Abstract
Stormwater particles often provide transport for metals and other contaminants, however only larger particles are effectively removed by typical best management practices. Fine particles and their associated constituents are more likely to reach receiving waters; this merits further investigation regarding the metal contribution of fine (dp<10 μm) and very fine (dp <1.5 μm) particles. Road associated particles were collected by vacuuming a road surface and by collecting highway stormwater runoff. A cell sorter was employed to sort road associated particles into four size ranges: 0.1–0.3, 0.3–0.5, 0.5–1.0, and 1.0–1.5 μm. These very fine particles, along with six particle size ranges (total range <2–63 μm) separated using a settling column, were analyzed for Al, Mn, Fe, Cr, Ni, Cu, Zn, and Pb using Inductively Coupled Plasma Mass Spectrometry (ICP-MS). Enrichment factors (EFs), calculated using Al as a basis to represent crustal contributions, were similar for the vacuumed road dust and the stormwater runoff. Fe and Mn were minimally depleted (0.1x) or near unity for all size ranges (Fe EF range 0.01–3.7; Mn EF range 0.02–10.6). Cr, Ni, Cu, Zn, and Pb were moderately (10x) to considerably (>100x) enriched for most size ranges; these metals were most enriched in the very fine fractions (max EF~4900 in Zn, 0.1–0.3 μm). Based on this preliminary study, a cell sorter is an acceptable means of fractionating aqueous particles of diameter 0.1–1.5 μm. In spite of their minimal relative mass contribution, the very fine particles are environmentally relevant due to their mobility and enrichment in potentially toxic metals..
Keywords: stormwater, particles, metals, enrichment factor
1. Introduction
Particles are ubiquitous in the environment and serve as vectors for metals in atmospheric and aquatic compartments. Above the North Sea, ten percent of rainwater particles (wet deposition) contained detectable heavy metals and this fraction was expected to increase over-land due to terrestrial sources (Jambers et al., 2000). Resuspension by Los Angeles Interstate 405 significantly increased atmospheric particle numbers and metal concentrations, however both returned to urban background levels via dry deposition within 100–150 m of the highway (Sabin et al., 2006). Both dry and wet deposition processes contribute particle bound metals to the aquatic environment, for example via stormwater (e.g., Tuccillo, 2006; Sansalone, 1996).
In a review of stormwater and sediment toxicity, urban stormwater toxicity was significantly attributed to metals through Toxicity Identification Evaluations (TIE) (Kayhanian et al., 2008; Burton et al., 2000). Multi-lane divided highway stormwater was considered severely toxic in 20% of evaluated cases, compared to only 1% of urban stormwater (Marsalek et al., 1999). Metal bioavailability, which is often correlated with toxicity, increased with decreasing particle size (Preciado and Li, 2006).
Particle size affects particle fate in an aqueous environment via its effect on dissolution, entrainment, and settling processes. Temperature, pH, and specific surface area (surface area/mass = 4πr2/mass = 3/ρr assuming spherical particles of radius r and density ρ) can each affect the equilibrium rate of particle dissolution. Particle settling is described by Stoke’s Law:
| Eq. 1 |
Where vst is the particle’s terminal settling velocity, g is gravitational acceleration, ρp and dp are the particle density and diameter respectively, ρw is water’s density, and CD is the drag coefficient (spherical particle assumed; CD= 24/NR where NR is the Reynold’s number). All other parameters being equal, small particles contribute more constituents per unit mass to the dissolved phase, require less critical tractive force to become entrained (Chien, 1954), and settle more slowly.
Background urban atmospheric particles contained over 50% total metal in the smallest size fraction measured (dp<6 μm) (Sabin et al., 2006). Atmospheric particles were 86.9% insoluble as metallic oxides, while the soluble fraction was predominantly organic (Ghio et al., 1999). A compilation of five studies on stormwater particle size distribution (PSD) and their associated metals revealed that metal concentrations increase with decreasing size (Li et al., 2006) which has also been observed by others (Herngren et al., 2006; Lin et al., 2005). Fine particles (dp<20 μm) settled more slowly than Stoke’s Law (Equation 1) predicted. The error increased with decreasing particle size and was attributed to electrostatic repulsion, density, and shape factors (Backstrom, 2002). Particle size affected best management practice (BMP) removal efficiencies. Vegetative filter strips effectively removed particles dp>8 μm (Han et al., 2005) and grass swales removed a majority of particles dp>13.5 μm (Backstrom, 2002). Simulated double compartment settling tank conditions revealed that tank sizing significantly affected particle removals; if the 33rd percentile storm were used to size the 24 hour storage tank, ~45% of particles 2<dp<10 μm were removed while almost all particles dp>40 μm were removed (Li et al., 2006). Petterson determined that particles with dp<10 μm were predominantly responsible for detention pond effluent specific surface area (Pettersson, 1998).
Air quality research has often included fractionation into sub-micron categories for compositional analysis and source characterization/apportionment (e.g., Birmili et al., 2006; Kupiainen et al., 2005; Lin et al., 2005; Zhou et al., 2005). While similar work has been conducted in water and stormwater research, the size-fractionation approach has not included very fine particles (dp<1.5 μm) to the same degree. Fergusson and Ryan (1984) investigated size fractionated (33<dp<963 μm) elemental composition for road dust from five cities. Large cities contained predominantly “non-crustal” elements while smaller cities were dominated by crustal contributions but most trace element concentrations increased with decreasing particle size. Westerlund and Viklander (2006) studied stormwater runoff PSD (4 to 120 μm) and the associated elemental composition for the snow and rainy seasons. The 6–9 μm snowmelt particle fraction contained the highest concentration of heavy metals; the 4–6 μm fraction contained the highest heavy metal concentration in the rain runoff. Tuccillo (2006) found stormwater Cu and Zn in the dissolved phase and in particles dp>5 μm, Pb and Cr in dp >5 μm, and Fe, Al, and Si in dp > 0.45 μm.
