Abstract
A phase III clinical trial assessed recurrence of adenomatous polyps after treatment for 36 months with difluoromethylornithine (DFMO) plus sulindac or matched placebos. Temporary hearing loss is a known toxicity of treatment with DFMO, thus a comprehensive approach was developed to analyze serial air conduction audiograms. The generalized estimating equation method estimated the mean difference between treatment arms regarding change in air conduction pure tone thresholds while accounting for within-subject correlation due to repeated measurements frequencies. Based on 290 subjects, there was an average difference of 0.50 dB between subjects treated with DFMO plus sulindac compared to those treated with placebo (95 percent confidence interval, −0.64 to 1.63 dB; P=0.39), adjusted for baseline values, age, and frequencies. In the normal speech range of 500 Hz to 3000 Hz, an estimated difference of 0.99 dB (−0.17 to 2.14 dB; P=0.09) was detected. Dose intensity did not add information to models. Follow-up air conduction performed at least 6 months after end of treatment showed an adjusted mean difference in hearing thresholds of 1.08 dB (−0.81to 2.96 dB; P=0.26) between treatment arms. There was no significant difference in the proportion of subjects in the DFMO plus sulindac group who experienced clinically significant hearing loss compared to the placebo group. The estimated attributable risk of ototoxicity from exposure to the drug is 8.4% (95% confidence interval, −2.0% to 18.8%; P=0.12). There is less than 2 dB difference in mean threshold for patients treated with DFMO plus sulindac compared to those treated with placebo.
Keywords: Difluoromethylornithine, air conduction audiograms, ototoxicity, generalized estimating equations, chemoprevention
Introduction
Removal of adenomas found during screening sigmoidoscopy or colonoscopy may prevent colorectal cancer (1), the second most common cause of cancer deaths in the U.S. (2). Difluoromethylornithine (DFMO) has been identified as a potent inhibitor of intestinal and colon carcinogenesis in animal models, especially in combination with non-steroidal anti-inflammatory drugs (NSAIDS) (3–5). DFMO and the non-specific NSAID sulindac also interact additively to prevent the growth and viability of human colon cancer cells (6). Results of a Phase III clinical chemoprevention trial demonstrated the efficacy of a low dose of DFMO plus sulindac, at a dose one-half the usual therapeutic dose. In the population of individuals at moderately high risk for sporadic adenomas, 41 percent of subjects receiving placebos developed recurrent adenomas compared to 12 percent of subjects receiving DFMO plus sulindac. There was a marked reduction of the recurrence of all adenomas in subjects receiving DFMO plus sulindac (70 percent decrease relative to those receiving placebo), advanced adenomas (92 percent decrease) and recurrence of greater than one adenoma (95 percent decrease) (7).
Temporary hearing loss is one of the known toxicities of treatment with DFMO (8–13). One study reported permanent hearing loss with higher doses than used in the current trial (14). In the Phase III clinical chemoprevention trial conducted by Meyskens and colleagues, self-reported hearing changes were not significantly different between the two groups. Although no evidence of a decrement in the normal speech range was documented, serial audiograms suggested a possible effect across a broader range of frequencies tested that was reversible in some cases (7). The details of the audiologic studies and comprehensive analyses are reported here. The statistical issues that have been addressed include the need for (1) appropriate adjustment for known sources of variation in hearing, (2) application of the generalized estimating equation (GEE) approach to the data to take into account the correlation between values across frequencies for individual subjects, hearing thresholds measured in left and right ears, and age adjustment, (3) estimation of the differences in hearing thresholds between final and baseline values and between frequencies, and (4) evaluation of the effect of treatment with DFMO plus sulindac on hearing loss.
Materials and Methods
Study Design
This study was a randomized, double-blind placebo-controlled trial to test whether the combination of a low dose of DFMO plus a low dose of Sulindac reduces the recurrence of colorectal adenomas detected by standard colonoscopy. The trial involved seven clinical sites in the United States. The human subjects committee at each site approved the study protocol and written informed consent was provided by all patients before enrollment. Quality control to promote uniform practice and protocol compliance included meetings before enrollment and site inspections during and after the trial. An independent Data and Safety Monitoring Board (DSMB) reviewed safety and efficacy data twice yearly.
Recruitment and Study Population
Eligibility required patients age 40–80 with a history of ≥ 1 resected adenoma of at least 3 mm within 5 years prior to study entry. Participants with > 20 dB sensorineural hearing loss above age-adjusted norms (15) assessed by pure tone audiometry at any frequency in the normal hearing range were ineligible. Additional eligibility criteria are reported elsewhere (7). A screening colonoscopy within 6 months of study entry was done and all polyps removed and pathologically examined. Before randomization to the agents, screening was done and included baseline history, physical examination, pure tone audiometry, and laboratory evaluations for baseline hematologic, renal and hepatic status. A one month placebo run-in period was used to assess compliance. To be randomized, participants had to demonstrate 80 percent adherence to the 1-month run-in medication. Three years after randomization, colonoscopies were performed. Gastroenterologists associated with the trial performed all study colonoscopies.
Safety evaluations were performed at return visits after the run-in and at 3, 6, 9, 12 and every 6 months through the end of the study. Pure tone audiograms were done at 18 and 36 months after randomization, or off study, and repeated 6 months later. Compliance with the protocol, including in-person and telephone visits, study medication, and blood draws, were monitored throughout the duration of the study.
