Abstract
Escherichia coli O157:H7 causes Shiga toxin (Stx)-mediated vascular damage, resulting in hemorrhagic colitis and the hemolytic uremic syndrome in humans. These infections are often foodborne, and healthy carrier cattle are a major reservoir of E. coli O157:H7. We were interested in knowing why cattle are tolerant to infection with E. coli O157:H7. Cattle tissues were examined for the Stx receptor globotriaosylceramide (Gb3), for receptivity to Stx binding in vitro, and for susceptibility to the enterotoxic effects of Stx in vivo. TLC was used to detect Gb3 in tissues from a newborn calf. Gb3 was detected by TLC in kidney and brain, but not in the gastrointestinal tract. Immunohistochemistry was used to define binding of Stx1 and Stx2 overlaid onto sections from cattle tissues. Stx1 and Stx2 bound to selected tubules in the cortex of the kidney of both newborn calves (n = 3) and adult cattle (n = 3). Stx did not bind to blood vessels in any of the six gastrointestinal and five extraintestinal organs examined. The lack of Gb3 and of Stx receptivity in the gastrointestinal tract raised questions about the toxicity of Stx in bovine intestine. We found that neither viable E. coli O157:H7 nor Stx-containing bacterial extracts were enterotoxic (caused fluid accumulation) in ligated ileal loops in newborn calves. The lack of vascular receptors for Stx provides insight into why cattle are tolerant reservoir hosts for E. coli O157:H7.
Cattle are a major reservoir for enterohemorrhagic Escherichia coli O157:H7 strains, which cause hemorrhagic colitis and hemolytic uremic syndrome (HUS) in humans (1–3). Transmission of E. coli O157:H7 from cattle to humans occurs via foodborne and waterborne routes (3–5). Although E. coli O157:H7 strains occur in other animals, cattle have the highest known prevalence (1, 2, 6). Therefore, knowledge of the ecologic relationship between E. coli O157:H7 and its bovine reservoir host is critical to understanding the epidemiology of the disease and ultimately to the rational development of programs to eliminate the organism from cattle. E. coli O157:H7 produces Shiga toxins (Stx1 and Stx2) that inhibit protein synthesis, leading to necrosis and/or apoptosis of receptor-bearing, susceptible, microvascular endothelial cells (7). Stx-mediated vascular damage in colon and kidney is thought to lead, respectively, to hemorrhagic colitis and HUS (8, 9). In addition to Stx, E. coli O157:H7 has other virulence attributes, such as attaching and effacing activity and hemolysin production, which are thought to contribute to hemorrhagic colitis and HUS (2, 10).
In contrast to humans, in whom the organism causes severe illness, most E. coli O157:H7-infected cattle remain free of disease and are tolerant of E. coli O157:H7 for most of their lives (11, 12). However, E. coli O157:H7 does cause fatal ileocolitis in newborn calves (13). It is unknown why cattle are susceptible to E. coli O157:H7-induced disease as neonates but are tolerant carriers of the infection for most of their lives. In this regard it is interesting to note that, in contrast to humans, neither clinically affected calves nor older carrier cattle with E. coli O157:H7 infections develop extraintestinal vascular lesions (11–13). Furthermore, calves clinically infected with other serotypes of Stx-producing E. coli do not develop extraintestinal vascular lesions or systemic manifestations of disease (14–16). The tolerance of adult cattle for E. coli O157:H7 infection and the lack of systemic vascular damage in calves and adult cattle with E. coli O157:H7 infection led us to hypothesize that cattle lack receptors for Stx. One alternative but related hypothesis is that newborn calves have receptors for Stx, which are confined to the intestine and disappear with age. If correct, these hypotheses would provide insight into why adult cattle are tolerant reservoir hosts for E. coli O157:H7.
The preferred receptor for Stx1 and Stx2 is globotriaosylceramide (Gb3) (17, 18). The preferred receptor for Stx2e (a variant of Stx2 produced by strains of E. coli that cause edema disease of swine) is globotetraosylceramide (17, 18). Humans, rabbits, and pigs have vascular receptors for Stx and develop Stx-mediated vascular damage (8, 17, 19–22). Rabbits also have Stx receptors on intestinal villous epithelial cells, and Stx is enterotoxic (impairs NaCl absorption, causing intestinal fluid accumulation and diarrhea) in rabbits (23, 24).