The objective of this study was to present a method to measure size-resolved particle-associated (0.1<dp<63 μm) metal content. This method was then used to examine the metal burden associated with vacuumed road dust and highway stormwater runoff particles with particular emphasis placed on those particles that are less likely to be removed by BMPs (e.g., particles with dp<10 μm).
2. Materials and Methods
2.1 Sample Collection
Samples were collected from two separate sources: vacuumed road dust from four low average daily traffic (ADT) roads and five storms from one high ADT highway site. Four road sample collection areas with similar average daily traffic (ADT) loads were identified in two California communities with four different adjacent land usage patterns: agricultural (posted speed limit 25 mph; 3,214 ADT) and commercial (45 mph, 1,943 ADT) in Davis, CA (pop. 60,000); industrial (35 mph, 4,246 ADT) and residential (45 mph, 5,502 ADT) in Stockton, CA (pop. 270,000).The California Air Resources Board (2003) estimated the following diesel particulate matter emissions: Yolo County (Davis) had 320 tons year−1 in 2003 (0.316 tons mi−2 year−1); San Joaquin county (Stockton) had 734 tons year−1 in 2003 (0.535 tons mi−2 year−1). Bradford et al. (1996) determined background concentrations in soils using uncontaminated field samples, composite surface to 0.5m depth. Davis’s representative soil was collected southwest of Dixon, CA (roughly 10 miles from Davis) and Stockton’s sample was collected northwest of Stockton. Elemental concentrations are shown in Table 1. Davis soil is enriched in Cr and Ni due to ultramafic-rock derived serpentine soils in the coast range mountain watershed.
Table 1.
Representative soil composition
| Location | Al % | Mn mg kg−1 | Fe% | Cr mg kg−1 | Ni mg kg−1 | Cu mg kg−1 | Zn mg kg− | Pb mg kg−1 |
|---|---|---|---|---|---|---|---|---|
| Davis (Dixon) | 7.5 | 674 | 4.5 | 397 | 212 | 41.5 | 119 | 18.9 |
| Stockton | 6.6 | 449 | 2 | 26 | 12 | 13.7 | 182 | 21.3 |
Road dust samples were collected according to EPA AP-42, Appendices C1 and C2 (1993a; 1993b). Briefly, at half mile intervals, one-foot wide strips of road, perpendicular to the flow of traffic, were identified for sample collection. Large, loose materials were collected by slow sweeping to avoid stirring-up finer particles, and then finer particles were vacuumed using a handheld portable vacuum into a pre-weighed filter. Only the area over which the vehicle wheels and carriage would pass was sampled. Filter bags were sealed by folding over the unused portion of the bag and binding with a rubber band. Incremental samples from the same road were combined and stored in glass jars with polyethylene screw caps. Cumulative swept samples were at least 400 g and cumulative vacuum samples were at least 200 g; filled vacuum filter mass was 3–5 times that of an empty filter. Road dust samples were dried overnight at 110°C to determine percent moisture and sieved through a 75 μm sieve to determine percent silt. Known samples were subjected to identical treatment to determine losses for each step; reported results include loss corrections. The vacuumed road particles were then mixed at 10mg mL−1 with a solution containing 20mM ammonium acetate (ICN Biochemicals, Irvine, CA, USA) and 20mM acetic acid (glacial, Seastar Chemicals, Burnaby, BC, Canada, baseline grade; pH~4.7) to simulate the conditions in stormwater using a metal-free synthetic rain; this suspension was used in all further analyses. Samples were inverted, let stand for one hour, inverted again, and stored for 6 hours. All storage periods were completed at 4°C in the dark; samples were subsequently processed promptly for further analysis.
Stormwater samples were collected as grab samples from a stormwater drainage along westbound Interstate 80 near Davis, CA (65 mph posted speed, 127,000 ADT, drainage area ~ 2000 m2). Suspended and dissolved solids concentrations and particle size distribution have been observed to vary during the course of an event (Sansalone et al., 1998; Li et al., 2005). The grab samples provided a “snapshot” of that variability, while the vacuumed road dust samples served as composite sample. Samples were collected and stored in glass jars at 4°C in the dark. The five storms included in this study are listed in Table 2. Rainfall was measured at a nearby USGS weather station and runoff volume was calculated assuming 100% imperviousness.
Table 2.
Storm characteristics
| Date | Total Rainfall (mm) | Antecedent Dry Period (days) | Runoff Volume (L) |
|---|---|---|---|
| 12/6/2003 | 9.6 | 18 | 4,719 |
| 12/10/2003 | 8.1 | 2 | 3,418 |
| 12/12/2003 | 2.0 | 1 | 470 |
| 2/2/2004 | 16.0 | 25 | 21,886 |
| 2/25/2003 | 47.5 | 22 | 73,422 |
2.2 Particle Size Distribution (PSD)
Suspended road dust and stormwater particle size distributions were determined using a laser-based optical particle counter (LiQuilaz®-S05-HF, Particle Measuring Systems, Inc., Boulder, Colorado) which quantified particle numbers mL−1 for 15 user defined size bins from 0.5–20 μm. Each size bin was denoted by its minimum diameter and extended up to the next bin size, such that the largest bin included all particles 20 μm and greater. Samples with total counts (total number mL−1 for all size bins) greater than 7,000 were appropriately diluted with double dieonized water (Milli-Q A10, Millipore, Billerica, MA, USA) to prevent undercounting of finer particles as described in the instrument manual. Particle size distributions were determined within one week of sample collection. Li et al. (2005) observed aggregation in samples held longer than 6 hrs, therefore the PSDs measured in this study probably do not represent the original PSDs.