Hearing assessment
Air conduction pure tone thresholds were obtained by audiologists using standard clinical protocol. Frequencies tested were 250 Hz, 500 Hz, 1000 Hz, 2000 Hz, 3000 Hz, 4000 Hz, 6000 Hz and 8000 Hz. The inter-octave frequencies of 3000 Hz and 6000 Hz were added to the usual clinical practice to capture changes at these critical frequencies (16). For audiometric testing, 5 dB steps were specified in the protocol as this has remained a standard since 1959 (17, 18). All audiograms were evaluated for change in thresholds by the study audiologist.
Study Treatment
DFMO was given orally at a single daily dose of 500 mg and sulindac was given orally at a single daily dose of 150 mg. Dose intensity of DFMO was estimated as the proportion of full dose that a participant took during the trial. The randomization used a blocked design and was stratified by clinical site and on the basis of the use (defined as 81 mg or less daily or 325 mg or less twice weekly) or nonuse of low dose aspirin at study entry.
Statistical Analysis
A total of 375 subjects were randomized. Of these, 290 participants had baseline and at least one repeat air conduction audiogram available for analysis. For pure tone thresholds, summary statistics were computed at each frequency for two treatment groups. Consistent with the approach taken in previous investigations, the average of pure tone threshold values from left and right ears was used for graphical and numeric analyses (13, 19, 20). For a given frequency, the available value was used if the threshold value was present for one ear, but missing for the other ear. For each treatment group, box plots were constructed to illustrate the variability in thresholds at each frequency and at each test time. For adverse events reporting, the NCI Common Terminology Criteria for Adverse Events, version 3.0 were utilized. A description of these criteria is available at http://ctep.cancer.gov/forms/CTCAEv3.pdf. The relative risk of hearing loss of at least 15 dB in any frequency across the entire range tested in the DFMO plus sulindac group versus that of placebo was assessed by log-binomial regression. The likelihood ratio test P-value is reported. The estimated attributable risk from exposure to the DFMO plus sulindac was calculated as the difference in the proportions of subjects in the two groups who experienced hearing loss of at least 15 dB in any frequency. The two-sample test of equality of proportions was applied and the 95% confidence interval for the difference in proportions was calculated. Similarly, the relative risk of hearing loss of at least 15 dB in the DFMO/sulindac group at two consecutive frequencies versus that of placebo was assessed by log-binomial regression.
Imputation and smoothing for Missing Threshold Values
For some subjects, not all frequencies had dB values recorded for one or both ears. Inspection of the data showed that although the research protocol specified that measurements were to be taken at 3000 Hz and 6000 Hz, pure tone thresholds were missing for both ears at 3000 Hz for 33 of 290 subjects and at 6000 Hz for 36 of 290 subjects. If threshold values were missing for both ears, multiple imputation was used to estimate the average at that threshold. Multiple imputation with the regression method was applied to impute 10 values for each missing threshold value (SAS 9.1, PROC IM). Locally weighted scatterplot smoothing (Lowess) was used to reduce within-subject variation across frequencies. Generalized cross-validation criterion was employed to select subject-specific smoothing parameters (21). In addition, the profiles of smoothed values were examined graphically per subject by treatment group.
Multiple Linear Regression
For each frequency (250 Hz to 8000 Hz), multiple linear regression analysis was applied with the observed pure tone threshold from the 18-month or end-of-treatment audiogram as the outcome variable, and predictors including baseline threshold value, age group (decade) and treatment group. When the end-of-treatment audiogram was available it was used as the outcome variable for the analysis. In most cases the end-of-treatment audiogram was obtained at approximately 36 months. Otherwise, the threshold values measured at the 18-month visit were used. Models with interaction between treatment, age group, and use of low dose aspirin at study entry were considered. For the outcome of hearing thresholds, at each frequency the estimated mean difference between treatment groups and 95% confidence intervals was computed from models adjusted for baseline hearing threshold and other covariates.
Generalized Estimating Equation (GEE)
In the audiology monitoring process, several pure tone tests were performed across a range of the frequencies. For analysis, previous studies have used either multiple regression analysis (11, 13) or repeated-measures analysis of variance (ANOVA) (12, 22–26). In contrast, to take into account the correlation between values across frequencies for individual subjects, the generalized estimating equation (GEE) method was applied with subjects as clusters, an exchangeable correlation structure, and a normal link function. The outcome variable was pure tone threshold, measured at the 18-month or end-of-treatment audiogram. Predictors included baseline threshold value, age group (decade), quartile of dose intensity, frequency, and treatment group. Models were examined that contained variables representing interactions between age groups, treatment groups, and frequencies, where frequencies were grouped into three levels: low (250 Hz to 500Hz), medium (1000 Hz to 4000 Hz), and high (5000 Hz to 8000 Hz). The estimated mean difference between treatment groups, and 95% confidence intervals, was computed from GEE models adjusted for baseline hearing threshold and other covariates. To examine goodness-of-fit of the GEE models, the marginal R2, was calculated (27, 28). Results from the 10 separate GEE models were combined (29).
Recovery from treatment
To examine recovery from treatment, mean pure tone thresholds at baseline were compared to those obtained from retesting at least 6 months after treatment was stopped. The mean (+/− one standard deviation) of the duration of the follow-up to the date of the end of therapy was calculated. For individual participants, the presence of clinically significant hearing loss was defined as sustained threshold elevations of at least 15 db above baseline at any frequency on both end-of-treatment and post-treatment audiograms. The proportion of patients with clinically significant hearing loss was computed for each treatment group.
