not affect membrane fluidity, but it significantly reduced the C48/80-induced increase in membrane fluidity. These results suggest that dantrolene is a direct neuronal plasma membrane stabilizer, possibly through the inhibition of phospholipase A2 67.
4. PROTEIN FOLDING
In an in vivo ischemia model of 90-minute middle cerebral artery (MCA) occlusion, Li et al. studied the effect of dantrolene on ER stress-mediated apoptotic signal pathway activation 68. Dantrolene significantly decreased infarct volume and DNA fragmentation assessed 24 hours after transient ischemia. At the same time, apoptotic markers were significantly reduced in the ischemic penumbra, but not the ischemic core. With dantrolene treatment, various markers of ER stress were significantly downregulated within the penumbra. These results suggest that dantrolene has its greatest effect within the penumbra and may decrease infarct volume by preventing protein misfolding.
5. NECROSIS
In a C. elegans model of necrosis, ryanodine receptors and Ca2+-release from the ER were demonstrated to play a critical role 69. In this in vivo model, dantrolene also rescued neuronal cell death. Likewise, in an in vivo mouse model of Alzheimer's disease where presenilin-1 mutation predisposes neurons to excitotoxic necrosis, dantrolene pretreatment also protected 80% of neurons from cell death measured by maintenance of plasma membrane integrity 70, 71. Dantrolene has been shown to protect other tissues from necrosis, such as heart, liver, and pancreas 72-75.
CHALLENGES IN THE TRANSLATION FROM BENCH TO BEDSIDE
A. EXPERIMENTAL MODELS: DANTROLENE DOES NOT ALWAYS WORK
The neuroprotective effect of dantrolene appears rather consistent across multiple cell and animal models of neurological injury that include excitotoxicity, oxygen glucose deprivation (OGD), global ischemia, forebrain ischemia, focal ischemia, seizure, and traumatic injury (see Table). Three reports showed no effect on neuronal injury 76-78. In the first study, a neurological score without histology was performed. The neurological score on a small number of animals might have been too insensitive to find differences 76. In the second study, only a low dose of dantrolene (10 μM) was used, which suggests that neuroprotection may be dose-dependent 78. In the third negative study, no change in neuronal viability was found for unclear reasons 77. This study stands in contrast to other studies with evidence for neuroprotection by dantrolene using the same in vitro hippocampal slice model 16, 17, 54, 79.
TABLE.
Studies testing the potential neuroprotective effect of dantrolene in animal or cell models are listed by injury model, animal model, timing of dantrolene administration in relation to the injury, dantrolene dose, findings, and the citation.
| Injury | Model | Timing of Dantrolene Relative to Injury | Dose of Dantrolene | Findings | Citation |
|---|---|---|---|---|---|
| Excitotoxicity (NMDA) | Cortical neuron culture | at the time of injury | 30 μM | iCa2+ reduced by 30% in presence of extracellular Ca2+ and 100% in absence of extracellular Ca2+; toxic effect of glutamate entirely prevented | 7 |
| Excitotoxicity (Glutamate, NMDA, QA, KA, AMPA) | Cortical neuron culture | at the time of injury | 30 μM | Dantrolene blocks LDH and calcium release by NMDA, QA and glutamate, no effect on KA or AMPA | 48 |
| Excitotoxicity (NMDA) | Cerebellar granule neuron culture | 45 min prior | 1-100 μM alone and in combination with nimodipine and ruthenium red (RuR) | dose-dependent neuroprotection at 5 and 15 min, alone and in combination with RuR and nimodipine | 44 |
| Excitotoxicity (NMDA,KA, QA) | Cerebellar granule neuron culture | Simultaneous | 50 μM | Dantrolene inhibited glutamate induced Ca2+-release | 45 |
| Excitotoxicity (NMDA) | Cerebellar granule neuron culture | at the time of injury | 5-30 μM | decrease in iCa2+ in response to NMDA and K+, proving that NMDA/K+ released Ca2+ from intracellular stores | 46 |
| Excitotoxicity (NMDA) | Mixed cortical and retinal cell cultures | at the time of injury | 30 μM | iCa2+ decreased by 56%; cell toxicity reduced by 50% | 49 |
| Excitotoxicity (NMDA) | Hippocampal neuron culture | 10-20 min prior | 10 μM | reduced NMDA-induced increased iCa2+ | 50 |
| Neurotoxicity by 3-HK | Cerebellar granule neuron culture, PC12 cells, and GT1-7 hypothalamic neurosecretory cells | 30 min prior | 120 μM | 3-HK induced cell death of PC12 and GT1-7 cells inhibited; marked increase in protein level Bcl-2 | 58 |
| HIV-1 infection (cytotoxicity by HIV coat protein gp 120) | Cortical synaptosomes | Immediately prior and 10 min during experiment | 10 μM | decreased Gp120-induced iCa2+ rise | 109 |
| Status epilepticus | Kainic acid 8 mg/kg, rat | 30-60 min after | 10 mg/kg i.p. (approx 40 μM) | CA3 protected and CA1 preserved at 4 days | 110 |
| Status epilepticus (140min) | Electrogenic limbic status epilepticus, rat | Simultaneous, 30 or 140 min after onset | 10 mg/kg i.p. (approx 40 μM) | early administration reduced injury in all areas of hippocampus, late administration reduced injury in CA3 region | 82 |
| Epilepsy | Rat | 15, 30, or 60 min prior | 62.5 mg/kg, 125 mg/kg, 250mg/kg, 500 m/kg, all i.p. (approx 250-2000 μM) | dose dependent reduction in limbic seizures | 84 |
| Epilepsy | EL mouse (mutant susceptible to convulsive seizures) | 60 min prior | 20, 40 and 80 mg/kg i.p (approx 80-320 μM) | Seizure suppression; no influence on NO levels at any dose | 111 |
| Epilepsy | Hippocampal slice, rat | n/a | 10-100 μM | Dose-dependent suppression of pilocarpine- and DHPG-induced ictal activity | 17 |
| Epilepsy | Entorhinal cortex and hippocampus slice, rat | 30 min prior and during | 30 μM | complete cell death prevention after 1, 3, or 12-14hrs; no effect on synaptic transmission or epileptic discharges | l6 |
| OGD(0-15min) | intracellular recordings in cortical slice, rat | 15 min prior | 10-30 μM | no effect on membrane depolarization or intracellular calcium increase | 112 |