2.3 Particle Fractionation
Samples were size-fractionated by two different methods: a cell sorter, also known as a flow cytometer, for particles of diameter 0.1–1.5 μm and a settling column for particles of 2–63 μm. After settling overnight to remove large particles, a flow cytometer (DykoCytomation MoFlo Cell Sorter) was employed to fractionate and count the very fine particles into four size bins: 0.1–0.3, 0.3–0.5, 0.5–1.0, and 1.0–1.5 μm. For each sample, duplicates of the 0.1–0.3 μm size fraction were collected for reproducibility analysis; additional duplicates in other size fraction were also included. The sample was injected into the cell sorter inside a sheath of 1.0 meq L−1 saline (Puratronic, Alfa Aesar, Ward Hill, MA, USA). The cell sorter diluted the sample by a factor of at least 125, but the exact dilution was not known. The flow cytometer sorted particles into user determined size ranges based on forward and side light scattering. Sample-free 1.0 meq L−1 saline was collected for analysis by the ICP-MS; this was used to correct for any sheath fluid metal contribution. Cell sorter size bins were calibrated and recovery was evaluated by analyzing a mixture of 0.2, 0.4, 0.75, and 1.25 μm polystyrene beads. Because the dilution factor was unknown, mass recovery and particle concentrations were also unknown.
Particles (2<dp<63 μm) were fractionated using a settling column at 22±0.5°C. Samples were withdrawn from the column at specified times and depths according to Stokes settling law (Eq. 1). Particle densities for the vacuumed/swept particles, determined by water displacement, were 2.27 g cm−3 for urban samples and 2.61 g cm−3 for rural samples. Samples were collected for the following size fractions: <63, <20, <15, <10, <5, and <2 μm. Size range concentrations were determined by difference; for example the 20–63 μm concentration was determined by [<63] – [<20], and so forth. Dissolved concentrations (0.5 μm, Whatman glass fiber) were subtracted from the <2 μm size fraction.
2.4 Sample Elemental Analysis: Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
Size-fractionated samples were stored overnight and a 1 mL subsample was combined with 4 mL 0.1 N nitric acid (pH<2, as specified in EPA method 6020) within six months of collection. Sample acidification disperses aggregated particles and desorbs surface-bound metals. The acidified samples were analyzed by ICP-MS (Agilent 7500i, Palo Alto, CA, USA) for Al, Mn, Fe, Cr, Ni, Cu, Zn, and Pb. In each batch, ICP-MS accuracy was verified with Standard Reference Materials (NIST 1640, Washington, DC or SLRS-4, NRC, Ottawa, Canada) with results within the certified concentration range for all relevant elements. The ICP-MS (equipped with a Babbington nebulizer and 2°C double-pass spray chamber) can determine dissolved constituents, as well as sub-1 μm particles or the outer ~1 μm shell of larger particles (Aeschliman et al., 2003; Houk et al., 1997). This means for very fine particles, the entire particle composition was included in the ICP-MS determined concentration. Larger particles contributed the bound constituents plus some portion of their actual mass, where the relative mass contribution was inversely proportional to particle size. Specific surface area decreases with increasing particle size; on a mass basis, both bound- and intra-particle constituent contributions will decrease with increasing particle size.
2.5 Data Analysis
Cell sorter samples were corrected for sheath fluid impurity metals and any resultant negative concentrations were assigned a zero concentration. Samples with associated relative standard deviation (RSD) greater than 60% were excluded from further data analysis.
Enrichment factors, which provide a convenient means of standardizing mass based comparisons, were calculated (Eq. 2).
| Eq. 2 |
Where X was the constituent of interest, and Y was a basis for normalization; a good normalizer is one that is ubiquitous and not influenced by external factors such as traffic related sources. In this research, that translated to a crustal element that had no significant anthropogenic source, which was best met by Al. Both X and Y had the units [constituent mass (particle mass)−1 = μg g−1], where the constituent concentration was determined by the ICP-MS. Particle masses for cell sorter samples were calculated using the cell sorter particle count, mid-point diameter of size range (e.g., 0.2 μm for the 0.1–0.3 μm size range) and the measured bulk densities (spherical particles assumed). Bulk and settling column masses were measured after drying in an oven at 105°C for 1 hr (standard method 2540).
Often global crustal ratios are employed as the reference condition, however here the bulk particle-constituent concentrations were selected as the reference condition. The rationale behind this selection was two fold: 1) in the case of zero particle removal, it represented the particle-associated constituents reaching the receiving water, and 2) it provided a more accurate basis of comparison for enrichment/depletion in a particular element and size fraction. The difference between the metal concentration of a filtered (0.5 μm; Whatman glass fiber) and an unfiltered sample, divided by the total suspended solids concentration, was used to calculate the bulk particle-constituent concentrations.
Note that this produced two discrepancies between the sample and the reference conditions. First, the reference condition was calculated based on 0.5 μm filtration process, however, the cell sorter selected particles for two size ranges, 0.1–0.3 and 0.3–0.5 μm, which were not explicitly included by filtration (0.5 μm), but may be removed by interception by and diffusion onto the filter. Secondly, the reference condition included particles >63 μm which were not included in any of the size fractions – although they are rare in stormwater and are mostly removed by sieving in the road silt determination.
An EF>1 indicated that element X was more concentrated (enriched) in the size fraction in question compared to the bulk suspended solids. If the size fraction in question was one that would be significantly removed by a BMP, removal of element X would be enhanced proportional to the EF. This also meant that if the size fraction in question was one that was poorly removed by a BMP (fine and very fine particles), then elements that were enriched in these size fractions would reach the receiving waters in higher concentrations than a simple particle mass calculation would predict. Conversely, an EF<1 indicated that the particle has less element X than did the bulk suspended solids, and it was therefore considered to be depleted with respect to that element.