Results
Descriptive and graphical results
Each of the 375 subjects enrolled in this Phase III clinical trial had baseline audiograms performed. Of these, 290 participants had repeated air conduction audiograms available for analysis (Table 1). At baseline there was no significant difference in average pure tone threshold for left and right ears (Score statistic P=0.82), thus the average of pure tone threshold values from both ears was used for graphical and numeric analyses. Shotland and colleagues present gender-specific tables of 95th percentiles for age-adjusted air conduction pure-tone thresholds, adjusted up to the nearest 5 dB increment (30). The values are adapted from information published by Morrel et al. (15). For any 2.5-year age range, they represent hearing levels in decibels in which at least 95% of the population have equal or better hearing. For each participant, values recorded at baseline were compared to age-adjusted air conduction pure-tone thresholds for 500Hz, 1000Hz, 2000Hz, and 4000Hz (15, 30). There were 33 of 151 (21.2%) in the DFMO plus sulindac group and 30 of 139 (21.6%) in the placebo group with hearing worse than the 95th percentile for at least one of these four frequencies.”
Table 1.
Number of randomized subjects in the analysis cohort
| Analysis Cohort and outcome audiogram | DFMO/Suliudac | Placebo | Total |
|---|---|---|---|
| End-of-treatment audiogram | 138 | 112 | 250 |
| Discontinued treatment prior to 36 months with off-treatment audiogram performed | 20 | 15 | 35 |
| 36-month audiogram performed | 118 | 97 | 215 |
| 18-Month audiogram | 13 | 27 | 40 |
| Discontinued treatment prior to 36 months with 18-month audiogram performed only | 5 | 6 | 11 |
| 18-month audiogram performed. Pending 36-month audiogram | 8 | 21 | 29 |
| Analysis Cohort Total | 151 | 139 | 290 |
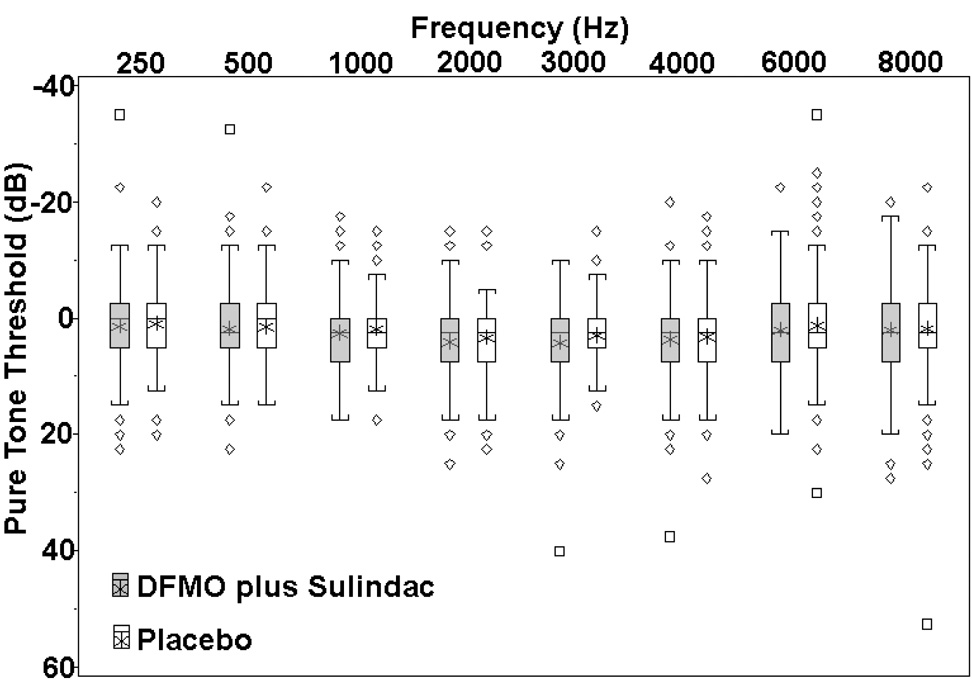
For each treatment group, the subject-specific differences between pure tone thresholds (final – baseline) are presented as boxplots in Figure 1. Frequencies are plotted on a log10 scale. To conform to clinical practice, positive values on the vertical axis indicate hearing loss and negative values indicate hearing improvement. Shown in reverse of the usual orientation, the box stretches from the 25th to the 75th percentile. The median is shown with a line across the box, and the mean is indicated with an asterisk. The audiogram values were not corrected for age.
Figure 1.
For each treatment group, the subject-specific differences between pure tone thresholds (outcome – baseline) are presented as boxplots. The box stretches from the 25th to the 75th percentile. The median is shown with a line across the box, and the mean with an asterisk. The whiskers indicate 1.5 times the interquartile range above the third and below the first quartiles, or to the upper or lower extreme values, whichever is closer. Values outside the whiskers were marked either as a diamond if the value was between 1.5 and 3 IQR or as a square if the value was farther away.