| OGD (40min) | Hippocampal slice, rat | 120 min prior or simultaneously or 120 min after | 10 μM | No effect | 77 |
| OGD(0-10min) | Hippocampal slice, gerbil | 60 min prior and during | 50 μM | Dantrolene reduced iCA2+ increase from ER | 79 |
| OGD (10-16 hours) | Neuroblastoma cell line | Simultaneous or 16 hours or 48 hours after | 40 and 80 μM | viability doubled at 48 hours; treatment after OGD achieves same effect as during OGD | 80 |
| OGD (40min) | Neonatal cortical slice | 0-40 or 40-120 min after | 20 μM | early but not late energy failure reduced | 54 |
| OGD (45 min) | Retinal cell culture, rat | 30 min prior and during | 100 μM | ganglion cell death prevented; 54% reduction of dead cells in the retinal layer | 47 |
| OGD (48 hrs) | Cortical neuron cell culture with presenlin-1 expression | 60 min prior | 10 μM | 80% of neurons rescued | 71 |
| Forebrain ischemia (20 min) | 4 vessel occlusion, rat | 15 min prior | 20 μM topical | Reduction of free fatty acids from ischemia and reperfusion | 113 |
| Forebrain ischemia (3 min) | Gerbil | 30 or 120 min after | 6μ1 of 1.6 mM intracerebroventricular (approx 10 μM in the brain); 0.4mM solution (approx 2.5 μM in brain) | 3 and 4.5 fold neuronal protection for 0.4 and 1.6mM. No effect at 120 min. | 81 |
| Forebrain ischemia (5 min) | 4 vessel occlusion, rat | 15 min after followed by infusion for 3 days | 33 μg intracerebroventricular (approx 50 μM) followed by 40 μg/day (approx 60 μM) | 50% CA1 cell loss reduction at 4 days; reduced TUNEL staining in the CA1 region. | 114 |
| Forebrain ischemia (10 min) | 4 vessel occlusion, rat | 15 min prior | 33 μg intracerebroventricular (approx 50 μM) | 40% more viable neurons in CA1 at 7 days. | 51 |
| Focal ischemia (90 min) | Middle cerebral artery occlusion, rat | Immediately after | 20 μg intracerebroventricular (approx 30 μM) | 65% infarct volume reduction at 24 hours; reduced ER stress markers and TUNEL staining in the penumbra | 68 |
| Focal ischemia (120 min) | Middle cerebral artery occlusion, rat | 30 min prior | 2 mg/kg (approx 8 μM) | No effect | 78 |
| Global cerebral ischemia (11 min) | Dog | Immediately prior | 4mg/kg intravenous (approx 16 μM) | No effect | 76 |
| Apoptosis (in vitro); global ischemia (in vivo) | GT1-7 hypothalamic neurosecretory cells (in vitro), gerbil (in vivo) | 30min prior (in vitro), Immediately after (in vivo) | 120 and 240 μM (in vitro); 10, 25, and 50 mg/kg intravenous (approx 40-200 μM) | Dose-dependent reduction of iCa2+, DNA-fragmentation and cell death (in vitro). dose-dependent increase of CA1 cells at 7 days (in vivo); 50mg/kg was toxic. | 15 |
| Apoptosis | PC12 cells | 120 min prior | 1 μM | Suppression of peroxide accumulation and protection against oxidative stress | 57 |
| Apoptosis (in vitro) / status epilepticus (in vivo) | Cerebellar granule neuron culture (in vitro) and rat (in vivo) | Simultaneous (in vitro), 60 min prior (in vivo) | 10 μM (in vitro); 10 mg/kg i.p. (approx 40 μM, in vivo) | cell death and apoptosis decreased at 3h and 24h (in vitro). CA1 region and cerebral cortex protected against apoptosis at 24hrs (in vivo) | 60 |
| Apoptosis (in vivo) | Hypoxic-ischemic neonatal rat brain | Immediately after | 1 mM intracerebroventricular | Lip/Cr ratio decreased (1H MR spectroscopy) at 24hrs; no effect on NAA/Cr ratio; infarct area decreased at 14d; no effect on TUNEL stain and survival rate at 14d. | 61 |
| Membrane fluidity | Neuroblastoma cell line | Immediately after | 10 μM | Dantrolene decreased Ca2+ mobilization by 40%, reduced increase in membrane fluidity and stabilized neuronal plasma cell membranes | 66 |
| Spinal cord injury (mechanical compression) | Ex-vivo spinal cord, rat | 15 min prior until 20min after | 10 μM | Electrophysiological recovery improved 1h after injury | 18 |
| Trauma | Hippocampal neuron culture | Simultaneously | 30 μM and 100 μM | Dose-dependent reduction in cell death | 115 |
Abbreviations: NMDA, N-methyl-D-aspartate; QA, quisqualate; KA, Kainate; AMPA, α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid; 3-HK, 3-hydroxykynurenine; HIV-1, human immunodeficiency virus type-1; gp120, glycoprotein; mGluR, metabotropic glutamate receptor; 1S,3R-ACPD, (1S,3R)-1-aminocyclopentane-1-3-dicarboxylic acid; OGD, oxygen glucose deprivation; PC, pheochromocytoma cells; RuR, ruthenium red; ip., intraperitoneal; iCa2+, intracellular calcium; LDH, lactate dehydrogenase; NO, nitric oxide; DHPG, dihydroxyphenylglycine; ER, endoplasmic reticulum; TUNEL, terminal deoxynucleotidyl transferase mediated dUTP nick end labeling; TTC, tetrazolium chloride; Lip/Cr, lipid/creatine; NAA/Cr, N-acetyl aspartate/creatine.
B. TIMING AND DOSING: WHEN AND HOW MUCH?
The neuroprotection provided by dantrolene has a narrow temporal therapeutic window. As in most other neuroprotective agents, timing is one of the major limitations in its application in the human. For in vitro neonatal rat brain or neuronal cell models, pretreatment, intra-injury treatment and post-treatment out to 40 minutes have shown consistent protection. However, experiments with analysis at later time points (either 40 minutes or 16 hours after the injury) did not show any neuroprotection 54, 80. Using in vivo brain ischemia or epilepsy models, dantrolene pre- and post-treatment extending out to 30 minutes appears neuroprotective, whereas delayed treatment at 120-140 minutes provides little or no protection 81, 82 (see Table).
The dose of dantrolene that achieves neuroprotection appears to be consistent among studies (see Table). Doses of 10-100 μM in cell culture or tissues are effective. In vitro and in vivo doses between 1-10 μM may or may not be effective 44, 46, 78, 81, 83. Doses exceeding 120 μM may be toxic to cells in culture 58, but in vivo doses as high as 1mM and 2mM were well tolerated in a brain ischemia and epilepsy model 61, 84. In another study of dose toxicity the dose at which caused 50% death (LD50) for dantrolene in rats was 500mg/kg (2mM) intraperitoneally 85.