The objectives of this study were to (i) assay tissues from a newborn calf for Gb3 expression, (ii) localize the binding of Stx overlaid onto tissue sections from newborn calves and adult cattle, and (iii) determine if Stx is enterotoxic in the ileum of newborn calves.
Materials and Methods
Animals.
Tissues from healthy colostrum-deprived newborn (<24 h old) male calves were used for Gb3 analysis (n = 1 calf) or immunohistochemical assays for Stx binding (n = 3 calves). Seven other healthy colostrum-deprived newborn male calves were fed antibiotic-free milk replacer and used in intestinal loop assays for enterotoxigenicity. Tissues from three healthy 2-year-old females (adult cattle) were used in immunohistochemical assays for Stx binding.
Bacterial Strains and Culture Conditions.
Enterohemorrhagic Escherichia coli O157:H7 strain 933 (American Type Culture Collection 43895) was provided by Alison O'Brien (25). A spontaneous streptomycin-resistant mutant of strain 933 was also used (26). Strain 933 produces Stx1 and Stx2, hybridizes to probes for eae (one of the genes encoding attaching/effacing activity) and CVD419 (a plasmid carrying genes for hemolysin) (10). E. coli 123 (serogroup O43:H28) is nonpathogenic, does not produce Stx, and is probe negative for eae and CVD419. Stock inocula containing approximately 109 colony-forming units per ml were prepared as described (27) and stored in 1.5-ml aliquots at −80°C until used.
Toxin Preparation.
Crude extracts containing Stx1 or Stx2 were produced from E. coli DH5α/pCKS112 and DH5α/pJES120, respectively (18, 28). The Stx-deficient strain DH5α was used as a control. All three strains were grown in Tryptic Soy Broth (Difco) for 18 h at 37°C on the shaker (200 rpm). Tryptic Soy Broth was supplemented with ampicillin (200 μg/ml) for E. coli DH5α/pCKS112 and DH5α/pJES120. Bacteria were subcultured for another 18 h in similar conditions. Bacterial cultures were centrifuged at 7,600 × g for 30 min at 4°C. Bacteria from 500 ml of culture were resuspended in 50 ml of PBS (pH 7.4) and sonicated three times for 1 min (total of 3 min, Power 7, alternative pulse) (heat systems and cell disruptor, model W-225R; Ultrasonics, Farmingdale, NY). The tubes containing the bacteria were held on ice after each sonication. Sonicates were clarified by centrifugation at 7,600 × g for 30 min at 4°C. Supernatants were dialyzed once against PBS (40 ml supernatant/4,000 ml PBS) overnight at 4°C in a Slide-A-Lyzer cassette (10,000 Da molecular mass cutoff), filtered through a 0.22-μm filter, and stored at −80°C. The crude extracts containing Stx were tested for cytotoxicity (Table 1) on Vero cells as described (18, 29). Purified Stx1 (0.6 μg/ml, 1 × 107 CD50/ml) was produced as reported (28).
Table 1.
Colony-forming units of E. coli and toxin titers (CD50/ml in Vero cell assays) of Stx containing crude extracts used, as inocula for ileal loops
| Strains and toxin crude extracts | Colony-forming units per milliliter* | Toxin (CD50/ml) |
|---|---|---|
| E. coli strain 933 (O157∶H7, Stx1+, Stx2+) | 3 × 108 | NA† |
| Streptomycin-resistant mutant of E. coli strain 933 | 5 × 108 | NA |
| E. coli strain 123 (non pathogenic) | 7 × 109 | NA |
| Stx1+ crude extract | NA | 1 × 108 |
| Stx2+ crude extract | NA | 1 × 107 |
| Stx− crude extract (negative control) | NA | 1 × 103 |
Viable E. coli.
NA, not applicable.
Antibodies.
Monoclonal mouse anti-Stx1 (13C4) and anti-Stx2 (11E10) IgG specific for the B subunit of the toxin were produced as reported (30, 31).
Glycolipid (Gb3) Assay.
