Abstract
For vertebrate olfactory signal transduction, a calcium-activated chloride conductance serves as a major amplification step. However, the molecular identity of the olfactory calcium-activated chloride channel (CaCC) is unknown. Here we report a proteomic screen for cilial membrane proteins of mouse olfactory sensory neurons (OSNs) that identified all the known olfactory transduction components as well as Anoctamin 2 (ANO2). Ano2 transcripts were expressed specifically in OSNs in the olfactory epithelium, and ANO2::EGFP fusion protein localized to the OSN cilia when expressed in vivo using an adenoviral vector. Patch-clamp analysis revealed that ANO2, when expressed in HEK-293 cells, forms a CaCC and exhibits channel properties closely resembling the native olfactory CaCC. Considering these findings together, we propose that ANO2 constitutes the olfactory calcium-activated chloride channel.
Keywords: Anoctamin, cilia, olfaction, signal transduction, TMEM16B
In the nervous system, chloride conductances often serve critical signaling roles, functioning as either inhibitory or excitatory signals. In vertebrate olfactory sensory neurons (OSNs), signaling by a calcium-activated chloride conductance in the cilia is the final step of a well-characterized signal transduction pathway (1, 2). Because of the active accumulation of chloride within OSNs (3–5), this chloride conductance (6–9) serves as an amplification step, accounting for up to 80% to 90% of the odorant-induced depolarizing current (10–12) and thus is critical for olfactory sensation. In this study, we identified Anoctamin 2 (ANO2) in a proteomic screen of OSN cilial membranes. We provide molecular and electrophysiological evidence indicating that ANO2 constitutes the long-sought olfactory calcium-activated chloride channel (CaCC) that may mediate signal amplification.
Results
ANO2 Is Present in a Preparation of Olfactory Cilial Membranes.
In rodents, the native olfactory CaCC is predicted to be 8 times more abundant than the olfactory cationic cyclic nucleotide-gated (CNG) channel (9). Because of this high abundance, we reasoned that a proteomic screen might be a viable approach to identify this channel. Therefore, we isolated OSN cilia from mouse olfactory mucosa, enriched the preparation for membrane proteins, and separated the proteins by SDS/PAGE (Fig. S1). To reduce the potential interference in the proteomic analysis by odorant receptors (ORs), which include more than 1000 different types and usually migrate at 35–55 kDa, we cut the gel into 3 slices: > 55 kDa, 35–55 kDa, and <35 kDa, and analyzed each slice by mass spectrometry. We performed 2 independent cilial membrane preparations and mass spectrometric screens. Only proteins that were identified by 2 or more peptides in both preparations were considered for further analysis.
Because most chloride channels have several transmembrane domains and therefore are of a larger molecular weight (Fig. S2), we initially focused our analysis on the > 55-kDa sample, in which 53 proteins were identified (Table 1 and Table S1). The robustness of this approach was confirmed by the identification of all known transmembrane and membrane-associated olfactory transduction components downstream of ORs within this range of molecular weights (Table 1). Other proteins arising from OSNs, e.g., the neural cell adhesion molecule 1, were found also. In addition, a number of proteins from dominant contaminating sources, i.e., axonemes (tubulin) and microvilli of sustentacular cells (cytochrome P450 2A; ref. 13) were present.
Table 1.
Proteins identified by mass spectrometry
| Symbol | Protein Name | GI Accession Number | Peptides Average (first, second) |
|---|---|---|---|
| ATP1A1 | sodium/potassium ATPase alpha-1 | 55976751 | 35 (30, 40) |
| P450R | NADPH cytochrome P450 reductase | 548338 | 30 (25, 35) |
| ANXA6 | annexin a6 | 113963 | 20.5 (19, 22) |
| AC3 | adenylyl cyclase type 3 | 25008337 | 18 (14, 22) |
| VIL2 | Ezrin | 32363497 | 14.5 (20, 9) |
| HSPA5 | glucose-regulated protein 78 | 2506545 | 14 (9, 19) |
| HSC70C | Heat-shock cognate 71-kDa protein 8 | 51702275 | 13 (10, 16) |
| NCAM1 | neural cell adhesion molecule 1 | 205830666 | 12.5 (6, 19) |
| ATP6V1A | vacuolar ATP synthase catalytic subunit a | 145559539 | 12.5 (16, 9) |
| ANPEP | aminopeptidase n | 31077182 | 12.5 (17, 8) |
| TBB3 | tubulin beta-3 | 20455323 | 11.5 (12, 11) |
| SLC27A2 | very-long-chain acyl-CoA synthetase | 3183203 | 11 (11, 11) |
| ENPL | Heat-shock protein 90-kDa beta 1 | 119362 | 11 (10, 12) |
| TMEM16B | transmembrane protein 16B (Anoctamin-2) | 81877094 | 10.5 (12, 9) |
| VOME | vomeromodulin | 81895337 | 10.5 (5, 16) |
| PDE1C | phosphodiesterase 1c | 57015315 | 10 (12, 8) |
| TBA1A | tubulin alpha-1 | 55977479 | 10 (11, 9) |