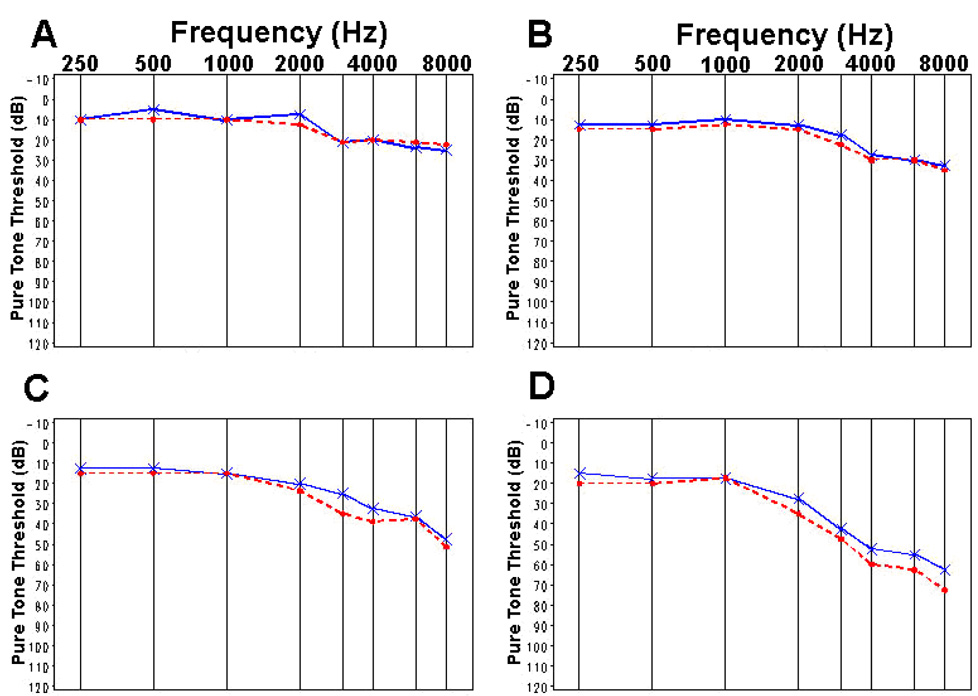
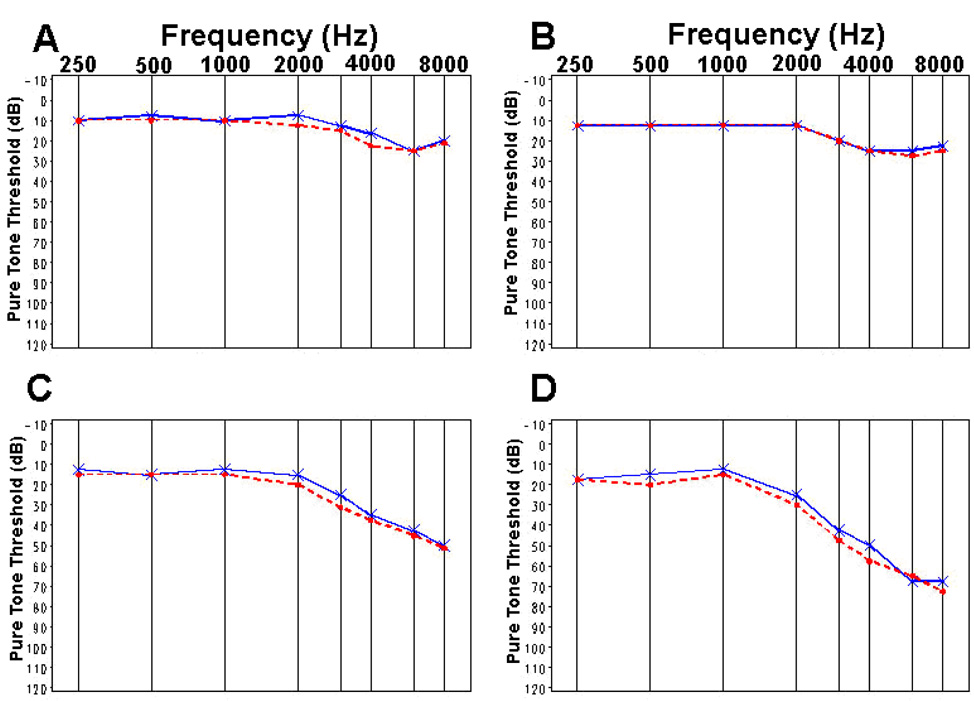
Figure 2 displays traditional audiogram graphs of the median threshold values at baseline and at 18- or 36-months for the DFMO plus sulindac group (N=151) versus placebo (N=139). Comparison of the median values is shown by age group (Fig. 3, Fig. 4). There were 42 of 151 (27.8%) subjects in the DFMO plus sulindac group who experienced clinically significant hearing loss, defined as at least 15 dB hearing loss from baseline in any frequency across the entire range tested, compared to 27 of 139 (19.4%) of subjects in the placebo group. The estimated attributable risk of ototoxicity from exposure to the drug is 8.4%. (95% confidence interval, −2.0% to 18.8%; binomial test P=0.12)
Figure 2.
Median pure tone threshold values for baseline (——) and final audiograms (– – –): (A) DFMO plus sulindac, (B) placebo.
Figure 3.
Median pure tone threshold values for baseline (——) and final (– – –) audiograms measured in patients treated with DFMO plus sulindac: A [40, 50) yr, B [50, 60) yr, C [60, 70) yr, D [70, 80) yr.
Figure 4.
Median pure tone threshold values for baseline (——) and final (– – –) audiograms measured in patients treated with placebo: A [40, 50) yr, B [50, 60) yr, C [60, 70) yr, D [70, 80) yr.
There were 14 of 151 (9.3%) in the DFMO plus sulindac group and 4 of 139 (2.9%) in the placebo group who experienced at least 15 dB hearing reduction from baseline in 2 or more consecutive frequencies across the entire range tested (Chi-square test P=0.02). The unadjusted relative risk of hearing loss for the DFMO plus sulindac treatment group was 3.2 with 95% interval of 1.09 to 9.55 relative to that of placebo.