C. ROUTE OF ADMINISTRATION
The ideal route of dantrolene administration - as with all neuroprotective drugs - remains an unsolved problem. Despite dantrolene's high lipid solubility, low molecular weight, and pKa of 7.7 there are suggestions that dantrolene has poor permeability of the blood brain barrier (BBB) based on an older oral dose C14-dantrolene experiments with monkeys 86. However, more recent evidence based on microdialysis and HPLC suggests that dantrolene penetrates the BBB with at tissue/plasma concentration ratio Kp of 6.4 at steady state87. This partion coefficient across the BBB is similar to oxycodone88. This new evidence is consistent with the finding that drowsiness is a common side-effect of dantrolene and is noted when blood drug levels are 0.64 μg/ml89 and dantrolene's ability to upregulate dopamine metabolism as measured by metabolites in human CSF90. Most experiments that reported successful neuroprotection used intracerebroventricular delivery, with doses as low as 2.5 μM 81, whereas two negative studies used intravenous delivery at low doses of either 8 or 16 μM 76, 78 (see Table). However, during neuronal injury the BBB frequently is not intact. Whether or not dantrolene has improved BBB penetration with brain injury requires further study. In fact, in an in vivo model of global cerebral ischemia dantrolene administered intravenously at doses 40-80 μM immediately following a five minute global cerebral ischemia was effective in protecting CA1 neurons, suggesting that not only the mode of delivery but also the injury state of the brain might play a role 15. Furthermore, other in vivo non-ischemic models of neuroprotection have used intraperitoneal delivery successfully at doses as low as 40μM (see Table).
POSSIBLE CLINICAL APPLICATIONS IN THE NICU
Neuroprotection continues to be challenging in clinical practice. As with the recent negative results of the Phase III trial of NXY-059 in ischemic stroke 91, taking promising therapeutic agents from the animal lab to the bedside can be disappointing when done prematurely. However, the field of neurocritical care encounters many different forms of neurological injury, and therefore neuroprotection is particularly interesting in the diseases seen in the NICU.
However, not all cell or animal models described in our article apply to clinical practice. Pre-injury administration might only be feasible pre-operatively prior to high-risk procedures during which brain injury might ensue (i.e. carotid endarterectomy, cerebral angiography, brain surgery). For all other brain injuries that cannot be foreseen, administration just minutes after the injury is unrealistic. Given that only 5% of all acute stroke patients receive the FDA approved tissue plasminogen activator within 3 hours 92, it is unlikely that a neuroprotective agent can be given sooner. As discussed above, the mode of administration remains an important unsolved problem and must be investigated. Intravenous administration is certainly the easiest way; however further study on BBB permeability comparing intravenous and intracerebroventricular administration is required in injured and uninjured brains of animals and possibly humans.
In addition to being potentially neuroprotective, dantrolene has several other pharmacologic effects that might be of additional benefit to patients with neurologic injuries. It inhibited stretch-induced smooth muscle contraction in rabbits 93. Recently, in an ex vivo rat basilar artery vasoconstriction model, dantrolene has been shown to inhibit serotonin induced vasoconstriction 94. Synergistic effects were seen with the combination of nimodipine and dantrolene. Other animal studies have confirmed synergistic neuroprotective effects of nimodipine and dantrolene 44, 83. We have observed dantrolene-mediated vasorelaxation in three of our own NICU patients [submitted to the Neurocritical Care Journal]. Whether dantrolene might be a potential treatment for cerebral vasoconstriction syndromes such as vasospasm after SAH, trauma or “Call-Fleming Syndrome” will require prospective human trials 95, 96.
Dantrolene has been shown to significantly reduce the gain of shivering and the shivering threshold in healthy adults, thereby reducing shivering thermogenesis by as much as 30 kcal/h 2. Induced normothermia or hypothermia has become a treatment modality to reduce fever burden in neurological injury 97-102. The use of therapeutic fever reduction is compromised by shivering as a thermoregulatory defense, which causes an increase in oxygen consumption and doubling of the metabolic rate 103-105. Drugs that are used to inhibit shivering may have side effects that restrict their use. Propofol, fentanyl, benzodiazepines or neuromuscular blocking agents might work well in limiting shivering, but are often avoided in order to preserve the neurologic exam. Meperidine may also alter the mental status, but most importantly can lower seizure threshold 106 as well as blood pressure, as can benzodiazepines and central alpha-2 agonists 107, 108. Although occasionally used by us as an add-on antishivering drug, dantrolene usually causes less sedation and muscle relaxation than the medications commonly used to treat shivering, and at the same time may have a dual pharmacodynamic benefit of neuroprotection as discussed above.
CONCLUSION
This review provides scientific evidence in animal studies for the neuroprotective potential of dantrolene. Further animal investigations are needed for timing, dose and route of administration. Prospective studies are necessary in order to determine whether dantrolene is effective in humans. Dantrolene has few side effects, and is generally well tolerated. In addition, dantrolene reduces shivering, and inhibits vasoconstriction mediated by serotonin with synergism in the presence of nimodipine. Dantrolene or other novel ryanodine receptor blockers might provide a promising new line of direction for neuroprotection with multiple application possibilities in the NICU.
Acknowledgments
Disclosures: Dr. John R. Sims is supported by NIH 1 K08 NS049241-01A2. None of the authors have any conflict of interest.