| Q8BH53 | hypothetical transmembrane protein | 81896083 | 9.5 (10, 9) |
| MYO6 | myosin-6 | 13431710 | 9 (4, 14) |
| ECHA | trifunctional enzyme subunit alpha, mitochondrial | 81874329 | 8 (5, 11) |
| STOM | stomatin | 122066246 | 7 (3, 11) |
| TERA | transitional endoplasmic reticulum ATPase | 146291078 | 7 (10, 4) |
| CNGA2 | cyclic nucleotide-gated channel A2 | 2493744 | 6.5 (5, 8) |
| CP2A5 | cytochrome P450 2a5 | 117196 | 5.5 (6, 5) |
| CP2A4 | cytochrome P450 2a4 | 117195 | 5.5 (5, 8) |
| 4F2HC | 4f2 cell-surface antigen heavy chain | 112804 | 5.5 (4, 7) |
| NKCC1 | sodium- (potassium)-chloride cotransporter 1 | 1709293 | 5.5 (4, 7) |
| CMC1 | mitochondrial aspartate glutamate carrier 1 | 47605479 | 5 (3, 7) |
| AL3B1 | aldehyde dehydrogenase 3b1 | 67460523 | 5 (7, 3) |
| CTNA1 | alpha-1 catenin | 117607 | 5 (4, 6) |
| AT1A2 | sodium/potassium ATPase alpha-2 | 66773992 | 4.5 (6, 3) |
| CLIC6 | chloride intracellular channel 6 | 46395841 | 4.5 (6, 3) |
| ALBU | serum albumin precursor | 5915682 | 4 (4, 4) |
| UD2A2 | UDP glucuronosyltransferase 2a2 | 81892490 | 4 (6, 2) |
| HS90A | Heat-shock protein 90-alpha | 1170384 | 4 (3, 5) |
| LRGUK | guanylate kinase domain-containing protein | 81905373 | 3.5 (4, 3) |
| DCDC2 | doublecortin domain-containing protein 2 | 71153322 | 3.5 (2, 5) |
| CNGB1.B | cyclic nucleotide-gated channel B1.b | 81895348 | 3.5 (4, 3) |
| TBB4 | tubulin beta-4 | 146345529 | 3.5 (5, 2) |
| AT1B1 | sodium/potassium-ATPase beta-1 | 114393 | 3 (2, 4) |
| ODP2 | dihydrolipoamide acetyltransferase homolog | 146325018 | 3 (3, 3) |
| AQP1 | aquaporin-1 | 543832 | 3 (3, 3) |
| AT12A | proton/potassium ATPase alpha chain 2 | 51338843 | 3 (2, 4) |
| CD36 | platelet glycoprotein 4 | 729081 | 3 (4, 2) |
| CLH | clathrin heavy chain | 66773801 | 3 (3, 3) |
| TKT | transketolase | 730956 | 3 (4, 2) |
| ANXA1 | annexin a1 | 113945 | 2.5 (3, 2) |
| HS71A | Heat-shock 70-kDa protein 1a | 56757667 | 2.5 (2, 3) |
| Q9WV19 | olfactory-specific cytochrome P450 2 g1 | 81907639 | 2 (2, 2) |
| ABCB6 | ATP-binding cassette, sub-family b, member 6 | 81917203 | 2 (2, 2) |
| NCKX4 | sodium/potassium/calcium exchanger 4 | 37081103 | 2 (2, 2) |
| Q9ESE4 | olfactor UDP glucuronosyltransferase | 81906316 | 2 (2, 2) |
| SO1A1 | sodium-independent organic anion- transporter 1 | 27734565 | 2 (2, 2) |
List of 53 proteins from the > 55-kDa sample. Listings in bold are proteins known to be involved in olfactory transduction (1, 2). Listings in italics are proteins either annotated to confer chloride conductance or with no known function. For the full list of identified proteins of all molecular weights, including the lower molecular weight olfactory transduction components (Gαolf and CNGA4), see Table S1.
Because we had found all of the known signal transduction components downstream of ORs, we reasoned that the olfactory CaCC likely should be included in this short list of 53 proteins. We thus narrowed the candidates for the olfactory CaCC down to 2 categories of proteins: those annotated to confer a chloride conductance and those predicted to be transmembrane proteins with unknown function. Only 3 proteins, CLIC6, TMEM16B (ANO2), and Q8BH53, were found in these 2 categories. CLIC6 is a member of a family of intracellular chloride channels (14). However, in situ hybridization showed that CLIC6 is expressed primarily in supporting cells in the olfactory epithelium (OE), but not in OSNs (Fig. S3). Q8BH53 (gi81896083) is a protein of unknown function. It is enriched in many ciliated cells (15), and might have a more general role in cilial structure or maintenance.
TMEM16B (ANO2) is 1 of 10 members of the Anoctamin family of proteins (16). ANO2 is predicted to have 8 transmembrane domains with both N and C termini in the cytoplasm, a topology consistent with many ion channels. A mutation in human TMEM16E (ANO5) causes gnathodiaphyseal dysplasia, a disorder characterized by bone calcification defects (17). Bone calcification defects have been noted for mutations in chloride channels (18). BLAST (http://www.ncbi.nlm.nih.gov/BLAST/) searches revealed that ANO family members are related to the yeast protein Ist2p. Mutants of Ist2p were reported to have increased tolerance to NaCl (19). These studies, as well as our identification of ANO2 in the olfactory cilial proteomic screen, pointed to ANO2 as a promising candidate for the olfactory CaCC.
Olfactory Sensory Neurons Express a Splice Variant of Ano2.
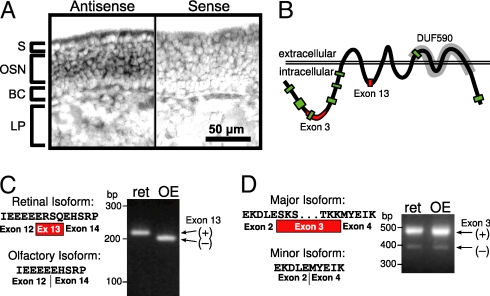
Ano2 transcripts are highly expressed in the olfactory system (GNF Symatlas and Genepaint), specifically in OSNs (20). Using independent in situ hybridization probes, we confirmed that Ano2 mRNA localized specifically to OSNs within the OE and not to the sustentacular or basal stem cell layers (Fig. 1A).
