Abstract
Background
Previous studies have defined vaccinia virus (VACV)-derived T cell epitopes in VACV-infected human leukocyte antigen-A*0201 (HLA-A2.1) transgenic (Tg) mice and A2.1-positive human Dryvax vaccinees. A total of 14 epitopes were detected in humans and 16 epitopes in A2.1 Tg mice; however, only two epitopes were independently reported in both systems. This limited overlap raised questions about the suitability of using HLA Tg mice as a model system to map human T cell responses to a complex viral pathogen. The present study was designed to investigate this issue in more detail.
Results
Re-screening the panel of 28 A2.1-restricted epitopes in additional human vaccinees and in A2.1 Tg mice revealed that out of the 28 identified epitopes, 13 were detectable in both systems, corresponding to a 46% concordance rate. Interestingly, the magnitude of responses in Tg mice against epitopes originally identified in humans is lower than for epitopes originally detected in mice. Likewise, responses in humans against epitopes originally detected in Tg mice are of lower magnitude.
Conclusion
These data suggest that differences in immunodominance patterns might explain the incomplete response overlap, and that with limitations; HLA Tg mice represent a relevant and suitable model system to study immune responses against complex pathogens.
Background
Model systems to study human responses to potential pathogens in general, and to complex viruses such as poxviruses in particular, are of considerable practical interest. They allow for characterization of immune responses directed against epitopes potentially capable of being recognized in humans in a rigorously controlled experimental situation, without having to rely on samples derived from exposed individuals. These issues are particularly relevant in the case of pathogens of bioterrorism concern, as well as emerging and re-emerging pathogens.
Several alternative approaches have been described to circumvent the need to use samples from infected humans. These include using mice re-colonized with human hematopoietic stem cells, primary in vitro restimulation utilizing peripheral blood mononuclear cells (PBMC) from healthy volunteers, and human leukocyte antigen (HLA) transgenic (Tg) mice. Murine/human chimeric systems appear to be associated with significant experimental variability, and responses of low magnitude and diversity compared to those observed in humans have been reported [1]. Primary in vitro restimulation systems are an attractive alternative, but are associated with limited throughput, high cost, labor intensiveness, and the lack of testing the impact of variables associated with host-pathogen interactions in a living organism.
Several groups have reported the development and validation of HLA Tg mice as a model system to study T cell responses restricted by human major histocompatibility complex (MHC) molecules [2-4]. It has been established that HLA Tg mice represent a fairly accurate model of human immune responses based on peptide immunizations. For example, Wenthworth and co-workers analyzed the repertoire of influenza-specific T cell responses obtained in HLA Tg mice with that obtained following primary induction of human cytotoxic T lymphocyte (CTL), and observed concordant results for approximately 85% of the peptides assayed [4].
The question of how suitable HLA Tg mice are for defining responses to complex pathogens and antigens has, however, not been addressed in detail. This issue is relevant because significant differences between humans and HLA Tg mice could exist at several levels. It is possible that co-expression of different MHC molecules might impact the TCR repertoire, in that certain specificities are lost while others are gained, as a function of the MHC alleles present [5]. Differences also exist in the cellular processing machinery operating in mice and humans at the level of the proteasome and TAP transport [6-8]. Discrepancies might also exist in the pattern of expression of viral antigens in infected cells of human and murine origin. Furthermore, it is currently unknown to what degree these differences might influence the global outcome of immune responses.
In our laboratory, we have characterized the T cell response to VACV observed in infected HLA-A*0201 (A2.1) Tg mice [5] and in human Dryvax vaccinees expressing A2.1 [9]. This enabled us to compare responses to a complex viral pathogen in the two different systems. We have identified a total of 28 different A2.1-restricted epitopes, and other investigators have described three additional epitopes (J8R11–19, A47L169–177, and B5R5–19) using A2-positive vaccinees [10-12]. Concordant results were found in some cases, but for the most part, epitopes detected in humans were not detected in mice, and vice versa. In the present study, we have further assessed the issue of limited epitope overlap observed in humans and HLA Tg mice.