Total lipid extracts from tissues of a newborn calf and purified glycolipid standards were processed on aluminum-backed silica TLC (TLC) plates (Silica Gel 60; Merck) with CHCl3-CH3OH-aqueous KCl (5:4:1) as described (18, 32). After chromatographic separation of glycolipids, the plates were coated with 0.1% polyisobutylmethacrylate (Polysciences), air dried, and sprayed with Tris-buffered saline–BSA [0.1 M Tris⋅HCl (pH 7.4), 0.15 M NaCl, 1% BSA)]. The plates were treated with 125I-labeled purified Stx-1 in Tris-buffered saline–BSA at 37°C for 2 h. After extensive washing, the plates were dried, and specific toxin binding to glycolipids was visualized by Molecular Dynamics analysis. Relative amounts of toxin-binding glycolipids in the extracts were estimated by comparison with serial dilutions of purified Gb3 separated on the same plates by densitometric scanning of images using image analysis software (is-1000 image analysis; Innotech Corp., San Leandro, CA).
Immunohistochemical Assay for Stx Binding.
Tissues were obtained from three newborn calves and three adult cows. Tissues were immediately embedded in OCT (Miles), frozen in liquid nitrogen, and then stored at −80°C. Six-micrometer frozen sections were cut and fixed in acetone at −20°C for 10 min to permeabilize the cellular membrane for antibody penetration. Sections were incubated for 10 min at 4°C in a cryoprotective storage medium (42.8 g sucrose/0.33 g anhydrous MgCl2/250 ml PBS, pH 7.2/250 ml glycerol) and stored at −80°C until they were used.
For in vitro binding of Stx, slides were rehydrated for 30 min in 0.1 M Tris⋅HCl at pH 7.8 (Tris) at 20°C. Sections were incubated for 2 min in cold (4°C) glacial acetic acid (20%) in PBS to block endogenous alkaline phosphatase. Tissues were rinsed extensively with Tris. Tissues were incubated with 200 μl of a 1:120 dilution of the purified Stx1 or 400 μl of the Stx2 crude extract for 1 h at 37°C in a humidified chamber. Sections were rinsed extensively with Tris. A modification of a biotin/streptavidin alkaline phosphatase detection system for mouse IgG (Histomark Biotin/Streptavidin Kit AP System; Kirkegaard & Perry Laboratories) was used. Briefly, slides were (i) covered with normal goat serum and incubated for 30 min; (ii) incubated with a 1:600 or 1:200 dilution of the ascites fluids containing the monoclonal 13C4 (anti-Stx1) or 11E10 (anti-Stx2) IgG, respectively, in Tris supplemented with 3% BSA for 1 h; (iii) rinsed extensively with Tris; (iv) incubated with biotinylated goat anti mouse IgG (H + L) for 1 h; (v) washed extensively in Tris; (vi) covered with streptavidin-phosphatase for 30 min; and (vii) rinsed extensively with Tris. All incubations took place at 37°C in a humidified chamber. Color was developed using Histomark Red (Kirkegaard & Perry) according to the instructions provided by the manufacturer. To assess nonspecific reactivity, negative control sections (no Stx exposure) were prepared from all tissues of all calves and adult cattle examined via immunohistochemistry. Sections of ileum from 6-week-old pigs were used as positive controls (17, 21).
Intestinal Loop Surgery and Enterotoxicity.
Newborn calves (n = 7) were given an i.v. preanesthetic treatment: guaifenesin (2.5 g), ketamine (50 mg), and xylaxine (2 mg) in 5% dextrose and 0.9% NaCl. Calves were anesthetized by inhalation of isoflurane. A laparotomy was performed, and 12 ligated loops per calf were created, starting 1 m cranial to the ileocecal valve (33). Loops were 30 cm long and alternated with 15-cm interloops. Two loops per calf were randomly inoculated with each of the following materials: 20 ml of Stx1 crude extract or Stx2 crude extract or Stx-deficient crude extract (negative control from strain DH5α), or 1.5 ml of bacterial culture containing E. coli strain 933 or streptomycin-resistant E. coli strain 933 or the nonpathogenic E. coli strain 123 (Table 1). Calves were allowed to recover and were given an analgesic (2.2 mg/kg of Banamine as the initial dose, followed by 1.1 mg/kg as a maintenance dose 8 h later). Calves were euthanized by i.v. injection of sodium pentobarbital 18 h after surgery. Enterotoxicity was evaluated by observation at necropsy for accumulation of fluid in the intestinal loops (33).
Results
Glycolipid (Gb3) Assay.