3. Results and Discussion
3.1 Particle Size Distribution
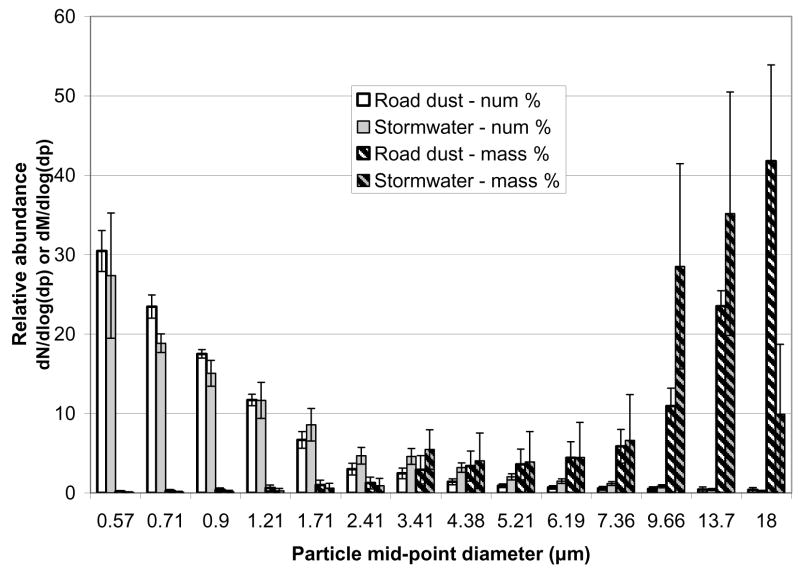
The particle size distributions, represented as number and mass percentages (dN/dlog(dp) and dM/dlog(dp)), for the vacuumed road dust and stormwater samples are presented in Figure 1. The vacuumed road dust and stormwater distributions were similar with overlapping standard deviations in most size ranges. However the stormwater contained considerably lower calculated mass fraction for 16<dp<20 μm. Both particle samples showed a distinct number bias toward the smaller diameter particles. By contrast, the large particles contributed a majority of the total calculated surface area and mass (constant density 2.3 g cm−3, spherical morphology assumed). This was similar to the findings of Sansalone et al. (1998), where 2<dp<9500 μm particles were studied. In that study, particles with diameters less than 25 μm were optically separated based on light obscuration. Specific surface area, both measured and calculated, generally increased with decreasing particle size, however total surface area peaked between 425–850 μm. In comparing measured vs. calculated specific surface areas, the trends were similar, but the difference was roughly 1000-fold (Sansalone et al., 1998).
Figure 1.
Particle size distributions based on number (measured) and mass (calculated, assuming spheres with density = 2.3 g cm−3)
Total particle counts for the stormwater samples ranged from 1.8 – 7.2 E +6 particles mL−1, with an average of 3.4 E +6 particles mL−1. Stormwater samples also contained varying dissolved fractions of elements (dissolved concentration/total concentration * 100%), averaged from all storms: Al (0.1%), Cr (8.6%), Mn (31.2%), Fe (0.3%), Ni (31.0%), Cu (54.6%), Zn (28.1%), and Pb (3.6%). The December 2003 storms, which all produced lower runoff volumes, had higher dissolved fractions for all metals compared to the higher volume February 2004 storms. Cr was an exception and had a consistent dissolved fraction for all storms. Relative elemental dissolved fractions and their dependence on rain event characteristics are consistent with Sansalone et al. (1996).
3.2 Cell Sorter Validity
Polystyrene calibration bead test and percent recovery results are shown in Table 3. Average relative percent error for all polystyrene bead recoveries was 14.6%. Note that the finest size fraction was recovered less well than the other size fractions, indicating reported values for the finest size fraction may under-represent true concentrations.
Table 3.
Cell sorter calibration
| Calibration bead diameter (μm) | Injected (%) | Recovered (%) |
|---|---|---|
| 0.2 | 43.5 | 35.9 |
| 0.4 | 46.1 | 49.5 |
| 0.7 | 8.84 | 10.5 |
| 1.25 | 1.55 | 1.78 |
Cell sorter data was corrected for the elemental contribution from the saline (Puratronic). Negative sample concentrations were set to zero as they represent insufficient sample signal. After accounting for saline contributions, 38 and 58 data points from stormwater and road dust respectively were negative. Mn was the least robust element considered and had 42 total negative saline corrected concentrations. Data point and enrichment factor exclusions, with their associated justifications, are shown in Table 4; the initial count represents all potential EF numerator data points, including duplicates. Data points were eliminated if the cell sorter did not detect any particle in a prescribed diameter range (ND), or if the ICP-MS five sample RSD% exceeded 60%. Enrichment factors were eliminated if the Al data point for a given set was 0 or had been eliminated. Eliminations were considered in the above-listed order – if a data point could be eliminated on multiple grounds, it is only included in the first count. Stormwater Mn was eliminated on 5 counts due to RSD%>60. Among the road dust RSD%>60 eliminations, the following were notable: Ni (13) and Cr (11).
Table 4.
Data eliminations
| Stormwater | Road Dust | ||
|---|---|---|---|
| Cell sorter | Cell sorter | Settling column | |
| Initial | 252 | 112 | 168 |
| No particles | 49 | 0 | 0 |
| Non-Al RSD%>60 | 26 | 35 | 5 |
|
| |||
| Al RSD%>60 | 0 | 26 | 0 |
| [Al] = 0 | 5 | 7 | 7 |
|
| |||
| Remaining | 172 | 44 | 156 |
Duplicate analysis was performed for each stormwater sample for the 0.1–0.3 μm size fraction, and the RSD% were averaged (n=12): Al (41.9%, range 14–88%), Cr (30.0%, 0.7–68%), Mn (36.8%, 5–118%), Fe (31.9%, 12–61%), Ni (32.8%, 0.7–72%), Cu (26.7%, 0.7–47%), Zn (30.1%, 0.4–67%), Pb (44.8%, 5–130%). Only a small volume was subjected to sorting and no particles were detected in 8/36 sample-size fractions. Sorting a larger volume would probably increase reproducibility. Based on duplicate analysis (intra- and inter-storm), negative concentrations (insignificant signal), and high ICP-MS determined RSD% (intra-sample), Mn and Pb were the least consistent elements measured in this study, they were also the least abundant and were thus influenced more by random noise. Al, Cu, Fe, Ni, Zn, and Cr were all fairly consistent.
3.3 Vacuumed Road Dust
Many similarities were seen between enrichment factors calculated for the two Davis sites, and likewise for the two Stockton sites; averages are shown in Table 5. While the actual EFs differ, many of the same EF patterns with regard to particle size and sample location were observed (data not shown). Elevated agricultural Zn EF was primarily driven by the EFAg, 0.1–0.3 = 4876, while the elevated industrial Ni EF was greatly influenced by EFInd, 0.1–0.3 = 623.
Table 5.