Abbreviations
- ADP
adenosine diphosphate
- AMPA
alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- ATP
adenosine triphosphate
- CaM
calmodulin
- CICR
calcium-induced calcium release
- CytC
Cytochrome C
- DNA
Deoxyribonucleic acid
- KA
kainic acid
- MtPTP
mitochondrial permeability transition pore
- NAD
Nicotinamide adenine dinucleotide
- NMDA
N-methyl-D-aspartic acid
- NO
nitric oxide
- NOS
nitric oxide synthase
- PARP
Poly (ADP-ribose) polymerase
- RyR
ryanodine receptor
- SERCA
Sarcoendoplasmic Reticulum Ca2+-ATPase
- UPR
unfolded protein response
- VGCC
voltage gated calcium channel
REFERENCES
- 1.Harrison GG. Control of the malignant hyperpyrexic syndrome in MHS swine by dantrolene sodium. British journal of anaesthesia. 1975;47(1):62–5. doi: 10.1093/bja/47.1.62. [DOI] [PubMed] [Google Scholar]
- 2.Lin CM, Neeru S, Doufas AG, et al. Dantrolene reduces the threshold and gain for shivering. Anesth Analg. 2004;98(5):1318–24. doi: 10.1213/01.ane.0000108968.21212.d7. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lydiatt JS, Hill GE. Treatment of heat stroke with dantrolene. JAMA. 1981;246(1):41–2. doi: 10.1001/jama.1981.03320010023020. [DOI] [PubMed] [Google Scholar]
- 4.Hadad E, Cohen-Sivan Y, Heled Y, Epstein Y. Clinical review: Treatment of heat stroke: should dantrolene be considered? Critical care (London, England) 2005;9(1):86–91. doi: 10.1186/cc2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall AP, Henry JA. Acute toxic effects of `Ecstasy' (MDMA) and related compounds: overview of pathophysiology and clinical management. British journal of anaesthesia. 2006;96(6):678–85. doi: 10.1093/bja/ael078. [DOI] [PubMed] [Google Scholar]
- 6.Graber MA, Hoehns TB, Perry PJ. Sertraline-phenelzine drug interaction: a serotonin syndrome reaction. The Annals of pharmacotherapy. 1994;28(6):732–5. doi: 10.1177/106002809402800610. [DOI] [PubMed] [Google Scholar]
- 7.Frandsen A, Schousboe A. Dantrolene prevents glutamate cytotoxicity and Ca2+ release from intracellular stores in cultured cerebral cortical neurons. Journal of neurochemistry. 1991;56(3):1075–8. doi: 10.1111/j.1471-4159.1991.tb02031.x. [DOI] [PubMed] [Google Scholar]
- 8.MacLennan DH, Duff C, Zorzato F, et al. Ryanodine receptor gene is a candidate for predisposition to malignant hyperthermia. Nature. 1990;343(6258):559–61. doi: 10.1038/343559a0. [DOI] [PubMed] [Google Scholar]
- 9.Marks AR, Tempst P, Hwang KS, et al. Molecular cloning and characterization of the ryanodine receptor/junctional channel complex cDNA from skeletal muscle sarcoplasmic reticulum. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(22):8683–7. doi: 10.1073/pnas.86.22.8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balshaw DM, Yamaguchi N, Meissner G. Modulation of intracellular calcium-release channels by calmodulin. The Journal of membrane biology. 2002;185(1):1–8. doi: 10.1007/s00232-001-0111-4. [DOI] [PubMed] [Google Scholar]
- 11.Giannini G, Conti A, Mammarella S, Scrobogna M, Sorrentino V. The ryanodine receptor/calcium channel genes are widely and differentially expressed in murine brain and peripheral tissues. The Journal of cell biology. 1995;128(5):893–904. doi: 10.1083/jcb.128.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fruen BR, Mickelson JR, Louis CF. Dantrolene inhibition of sarcoplasmic reticulum Ca2+ release by direct and specific action at skeletal muscle ryanodine receptors. The Journal of biological chemistry. 1997;272(43):26965–71. doi: 10.1074/jbc.272.43.26965. [DOI] [PubMed] [Google Scholar]
- 13.Zhao F, Li P, Chen SR, Louis CF, Fruen BR. Dantrolene inhibition of ryanodine receptor Ca2+ release channels. Molecular mechanism and isoform selectivity. The Journal of biological chemistry. 2001;276(17):13810–6. doi: 10.1074/jbc.M006104200. [DOI] [PubMed] [Google Scholar]
- 14.Mody I, MacDonald JF. NMDA receptor-dependent excitotoxicity: the role of intracellular Ca2+ release. Trends in pharmacological sciences. 1995;16(10):356–9. doi: 10.1016/s0165-6147(00)89070-7. [DOI] [PubMed] [Google Scholar]
- 15.Wei H, Perry DC. Dantrolene is cytoprotective in two models of neuronal cell death. Journal of neurochemistry. 1996;67(6):2390–8. doi: 10.1046/j.1471-4159.1996.67062390.x. [DOI] [PubMed] [Google Scholar]
- 16.Pelletier MR, Wadia JS, Mills LR, Carlen PL. Seizure-induced cell death produced by repeated tetanic stimulation in vitro: possible role of endoplasmic reticulum calcium stores. Journal of neurophysiology. 1999;81(6):3054–64. doi: 10.1152/jn.1999.81.6.3054. [DOI] [PubMed] [Google Scholar]
- 17.Rutecki PA, Sayin U, Yang Y, Hadar E. Determinants of ictal epileptiform patterns in the hippocampal slice. Epilepsia. 2002;43(Suppl 5):179–83. doi: 10.1046/j.1528-1157.43.s.5.34.x. [DOI] [PubMed] [Google Scholar]
- 18.Thorell WE, Leibrock LG, Agrawal SK. Role of RyRs and IP3 receptors after traumatic injury to spinal cord white matter. Journal of neurotrauma. 2002;19(3):335–42. doi: 10.1089/089771502753594909. [DOI] [PubMed] [Google Scholar]
- 19.Dykes MH. Evaluation of a muscle relaxant: dantrolene sodium (Dantrium) Jama. 1975;231(8):862–4. [PubMed] [Google Scholar]
- 20.Lietman PS, Haslam RH, Walcher JR. Pharmacology of dantrolene sodium in children. Archives of physical medicine and rehabilitation. 1974;55(8):388–92. [PubMed] [Google Scholar]
- 21.Meyler WJ, Mols-Thurkow HW, Wesseling H. Relationship between plasma concentration and effect of dantrolene sodium in man. European journal of clinical pharmacology. 1979;16(3):203–9. doi: 10.1007/BF00562062. [DOI] [PubMed] [Google Scholar]
- 22.Flewellen EH, Nelson TE, Jones WP, Arens JF, Wagner DL. Dantrolene dose response in awake man: implications for management of malignant hyperthermia. Anesthesiology. 1983;59(4):275–80. doi: 10.1097/00000542-198310000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Ellis KO, Wessels FL. Muscle relaxant properties of the identified metabolites of dantrolene. Naunyn-Schmiedeberg's archives of pharmacology. 1978;301(3):237–40. doi: 10.1007/BF00507042. [DOI] [PubMed] [Google Scholar]