Glycolipids were extracted from the kidney, ileum, cecum, rectum, brainstem, cerebrum, and cerebellum of a newborn calf. Glycolipid levels were analyzed to determine whether a minimum concentration of Gb3 receptor (25 pmol/mg tissue) was present in each extract. Kidney, brainstem, cerebrum, and cerebellum all contained more than 25 pmol Gb3/mg tissue. Thus cattle do produce the preferred receptor for Stx. However, ileum, cecum, and rectum did not contain detectable amounts of this receptor. The extraction and quantitation of Stx-1-binding glycolipids were performed in two independent experiments, and the results were the same for the two experiments. The TLC assay used here routinely detects as little as 10 pmol Gb3/mg tissue (32), and the negative tissues in this assay (ileum, cecum, and rectum) did not express even this minimum amount of receptor.
Stx Binding to Tissues.
Frozen tissue sections from cerebrum, lung, liver, spleen, kidney, abomasum, jejunum, proximal ileum, distal ileum, cecum, proximal colon, spiral colon, distal colon, and rectum from calves (n = 3) and adult cattle (n = 3) were incubated with either purified Stx1 or Stx2 crude extract. Immunohistochemistry was used to detect bound Stx. Stx1 and Stx2 binding was detected in renal tubular epithelium, but not in any of the other bovine tissues. Thus, except for cerebrum, immunohistochemical detection of Stx binding to tissue sections correlated with Gb3 production detected by TLC. Vascular binding of Stx was not detected in any of the bovine tissues.
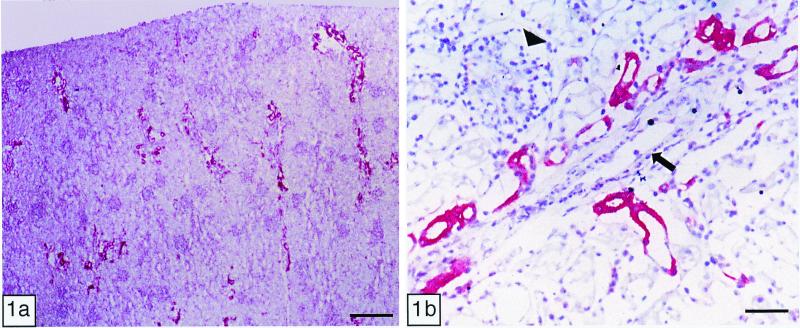
In calves, Stx1 and Stx2 bound to convoluted tubules and collecting ducts within the renal cortex, but rarely to the medulla (Fig. 1a). The binding was particularly intense on convoluted tubules near glomeruli. Stx binding to epithelial cells occurred at the cell membrane (luminal and adluminal face) and/or within the cytoplasm. Stx binding was similar in location, but less intense in the kidneys of adults. Stx binding to glomerular or extraglomerular blood vessels from either age group was not detected (Fig. 1b).
Figure 1.
Immunohistochemistry of Stx1 binding in vitro: newborn calf kidney. (a) Binding of Stx1 was detected mainly in the cortex. (Bar = 400 μm.) (b) Within the renal cortex, Stx1 bound multifocally to convoluted tubules and collecting ducts. No Stx1 binding to glomeruli (arrowhead) or blood vessels (arrow) was observed. (Bar = 70 μm.)
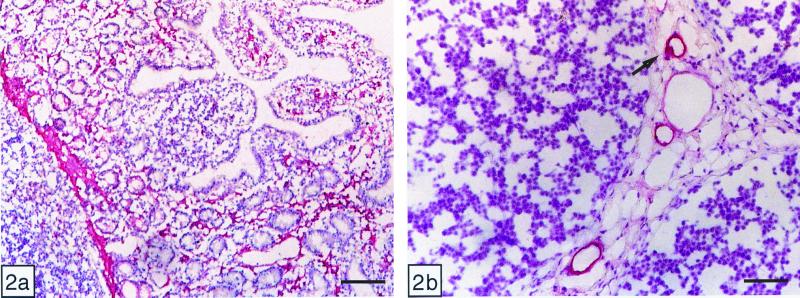
Nonspecific reactivity, assessed by omission of the incubation with Stx (negative control), was not observed in any of the tissues. Positive controls for Stx binding were sections of pig ileum (Fig. 2). The results were similar to those reported by others for Stx2e binding (21). In contrast to the pattern observed in cattle, both Stx1 and Stx2 bound to capillaries and mononuclear cells within the lamina propria, to smooth muscle cells of the muscularis mucosae, and to endothelial and smooth muscle cells of blood vessels within the lamina propria and submucosa. There was also moderate Stx binding to some crypt enterocytes.