Sample specific enrichment factor averages
| Location | Average EF | |||||||
|---|---|---|---|---|---|---|---|---|
| Adjacent land use | Mn | Fe | Cr | Ni | Cu | Zn | Pb | |
| Davis | Agriculture | 0.08 | 0.33 | 137.48 | 6.16 | 98.93 | 735.37 | 14.78 |
| Davis | Commericial | 0.16 | 0.20 | 605.26 | 3.64 | 43.40 | 5.98 | 13.04 |
| Stockton | Industrial | 0.03 | 0.14 | 502.75 | 87.65 | 35.18 | 83.48 | 18.89 |
| Stockton | Residental | 0.05 | 0.12 | 521.54 | 1.21 | 4.90 | 16.40 | 4.97 |
As is shown in Table 1, the two locations are significantly different in background soil composition. When the background soil has a comparatively elevated constituent concentration, the effect of an external contribution would be somewhat muted and the EF depressed. This was illustrated in the following: Davis had far higher concentrations of soil Cr and a depressed CrAg; conversely, Stockton had a lower background Ni concentrations and an elevated NiInd. The abnormally high agricultural Zn EF was primarily due to an extraordinarily high EF in the agricultural 0.1–0.3 μm size fraction. This may have been due to Zn containing pesticides (e.g. zinc diethyldithiocarbamate (trade name Ziram) or zinc ion with manganese ethylenebis dithiocarbamate (Mancozeb) both of which were in the top 100 pesticides used in CA statewide for 2003 and 2004 (California Department of Pesticide Regulation, 2003; California Department of Pesticide Regulation, 2004)) used on the adjacent agricultural lands.
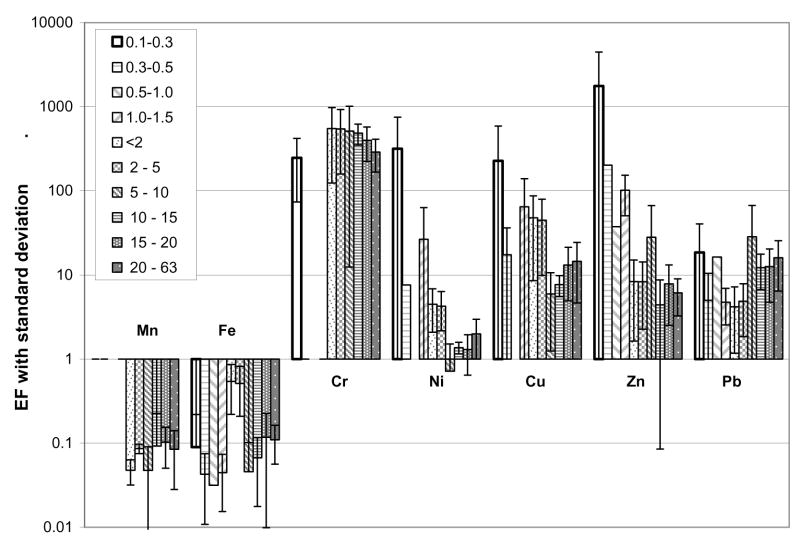
Enrichment factors were calculated for all data sets and the four samples were averaged (Figure 2). For the purposes of this discussion, when the standard deviation range does not include EF = 1, the enrichment was considered significant, with the enrichment (depletion) considered more noteworthy the larger the deviation from unity. Using this reasoning the following definitions were applied: 0.1<EF<1 or 1<EF<10 = minimal depletion/enrichment; 0.01<EF<0.1 or 10<EF<100 = moderate depletion/enrichment; EF>100 = considerably enriched. Based on average California soil composition (Bradford et al., 1996), Mn and Fe (0.646% and 3.7% respectively) were referred to as “crustal” while Cr (122 mg kg−1), Ni (57 mg kg−1), Cu (26.7 mg kg−1), Zn (149 mg kg−1), and Pb (23.9 mg kg−1) were referred to as “non-crustal” or “anthropogenic.” Few statistically significant differences in enrichment factors were observed when comparing different particle classes for a given element but there were clear differences among the different elements, and standard deviations aside, size correlated trends were observed within a particular element’s EFs, notably for Ni, Cu, and Zn. Both Mn and Fe were minimally or moderately depleted for all size ranges while Cr, Ni, Cu, Zn, and Pb were all enriched to varying degrees. Cr was considerably enriched, with the standard deviation range never extending down to 10, for all size fractions. While some standard deviations extended down to unity, Cr, Ni, Cu, Zn, and to a lesser degree Pb, were all considerably enriched in the finest size fraction (0.1–0.3 μm); Ni, Cu, and Zn all were most enriched in the 0.1–0.3 μm size fraction. Assuming that most of the particles in the bulk solution were crustally derived, the fine and very fine particles were composed mostly of non-crustal materials, as evidenced by the depletion of the crustal elements (Mn and Fe) and the enrichment of the non-crustal elements (Cr, Ni, Cu, Zn, and Pb). This size-composition relationship was an extension of the trends observed by others in larger particle size classes (Fergusson and Ryan, 1984; Westerlund and Viklander, 2006).
Figure 2.
Vacuumed road dust average enrichment factor
It should be noted that since these samples were dry when vacuumed, everything associated with the collected particles was included in the bulk solution. This was in contrast to particles subjected to a real rain event. If the desorption kinetics are faster than the particle entrainment rate, then some or all particle-bound constituents would desorb and contribute to the dissolved concentration. Additionally, vacuuming may not equally represent all particle size fraction in the same manner that storm water runoff does; particle size distributions from the two methods indicated that in this case, there was not a large discrepancy (Figure 1). If one were to collect a sample that was representative of the entire storm, the bulk entire storm sample should match a vacuumed road dust sample, subjected to synthetic rain (assuming equal entrainment and equal aqueous chemical conditions). However, storm water sampling was conducted in discrete samples, which does not represent the entire storm; a particle and its previously associated constituents may or may not have been present in the same discrete stormwater sample. Equally, particles not included in the discrete stormwater sample may have contributed to dissolved constituent concentrations in the bulk solution.