- 24.Faling LJ, Petusevsky ML, Snider GL. Nitrofurantoin and dantrolene; liver and lung. Annals of internal medicine. 1980;93(1):151. doi: 10.7326/0003-4819-93-1-151_2. [DOI] [PubMed] [Google Scholar]
- 25.Chan CH. Dantrolene sodium and hepatic injury. Neurology. 1990;40(9):1427–32. doi: 10.1212/wnl.40.9.1427. [DOI] [PubMed] [Google Scholar]
- 26.Durham JA, Gandolfi AJ, Bentley JB. Hepatotoxicological evaluation of dantrolene sodium. Drug and chemical toxicology. 1984;7(1):23–40. doi: 10.3109/01480548409014171. [DOI] [PubMed] [Google Scholar]
- 27.Yamagishi F, Komoda T, Ohnishi K, Itoh S. Protective effect of dantrolene sodium on carbon tetrachloride induced liver injury in the rat. Research communications in chemical pathology and pharmacology. 1993;82(2):237–40. [PubMed] [Google Scholar]
- 28.Sorensen EM, Acosta D. Comparison of dantrolene sodium with erythromycin estolate using primary cultures of rat hepatocytes. Drug and chemical toxicology. 1985;8(4):219–37. doi: 10.3109/01480548509038647. [DOI] [PubMed] [Google Scholar]
- 29.Petusevsky ML, Faling LJ, Rocklin RE, et al. Pleuropericardial reaction to treatment with dantrolene. Jama. 1979;242(25):2772–4. [PubMed] [Google Scholar]
- 30.Mahoney JM, Bachtel MD. Pleural effusion associated with chronic dantrolene administration. The Annals of pharmacotherapy. 1994;28(5):587–9. doi: 10.1177/106002809402800507. [DOI] [PubMed] [Google Scholar]
- 31.Felz MW, Haviland-Foley DJ. Eosinophilic pleural effusion due to dantrolene: resolution with steroid therapy. Southern medical journal. 2001;94(5):502–4. [PubMed] [Google Scholar]
- 32.Saltzman LS, Kates RA, Corke BC, Norfleet EA, Heath KR. Hyperkalemia and cardiovascular collapse after verapamil and dantrolene administration in swine. Anesth Analg. 1984;63(5):473–8. [PubMed] [Google Scholar]
- 33.Freysz M, Timour Q, Bernaud C, Bertrix L, Faucon G. Cardiac implications of amlodipine-dantrolene combinations. Canadian journal of anaesthesia = Journal canadien d'anesthesie. 1996;43(1):50–5. doi: 10.1007/BF03015958. [DOI] [PubMed] [Google Scholar]
- 34.Benveniste H, Drejer J, Schousboe A, Diemer NH. Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. Journal of neurochemistry. 1984;43(5):1369–74. doi: 10.1111/j.1471-4159.1984.tb05396.x. [DOI] [PubMed] [Google Scholar]
- 35.Small DL, Buchan AM. Mechanisms of cerebral ischemia: intracellular cascades and therapeutic interventions. Journal of cardiothoracic and vascular anesthesia. 1996;10(1):139–46. doi: 10.1016/s1053-0770(96)80189-3. [DOI] [PubMed] [Google Scholar]
- 36.Kristian T, Siesjo BK. Calcium in ischemic cell death. Stroke. 1998;29(3):705–18. doi: 10.1161/01.str.29.3.705. [DOI] [PubMed] [Google Scholar]
- 37.Somlyo AP, Bond M, Somlyo AV. Calcium content of mitochondria and endoplasmic reticulum in liver frozen rapidly in vivo. Nature. 1985;314(6012):622–5. doi: 10.1038/314622a0. [DOI] [PubMed] [Google Scholar]
- 38.Carafoli E. Intracellular calcium homeostasis. Annual review of biochemistry. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- 39.Kohno K, Higuchi T, Ohta S, Kohno K, Kumon Y, Sakaki S. Neuroprotective nitric oxide synthase inhibitor reduces intracellular calcium accumulation following transient global ischemia in the gerbil. Neuroscience letters. 1997;224(1):17–20. doi: 10.1016/s0304-3940(97)13459-0. [DOI] [PubMed] [Google Scholar]
- 40.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nature reviews. 2003;4(7):552–65. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 41.Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes & development. 1999;13(10):1211–33. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- 42.Welihinda AA, Tirasophon W, Kaufman RJ. The cellular response to protein misfolding in the endoplasmic reticulum. Gene expression. 1999;7(46):293–300. [PMC free article] [PubMed] [Google Scholar]
- 43.Syntichaki P, Tavernarakis N. The biochemistry of neuronal necrosis: rogue biology? Nature reviews. 2003;4(8):672–84. doi: 10.1038/nrn1174. [DOI] [PubMed] [Google Scholar]
- 44.Duzenli S, Bakuridze K, Gepdiremen A. The effects of ruthenium red, dantrolene and nimodipine, alone or in combination, in NMDA induced neurotoxicity of cerebellar granular cell culture of rats. Toxicol In Vitro. 2005;19(5):589–94. doi: 10.1016/j.tiv.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 45.Bouchelouche P, Belhage B, Frandsen A, Drejer J, Schousboe A. Glutamate receptor activation in cultured cerebellar granule cells increases cytosolic free Ca2+ by mobilization of cellular Ca2+ and activation of Ca2+ influx. Experimental brain research Experimentelle Hirnforschung. 1989;76(2):281–91. doi: 10.1007/BF00247888. [DOI] [PubMed] [Google Scholar]
- 46.Simpson PB, Challiss RA, Nahorski SR. Involvement of intracellular stores in the Ca2+ responses to N-Methyl-D-aspartate and depolarization in cerebellar granule cells. Journal of neurochemistry. 1993;61(2):760–3. doi: 10.1111/j.1471-4159.1993.tb02184.x. [DOI] [PubMed] [Google Scholar]
- 47.Massote PD, Pinheiro AC, Fonseca CG, et al. Protective Effect of Retinal Ischemia by Blockers of Voltage-dependent Calcium Channels and Intracellular Calcium Stores. Cell Mol Neurobiol. 2008 doi: 10.1007/s10571-007-9243-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frandsen A, Schousboe A. Mobilization of dantrolene-sensitive intracellular calcium pools is involved in the cytotoxicity induced by quisqualate and N-methyl-D-aspartate but not by 2-amino-3-(3-hydroxy-5-methylisoxazol-4-yl)propionate and kainate in cultured cerebral cortical neurons. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(7):2590–4. doi: 10.1073/pnas.89.7.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lei SZ, Zhang D, Abele AE, Lipton SA. Blockade of NMDA receptor-mediated mobilization of intracellular Ca2+ prevents neurotoxicity. Brain research. 1992;598(12):196–202. doi: 10.1016/0006-8993(92)90183-a. [DOI] [PubMed] [Google Scholar]