Comparison of Pure Tone Thresholds across Frequencies
Multiple linear regression analyses for each frequency showed some evidence of interaction between treatment and pretrial use of low dose aspirin at 2000 Hz, but not for other frequencies. For the regression models with main effects of threshold, age group, and treatment group, Table 2 gives parameter estimates and 95% confidence intervals for predictors. These analyses were based on the average threshold measured in left and right ears, without imputation for missing values. For frequencies of 250 to 2000 Hz and 4000 Hz, data from the entire cohort of 290 subjects were analyzed. Threshold data were analyzed from 257 subjects measured at 3000 Hz, 254 subjects measured at 6000 Hz, and 288 subjects measured at 8000 Hz. Parameter estimates for variables representing age group and treatment with DFMO plus sulindac indicate the estimated mean difference in pure tone threshold compared to that of the reference group. Adjusted for baseline hearing threshold and age, 95% confidence intervals for mean hearing thresholds include zero for each frequency. Thus there was insufficient evidence of a difference in mean hearing threshold between treatment groups for each frequency. However, statistically significant hearing loss was experienced for patients who were 60 to 80 years of age, compared to those who were 40 to 50 years old. For example, on average subjects who were 70 to 80 years of age, experienced 5.3 dB greater hearing loss at a pure tone frequency of 2000 Hz, compared to the youngest group of patients and adjusted for baseline audiometry values, and treatment.
Table 2.
Multiple regression parameter estimates and 95% confidence intervals for model predictors based on observed hearing thresholds for the average of left and right ears. *
| Frequency (Hz) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 250 N = 290 |
500 N = 290 |
1000 N = 290 |
2000 N = 290 |
3000 N = 257 |
4000 N = 290 |
6000 N = 254 |
8000 N = 288 |
|
| Intercept | 4.582 (1.84,7.33) | 3.987 (1.35,6.62) | 2.777 (0.49,5.07) | 2.593 (0.02,5.17) | 2.145 (−0.55,4.84) | 4.208 (1.10,7.31) | 2.745 (−0.72,6.21) | 4.106 (0.53,7.68) |
| Baseline | 0.641 (0.54,0.74) | 0.734 (0.65,0.82) | 0.867 (0.80,0.94) | 0.903 (0.85,0.96) | 0.946 (0.90,0.99) | 0.931 (0.89,0.97) | 0.889 (0.84,0.94) | 0.875 (0.83,0.92) |
| Age group 4 [70, 80) yrs | 3.229 (0.07,6.39) | 3.550 (0.40,6.70) | 2.383 (−0.31,5.08) | 5.258 (2.15,8.37) | 3.440 (0.12,6.76) | 2.658 (−1.18,6.49) | 5.127 (0.77,9.48) | 4.368 (−0.12,8.85) |
| Age group 3 [60, 70) yrs | 1.695 (−1.01,4.40) | 1.232 (−1.51,3.98) | 1.095 (−1.25,3.44) | 2.813 (0.15,5.48) | 3.307 (0.56,6.05) | 2.403 (−0.79,5.60) | 3.582 (0.03,7.14) | 3.768 (0.04,7.49) |
| Age group 2 [50, 60) yrs | 0.276 (−2.44,2.99) | 0.127 (−2.60,2.85) | −0.150 (−2.49,2.19) | 1.119 (−1.53,3.77) | 1.094 (−1.64,3.83) | 0.319 (−2.84,3.47) | 2.198 (−1.28,5.67) | 2.653 (−0.96,6.27) |
| Age group 1 [40, 50) yrs | - | - | - | - | - | - | - | - |
| DFMO plus sulindac (N=151) | 0.808 (−0.66,2.28) | 0.596 (−0.87,2.06) | 1.076 (−0.19,2.34) | 1.056 (−0.38,2.49) | 1.455 (−0.08,2.99) | 0.365 (−1.34,2.07) | 0.694 (−1.26,2.65) | 0.186 (−1.77,2.14) |
| Placebo (N=139) | - | - | - | - | - | - | - | - |
Comparison reference groups were age group 1 [40, 50) yrs and placebo treatment group.
As described previously, for each subject multiple imputation was applied to impute threshold values missing from both ears at the same frequency. Data from the cohort of 290 were analyzed using the GEE method applied to each of 10 individual datasets. Dose intensity did not add information to models containing main effect predictors. For models including baseline threshold value, age group, treatment, an 8-level categorical variable representing frequency, and treatment by frequency interaction, no significant interactions were found. The distribution of score statistic p-values, ordered from lowest to highest, was 0.158, 0.275, 0.276, 0.296, 0.316, 0.326, 0.355, 0.436, 0.465, and 0.515. Thus, for the GEE models of main effects, results from the 10 datasets were combined to estimate parameter values and 95% confidence intervals (29). As displayed in Table 3, on average, subjects in the DFMO plus sulindac group did not have statistically significantly greater hearing loss than those in the placebo group. The estimated mean difference in hearing thresholds was 0.50 dB higher in those taking DFMO plus sulindac than those taking placebo (95 percent confidence interval (C.I.), −0.64 to 1.63 dB; P=0.39). For the placebo group, the estimated means (95% C.I.) are as follows: baseline, 25.3 dB (23.5 to 27.2); 18 months, 26.1 dB (24.3, 28.0 dB); 36 months, 27.6 dB (25.7, 29.5 dB); and follow-up 29.8 dB, (27.7, 31.9 dB). Within the normal speech range of 500 Hz to 3000 Hz, on average, subjects in the DFMO plus sulindac group experienced 0.99 dB greater hearing loss than the subjects in the placebo group (95 percent C.I., −0.17 to 2.14 dB; P=0.09).
Table 3.