Standard deviations could likely have been decreased by increasing the number of samples and collecting all samples from the same general area, i.e. all Davis or all Stockton.
3.4 Stormwater Samples
Size fractionated concentrations for the 12/12/2003 storm, selected for illustration because it had a complete data set, are shown in Table 6. Note that concentrations in Table 6 are presented as μg constituent g−1 solid (TSS), and are not based on a stormwater volume concentration. From Table 6, it can be seen that the general trend was a decrease in constituent concentration for increasing particle size for all elements. This trend was also seen in the other storms and in the vacuumed road dust samples; however, in the road dust samples, the trend did not persist in the larger size fractions for many of the elements.
Table 6.
2/2/2004 very fine particle constituent concentrations
| Al | Mn | Fe | Cr | Ni | Cu | Zn | Pb | |
|---|---|---|---|---|---|---|---|---|
| Particle diameter (μm) | mg constituent g−1 solida | |||||||
| 0.1–0.3 | 179.5 | 8.5 | 329.0 | 86.0 | 64.5 | 42.0 | 903.8 | 13.4 |
| 0.3–0.5 | 45.1 | 2.0 | 119.9 | 22.9 | 12.0 | 9.1 | 203.3 | 1.8 |
| 0.5–1.0 | 8.5 | 0.3 | 10.6 | 2.2 | 1.8 | 1.3 | 29.5 | 0.2 |
| 1.0–1.5 | 3.9 | 0.3 | 8.4 | 1.6 | 1.5 | 0.8 | 19.0 | 0.1 |
TSS was calculated assuming spherical morphology and density = 2.3 g cm−3
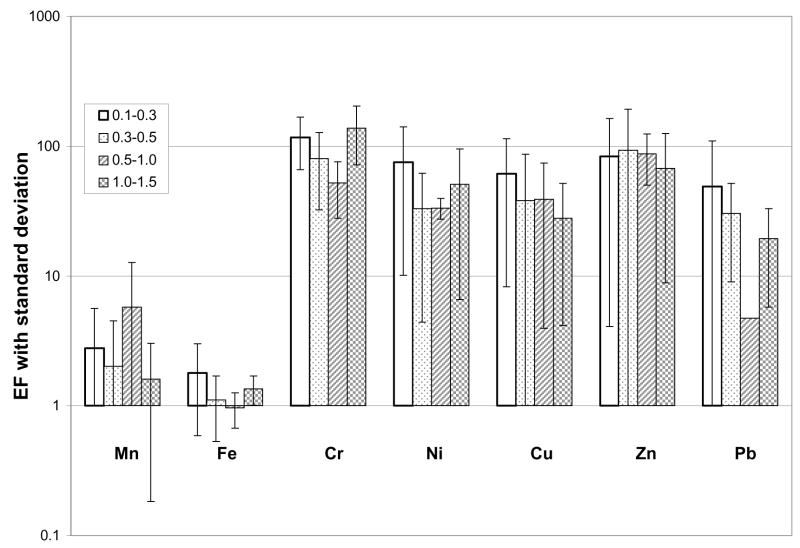
Stormwater elemental concentrations measured by ICP-MS include the bulk concentration that desorbs from all the particles exposed to the stormwater (that occur kinetically fast enough) under environmental conditions, plus the constituents that desorb more slowly under environmental conditions only from those particles that were collected (desorption was slower, and therefore occurred in the collection container under environmental conditions; due to the small amount of fluid compared to the amount of solids collected, this can probably be neglected), plus those constituents that desorbed under artificial conditions only from the select particle size fraction. The last fraction included all constituents bound to the particle surface as well as a portion of the particle itself which is dissolved by the acidification process. Due to increased specific surface area in smaller diameter particles, the relative contribution from this dissolution was greater for finer particles, and included the entire particle for a small enough diameter. The average enrichment factor results from five storms, all collected from the Davis I-80 location, are presented in Figure 3.
Figure 3.
Stormwater average enrichment factors
The anthropogenic constituents were present mostly in the moderate enrichment range. Cr, Ni, Cu, and Pb were all enriched to the greatest extent in the finest size fraction (0.1–0.3 μm). As in the case of particles collected dry, Mn and Fe had EFs close to unity, but this time they were minimally enriched whereas in the road dust samples they were minimally depleted. This difference could be attributed to the difference in the size fractionated aluminum concentration. In the road dust samples, the Al concentration was consistently ~35% of the total measured constituents. Contrast that to the stormwater particles, where the bulk Al concentration was ~35% of the measured constituents, but the Al concentration in the very fine particle size fractions (0.1 – 1.5 μm) was ~12% of the total; this served to increase all EF values in the finer size classes. In spite of this difference, the stormwater EF values for the anthropogenic constituents were consistently less than the vacuumed road dust, and were consistently more than the vacuumed road dust crustal EF values (vacuumed road dust >2 μm Al concentration was still ~35% of the total elements analyzed).
To account for antecedent dry period, the 12/6/03, 2/2/04, and 2/25/04 storms were grouped together as extended antecedent dry period (18+ days), and the 12/10/03 and 12/12/03 storm were grouped as limited antecedent dry period (1–2 days). The limited antecedent dry period storms had 4 of 8 size categories as non-detects (2 storms with 4 size categories each), while the extended antecedent dry period storms had only 2 of 12 size categories as non-detects. Due to non-detected size categories in the limited dry period storms, comparison was only possible for the 0.1–0.3 and 1.0–1.5 μm size ranges. The elemental mass concentrations (μg constituent g−1 solid) in the finest fraction (0.1–0.3 μm) were of similar magnitudes, with the exception of Pb which was an order of magnitude higher in the extended dry period storms. In the 1.0–1.5 μm size range, all monitored elements were 1–2 orders of magnitude higher in the limited dry period storm. However, a comparison of the enrichment factors revealed some distinct differences: the extended dry period storms were more enriched than the limited dry period storm in Pb in the finest size fractions, while Cr, Ni, and Cu were more enriched (>2-fold) in both the 0.1–0.3 and 1.0–1.5 μm fractions in the limited than the extended dry period storms.