- 50.Segal M, Manor D. Confocal microscopic imaging of [Ca2+]i in cultured rat hippocampal neurons following exposure to N-methyl-D-aspartate. The Journal of physiology. 1992;448:655–76. doi: 10.1113/jphysiol.1992.sp019063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakayama R, Yano T, Ushijima K, Abe E, Terasaki H. Effects of dantrolene on extracellular glutamate concentration and neuronal death in the rat hippocampal CA1 region subjected to transient ischemia. Anesthesiology. 2002;96(3):705–10. doi: 10.1097/00000542-200203000-00029. [DOI] [PubMed] [Google Scholar]
- 52.Eliasson MJ, Sampei K, Mandir AS, et al. Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nature medicine. 1997;3(10):1089–95. doi: 10.1038/nm1097-1089. [DOI] [PubMed] [Google Scholar]
- 53.Zhang J, Dawson VL, Dawson TM, Snyder SH. Nitric oxide activation of poly(ADP-ribose) synthetase in neurotoxicity. Science. 1994;263(5147):687–9. doi: 10.1126/science.8080500. [DOI] [PubMed] [Google Scholar]
- 54.Tasker RC, Sahota SK, Cotter FE, Williams SR. Early postischemic dantrolene-induced amelioration of poly(ADP-ribose) polymerase-related bioenergetic failure in neonatal rat brain slices. J Cereb Blood Flow Metab. 1998;18(12):1346–56. doi: 10.1097/00004647-199812000-00009. [DOI] [PubMed] [Google Scholar]
- 55.Moubarak RS, Yuste VJ, Artus C, et al. Sequential Activation of Poly(ADP-Ribose) Polymerase 1, Calpains, and Bax Is Essential in Apoptosis-Inducing Factor-Mediated Programmed Necrosis. Molecular and cellular biology. 2007;27(13):4844–62. doi: 10.1128/MCB.02141-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nath R, Raser KJ, Hajimohammadreza I, Wang KK. Thapsigargin induces apoptosis in SH-SY5Y neuroblastoma cells and cerebrocortical cultures. Biochemistry and molecular biology international. 1997;43(1):197–205. doi: 10.1080/15216549700203971. [DOI] [PubMed] [Google Scholar]
- 57.Guo Q, Sopher BL, Furukawa K, et al. Alzheimer's presenilin mutation sensitizes neural cells to apoptosis induced by trophic factor withdrawal and amyloid beta-peptide: involvement of calcium and oxyradicals. J Neurosci. 1997;17(11):4212–22. doi: 10.1523/JNEUROSCI.17-11-04212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wei H, Leeds P, Chen RW, et al. Neuronal apoptosis induced by pharmacological concentrations of 3-hydroxykynurenine: characterization and protection by dantrolene and Bcl-2 overexpression. Journal of neurochemistry. 2000;75(1):81–90. doi: 10.1046/j.1471-4159.2000.0750081.x. [DOI] [PubMed] [Google Scholar]
- 59.Kim BC, Kim HT, Mamura M, Ambudkar IS, Choi KS, Kim SJ. Tumor necrosis factor induces apoptosis in hepatoma cells by increasing Ca(2+) release from the endoplasmic reticulum and suppressing Bcl-2 expression. The Journal of biological chemistry. 2002;277(35):31381–9. doi: 10.1074/jbc.M203465200. [DOI] [PubMed] [Google Scholar]
- 60.Popescu BO, Oprica M, Sajin M, et al. Dantrolene protects neurons against kainic acid induced apoptosis in vitro and in vivo. Journal of cellular and molecular medicine. 2002;6(4):555–69. doi: 10.1111/j.1582-4934.2002.tb00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gwak M, Park P, Kim K, et al. The effects of dantrolene on hypoxic-ischemic injury in the neonatal rat brain. Anesth Analg. 2008;106(1):227–33. doi: 10.1213/01.ane.0000287663.81050.38. table of contents. [DOI] [PubMed] [Google Scholar]
- 62.Gupta S. Molecular signaling in death receptor and mitochondrial pathways of apoptosis (Review) International journal of oncology. 2003;22(1):15–20. [PubMed] [Google Scholar]
- 63.Bucholz J, Tsai H, Duckles SP. Intracellular calcium release induced by caffeine is buffered by mitochondria in superior cervical ganglion cells. FASEB J. 1996;10:A 142. [Google Scholar]
- 64.Hermes-Lima M. How do Ca2+ and 5-aminolevulinic acid-derived oxyradicals promote injury to isolated mitochondria? Free radical biology & medicine. 1995;19(3):381–90. doi: 10.1016/0891-5849(95)00015-p. [DOI] [PubMed] [Google Scholar]
- 65.Saunders R, Szymczyk KH, Shapiro IM, Adams CS. Matrix regulation of skeletal cell apoptosis III: Mechanism of ion pair-induced apoptosis. J Cell Biochem. 2006 doi: 10.1002/jcb.21001. [DOI] [PubMed] [Google Scholar]
- 66.Hayashi T, Kagaya A, Motohashi N, Yamawaki S. Possible mechanism of dantrolene stabilization of cultured neuroblastoma cell plasma membranes. Journal of neurochemistry. 1994;63(5):1849–54. doi: 10.1046/j.1471-4159.1994.63051849.x. [DOI] [PubMed] [Google Scholar]
- 67.Bronner C, Wiggins C, Monte D, et al. Compound 48/80 is a potent inhibitor of phospholipase C and a dual modulator of phospholipase A2 from human platelet. Biochimica et biophysica acta. 1987;920(3):301–5. doi: 10.1016/0005-2760(87)90108-1. [DOI] [PubMed] [Google Scholar]
- 68.Li F, Hayashi T, Jin G, et al. The protective effect of dantrolene on ischemic neuronal cell death is associated with reduced expression of endoplasmic reticulum stress markers. Brain research. 2005;1048(12):59–68. doi: 10.1016/j.brainres.2005.04.058. [DOI] [PubMed] [Google Scholar]
- 69.Xu K, Tavernarakis N, Driscoll M. Necrotic cell death in C. elegans requires the function of calreticulin and regulators of Ca(2+) release from the endoplasmic reticulum. Neuron. 2001;31(6):957–71. doi: 10.1016/s0896-6273(01)00432-9. [DOI] [PubMed] [Google Scholar]
- 70.Guo Q, Fu W, Sopher BL, et al. Increased vulnerability of hippocampal neurons to excitotoxic necrosis in presenilin-1 mutant knock-in mice. Nature medicine. 1999;5(1):101–6. doi: 10.1038/4789. [DOI] [PubMed] [Google Scholar]