Fig. 1.
Analysis of ANO2 transcripts. (A) In situ hybridization for Ano2 in mouse OE. BC, basal cells; LP, lamina propria; OSN, olfactory sensory neurons; S, sustentacular cells. (B) Schematic of ANO2 predicted transmembrane topology. Green boxes indicate segments identified by mass spectrometry. Red boxes indicate segments encoded by exon 3 and the retinal exon 13. Gray highlights a conserved domain (DUF590) in all Anoctamin family members. (C) Exon 13 is present in the retinal isoform of Ano2 but is absent in the olfactory isoform. (Right) RT-PCR analysis with primers spanning the Exon 13 site. ret, retinal cDNA; OE, olfactory epithelial cDNA. (D) The major Ano2 isoforms in both retinal cells and OSNs contain exon 3, which is spliced out in a minor isoform. (Right) RT-PCR analysis with primers spanning the exon 3 site.
To date, mouse Ano2 mRNA sequences available from online databases are derived from retinal cDNA. The Ensembl database predicts several Ano2 transcript variants with different transcription initiation sites. We characterized mouse olfactory Ano2 transcripts. The longest ORF obtained by RT-PCR was 2,730 bases, made of 24 exons (see SI Methods for details). We found that the retinal exon 13, which encodes 4 amino acids in the predicted first intracellular loop, is absent in the olfactory-specific isoform (Fig. 1 B and C), suggesting that OSNs express a splice variant. The functional significance of these 4 missing amino acids remains to be determined. We also found that ANO2 exon 3, which encodes 33 amino acids in the predicted N-terminal cytoplasmic domain, is lacking in a minority of transcripts in both OSNs and retinal cells (Fig. 1D). Translation of the major isoform of the olfactory-specific Ano2 ORF yields 909 amino acids, with a predicted molecular weight of 104 kDa.
ANO2::EGFP Fusion Protein Traffics to Olfactory Cilia.
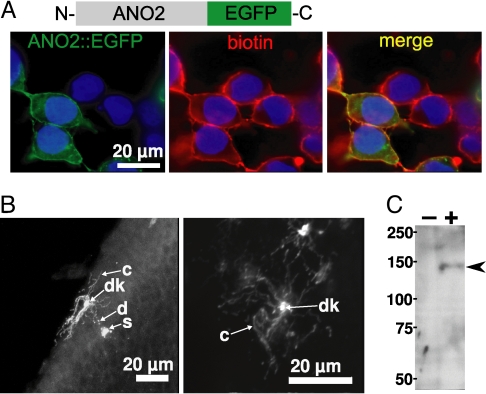
If ANO2 functions as the olfactory CaCC, it should be located in the OSN cilia. To determine whether the ANO2 protein found in the proteomic screen was indeed arising from OSN cilial membranes, we monitored the subcellular localization of GFP-tagged ANO2 proteins. We generated expression constructs for N- or C-terminally EGFP-tagged ANO2 (EGFP::ANO2 or ANO2::EGFP) and expressed them in HEK-293 cells. ANO2::EGFP localized to the plasma membrane (Fig. 2A). However, EGFP::ANO2 remained intracellular (Fig. S4). These data suggest that the signal sequence for trafficking of ANO2 to the plasma membrane may reside at the N terminus, in contrast to the yeast Ist2p that was reported to traffic to the plasma membrane by a C-terminal signal sequence (21).
Fig. 2.
Subcellular localization of ANO2::EGFP fusion proteins. (A) Anti-GFP immunostaining of HEK-293 cells transfected with the ANO2::EGFP plasmid. (Left) GFP staining shown in green. (Center) Cell surface labeling by biotinylation shown in red. (Right) The merge. Cell nuclei were counterstained with DAPI shown in blue. (B) Immunostaining of OE infected with an adenovirus expressing ANO2::EGFP using an anti-GFP antibody. (Left) Side view of a virus-infected OSN. (Right) En face view of a virus-infected OSN. c, cilia; d, dendrite; dk, dendritic knob; s, soma. (C) Western blot analysis using an anti-GFP antibody on olfactory mucosal tissues either infected with the ANO2::EGFP adenovirus (+) or not infected (−). Arrowhead points to the sole ≈130-kDa band present in the infected tissue.
We then generated an adenoviral vector to deliver DNA encoding ANO2::EGFP to OSNs in vivo. Low doses of virus were used to infect cells in the OE sparsely, allowing the clear visualization of individual OSNs. ANO2::EGFP localized primarily in the cilia and dendritic knobs of OSNs (Fig. 2B, Movie S1), a morphology reminiscent of those labeled by individual odorant receptor antibodies (22). Additionally, puncta of ANO2::EGFP were apparent in the dendrite, and there was perinuclear fluorescence in the OSN soma. No fluorescence was observed in the OSN axons. Western blot analysis confirmed that the ANO2::EGFP fusion protein of the expected size was present in the infected OE (Fig. 2C).
ANO2 Exhibits Channel Properties Similar to the Native Olfactory CaCC.