Parameter estimates and 95% confidence intervals from GEE models applied to hearing thresholds for the average of left and right ears measured in 290 subjects, with multiple imputation for missing thresholds.
| Parameter | Estimate | 95% Confidence | Limits | P-value |
|---|---|---|---|---|
| Intercept | 0.811 | −0.864 | 2.486 | 0.171 |
| Smoothed Baseline | 0.837 | 0.797 | 0.877 | <.00001 |
| Age [70, 80) | 4.766 | 2.436 | 7.096 | <.00001 |
| Age [60, 70) | 2.949 | 1.140 | 4.757 | 0.0007 |
| Age [50, 60) | 0.912 | −0.733 | 2.558 | 0.139 |
| Age [40, 50) | - | - | - | - |
| DFMO/Sulindac | 0.498 | −0.636 | 1.632 | 0.195 |
| Placebo | - | - | - | - |
| Frequency at 8000 | 5.759 | 4.039 | 7.479 | <.00001 |
| Frequency at 6000 | 5.282 | 3.480 | 7.085 | <.00001 |
| Frequency at 4000 | 5.598 | 4.310 | 6.886 | <.00001 |
| Frequency at 3000 | 4.712 | 3.503 | 5.921 | <.00001 |
| Frequency at 2000 | 3.184 | 2.402 | 3.966 | <.00001 |
| Frequency at 1000 | 1.598 | 0.933 | 2.264 | <.00001 |
| Frequency at 500 | 0.533 | 0.311 | 0.754 | <.00001 |
| Frequency at 250 | - | - | - | - |
Recovery from treatment
There were 122 of 290 (42.1%) of subjects with follow-up audiometry measurements made at least six months after treatment was stopped. The mean time of the follow-up exam was 2.14 (± 1.26 SD) years after treatment was stopped. On average, thresholds measured in the DMO plus sulindac group were 1.08 dB greater than for subjects in the placebo group (95 percent C.I. −0.81 to 2.96 dB; P=0.26), adjusted for baseline values, age, and differences between frequencies. Relative to thresholds measured at the end of treatment, the adjusted mean difference in hearing thresholds was 0.79 dB (−0.94 to 2.53 dB; P=0.37). There were 42 of 122 (34%) of participants who sustained threshold elevations of at least 15 db above baseline at any frequency on both end-of-treatment and post-treatment audiograms; 25 of 63 (40%) in the DFMO plus sulindac group as compared to 17 of 59 (29%) in the placebo group. These proportions were not statistically significantly different (Chi-square test P=0.21). The estimated relative risk of clinically significant hearing loss in patients treated with low doses of DFMO plus sulindac was 1.6 with 95% interval of 0.96 to 2.62 relative to those taking placebo, adjusted for age and pre-treatment thresholds at each frequency.
Discussion
Treatment groups were similar with regard to time between randomization until performance of the outcome audiogram. Based on new quantitative evaluation of pure tone audiograms, mean hearing thresholds did not differ between those treated with DFMO plus sulindac or placebo for each frequency. Adjusting for baseline threshold, age, and frequencies, the average difference of 0.50 dB between treatment groups was not statistically significantly different from zero (95 percent CI, −0.64 to 1.63 dB; P=0.39) and was less than the instrument error of + 5 dB. Similarly, in the normal speech range of 500 Hz to 3000 Hz, there was no significant difference in mean hearing thresholds (P=0.09). Models showed no significant association between dose intensity and hearing thresholds. Hearing loss was not a function of increasing dose intensity. Of 290 subjects, 122 had follow-up air conduction testing at least 6 months after stopping treatment and the mean difference between treatment groups in average hearing threshold was 1.08 dB (95 percent CI, −0.81 to 2.96 dB; P=0.26), adjusted for baseline values, age, and differences between frequencies. There was a mean difference of 0.79 dB (−0.94 to 2.53 dB) between treatment groups relative to thresholds measured at the end of treatment (P=0.37).
Analyses were done using the GEE method. Advantages are that unbalanced data can be analyzed, an empirical sandwich estimator criteria is applied to model the error structure, and the GEE model is relatively insensitive to possible misspecification of the covariance structure as compared to the general linear mixed model (31). This procedure fits a population-averaged response as a function of covariates without explicitly accounting for subject to subject heterogeneity. The regression coefficients have interpretation for the population rather than for any individual. The population-averaged response for a given covariate value is directly estimable from observations without assumptions about the heterogeneity across individuals in the parameters, thus parameters are in this sense one step closer to the data than subject-specific parameters (32).
For assessment of toxicity in clinical trials, analysis of longitudinal audiometry evaluations across frequencies is necessary to estimate and compare the degree of difference between treatment groups. On average, there is less than 2 dB difference in pure tone threshold for those taking DFMO plus sulindac compared to those taking placebo. Two dB is barely discernable as an intensity change by individuals with normal hearing (33). These results are important because DFMO is known to cause clinically significant ototoxicity (8–12, 34), which might preclude it from application in a cancer prevention setting. In the current trial the dose of DFMO was approximately one-fiftieth of the doses used in therapeutic trials and one-fourth the dosages used in earlier types of prevention studies. The modest ototoxic effects of DFMO-containing treatment observed in this trial were likely a consequence of the low dose of DFMO administered. While it is true that humans lose hearing acuity with age, the ototoxicity associated with treatment in this study does not seem to be age-related. Rather, treatment associated ototoxicity appears to be associated with a subset of patients and may be related to genetic factors affecting the biochemical pathway targeted by the treatment (35).
A limitation of the research was that approximately 12% of subjects did not have pure tone thresholds recorded at 3000 Hz and 6000 Hz. However, multiple imputation was used to impute the missing values and parameter estimates from models with- and without imputed values were similar. Clinically, factors such as aging, family history of hearing loss, and noise exposure are known to accelerate hearing loss (36). Further research is needed to examine environmental and genetic factors that may potentiate hearing loss in combination with the use of DFMO.