For both the vacuumed road dust samples and the stormwater runoff samples, the surface area distribution was biased toward the large particles while the very fine particles were enriched in anthropogenic elements to a greater extent than the larger particles. This was counter to the expectation, as typically surface binding is roughly proportional to available surface area. Since this trend was not observed in the data, either there are more/stronger surface sites available on the very fine particles (e.g. because they contain more organic carbon which can sequester metals), or the very fine particles, which were completely analyzed by the ICP-MS, were composed of anthropogenic elements, which indicates an anthropogenic source. Normally particles themselves are not considered to be as environmentally relevant, but on the basis of size and reactivity, very fine particles are environmentally important.
Consider the following simplified example. For the case of this hypothetical situation, assume that if no BMP were in place, then 100% of the particles and their associated constituents would reach the receiving water. Now, assume that there is a settling pond where all particles dp>10 μm settle, 50% of particles 2<dp<10 μm settle, and 0% of particles dp<2 μm settle, which is to say they all reach the receiving waters. Roughly based on this study, the respective mass fractions of the three particle size classes are 0.915, 0.08, and 0.005. For the sake of this example, assume that the elemental constituents on the particles have the following enrichment factors: EFd<2μm = 50; EF2<d<10 μm = 10. Only 4.5% of the initial particle mass would reach the receiving waters, however 65% of the particle-bound constituent would be associated with the particles that are not eliminated by the BMP. Clearly, these very fine particles are environmentally relevant, as they carry a far greater load of anthropogenic constituents that are more difficult to remove by BMPs. These metals may then desorb in the receiving water where particle concentrations are typically lower, resulting in metal-related toxicity as observed in numerous previous studies (e.g., Kayhanian et al., 2008).
4. Conclusions
Much of the heavy metal load reaching a receiving water does so in a particle-bound fashion with variations in degree depending on the constituent. On a particle mass basis, anthropogenic constituents are increasingly associated with decreasing particle size. A flow cytometer was used to size fractionate the very fine particles, which were then analyzed by an ICP-MS for associated heavy metals. This method revealed that crustal elements (Fe and Mn) had EFs close to unity or were minimally depleted for all size fractions. By contrast, the anthropogenic constituents Cr, Ni, Cu, Zn, and Pb were moderately to considerably enriched (10–100+ fold compared to the bulk solution concentration) for most size fractions. For the anthropogenic constituents, enrichment increased with decreasing particle size. As the simplified example above demonstrates, even though these very fine particles represent only a small mass fraction of the TSS, they can carry a disproportionately large heavy metal load.
Acknowledgments
This research was conducted with financial support from the California Department of Transportation under contracts 43A0073 and 43A0168, the UC Davis College of Engineering (TOPS Fellowships to E.R. McKenzie and C.M. Wong), and grant number 5 P42 ES004699 from the National Institute of Environmental Health Sciences (NIEHS), NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH. We thank Carol Oxford for her help in conducting the flow cytometer portion of this study.
Footnotes
Suggested Reviewers
Dr. Robert M. Roseen, Director, UNH Stormwater Center, Environmental Research Group, University of New Hampshire, 35 Colovos Road, Durham, NH 03824, Robert.roseen@unh.edu, Phone: 603-862-4024, Fax: 310-206-5476
Professor, Shirley E. Clark, School of Science and Engineering, Penn State University, 777 W. Harrisburg Pike TL-103, Middletown, PA 17507, seclark@psu.edu, Phone: (717) 948-6127, Fax: (717) 948-6580
Mary Ellen Tuccillo, U.S. Environmental Protection Agency, Urban Watershed Management Branch, 2890 Woodbridge Ave., Edison, NJ, 08837, 306-C South 3rd Ave. Highland, Park, NJ 08904. E-mail address: metuccillo@yahoo.com.
Camilla Westerlund, Division of Sanitary Engineering, Lulea° University of Technology, S-971 87, Lulea°, Sweden, Tel.: +46 920 491494, fax: +46 920 491493. E-mail address: cam@sb.luth.se
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Erica R. McKenzie, ermckenzie@ucdavis.edu, Civil and Environmental Engineering – University of California Davis, One Shields Avenue, 2001 Engineering Unit III, Davis, CA, 95616
Carol M. Wong, crlwong@ucdavis.edu, Civil and Environmental Engineering – University of California Davis, One Shields Avenue, 2001 Engineering Unit III, Davis, CA, 95616
Peter G. Green, pggreen@ucdavis.edu, Civil and Environmental Engineering – University of California Davis, One Shields Avenue, 2001 Engineering Unit III, Davis, CA, 95616
Masoud Kayhanian, mdkayhanian@ucdavis.edu, Civil and Environmental Engineering – University of California Davis, One Shields Avenue, 2001 Engineering Unit III, Davis, CA, 95616.