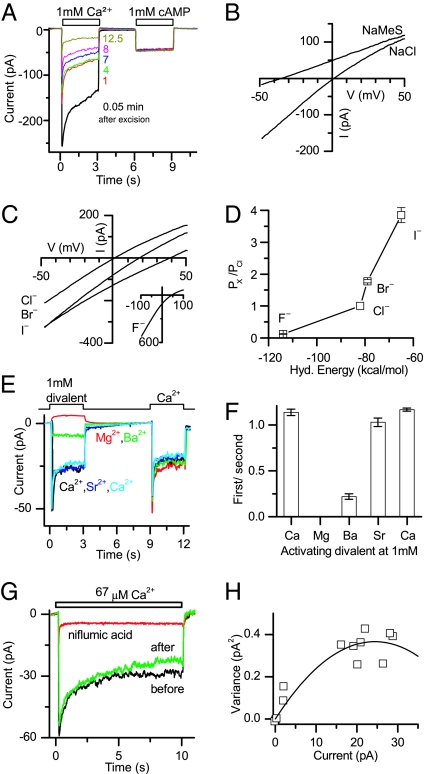
Recently, ANO1 (TMEM16A), a paralog of ANO2, has been found to form a CaCC when heterologously expressed (23–25). ANO2 also was shown to confer calcium-activated conductance to Xenopus oocytes but has not been characterized (25). We expressed the olfactory form of mouse ANO2 in HEK-293 cells and conducted patch-clamp analysis. The cells were co-transfected with a plasmid expressing the olfactory CNG channel subunit CNGA2, enabling us to activate selectively either a cationic cAMP-gated conductance or a putative Ca2+-activated anion conductance in the patch. The cAMP-gated conductance aided in identifying the inside-out patch configuration and served as a control of patch integrity over time (see Fig. 3A). Experiments were performed in 140-mM symmetrical NaCl solutions. The patches were stimulated with Ca2+ or cAMP at concentrations that yield a maximal open probability for the native olfactory CaCC and a CNGA2 homomeric channel, respectively (9, 26).
Fig. 3.
Biophysical properties of ANO2 channel. (A) Heterologously expressed ANO2 confers a Ca2+-activated conductance that runs down over time. Recording of an inside-out patch exposed to 1 mM Ca2+ followed by 1 mM cAMP. Repeated Ca2+ and cAMP application demonstrates the progressive reduction (“rundown”) of the Ca2+-activated current while the CNG current remains constant. The times beside each trace denote the minutes after patch excision. Holding potential was −40 mV. For quantification of the rundown, see Fig. S5. (B) I−V relationships of ANO2. The channel was activated with 67 μM and 100 μM Ca2+, respectively. NaCl trace, symmetrical NaCl solutions; NaMeS trace, methanesulfonate-negative replacement of Cl− in bath. (C and D) Halide permeability of the ANO2 channel. (C) I−V relationships from a patch where the bath solution contained 140 mM NaCl, NaBr, or NaI. The pipette contained 140 mM NaCl. (Inset) I–V relationships from a patch where the pipette solution contained 130 mM NaF and 10 mM NaCl. The bath contained 140 mM NaCl and 67 μM Ca2+. (D) Halide permeability as a function of hydration energy. (E and F) Activation of ANO2 by divalent cations. (E) Current traces of ANO2-containing patches activated by 1 mM Ca2+, Mg2+, Ba2+ and Sr2+. The small shift in current observed during Mg2+ application is not understood but might be caused by a change in seal resistance. (F) The ratio of the first peak response to the second. The current elicited by each test divalent was normalized to the Ca2+-elicited current recorded in close time proximity to minimize the effect of the rundown. (G) Niflumic acid (300 μM) greatly reduces ANO2 Ca2+-activated current. This inhibition is reversible. (H) Noise analysis of the data in Fig. 4A. The variance (see Material and Methods) was plotted against the current and fitted with σ2 = iI − I2/N, with i = 0.03 pA and n = 1640.
To test whether ANO2 confers a Ca2+-activated conductance to HEK-293 membranes, the patch was exposed to 1 mM Ca2+ immediately following excision. Indeed, Ca2+ elicited a rapidly peaking current (Fig. 3A). The current showed marked inactivation during the 3-s Ca2+ exposure and terminated rapidly after Ca2+ was removed. Repeated stimulation revealed a continuing rundown of peak current over time (Fig. 3A and Fig. S5). This current rundown was not caused by compromised patch integrity (e.g., patch size and access), because the cAMP-elicited current, which also was recorded following each Ca2+ stimulation, remained stable during the entire recording duration (Fig. 3A and Fig. S5). Rundown of the Ca2+-activated Cl− current over time is a well-documented property of the native olfactory CaCC (9). We analyzed the rundown of the ANO2 Ca2+-activated current in 11 patches that had sufficiently large Ca2+-activated currents and were stable for at least 12 min as judged by the cAMP-activated current. The ANO2 Ca2+-activated current exhibited 56% rundown over the time course of the experiment (Fig. S5), similar to the 52% rundown observed in the native olfactory CaCC (9). In the control experiments, in which HEK-293 cells were transfected with plasmids encoding CNGA2 and EGFP, only small and noisy calcium-activated currents were observed occasionally. These currents were activated with a considerable delay of a few seconds and disappeared within 1–2 min.
To investigate if the channel formed by ANO2 is indeed a Cl− channel, we recorded current–voltage (I–V) relationships. In symmetrical NaCl solutions, the ANO2 channel current reversed at a potential close to 0 mV (Fig. 3B), with slight inward rectification similar to the native olfactory CaCC (9). When 130 mM (of 140 mM) NaCl in the bath was replaced by Na-methanesulfonate, the reversal potential shifted to −34.6 ± 1.7 mV (n = 6), demonstrating that this current is indeed carried by Cl−. The relative permeability PMeS/PCl was determined to be 0.17 ± 0.02.