Figure 2.
Immunohistochemistry of Stx1 binding in vitro: pig ileum (positive control). (a) Mucosa: Stx1 bound to crypt enterocytes, mononuclear cells, smooth muscle cells (muscularis mucosa), and capillaries. (Bar = 160 μm.) (b) Peyer's patches: Stx1 bound to endothelial and smooth muscle cells of small and medium-sized blood vessels. (Bar = 70 μm.)
Enterotoxicity.
Fluid accumulation was not observed in any of the ileal loops examined 18 h after inoculation.
Discussion
Escherichia coli O157:H7 is a major cause of HUS in children and the elderly (3, 34). HUS is characterized by occlusion of the renal glomerular microvasculature with fibrin and platelets (8). In human kidneys, Gb3 is principally expressed in the cortex, and Gb3 expression is higher in adults than in children (19). Stx binds to distal convoluted tubules and collecting ducts of the juxtaglomerular region in both infants and adults, but more intensely in the latter (35). However, in children, Stx also binds to glomerular and occasionally extraglomerular capillaries. This differential localization of Stx binding to the pediatric renal glomerulus may explain the comparatively high susceptibility of children to HUS. Our results demonstrated that in calves and adult cattle, renal binding of Stx was similar in location to that of adult humans. Stx binding to glomeruli or extraglomerular blood capillaries, as occurs in children, was not demonstrated in either newborn or adult cattle.
Glycolipid analysis of calf tissues by TLC demonstrated Gb3 in kidney and brain but not ileum or large intestine. These results were consistent with the assumption that the receptivity of renal tubules demonstrated via immunohistochemistry was mediated by Gb3. The TLC results are also consistent with the immunohistochemical data indicating a lack of toxin binding to sections from ileum and large intestine. It is interesting, however, that we were unable to detect toxin binding to sections from cerebrum, despite clear TLC evidence that cerebrum contained Gb3. We speculate that immunohistochemistry may have been less sensitive than TLC. If Gb3 were diffusely distributed in cerebrum, rather than concentrated in a subpopulation of cells, as appeared to be the case in kidney, one would expect immunohistochemistry to be comparatively less sensitive than TLC. The lipid matrix and manner in which the Gal 1–4 Gal binding site of Gb3 is presented influences the binding of toxin to the Gb3 glycolipid (36). Thus it is conceivable that the membrane lipids in the sections of cerebrum used for immunohistochemistry altered the presentation of Gal 1–4 Gal by Gb3, inhibiting toxin binding, yet when glycolipid was extracted and separated on TLC, the presentation of this disaccharide favored Stx binding.
Stx1 and Stx2 binding to blood vessels was not demonstrated in the kidneys, gastrointestinal tract, or any of the other bovine tissues examined. This finding is in contrast to previous studies of Stx binding in rabbits, pigs, and humans (20–22, 35). In rabbits Stx1 binds to the microvasculature of the gastrointestinal tract and central nervous system, with subsequent endothelial injury and microvascular thrombosis of the affected organs. Lesions do not develop in organs where Gb3 expression is lacking. In pigs, Stx2e binds to endothelial cells and smooth muscle cells of arteries and veins in organs that are usually affected by the disease and to enterocytes in the ileal crypts (21). We found that Stx1 and Stx2 bound to blood vessels in the sections of pig ileum used as positive controls in the current study (Fig. 2).
Neither viable Stx-producing E. coli nor Stx-containing extracts caused fluid accumulation in the ileum of newborn calves. In contrast, Stx doses of 104 to 105 CD50 per loop cause fluid accumulation in ligated ileal loops in rabbits (37). However, we found that Stx doses of 108 to 109 CD50 per loop did not cause fluid accumulation in ileal loops of calves. Thus calves are apparently at least 1000-fold more resistant to the enterotoxic effects of Stx than are rabbits. This lack of enterotoxicity may be due to the lack of Stx binding to enterocytes and blood vessels within the calf ileum. In aggregate, the results are consistent with prior evidence suggesting that Stx is not required for E. coli O157:H7 to be pathogenic in neonatal calves (38).
Our results demonstrated Stx binding and/or Gb3 expression in selected renal tubules and brain. However, there is no evidence that these tissues are damaged in calves or adult cattle infected with E. coli O157:H7 or other Stx-producing E. coli (11–16). It would be interesting to determine whether this apparent lack of effect is due to the fact that these tissues are not exposed to toxin during infection, or that they do not bind toxin in vivo, or that they are resistant to the toxic effects of Stx that follow binding (36), or that damage to these tissues occurs but has not been recognized.