- 71.Mattson MP, Zhu H, Yu J, Kindy MS. Presenilin-1 mutation increases neuronal vulnerability to focal ischemia in vivo and to hypoxia and glucose deprivation in cell culture: involvement of perturbed calcium homeostasis. J Neurosci. 2000;20(4):1358–64. doi: 10.1523/JNEUROSCI.20-04-01358.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zucchi R, Yu G, Ghelardoni S, Ronca F, Ronca-Testoni S. A3 adenosine receptor stimulation modulates sarcoplasmic reticulum Ca(2+) release in rat heart. Cardiovascular research. 2001;50(1):56–64. doi: 10.1016/s0008-6363(00)00318-7. [DOI] [PubMed] [Google Scholar]
- 73.Yu G, Zucchi R, Ronca-Testoni S, Ronca G. Protection of ischemic rat heart by dantrolene, an antagonist of the sarcoplasmic reticulum calcium release channel. Basic research in cardiology. 2000;95(2):137–43. doi: 10.1007/s003950050175. [DOI] [PubMed] [Google Scholar]
- 74.Lopez-Neblina F, Toledo-Pereyra LH, Toledo AH, Walsh J. Ryanodine receptor antagonism protects the ischemic liver and modulates TNF-alpha and IL-10. The Journal of surgical research. 2007;140(1):121–8. doi: 10.1016/j.jss.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 75.Husain SZ, Prasad P, Grant WM, Kolodecik TR, Nathanson MH, Gorelick FS. The ryanodine receptor mediates early zymogen activation in pancreatitis. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(40):14386–91. doi: 10.1073/pnas.0503215102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kross J, Fleischer JE, Milde JH, Gronert GA. No dantrolene protection in a dog model of complete cerebral ischaemia. Neurological research. 1993;15(1):37–40. doi: 10.1080/01616412.1993.11740104. [DOI] [PubMed] [Google Scholar]
- 77.Martinez-Sanchez M, Striggow F, Schroder UH, Kahlert S, Reymann KG, Reiser G. Na(+) and Ca(2+) homeostasis pathways, cell death and protection after oxygen-glucose-deprivation in organotypic hippocampal slice cultures. Neuroscience. 2004;128(4):729–40. doi: 10.1016/j.neuroscience.2004.06.074. [DOI] [PubMed] [Google Scholar]
- 78.Kim JH, Kim SH, Min KTM, Kim MH, Song SK, Lee BH. Comparison of the Cerebral Protective Effects of Thiopental, Propofol and Dantrolene on Focal Cerebral Ischemia Induced by Temporary Middle Cerebral Artery Occlusion in the Rat Under the Monitoring of Compressed Spectral Array. J Korean Neurosurg Soc. 2002;32:239–45. [Google Scholar]
- 79.Mitani A, Yanase H, Sakai K, Wake Y, Kataoka K. Origin of intracellular Ca2+ elevation induced by in vitro ischemia-like condition in hippocampal slices. Brain research. 1993;601(12):103–10. doi: 10.1016/0006-8993(93)91700-3. [DOI] [PubMed] [Google Scholar]
- 80.Wang C, Nguyen HN, Maguire JL, Perry DC. Role of intracellular calcium stores in cell death from oxygen-glucose deprivation in a neuronal cell line. J Cereb Blood Flow Metab. 2002;22(2):206–14. doi: 10.1097/00004647-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 81.Zhang L, Andou Y, Masuda S, Mitani A, Kataoka K. Dantrolene protects against ischemic, delayed neuronal death in gerbil brain. Neuroscience letters. 1993;158(1):105–8. doi: 10.1016/0304-3940(93)90623-s. [DOI] [PubMed] [Google Scholar]
- 82.Niebauer M, Gruenthal M. Neuroprotective effects of early vs. late administration of dantrolene in experimental status epilepticus. Neuropharmacology. 1999;38(9):1343–8. doi: 10.1016/s0028-3908(99)00059-3. [DOI] [PubMed] [Google Scholar]
- 83.GepdIremen A, Duzenl IS, Hacimuftuoglu A, Suleyman H, Oztas S. The effects of dantrolene alone or in combination with nimodipine in glutamate-induced neurotoxicity in cerebellar granular cell cultures of rat pups. Pharmacol Res. 2001;43(3):241–4. doi: 10.1006/phrs.2000.0770. [DOI] [PubMed] [Google Scholar]
- 84.Tizzano JP, Griffey KI, Johnson JA, Fix AS, Helton DR, Schoepp DD. Intracerebral 1S,3R-1-aminocyclopentane-1,3-dicarboxylic acid (1S,3R-ACPD) produces limbic seizures that are not blocked by ionotropic glutamate receptor antagonists. Neuroscience letters. 1993;162(12):12–6. doi: 10.1016/0304-3940(93)90548-y. [DOI] [PubMed] [Google Scholar]
- 85.Borowicz KK, Gasior M, Kleinrok Z, Czuczwar SJ. Influence of isradipine, niguldipine and dantrolene on the anticonvulsive action of conventional antiepileptics in mice. European journal of pharmacology. 1997;323(1):45–51. doi: 10.1016/s0014-2999(97)00020-4. [DOI] [PubMed] [Google Scholar]
- 86.Wuis EW, Rijntjes NV, Van der Kleijn E. Whole-body autoradiography of 14C-dantrolene in the marmoset monkey. Pharmacology & toxicology. 1989;64(1):156–8. doi: 10.1111/j.1600-0773.1989.tb00621.x. [DOI] [PubMed] [Google Scholar]
- 87.Enokizono J, Kusuhara H, Ose A, Schinkel AH, Sugiyama Y. Quantitative investigation of the role of breast cancer resistance protein (Bcrp/Abcg2) in limiting brain and testis penetration of xenobiotic compounds. Drug metabolism and disposition: the biological fate of chemicals. 2008;36(6):995–1002. doi: 10.1124/dmd.107.019257. [DOI] [PubMed] [Google Scholar]
- 88.Bostrom E, Hammarlund-Udenaes M, Simonsson US. Blood-brain barrier transport helps to explain discrepancies in in vivo potency between oxycodone and morphine. Anesthesiology. 2008;108(3):495–505. doi: 10.1097/ALN.0b013e318164cf9e. [DOI] [PubMed] [Google Scholar]
- 89.Meyler WJ, Bakker H, Kok JJ, Agoston S, Wesseling H. The effect of dantrolene sodium in relation to blood levels in spastic patients after prolonged administration. Journal of neurology, neurosurgery, and psychiatry. 1981;44(4):334–9. doi: 10.1136/jnnp.44.4.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nisijima K, Ishiguro T. Does dantrolene influence central dopamine and serotonin metabolism in the neuroleptic malignant syndrome? A retrospective study. Biological psychiatry. 1993;33(1):45–8. doi: 10.1016/0006-3223(93)90277-k. [DOI] [PubMed] [Google Scholar]