The native olfactory CaCC has been documented to exhibit varying permeabilities to different halide ions (9). We therefore investigated the halide permeability of the ANO2 channel by equimolar replacement of bath NaCl by NaBr or NaI. This replacement progressively shifted the I–V relationships to the right and the reversal potentials to more positive values (Fig. 3C), indicating that the ANO2 channel is more permeable to both Br− and I− than it is to Cl−. In the case of F−, to avoid Ca2+ chelation by fluoride in the bath, 130 mM NaCl was replaced equimolarly with NaF in the pipette solution. The reversal potential was shifted to more positive values (Fig. 3C Inset), now indicating that the ANO2 channel is less permeable to F− than to Cl−. The reversal potentials and the Goldman-Hodgkin-Katz equation yielded a permeability sequence of I− > Br− > Cl− > F− and permeability ratios PX/PCl of 3.85:1.78:1:0.12 (n = 6 for Br− and I−, n = 7 for F−), which are inversely related to the hydration energies (Fig. 3D). These relative halide permeabilities are very similar to the native olfactory CaCC (9) (Table S2).
The native olfactory CaCC has been documented to exhibit varying sensitivities to different divalent cations (9). We tested the activation of the ANO2 channel by other divalents. Because ANO2 currents showed rundown, we first exposed the patch to the test divalent at 1 mM followed by an exposure to 1 mM Ca2+ (Fig. 4E). This procedure enabled us to normalize the response of the test divalent to the Ca2+-evoked current in each trial. Sr2+ activated the ANO2 channel almost as well as Ca2+, Ba2+ was only a poor agonist, and no current was observed upon Mg2+ application (Fig. 4G, n = 5). These observations for the ANO2 channel are consistent with those for the native olfactory CaCC (9) (Table S2).
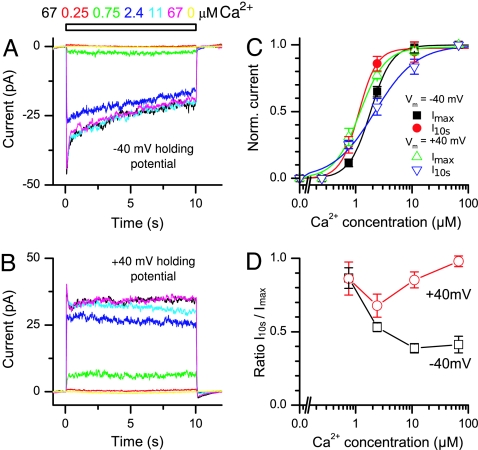
Fig. 4.
Ca2+ Dose dependency and inactivation kinetics of the ANO2 channel. (A and B) A patch was exposed for 10 s to a series of Ca2+ concentrations at −40 mV (A) and +40 mV (B). (C) The peak current and the current at 10 s were plotted against the Ca2+ concentration for both −40-mV and +40-mV holding voltages and fitted with Hill functions. (D) The ratio of the current at 10 s to the peak current showed a marked decline at −40 mV with increasing Ca2+ concentration but not at +40 mV.
We also tested the sensitivity of the ANO2 channel to niflumic acid, a routinely used blocker of the native olfactory CaCC (8, 9). Niflumic acid (300 μM) substantially reduced patch currents by 77% ± 5% (n = 8) when applied simultaneously with Ca2+ (Fig. 3G).
We attempted to obtain the single-channel conductance for the ANO2 channel. Because the low conductance of individual ANO2 channels hinders direct measurement, we used noise analysis (Fig. 3H). The single-channel conductance was estimated to be 0.8 ± 0.1 picosiemens (pS) (n = 5) by the parabolic fit and 1.22 ± 0.08 pS by the more accurate linear fit (see Materials and Methods). These values are similar to that of the native olfactory CaCC, 1.27 pS (9) (Table S2).
We further investigated the sensitivity of ANO2 to Ca2+ by exposing patches for 10 s to increasing Ca2+ concentrations at both −40-mV and +40-mV holding potentials (Fig. 4 A and B). The experiments were performed ≈10 min after patch excision to allow completion of the more rapid phase of the current rundown. We determined the dose-dependency (Fig. 4C) for the peak current (Imax) and for the current at 10 s (I10s), similar to the analysis performed for the native olfactory CaCC (9). The current at 10 s was used to evaluate a change in Ca2+ sensitivity during the inactivation. The data were fit with Hill functions. When comparing Imax, the K1/2 were 1.83 μM (n = 5) at −40 mV (Fig. 4C, black curve) and 1.18 μM (n = 4) at +40 mV (Fig. 4C, green curve), indicating a higher apparent Ca2+ affinity at positive holding potentials. This finding is consistent with observations in the native olfactory CaCC (9). Hill coefficients were 2.3 at −40 mV and 1.92 at +40 mV. However, when comparing the current after inactivation (I10s), the dose dependency showed the opposite trend. The K1/2 were 1.12 μM at −40 mV (Fig. 4C, red curve) and 2.18 μM at +40mV (Fig. 4C, blue curve), indicating that after inactivation ANO2 actually was more sensitive to Ca2+ at −40 mV that at +40 mV. The dose–response also was much shallower at +40 mV (Hill coefficients were 2.67 at −40 mV and 1.18 at +40 mV). Overall, the Ca2+ sensitivities of the ANO2 channel are comparable to those of the native olfactory CaCC (4, 9) (Table S2).