The absence of systemic vascular lesions in newborn and adult cattle infected with E. coli O157:H7, combined with the lack of immunohistochemically detectable vascular receptors for Stx reported here, leads us to suspect that cattle of all ages are inherently resistant to Stx. Studies to determine the susceptibility of cattle to intravenously administered Stx are needed. Current evidence is consistent with the hypothesis that species differences in the tissue level of Gb3 expression and Stx binding explain, in part, why E. coli O157:H7 infections cause hemorrhagic colitis and HUS in humans but usually remain asymptomatic in the bovine reservoir host.
Acknowledgments
We are grateful to S. Booher, I. Matise, and P. Navarro for technical assistance and helpful discussions. This work was supported in part by the Frank K. Ramsey endowment and Grant R0IA141328 from the National Institutes of Health.
Abbreviations
- Stx
Shiga toxin
- HUS
hemolytic uremic syndrome
- Gb3
globotriaosylceramide
- TLC
thin layer chromatography
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.190329997.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.190329997
References
- 1.Elder R O, Keen J E, Siragusa G R, Barkocy-Gallagher G A, Koohmaraie M, Laegreid W W. Proc Natl Acad Sci USA. 2000;97:2999–3003. doi: 10.1073/pnas.060024897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gansheroff L J, O'Brien A D. Proc Natl Acad Sci USA. 2000;97:2959–2961. doi: 10.1073/pnas.97.7.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffin P M, Tauxe R V. Epidemiol Rev. 1991;13:60–98. doi: 10.1093/oxfordjournals.epirev.a036079. [DOI] [PubMed] [Google Scholar]
- 4.Hancock D D, Besser T E, Rice D H. In: Escherichia coli O157:H7 and Other Shiga Toxin-Producing E. coli Strains. Kaper J B, O'Brien A D, editors. Washington, DC: Am. Soc. Microbiol.; 1998. pp. 85–91. [Google Scholar]
- 5.Pebody R G, Furtado C, Rojas A, McCarthy N, Nylen G, Ruutu P, Leino T, Chalmers R, de Jong B, Donnelly M, et al. Epidemiol Infect. 1999;123:217–223. doi: 10.1017/s0950268899002940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beutin L, Geier D, Steinruck H, Zimmermann S, Scheutz F. J Clin Microbiol. 1993;31:2483–2488. doi: 10.1128/jcm.31.9.2483-2488.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Obrig T G. In: Escherichia coli O157:H7 and Other Shiga Toxin-Producing E. coli Strains. Kaper J B, O'Brien A D, editors. Washington, DC: Am. Soc. Microbiol.; 1998. pp. 303–311. [Google Scholar]
- 8.Richardson S E, Karmali M A, Becker L E, Smith C R. Hum Pathol. 1988;19:1102–1108. doi: 10.1016/s0046-8177(88)80093-5. [DOI] [PubMed] [Google Scholar]
- 9.Kelly J K, Oryshak A, Wenetsek M, Grabiec J, Handy S. Am J Surg Pathol. 1990;14:87–92. doi: 10.1097/00000478-199001000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Tzipori S, Karch H, Wachsmuth K I, Robins-Browne R M, O'Brien A D, Lior H, Cohen M L, Smithers J, Levine M M. Infect Immun. 1987;55:3117–3125. doi: 10.1128/iai.55.12.3117-3125.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cray W C, Jr, Moon H W. Appl Environ Microbiol. 1995;61:1586–1590. doi: 10.1128/aem.61.4.1586-1590.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown C A, Harmon B G, Zhao T, Doyle M P. Appl Environ Microbiol. 1997;63:27–32. doi: 10.1128/aem.63.1.27-32.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dean-Nystrom E A, Bosworth B T, Cray W C, Jr, Moon H W. Infect Immun. 1997;65:1842–1848. doi: 10.1128/iai.65.5.1842-1848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall G A, Reynolds D J, Chanter N, Morgan J H, Parsons K R, Debney T G, Bland A P, Bridger J C. Vet Pathol. 1985;22:156–163. doi: 10.1177/030098588502200210. [DOI] [PubMed] [Google Scholar]