- 91.Shuaib A, Lees KR, Lyden P, et al. NXY-059 for the treatment of acute ischemic stroke. N Engl J Med. 2007;357(6):562–71. doi: 10.1056/NEJMoa070240. [DOI] [PubMed] [Google Scholar]
- 92.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117(4):e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 93.Nakayama K, Suzuki S, Sugi H. Physiological and ultrastructural studies on the mechanism of stretch-induced contractile activation in rabbit cerebral artery smooth muscle. The Japanese journal of physiology. 1986;36(4):745–60. doi: 10.2170/jjphysiol.36.745. [DOI] [PubMed] [Google Scholar]
- 94.Sims JR, Salomone S. Dantrolene inhibits serotonin and endothelin-1 vasoconstriction in the rat basilar artery. Neurocritical care. 2007;6(3):267–A107. [Google Scholar]
- 95.Call GK, Fleming MC, Sealfon S, Levine H, Kistler JP, Fisher CM. Reversible cerebral segmental vasoconstriction. Stroke. 1988;19(9):1159–70. doi: 10.1161/01.str.19.9.1159. [DOI] [PubMed] [Google Scholar]
- 96.Calabrese LH, Dodick DW, Schwedt TJ, Singhal AB. Narrative review: reversible cerebral vasoconstriction syndromes. Annals of internal medicine. 2007;146(1):34–44. doi: 10.7326/0003-4819-146-1-200701020-00007. [DOI] [PubMed] [Google Scholar]
- 97.Dietrich WD. The importance of brain temperature in cerebral injury. Journal of neurotrauma. 1992;9(Suppl 2):S475–85. [PubMed] [Google Scholar]
- 98.Azzimondi G, Bassein L, Nonino F, et al. Fever in acute stroke worsens prognosis. A prospective study. Stroke. 1995;26(11):2040–3. doi: 10.1161/01.str.26.11.2040. [DOI] [PubMed] [Google Scholar]
- 99.Reith J, Jorgensen HS, Pedersen PM, et al. Body temperature in acute stroke: relation to stroke severity, infarct size, mortality, and outcome. Lancet. 1996;347(8999):422–5. doi: 10.1016/s0140-6736(96)90008-2. [DOI] [PubMed] [Google Scholar]
- 100.Ginsberg MD, Busto R. Combating hyperthermia in acute stroke: a significant clinical concern. Stroke. 1998;29(2):529–34. doi: 10.1161/01.str.29.2.529. [DOI] [PubMed] [Google Scholar]
- 101.Fernandez A, Schmidt JM, Claassen J, et al. Fever after subarachnoid hemorrhage. Risk factors and impact on outcome. Neurology. 2007 doi: 10.1212/01.wnl.0000258543.45879.f5. [DOI] [PubMed] [Google Scholar]
- 102.Wartenberg KE, Schmidt JM, Claassen J, et al. Impact of medical complications on outcome after subarachnoid hemorrhage. Crit Care Med. 2006;34(3):617–23. doi: 10.1097/01.ccm.0000201903.46435.35. quiz 24. [DOI] [PubMed] [Google Scholar]
- 103.Lopez M, Sessler DI, Walter K, Emerick T, Ozaki M. Rate and gender dependence of the sweating, vasoconstriction, and shivering thresholds in humans. Anesthesiology. 1994;80(4):780–8. doi: 10.1097/00000542-199404000-00009. [DOI] [PubMed] [Google Scholar]
- 104.Ralley FE, Wynands JE, Ramsay JG, Carli F, MacSullivan R. The effects of shivering on oxygen consumption and carbon dioxide production in patients rewarming from hypothermic cardiopulmonary bypass. Canadian journal of anaesthesia = Journal canadien d'anesthesie. 1988;35(4):332–7. doi: 10.1007/BF03010851. [DOI] [PubMed] [Google Scholar]
- 105.Horvath SM, Spurr GB, Hutt BK, Hamilton LH. Metabolic cost of shivering. Journal of applied physiology. 1956;8(6):595–602. doi: 10.1152/jappl.1956.8.6.595. [DOI] [PubMed] [Google Scholar]
- 106.Modica PA, Tempelhoff R, White PF. Pro- and anticonvulsant effects of anesthetics (Part II) Anesth Analg. 1990;70(4):433–44. doi: 10.1213/00000539-199004000-00016. [DOI] [PubMed] [Google Scholar]
- 107.Buggy D, Higgins P, Moran C, O'Donovan F, McCarroll M. Clonidine at induction reduces shivering after general anaesthesia. Canadian journal of anaesthesia = Journal canadien d'anesthesie. 1997;44(3):263–7. doi: 10.1007/BF03015363. [DOI] [PubMed] [Google Scholar]
- 108.Bicer C, Esmaoglu A, Akin A, Boyaci A. Dexmedetomidine and meperidine prevent postanaesthetic shivering. Eur J Anaesthesiol. 2006;23(2):149–53. doi: 10.1017/S0265021505002061. [DOI] [PubMed] [Google Scholar]
- 109.Nath A, Padua RA, Geiger JD. HIV-1 coat protein gp120-induced increases in levels of intrasynaptosomal calcium. Brain research. 1995;678(12):200–6. doi: 10.1016/0006-8993(95)00185-s. [DOI] [PubMed] [Google Scholar]
- 110.Berg M, Bruhn T, Frandsen A, Schousboe A, Diemer NH. Kainic acid-induced seizures and brain damage in the rat: role of calcium homeostasis. Journal of neuroscience research. 1995;40(5):641–6. doi: 10.1002/jnr.490400509. [DOI] [PubMed] [Google Scholar]
- 111.Nagatomo I, Hashiguchi W, Tominaga M, Akasaki Y, Uchida M, Takigawa M. Effects of MK-801, dantrolene, and FK506 on convulsive seizures and brain nitric oxide production in seizure-susceptible EL mice. Brain research. 2001;888(2):306–10. doi: 10.1016/s0006-8993(00)03101-2. [DOI] [PubMed] [Google Scholar]
- 112.Pisani A, Calabresi P, Tozzi A, D'Angelo V, Bernardi G. L-type Ca2+ channel blockers attenuate electrical changes and Ca2+ rise induced by oxygen/glucose deprivation in cortical neurons. Stroke. 1998;29(1):196–201. doi: 10.1161/01.str.29.1.196. discussion 2. [DOI] [PubMed] [Google Scholar]
- 113.Phillis JW, Diaz FG, O'Regan MH, Pilitsis JG. Effects of immunosuppressants, calcineurin inhibition, and blockade of endoplasmic reticulum calcium channels on free fatty acid efflux from the ischemic/reperfused rat cerebral cortex. Brain research. 2002;957(1):12–24. doi: 10.1016/s0006-8993(02)03578-3. [DOI] [PubMed] [Google Scholar]
- 114.Yano T, Nakayama R, Imaizumi T, Terasaki H, Ushijima K. Dantrolene ameliorates delayed cell death and concomitant DNA fragmentation in the rat hippocampal CA1 neurons subjected to mild ischemia. Resuscitation. 2001;50(1):117–25. doi: 10.1016/s0300-9572(00)00369-5. [DOI] [PubMed] [Google Scholar]
- 115.Yoon KW, Mitchell HL, Broder LD, Brooker RW, Delisle RK. Trauma-induced neurotoxicity in rat hippocampal neurons. Stroke. 1996;27(1):122–6. doi: 10.1161/01.str.27.1.122. [DOI] [PubMed] [Google Scholar]