Results and discussion
Additional testing in A2.1 Tg mice reveals a more extensive repertoire overlap
We previously detected 14 different epitopes utilizing PBMC from A2.1-positive human volunteers vaccinated with Dryvax (Figure 1A) [9]. Of these epitopes, only two were also independently identified in VACV-infected A2.1 Tg mice [5,13-16]. Based on these results, Terajima and Ennis hypothesized that the large number of potentially immunogenic peptides encoded by a complex pathogen such as VACV might explain the incomplete overlap observed [17]. Herein, we addressed this issue in more detail.
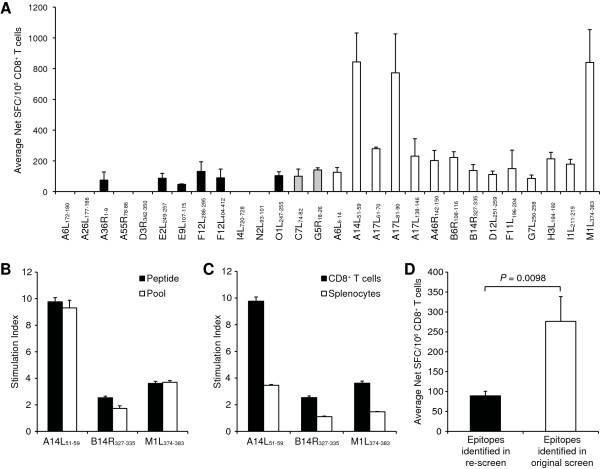
Figure 1.
Testing A2.1-restricted VACV T cell epitopes in A2.1 Tg mice using purified CD8+ T cells and individual peptides. (A) Splenic CD8+ cells were purified from VACV-infected A2.1 Tg mice and incubated with peptide-pulsed Jurkat-A2.1/Kb cells, after which number of IFN-γ-producing cells was enumerated in an ELISPOT assay. Shown are the average net SFC/106 CD8+ T cells in response to each peptide; black bars, epitopes identified by Oseroff et al. [9] in A2.1 positive donors and recognized in Tg mice upon re-screening; white bars, peptides identified by Pasquetto et al. [5] in Tg mice; grey bars, epitopes identified in both studies. (B) The specific T cell response to three different epitopes (B14R327–335, M1L374–383, and A14L51–59) was assessed 7 days after VACV infection in A2.1 Tg mice. Shown is the stimulation index for the epitopes tested either as individual peptides (black bars) or in pools of ten peptides (white bars). (C) Shown is the stimulation index for the epitope-specific responses by using either CD8+ T cells (black bars) or splenocytes (white bars). (D) Shown are the average net SFC/106 CD8+ T cells in response to groups of epitopes recognized in Tg mice upon re-screening (black bar) and identified in Tg mice in the original study by Pasquetto et al. [5] (white bar). The unpaired t test with Welch's correction was used to determine if differences were significant. For all panels, average data are shown from at least two independent experiments. For each individual experiment samples are tested in triplicate. Error bars indicate SEM.
First, A2.1 Tg mice were inoculated with VACV and the reactivity against each of the 28 individual A2.1-restricted epitopes was evaluated. Responses were detected for all epitopes previously reported in HLA Tg mice (Figure 1A; white and grey bars). In addition, responses were detected for 6 peptides that had been originally described in A2.1-positive humans [9,14,18], but not reported in studies utilizing Tg mice [5,15] (Figure 1A; black bars). Altogether, responses directed against 8 of 14 (57%) epitopes reported in humans were now also detected in A2.1 Tg mice.