The kinetics of ANO2 channel inactivation were found to be quite different at positive and negative holding potentials. At −40 mV and at high Ca2+ concentrations, ANO2 currents typically showed a rapid inactivation during the first 1 s of Ca2+ application followed by a steady decline thereafter (Fig. 4A). These inactivation kinetics are comparable to the native olfactory CaCC (9). However, the steady secondary decline of the ANO2 current was minimal at +40 mV (Fig. 4B), unlike the native olfactory CaCC, which also displays a steady secondary inactivation at +40 mV (9). We quantified the level of inactivation during Ca2+ stimulation by dividing I10s by its respective Imax (Fig. 4D) for each Ca2+ concentration. At low Ca2+ levels, currents showed little inactivation regardless of holding potential. Inactivation became more apparent and severe at higher Ca2+ concentrations at −40 mV, whereas at +40 mV the I10s/Imax ratio decreased only slightly with increasing Ca2+ concentrations and recovered to almost 1 at saturating Ca2+ concentrations. For the ANO2 channel, the difference in inactivation kinetics between positive and negative holding potentials partly explains the reduction in the Hill coefficient for the I10s dose–response at +40 mV in Fig. 4C. Note that the rapid inactivation of the current at the onset of Ca2+ exposure was observed in some, but not all, patches obtained from native OSNs (9), probably because patches from OSNs often contain long, thin cilia that slow solution exchange and thus slow the activation of the current.
Taken together, the patch-clamp experiments on heterologously expressed ANO2 channels show that ANO2 forms a calcium-activated chloride channel with properties remarkably similar to the native olfactory CaCC in terms of rundown, slight permeability to Na-methanesulfonate, halide permeability sequence, divalent cation activation, inhibition by niflumic acid, single-channel conductance, biphasic inactivation at negative holding potentials, and Ca2+ sensitivity (4, 8, 9). Table S2 shows a comparison of ANO2 and native olfactory CaCC properties.
Discussion
In this study, we identified ANO2 from a proteomic screen of an olfactory cilial membrane preparation. The nature of mass spectrometry-based proteomics is biased toward the identification of abundant proteins. Therefore, the identification of all known olfactory transduction components downstream of ORs in our proteomic screen exemplifies that OSN cilia are a highly specialized compartment for sensory signaling. Although we intentionally separated a gel band for 35- to 55-kDa proteins with the consideration of the presence of a large number of ORs, mass spectrometry did not identify any ORs in any fraction (Table S1), presumably because of the high diversity and very low abundance of individual ORs in the preparation. We tailored our proteomic analysis toward the identification of CaCC candidate(s), based on the hypotheses that the olfactory CaCC is enriched in the cilial membrane and that the CaCC might have a relatively high molecular weight. Using these criteria, we were able to focus on a small list of proteins to select ANO2 as a CaCC candidate. Previously, 2 proteomic analyses of rat olfactory cilial preparations were reported. In the first report, ANO2 and even adenylyl cyclase III (ACIII) were not found (27). In the second report, ANO2 was found among 377 proteins (28).
The close similarity of ANO2 channel properties to the native olfactory CaCC strongly suggests that ANO2 is a major, if not sole, component of the olfactory CaCC. However, because we found a few minor differences, we cannot rule out the possibility that an additional regulatory subunit is present in the native channel, as has been suggested for ANO1 (25). Nevertheless, ANO2 most likely is the pore-forming subunit. Although RT-PCR analysis of nasal tissue, which contains various cell types, for all Ano family members shows amplification of a few Ano genes (Fig. S6 and Table S3 ), ANO2 was the only ANO protein found in our proteomic screen. Additionally, no other Ano family member transcripts have been found to be OSN-specific (20). Bestrophin 2 (Best2) has been proposed as a candidate olfactory CaCC (29). However, we did not detect any peptides matching Best2 protein in our screen of olfactory cilial membranes. Also, some channel properties of Best2 differ from the native olfactory CaCC. Further, Best2−/− mice have been shown to perform an olfactory behavior test normally (30).
The gating mechanism of the native olfactory CaCC is unknown. Although calcium alone is sufficient to open the native olfactory CaCC in patch-clamp experiments (9), the loss of Cl− channel activity over time after patch excision (“rundown”) is consistent with the loss of a modulatory factor. A study performed using the OSN-like cell line Odora implicated calmodulin in the gating mechanism of the native olfactory CaCC (31). Further, other regulatory mechanisms affecting the olfactory CaCC, such as phosphorylation and membrane trafficking, have not yet been explored. With the identification of ANO2 as the major component of the olfactory CaCC, these questions can be addressed directly by site-directed mutagenesis and protein-interaction studies.
Different members of channel families often have different biophysical properties, making them more suited for their roles in their specific cell type. Comparison of heterologously expressed ANO1 and ANO2 channels shows substantial differences in their channel properties, even though they share a similar order of relative halide permeabilities (23, 25). ANO1 channels seem to have a higher sensitivity to Ca2+, certainly at positive holding potentials (23, 24). ANO1 channels also have much slower opening and closing kinetics (23) when exposed to Ca2+. A channel with such slow kinetics acts like a low-pass filter, a characteristic possibly better suited for secretion but not for rapidly signaling in neurons. Finally, the single-channel conductance for ANO1 was reported to be 8.3 pS (23), a value around 8 times larger than we found for ANO2 and for the native olfactory CaCC (9). A small chloride-channel conductance is important in olfactory transduction to convey the low-noise amplification of the primary CNG current (11). Thus, the ANO2 channel is well suited for its role in olfactory signal transduction.
It is tempting to speculate that animals lacking ANO2 may have significantly reduced sensitivity to odors. In humans, the full-length ANO2 is not present in patients who have von Willebrand's disease type 3, where a 253-kb deletion in chromosome 22 results in the deletion of the gene encoding von Willebrand's factor—a protein involved in blood clot formation—and the deletion of the N terminus of the neighboring Ano2 gene (32). In an interview with the widow of a patient homozygous for this mutation, it was found that “1) her husband never complained about the meals even if they were burnt. 2) When [the patient] was cooking he was possibly not noticing when the potatoes were burnt. 3) [His] use of perfume was sometimes so extreme that his wife wondered how he could stand it. 4) He never mentioned the smell of flowers or ‘rural’ smells.” (Drs. Roswitha Eisert and Reinhard Schneppenheim, personal communication).