While the evidence for significant ototoxicity of DFMO at doses in excess of 1.0 gm/m2 is compelling, case reports of DFMO-induced ototoxicity at lower doses (14) should be considered in light of the analysis presented here. The present evaluation of DFMO-associated ototoxicity in a randomized trial using quantitative audiologic endpoints documents age-related variation in audiologic parameters and places ototoxicity induced by daily oral DFMO doses of 500 mg in a quantitative context. This statistical approach complements and enhances evaluation of serial air conduction audiograms. These analyses do suggest a biological effect on hearing relevant to DFMO even at the low dose used, but the effect is subclinical. Ototoxicity at this low dose is much less than expected and only occurs in a small subset (less than 10%) of patients.
Acknowledgements
We thank clinical and research staff, Angela Garcia, Rachel Gonzalez, Yuhong Huang, Westley Lagerberg, Sharon Maxwell, and Lu Wu, for their invaluable assistance with the study.
This work was supported in part by NCI contract NO1-CN75019 (FLM, CEM) and grants CA59024 (FLM), CA88078 (FLM, CEM) and CA23074, CA95060 (EWG) and CA 63640 (CHH).
Footnotes
Participating Institutions and Investigators
Chao Family Comprehensive Cancer Center, University of California Irvine, CA: Frank L. Meyskens, Jr., M.D. (Principal Investigator), Gregory Albers, M.D., Sharon Fujikawa-rooks, Ph.D., Philip M. Carpenter, M.D., Daniel L. Gillen, Ph.D., Christine E. McLaren, Ph.D., Daniel Pelot, M.D.
Arizona Cancer Center, University of Arizona, AZ: Eugene W. Gerner, Ph.D., Steven Goldschmid, M.D., Peter Lance, M.D.
Denver Department of Veteran Affairs Medical Center and University of Colorado: Dennis J. Ahnen, M.D.
Department of Veterans Affairs Long Beach Healthcare System, CA: Jayashri Kidao, M.D.
Kaiser Permanente, Sacramento, CA: Michael J. Lawson, M.D.
Loma Linda University, CA: John McCracken, M.D.
University of Michigan, Ann Arbor, CA: D. Kim Turgeon, M.D.
University of Kansas, Kansas City, CA: Curt H. Hagedorn, M.D.
VA Loma Linda Healthcare System, CA: Ronald Griffen, M.D.
References
- 1.Winawer SJ, Zauber AG, Ho MN, et al. The National Polyp Study Workgroup. Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 2.Minino AM, Heron MP, Murphy SL, Kochanek KD. Deaths: final data for 2004. Natl Vital Stat Rep. 2007;55:1–119. [PubMed] [Google Scholar]
- 3.Gerner EW, Meyskens FL, Jr, Goldschmid S, Lance P, Pelot D. Rationale for, and design of, a clinical trial targeting polyamine metabolism for colon cancer chemoprevention. Amino Acids. 2007;33:189–195. doi: 10.1007/s00726-007-0515-2. [DOI] [PubMed] [Google Scholar]
- 4.Meyskens FL, Jr, Gerner EW. Development of difluoromethylornithine (DFMO) as a chemoprevention agent. Clin Cancer Res. 1999;5:945–951. [PubMed] [Google Scholar]
- 5.Nigro ND, Bull AW, Boyd ME. Inhibition of intestinal carcinogenesis in rats: effect of difluoromethylornithine with piroxicam or fish oil. J Natl Cancer Inst. 1986;77:1309–1313. [PubMed] [Google Scholar]
- 6.Lawson KR, Ignatenko NA, Piazza GA, Cui H, Gerner EW. Influence of K-ras activation on the survival responses of Caco-2 cells to the chemopreventive agents sulindac and difluoromethylornithine. Cancer Epidemiol Biomarkers Prev. 2000;9:1155–1162. [PubMed] [Google Scholar]
- 7.Meyskens FL, Jr, McLaren CE, Pelot D, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: A randomized placebo controlled, double-blind trial. Cancer Prev Res. 2008;1 doi: 10.1158/1940-6207.CAPR-08-0042. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abeloff MD, Rosen ST, Luk GD, Baylin SB, Zeltzman M, Sjoerdsma A. Phase II trials of alpha-difluoromethylornithine, an inhibitor of polyamine synthesis, in advanced small cell lung cancer and colon cancer. Cancer Treat Rep. 1986;70:843–845. [PubMed] [Google Scholar]
- 9.Meyskens FL, Kingsley EM, Glattke T, Loescher L, Booth A. A phase II study of alpha-difluoromethylornithine (DFMO) for the treatment of metastatic melanoma. Invest New Drugs. 1986;4:257–262. doi: 10.1007/BF00179593. [DOI] [PubMed] [Google Scholar]
- 10.Croghan MK, Booth A, Meyskens FL., Jr A phase I trial of recombinant interferon-alpha and alpha-difluoromethylornithine in metastatic melanoma. J Biol Response Mod. 1988;7:409–415. [PubMed] [Google Scholar]
- 11.Croghan MK, Aickin MG, Meyskens FL. Dose-related alpha-difluoromethylornithine ototoxicity. Am J Clin Oncol. 1991;14:331–335. doi: 10.1097/00000421-199108000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Pasic TR, Heisey D, Love RR. alpha-difluoromethylornithine ototoxicity. Chemoprevention clinical trial results. Arch Otolaryngol Head Neck Surg. 1997;123:1281–1286. doi: 10.1001/archotol.1997.01900120031004. [DOI] [PubMed] [Google Scholar]
- 13.Doyle KJ, McLaren CE, Shanks JE, Galus CM, Meyskens FL. Effects of difluoromethylornithine chemoprevention on audiometry thresholds and otoacoustic emissions. Arch Otolaryngol Head Neck Surg. 2001;127:553–558. doi: 10.1001/archotol.127.5.553. [DOI] [PubMed] [Google Scholar]
- 14.Lao CD, Backoff P, Shotland LI, et al. Irreversible ototoxicity associated with difluoromethylornithine. Cancer Epidemiol Biomarkers Prev. 2004;13:1250–1252. [PubMed] [Google Scholar]
- 15.Morrell CH, Gordon-Salant S, Pearson JD, Brant LJ, Fozard JL. Age- and gender-specific reference ranges for hearing level and longitudinal changes in hearing level. J Acoust Soc Am. 1996;100:1949–1967. doi: 10.1121/1.417906. [DOI] [PubMed] [Google Scholar]
- 16.Campbell KC, Durrant J. Audiologic monitoring for ototoxicity. Otolaryngol Clin North Am. 1993;26:903–914. [PubMed] [Google Scholar]
- 17.Carhart R, Jerger J. Preferred method of clinical determination of pure-tone threholds. J Speech Hearing Dis. 1959;24:330–345. [Google Scholar]
- 18.American Speech-Language-Hearing Association. Guidelines for Manual Pure-Tone Threshold Audiometry [Guidelines] 2005 Available from www.asha.org/policy.