The present epitope screen differs in two experimental settings from the original study by Pasquetto et al. [5]. First, individual peptides were used here instead of peptide pools. Second, the initial epitope identification used splenocytes as effector cells [5], whereas the present study utilized purified CD8+ T cells. Herein we examined the impact of these two experimental differences. When three representative epitopes (A14L51–59, B14R327–335, and M1L374–383) were tested either as individual peptides or in pools of 10 peptides, similar response magnitudes were observed (Figure 1B). We also tested the response to the same 3 epitopes using either purified CD8+ T cells or splenocytes, and found that the higher non-specific interferon (IFN)-γ production obtained when using splenocytes led to a reduced stimulation index, and thereby decreased sensitivity (Figure 1C).
We observed that the average responses against these epitopes not initially identified in Tg mice were ~3 times lower than those against the epitopes identified in the original screen (P = 0.0098; Figure 1D). Although the 3 dominant epitopes (A14L51–59, A17L81–90, and M1L347–383) contribute to a significant portion of the higher response identified in mice, exclusion of these 3 epitopes still results in a significant difference in the magnitude of the response for the epitopes identified in humans versus mouse (P = 0.0012). Taken together, these results suggest that while 8 of 14 (57%) peptides reported in humans can also be detected in A2.1 Tg mice, differences in the patterns of immunodominance might render them less prominent and consequently make their detection more difficult.
The mice utilized by Pasquetto et al. were A2.1 bxd mice [5]. To examine whether expression of endogenous mouse MHC molecules might also contribute to the incomplete overlap, we tested the reactivity of the 14 epitopes identified in humans using CD8+ T cells from VACV-infected HHD A2.1 mice, which do not express endogenous mouse MHC [19]. However, no additional epitopes were recognized in the HHD mice, and the response magnitude and immunodominance pattern was similar to that in A2.1 bxd mice (data not shown).
Testing of a broader panel of human vaccinees reveals a more extensive overlap
As described above, a total of 16 epitopes were initially identified in HLA Tg mice [5,13,15]. Of these, only two (13%) were also independently identified in human vaccinees (Figure 1A). Most of the candidate peptides were tested in only 6 donors, with each individual donor yielding a rather distinct pattern of epitope recognition. While some epitopes were identified in multiple donors, most of the epitopes were only detected in 1 out of the 6 donors investigated [9].
To test the hypothesis that the epitopes not initially detected in humans were associated with lower and/or less frequent responses, we examined the responses to the set of 16 A2.1-restricted epitopes identified in Tg mice using PBMC from 8 different A2-positive vaccinees. The samples tested included frozen cell aliquots from 5 of the donors tested in the original study of Oseroff et al. [5], as well as PBMC from 3 additional A2-positive vaccinees. PBMC were cultured in the presence of individual peptides, and the numbers of IFN-γ-producing cells were enumerated in an enzyme-linked immunosorbent spot (ELISPOT) assay. The same two peptides previously identified as positive in these donors were also re-identified in the course of the present experiments, and responses to an additional 5 peptides originally identified in A2.1 Tg mice, but not identified in the human donor screen of Oseroff et al. [9], were detected. Similar to what we observed in the mouse experiments, responses to the newly detected epitopes were in general lower than to epitopes identified in the initial screen (see Additional file 1). Overall, responses directed against 7 of 16 (44%) peptides initially identified in HLA Tg mice, were also detected in humans. Considering the large donor-to-donor variation observed, it is likely that testing of additional donors would further increase the overlap between the two systems.
Conclusion
In summary, of the 28 epitopes identified in either humans or mice, in this study we showed that 13 were detected in both systems, with an overall concordance rate of 46% (Table 1). This degree of overlap is, albeit incomplete, much greater than previously detected. In a similar study, a 68% concordance rate was observed between human immunodeficiency virus type 1 (HIV-1)-specific CD8+ T cell epitopes recognized in both HLA-A2.1 Tg mice and HLA-A2 HIV-1 positive patients [20]. A higher degree of overlap was likely detected because HIV-1 epitope recognition in A2.1 Tg mice was evaluated following peptide immunization and not natural infection, a larger cohort of donors were tested, and HIV-1 has a significantly smaller genome than VACV. These data suggest that while the Tg mouse system is suitable to model immune responses to complex pathogens, caution should be taken in interpreting the results obtained.