In addition to mediating amplification in the olfactory system, ANO2 might play roles in other neuronal systems. For instance, ANO2 transcripts have been found in the retina (NCBI), and in brain regions such as the inferior olivary complex of the brainstem and the cerebral lateral septal nucleus (Allen Brain Atlas). Other ANO family members have even broader expression in several tissues and across development (23–25, 33, 34) and have been proposed as mediators of physiological functions ranging from epithelial secretion to smooth muscle contraction (16). The role of ANO2 in the olfactory system will elucidate the diverse functions of calcium-activated chloride conductance brought about by this recently characterized protein family.
Materials and Methods
Mice were handled and euthanized with methods approved by the Animal Care and Use Committees of The Johns Hopkins University.
Olfactory Cilial Membrane Preparation and Protein Analysis by LC-MS/MS.
Olfactory cilia from 30 mice were isolated by the calcium shock method (35). The cilia were treated with hypotonic solution (36) followed by ultracentrifugation over a sucrose gradient to isolate membranes. The membrane-enriched sucrose fractions were pooled, and the proteins were separated by SDS/PAGE. The gel was cut into 3 sections and stored in water. The slices were processed and analyzed by LC-MS/MS by the Taplin Mass Spectrometry Facility (Harvard University). For details, see SI Methods.
Molecular Characterization of Ano2.
For details on RT-PCR, in situ hybridizations, fusion protein expression, and viral construction, see SI Methods.
Patch-Clamp Analysis.
Mouse olfactory ANO2 was co-expressed with CNGA2 in HEK-293 cells. Recordings from inside-out patches (see SI Methods for details) were performed essentially as described (9). For estimation of single-channel conductance by noise analysis, the data were fit with the parabolic function σ2 = iI − I2/N, where i is the single-channel conductance, N is the total number of channels in the patch, and I is the mean current (37), assuming the channel has no subconductances. Because the current showed rapid inactivation after its peak, the variance for high current values could not be determined reliably: digital high-pass filtering introduced wave-like artifacts during fast current changes (“ringing”). Also, the calculation of the mean current during the 0.5-s variance measurement interval would be questionable during rapidly changing currents. Typically, only after the current settled to around half its maximal value could the variance be determined. This limitation affected our ability to fit the entire parabola, but nevertheless the single-channel conductance was determined. A more accurate calculation was performed by fitting only small current values (typically the first 2 data points) with the linear function σ2 = iI.
Supplementary Material
Acknowledgments.
We thank Drs. Roswitha Eisert and Reinhard Schneppenheim for their interview of the widow of the late patient who had the VWF mutation regarding his olfactory abilities. We thank K. Cunningham, D. Fambrough, S. Hattar, E. Kozub, and R. Kuruvilla for suggestions and discussions and H. Ozdener for help with cell culture. We thank the members of the Hattar-Kuruvilla-Zhao mouse tri-lab of the Johns Hopkins Department of Biology for discussions. This work was supported by National Institutes of Health Grant DC007395, the Monell Chemical Senses Center, and a Morley Kare Fellowship (to J.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0903304106/DCSupplemental.
References
- 1.Firestein S. How the olfactory system makes sense of scents. Nature. 2001;413:211–218. doi: 10.1038/35093026. [DOI] [PubMed] [Google Scholar]
- 2.Kleene SJ. The electrochemical basis of odor transduction in vertebrate olfactory cilia. Chem Senses. 2008;33:839–859. doi: 10.1093/chemse/bjn048. [DOI] [PubMed] [Google Scholar]
- 3.Kaneko H, Putzier I, Frings S, Kaupp UB, Gensch T. Chloride accumulation in mammalian olfactory sensory neurons. J Neurosci. 2004;24:7931–7938. doi: 10.1523/JNEUROSCI.2115-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reisert J, Lai J, Yau KW, Bradley J. Mechanism of the excitatory Cl− response in mouse olfactory receptor neurons. Neuron. 2005;45:553–561. doi: 10.1016/j.neuron.2005.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nickell WT, Kleene NK, Kleene SJ. Mechanisms of neuronal chloride accumulation in intact mouse olfactory epithelium. J Physiol (Paris) 2007;583:1005–1020. doi: 10.1113/jphysiol.2007.129601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleene SJ, Gesteland RC. Calcium-activated chloride conductance in frog olfactory cilia. J Neurosci. 1991;11:3624–3629. doi: 10.1523/JNEUROSCI.11-11-03624.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurahashi T, Yau K-W. Co-existence of cationic and chloride components in odorant-induced current of vertebrate olfactory receptor cells. Nature. 1993;363:71–74. doi: 10.1038/363071a0. [DOI] [PubMed] [Google Scholar]
- 8.Kleene SJ. Origin of the chloride current in olfactory transduction. Neuron. 1993;11:123–132. doi: 10.1016/0896-6273(93)90276-w. [DOI] [PubMed] [Google Scholar]
- 9.Reisert J, Bauer PJ, Yau KW, Frings S. The Ca-activated Cl channel and its control in rat olfactory receptor neurons. J Gen Physiol. 2003;122:349–363. doi: 10.1085/jgp.200308888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lowe G, Gold GH. Nonlinear amplification by calcium-dependent chloride channels in olfactory receptor cells. Nature. 1993;366:283–286. doi: 10.1038/366283a0. [DOI] [PubMed] [Google Scholar]