- 19.Munro KJ, Buttfield LM. Comparison of real-ear to coupler difference values in the right and left ear of adults using three earmold configurations. Ear Hear. 2005;26:290–298. doi: 10.1097/00003446-200506000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Perez-Abalo MC, Savio G, Torres A, Martin V, Rodriguez E, Galan L. Steady state responses to multiple amplitude-modulated tones: an optimized method to test frequency-specific thresholds in hearing-impaired children and normal-hearing subjects. Ear Hear. 2001;22:200–211. doi: 10.1097/00003446-200106000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Craven P, Wahba G. Smoothing Noisy Data with Spline Functions. Estimating the Correct Degree of Smoothing by the Method of Generalized Cross-Validation. Numerische Mathematik. 1979;31:377–403. [Google Scholar]
- 22.Grolman W, Tange RA, de Bruijn AJ, Hart AA, Schouwenburg PF. A retrospective study of the hearing results obtained after stapedotomy by the implantation of two Teflon pistons with a different diameter. Eur Arch Otorhinolaryngol. 1997;254:422–424. doi: 10.1007/BF02439972. [DOI] [PubMed] [Google Scholar]
- 23.Krishnan A. Human frequency-following responses: representation of steady-state synthetic vowels. Hear Res. 2002;166:192–201. doi: 10.1016/s0378-5955(02)00327-1. [DOI] [PubMed] [Google Scholar]
- 24.Mo L, Stapells DR. The effect of brief-tone stimulus duration on the brain stem auditory steady-state response. Ear Hear. 2008;29:121–133. doi: 10.1097/AUD.0b013e31815d6343. [DOI] [PubMed] [Google Scholar]
- 25.Stenstrom R, Pless IB, Bernard P. Hearing thresholds and tympanic membrane sequelae in children managed medically or surgically for otitis media with effusion. Arch Pediatr Adolesc Med. 2005;159:1151–1156. doi: 10.1001/archpedi.159.12.1151. [DOI] [PubMed] [Google Scholar]
- 26.Thai-Van H, Micheyl C, Norena A, Collet L. Local improvement in auditory frequency discrimination is associated with hearing-loss slope in subjects with cochlear damage. Brain. 2002;125:524–537. doi: 10.1093/brain/awf044. [DOI] [PubMed] [Google Scholar]
- 27.Ballinger GA. Using Generalized Estimating Equations for Longitudinal Data Analysis. Organizational Research Methods. 2004;7:127–150. [Google Scholar]
- 28.Zheng B. Summarizing the goodness of fit of generalized linear models for longitudinal data. Stat Med. 2000;19:1265–1275. doi: 10.1002/(sici)1097-0258(20000530)19:10<1265::aid-sim486>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 29.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Hoboken, New Jersey: John Wiley & Sons, Inc.; [Google Scholar]
- 30.Shotland LI, Ondrey FG, Mayo KA, Viner JL. Recommendations for cancer prevention trials using potentially ototoxic test agents. J Clin Oncol. 2001;19:1658–1663. doi: 10.1200/JCO.2001.19.6.1658. [DOI] [PubMed] [Google Scholar]
- 31.Overall JE, Tonidandel S. Robustness of Generalized Estimating Equation (GEE) Tests of Significance against Misspecification of the Error Structure Model. Biometrical Journal. 2004;46:203–213. [Google Scholar]
- 32.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–1060. [PubMed] [Google Scholar]
- 33.Jerger J, Shedd J, Harford E. On the detection of extremely small changes in sound intensity. Arch Otolaryngol. 1959;69:200–211. doi: 10.1001/archotol.1959.00730030206015. [DOI] [PubMed] [Google Scholar]
- 34.Schweitzer VG. Ototoxicity of chemotherapeutic agents. Otolaryngol Clin North Am. 1993;26:759–789. [PubMed] [Google Scholar]
- 35.Zell JA, McLaren CE, Gerner EW, Meyskens FL. Ornitine decarboxylase (ODC)-1 gene polymorphism effects on baseline tissue polyamine levels and adenoma recurrence in a radomized phase III adenoma prevention trial of DFMO + sulindac versus placebo. J Clin Oncol. 2008;26 abstract 1502. [Google Scholar]
- 36.Rosler G. Progression of hearing loss caused by occupational noise. Scand Audiol. 1994;23:13–37. doi: 10.3109/01050399409047483. [DOI] [PubMed] [Google Scholar]