Table 1.
Summary of overlap between A2.1-restricted VACV T cell epitope recognition in HLA Tg mice and human systems
| Epitope | Sequence | Original Identification | Reference |
Average Net SFC/106 Cells |
|
| Tg Mice | Human | ||||
| A6L172–180 | ILSDENYLL | Human | [9] | - | 106 |
| A26L177–186 | YLYTEYFLFL | Human | [9] | - | 164 |
| A36R1–9 | MMLVPLITV | Human | [9] | 75 | 49 |
| A55R78–86 | YIYGIPLSL | Human | [9] | - | 94 |
| D3R342–350 | FLVIAINAM | Human | [9] | - | 195 |
| E2L249–257 | KIDYYIPYV | Human | [9] | 87 | 192 |
| E9L107–115 | FLNISWFYI | Human | [9] | 48 | 132 |
| F12L286–295 | NLFDIPLLTV | Human | [9] | 132 | 252 |
| F12L404–412 | FLTSVINRV | Human | [9] | 90 | 73 |
| I4L720–728 | SMHFYGWSL | Human | [9] | - | 102 |
| N2L93–101 | YVNAILYQI | Human | [9] | - | 563 |
| O1L247–255 | GLNDYLHSV | Human | [9] | 104 | 77 |
| C7L74–82 | KVDDTFYYV | Human + Tg mice | [5,9,14,15,18] | 100 | 178 |
| G5R18–26 | ILDDNLYKV | Human + Tg mice | [5,9,14] | 141 | 271 |
| A6L6–14 | VLYDEFVTI | Tg mice | [5] | 126 | - |
| A14L51–59 | FILGIIITV | Tg mice | [5] | 844 | 60 |
| A17L61–70 | RTLLGLILFV | Tg mice | [5] | 279 | - |
| A17L81–90 | ILMIFISSFL | Tg mice | [5] | 773 | 42 |
| A17L138–146 | QIFNIISYI | Tg mice | [13] | 231 | - |
| A46R142–150 | GLFDFVNFV | Tg mice | [5] | 202 | - |
| B6R108–116 | LMYDIINSV | Tg mice | [5] | 222 | - |
| B14R327–335 | HVDGKILFV | Tg mice | [5] | 137 | - |
| D12L251–259 | RVYEALYYV | Tg mice | [5] | 112 | 63 |
| F11L196–204 | FLIVSLCPT | Tg mice | [5] | 150 | - |
| G7L250–258 | YLPEVISTI | Tg mice | [5] | 86 | - |
| H3L184–192 | SLSAYIIRV | Tg mice | [5,16] | 213 | - |
| I1L211–219 | RLYDYFTRV | Tg mice | [5] | 179 | 115 |
| M1L374–383 | IIIPFIAYFV | Tg mice | [5] | 840 | 91 |
Despite the more extensive and sensitive system used in the current study, approximately one half of the epitopes recognized specifically in one system were not recognized in the other. Differences in TCR repertoire between mouse and human might account for the different epitope recognition. However, peptide immunizations of the human epitopes in A2.1 Tg mice only failed in 2 of 14 instances to generate a T cell response. This is similar to the 85% success rate previously reported by Wentworth et al. [4], and suggests that repertoire differences account for a relatively minor percentage of the discrepancies. Differences in the processing apparatus and antigen presentation in murine versus human dendritic cell (DC) subsets and other antigen-presenting cells (APCs) involved in priming CTL immunity might also explain the differential recognition of some epitopes [7]. In mice, multiple DC subsets have been described, however, CD8+ DCs are considered the primary DC subset involved in directly activating naïve CD8+ T cells during VACV infection [21]. Recently, the CD8- DC subset has also shown to play an important role [22]. In humans, various cell types are susceptible to VACV infection, including dermal DCs, Langerhans cells, and macrophages [23,24], but the primary APC responsible for naïve CD8+ T cell priming is unknown. As the peptides identified in the Tg mice were effectively processed in VACV-infected human APCs [5], differences in processing appear an unlikely explanation. Furthermore, while mice were immunized i.p. and humans were vaccinated by dermal scarification, we recently reported that either route generates similar T cell responses, and only minor differences in magnitude of responses were observed in mice [25].