Thomas M. Young, tyoung@ucdavis.edu, Civil and Environmental Engineering – University of California Davis, One Shields Avenue, 2001 Engineering Unit III, Davis, CA, 95616, Phone: (530) 754-9399, Fax: (530) 752-7872
References
- Aeschliman DB, Bajic SJ, Baldwin DP, Houk RS. High-speed digital photographic study of an inductively coupled plasma during laser ablation: comparison of dried solution aerosols from a microconcentric nebulizer and solid particles from laser ablation. J Anal At Spectrom. 2003;18:1008–1014. [Google Scholar]
- Backstrom M. Sediment transport in grassed swales during simulated runoff events. Water Sci Technol. 2002;45:41–49. [PubMed] [Google Scholar]
- Benjamin M, Bhargava V, Cox P, Johnson M, Lin A, Look D, et al. THE 2003 CALIFORNIA ALMANAC OF EMISSIONS AND AIR QUALITY. California Air Resources Board - Planning and Technical Support Division; 2003. [Google Scholar]
- Birmili W, Allen AG, Bary F, Harrison RM. Trace metal concentrations and water solubility in size-fractionated atmospheric particles and influence of road traffic. Environ Sci Technol. 2006;40:1144–1153. doi: 10.1021/es0486925. [DOI] [PubMed] [Google Scholar]
- Bradford GR, Chang AC, Page AL, Bakhtar D, Frampton JA, Wright H. Background Concentrations of Trace and Major Elements in California Soils. The University of California - Kearney Foundation for Soil Science; 1996. [Google Scholar]
- Burton GA, Pitt R, Clark S. The role of traditional and novel toxicity test methods in assessing stormwater and sediment contamination. Crit Rev Environ Sci Technol. 2000;30:413–447. [Google Scholar]
- California Department of Pesticide Regulation. Top 100 pesticides used statewide (all sites combined) in 2003. Pesticide Use Reporting (PUR) 2003 [Google Scholar]
- California Department of Pesticide Regulation. Top 100 pesticides used statewide (all sites combined) in 2004. Pesticide Use Reporting (PUR) 2004 [Google Scholar]
- Chien N. The Present Status of Research on Sediment Transport. Trans ASCE. 1956;121:833–884. [Google Scholar]
- Fergusson JE, Ryan DE. The Elemental Composition of Street Dust from Large and Small Urban Areas Related to City Type, Source and Particle-Size. Sci Total Environ. 1984;34:101–116. [Google Scholar]
- Han J, Wu JS, Allan C. Suspended sediment removal by vegetative filter strip treating highway runoff. J Environ Sci Health, Pt A: Toxic/Hazard Subst Environ Eng. 2005;40:1637–1649. doi: 10.1081/ese-200060683. [DOI] [PubMed] [Google Scholar]
- Herngren L, Goonetilleke A, Ayoko GA. Analysis of heavy metals in road-deposited sediments. Anal Chim Acta. 2006;571:270–278. doi: 10.1016/j.aca.2006.04.064. [DOI] [PubMed] [Google Scholar]
- Houk RS, Winge RK, Chen XS. High speed photographic study of wet droplets and solid particles in the inductively coupled plasma. J Anal At Spectrom. 1997;12:1139–1148. [Google Scholar]
- Jambers W, Dekov V, Van Grieken R. Single particle and inorganic characterization of rainwater collected above the North Sea. Sci Total Environ. 2000;256:133–150. doi: 10.1016/s0048-9697(00)00477-0. [DOI] [PubMed] [Google Scholar]
- Kayhanian M, Stransky C, Bay S, Laud SL, Stenstrom MK. Toxicity of urban highway runoff with respect to storm duration. Sci Total Environ. 2008;389:386–406. doi: 10.1016/j.scitotenv.2007.08.052. [DOI] [PubMed] [Google Scholar]
- Kupiainen KJ, Tervahattu H, Raisanen M, Makela T, Aurela M, Hillamo R. Size and composition of airborne particles from pavement wear, tires, and traction sanding. Environ Sci Technol. 2005;39:699–706. doi: 10.1021/es035419e. [DOI] [PubMed] [Google Scholar]
- Li YX, Lau SL, Kayhanian M, Stenstrom MK. Particle size distribution in highway runoff. J Environ Eng-ASCE. 2005;131:1267–1276. [Google Scholar]
- Li YX, Lau SL, Kayhanian M, Stenstrom MK. Dynamic characteristics of particle size distribution in highway runoff: Implications for settling tank design. J Environ Eng-ASCE. 2006;132:852–861. [Google Scholar]
- Lin CC, Chen SJ, Huang KL, Hwang WI, Chang-Chien GP, Lin WY. Characteristics of metals in nano/ultrafine/fine/coarse particles collected beside a heavily trafficked road. Environ Sci Technol. 2005;39:8113–8122. 468. doi: 10.1021/es048182a. [DOI] [PubMed] [Google Scholar]
- Marsalek J, Rochfort Q, Brownlee B, Mayer T, Servos M. An exploratory study of urban runoff toxicity. Water Sci Technol. 1999;39:33–39. [Google Scholar]
- Pettersson TJR. Water quality improvement in a small stormwater detention pond. Water Sci Technol. 1998;38:115–122. [Google Scholar]
- Preciado HF, Li LY. Evaluation of metal loadings and bioavailability in air, water and soil along two highways of British Columbia, Canada. Water, Air, Soil Pollut. 2006;172:81–108. [Google Scholar]
- Sabin LD, Hee Lim J, Teresa Venezia M, Winer AM, Schiff KC, Stolzenbach KD. Dry deposition and resuspension of particle-associated metals near a freeway in Los Angeles. Atmos Environ. 2006;40:7528–7538. [Google Scholar]
- Sansalone JJ, Buchberger SG, AlAbed SR. Fractionation of heavy metals in pavement runoff. Sci Total Environ. 1996;190:371–378. [Google Scholar]
- Sansalone JJ, Koran JM, Smithson JA, Buchberger SG. Physical characteristics of urban roadway solids transported during rain events. J Environ Eng-ASCE. 1998;124:427–440. [Google Scholar]
- Tuccillo ME. Size fractionation of metals in runoff from residential and highway storm sewers. Sci Total Environ. 2006;355:288–300. doi: 10.1016/j.scitotenv.2005.03.003. [DOI] [PubMed] [Google Scholar]
- U.S. EPA. APPENDIX C.1 - PROCEDURES FOR SAMPLING SURFACE/BULK DUST LOADING. AP 42. 5. I. 1993. p. 484. [Google Scholar]
- U.S. EPA. APPENDIX C.2 - PROCEDURES FOR LABORATORY ANALYSIS OF SURFACE/BULK DUST LOADING SAMPLES. AP 42. 5. I. US: EPA; 1993. [Google Scholar]
- Westerlund C, Viklander M. Particles and associated metals in road runoff during snowmelt and rainfall. Sci Total Environ. 2006;362:143–156. doi: 10.1016/j.scitotenv.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Zhou LM, Hopke PK, Stanier CO, Pandis SN, Ondov JM, Pancras JP. Investigation of the relationship between chemical composition and size distribution of airborne particles by partial least squares and positive matrix factorization. J Geophys Res-Atmos. 2005;110 doi: 10.1029/2004JD005050. [DOI] [Google Scholar]