- 11.Kleene SJ. High-gain, low-noise amplification in olfactory transduction. Biophys J. 1997;73:1110–1117. doi: 10.1016/S0006-3495(97)78143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boccaccio A, Menini A. Temporal development of cyclic nucleotide-gated and Ca2+ -activated Cl− currents in isolated mouse olfactory sensory neurons. J Neurophysiol. 2007;98:153–160. doi: 10.1152/jn.00270.2007. [DOI] [PubMed] [Google Scholar]
- 13.Su T, Ding X. Regulation of the cytochrome P450 2A genes. Toxicol Appl Pharmacol. 2004;199:285–294. doi: 10.1016/j.taap.2003.11.029. [DOI] [PubMed] [Google Scholar]
- 14.Nishizawa T, Nagao T, Iwatsubo T, Forte JG, Urushidani T. Molecular cloning and characterization of a novel chloride intracellular channel-related protein, parchorin, expressed in water-secreting cells. J Biol Chem. 2000;275:11164–11173. doi: 10.1074/jbc.275.15.11164. [DOI] [PubMed] [Google Scholar]
- 15.Gherman A, Davis EE, Katsanis N. The ciliary proteome database: An integrated community resource for the genetic and functional dissection of cilia. Nat Genet. 2006;38:961–962. doi: 10.1038/ng0906-961. [DOI] [PubMed] [Google Scholar]
- 16.Hartzell HC, Yu K, Xiao Q, Chien LT, Qu Z. Anoctamin/TMEM16 family members are Ca2+-activated Cl− channels. J Physiol. 2008;587:2127–2139. doi: 10.1113/jphysiol.2008.163709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsutsumi S, et al. The novel gene encoding a putative transmembrane protein is mutated in gnathodiaphyseal dysplasia (GDD) Am J Hum Genet. 2004;74:1255–1261. doi: 10.1086/421527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kornak U, et al. Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell. 2001;104:205–215. doi: 10.1016/s0092-8674(01)00206-9. [DOI] [PubMed] [Google Scholar]
- 19.Entian KD, et al. Functional analysis of 150 deletion mutants in Saccharomyces cerevisiae by a systematic approach. Mol Gen Genet. 1999;262:683–702. doi: 10.1007/pl00013817. [DOI] [PubMed] [Google Scholar]
- 20.Yu T-T, et al. Differentially expressed transcripts from phenotypically identified olfactory sensory neurons. J Comp Neurol. 2005;483:251–262. doi: 10.1002/cne.20429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Juschke C, Wachter A, Schwappach B, Seedorf M. SEC18/NSF-independent, protein-sorting pathway from the yeast cortical ER to the plasma membrane. J Cell Biol. 2005;169:613–622. doi: 10.1083/jcb.200503033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strotmann J, Levai O, Fleischer J, Schwarzenbacher K, Breer H. Olfactory receptor proteins in axonal processes of chemosensory neurons. J Neurosci. 2004;24:7754–7761. doi: 10.1523/JNEUROSCI.2588-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang YD, et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455:1210–1215. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- 24.Caputo A, et al. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 25.Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134:1019–1029. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradley J, Reuter D, Frings S. Facilitation of calmodulin-mediated odor adaptation by cAMP-gated channel subunits. Science. 2001;294:2176–2178. doi: 10.1126/science.1063415. [DOI] [PubMed] [Google Scholar]
- 27.Mayer U, et al. proteomic analysis of a membrane preparation from rat olfactory sensory cilia. Chem Senses. 2008;33:145–162. doi: 10.1093/chemse/bjm073. [DOI] [PubMed] [Google Scholar]
- 28.Mayer U, et al. The proteome of rat olfactory sensory cilia. Proteomics. 2009;9:322–334. doi: 10.1002/pmic.200800149. [DOI] [PubMed] [Google Scholar]
- 29.Pifferi S, et al. Bestrophin-2 is a candidate calcium-activated chloride channel involved in olfactory transduction. Proc Natl Acad Sci USA. 2006;103:12929–12934. doi: 10.1073/pnas.0604505103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bakall B, et al. Bestrophin-2 is involved in the generation of intraocular pressure. Invest Ophthalmol Visual Sci. 2008;49:1563–1570. doi: 10.1167/iovs.07-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaneko H, Mohrlen F, Frings S. Calmodulin contributes to gating control in olfactory calcium-activated chloride channels. J Gen Physiol. 2006;127:737–748. doi: 10.1085/jgp.200609497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneppenheim R, et al. A common 253-kb deletion involving VWF and TMEM16B in German and Italian patients with severe von Willebrand disease type 3. Journal of Thrombosis and Haemostasis. 2007;5:722–728. doi: 10.1111/j.1538-7836.2007.02460.x. [DOI] [PubMed] [Google Scholar]
- 33.Rock JR, Harfe BD. Expression of TMEM16 paralogs during murine embryogenesis. Dev Dyn. 2008;237:2566–2574. doi: 10.1002/dvdy.21676. [DOI] [PubMed] [Google Scholar]
- 34.Gritli-Linde A, et al. Expression patterns of the Tmem16 gene family during cephalic development in the mouse. Gene Expression Patterns. 2009;9:178–191. doi: 10.1016/j.gep.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Anholt RR, Aebi U, Snyder SH. A partially purified preparation of isolated chemosensory cilia from the olfactory epithelium of the bullfrog, Rana catesbeiana. J Neurosci. 1986;6:1962–1969. doi: 10.1523/JNEUROSCI.06-07-01962.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adoutte A, et al. Biochemical studies of the excitable membrane of Paramecium tetraurelia. III. Proteins of cilia and ciliary membranes. J Cell Biol. 1980;84:717–738. doi: 10.1083/jcb.84.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeFelice LJ. Introduction to Membrane Noise. New York: Plenum Press; 1981. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.