- 15.Moxley R A, Francis D H. Infect Immun. 1986;53:339–346. doi: 10.1128/iai.53.2.339-346.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoonderwoerd M, Clarke R C, van Dreumel A A, Rawluk S A. Can J Vet Res. 1988;52:484–487. [PMC free article] [PubMed] [Google Scholar]
- 17.Boyd B, Tyrrell G, Maloney M, Gyles C, Brunton J, Lingwood C. J Exp Med. 1993;177:1745–1753. doi: 10.1084/jem.177.6.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samuel J E, Perera L P, Ward S, O'Brien A D, Ginsburg V, Krivan H C. Infect Immun. 1990;58:611–618. doi: 10.1128/iai.58.3.611-618.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyd B, Lingwood C A. Nephron. 1989;51:207–210. doi: 10.1159/000185286. [DOI] [PubMed] [Google Scholar]
- 20.Richardson S E, Rotman T A, Jay V, Smith C R, Becker L E, Petric M, Olivieri N F, Karmali M A. Infect Immun. 1992;60:4154–4167. doi: 10.1128/iai.60.10.4154-4167.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waddell T E, Coomber B L, Gyles C L. Can J Vet Res. 1998;62:81–86. [PMC free article] [PubMed] [Google Scholar]
- 22.Zoja C, Corna D, Farina C, Sacchi G, Lingwood C, Doyle M P, Padhye V V, Abbate M, Remuzzi G. J Lab Clin Med. 1992;120:229–238. [PubMed] [Google Scholar]
- 23.Kandel G, Donohue-Rolfe A, Donowitz M, Keusch G T. J Clin Invest. 1989;84:1509–1517. doi: 10.1172/JCI114327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pai C H, Kelly J K, Meyers G L. Infect Immun. 1986;51:16–23. doi: 10.1128/iai.51.1.16-23.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Brien A D, Melton A R, Schmitt C K, McKee M L, Batts M L, Griffin D E. J Clin Microbiol. 1993;31:2799–2801. doi: 10.1128/jcm.31.10.2799-2801.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindgren S W, Melton A R, O'Brien A D. Infect Immun. 1993;61:3832–3842. doi: 10.1128/iai.61.9.3832-3842.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarmiento J I, Casey T A, Moon H W. Am J Vet Res. 1988;49:1154–1159. [PubMed] [Google Scholar]
- 28.Tesh V L, Ramegowda B, Samuel J E. Infect Immun. 1994;62:5085–5094. doi: 10.1128/iai.62.11.5085-5094.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gentry M K, Dalrymple J M. J Clin Microbiol. 1980;12:361–366. doi: 10.1128/jcm.12.3.361-366.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perera L P, Marques L R, O'Brien A D. J Clin Microbiol. 1988;26:2127–2131. doi: 10.1128/jcm.26.10.2127-2131.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strockbine N A, Marques L R, Holmes R K, O'Brien A D. Infect Immun. 1985;50:695–700. doi: 10.1128/iai.50.3.695-700.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramegowda B, Samuel J E, Tesh V L. J Infect Dis. 1999;180:1205–1213. doi: 10.1086/314982. [DOI] [PubMed] [Google Scholar]
- 33.Smith H W, Halls S. J Pathol Bacteriol. 1967;93:499–529. doi: 10.1002/path.1700930211. [DOI] [PubMed] [Google Scholar]
- 34.Rowe P C, Orrbine E, Wells G A, McLaine P N. J Pediatr. 1991;19:218–222. doi: 10.1016/s0022-3476(05)80730-9. [DOI] [PubMed] [Google Scholar]
- 35.Lingwood C A. Nephron. 1994;66:21–28. doi: 10.1159/000187761. [DOI] [PubMed] [Google Scholar]
- 36.Lingwood C A. Trends Microbiol. 1996;4:147–153. doi: 10.1016/0966-842x(96)10017-2. [DOI] [PubMed] [Google Scholar]
- 37.Keenan K P, Sharpnack D D, Collins H, Formal S B, O'Brien A D. Am J Pathol. 1986;125:69–80. [PMC free article] [PubMed] [Google Scholar]
- 38.Dean-Nystrom E A, Bosworth B T, O'Brien A D, Moon H W. In: Escherichia coli O157:H7 in Farm Animals. Stewart C S, Flint H J, editors. Wallingford, U.K.: CABI Publishing; 1999. pp. 51–58. [Google Scholar]