Overall, our data suggest that while about half of the epitopes are recognized both in humans and Tg mice, the actual magnitudes vary, and thus differences in the immunodominance pattern contribute to the degree of overlap in the responses observed. Discrepancies in the kinetics of viral antigen expression in infected cells of human and murine origin might impact immunodominance [26]. Interestingly, a recent study mapping HLA-A2.1-restricted epitopes derived from modified VACV Ankara-infected human B cells by differential HPLC-coupled mass spectrometry found epitopes predominantly derived from early gene products [27]. However, we identified A2.1-restricted epitopes in HLA Tg mice and human vaccinees from gene products expressed both early and late during the viral life cycle [28]. Despite having comparable T cell repertoires, the differences in immunodominance might be due to distinct naïve CD8+ T cell precursor frequencies in the two systems. We have recently demonstrated that precursor frequencies shape the CD8+ T cell immunodominance hierarchy following LCMV infection [29], and therefore this might also apply to a more complex virus, such as VACV. These conclusions are in agreement with those drawn from a recent study mapping the T cell responses in VACV-immune individuals, where it was pointed out that an epitope might be considered immunodominant if recognized by both humans and HLA Tg mice [30]. In summary, the present study suggests that, with limitations, HLA Tg mice represent a relevant and suitable model system for identification and validation of T cell epitopes recognized during the course of complex viral infection in humans.
Methods
Peptides
Peptides were synthesized by A and A Labs and purified to >95% homogeneity by reverse-phase high-performance liquid chromatography (HPLC). Purity was determined using analytical reverse-phase HPLC and amino acid analysis, sequencing, and/or mass spectrometry. Peptides were radio-labeled with the chloramine T method as described [31].
Characteristics of study population
Healthy males and females between 18 and 59 years of age were used in this study. Exclusion criteria were body weight of < 45.4 kg and established pregnancy. Recruited donors were vaccinated by arm scarification with a VACV (Dryvax) vaccination as a prophylactic measure either because of their potential exposure to VACV in a laboratory or hospital setting or because of their enrollment into military and health worker vaccination program. The blood draw was performed within 1 year of the Dryvax vaccination. All experiments carried out with human subjects were in compliance with the Helsinki Declaration. Institutional Review Board approval (FWA# 00000032) and appropriate informed consent were obtained for this study.
PBMC isolation and HLA typing
PBMC were isolated from heparinized blood by gradient centrifugation with a Histopaque-1077 (Sigma-Aldrich, St. Louis, MO) [32], suspended in fetal bovine serum containing 10% dimethyl sulfoxide, and cryo-preserved in liquid nitrogen. Donor's PBMC were typed for HLA-A by high-resolution polymerase chain reaction (Forensic Analytical Molecular Genetics, San Francisco, CA).
Mice
A2.1 Tg mice used in this study were the F1 generation derived from crossing homozygous Tg mice (H-2b haplotype) expressing a chimeric gene (A2.1/Kb) consisting of the α1 and α2 domains of HLA and the α3 domain of H-2Kb with BALB/c mice (The Jackson Laboratory, Bar Harbor, ME) [4,33,34]. The HHD A2.1 strain [19] was kindly provided by Dr. Jack Bennink (NIAID, NIH, Bethesda, MD). All mice were bred and maintained following NIH guidelines and Institutional Animal Care and Use Committee-approved animal protocols (AAALAC# 000840 and OLAW# A3779-01).
Viruses and infection
The Western Reserve strain of VACV was obtained from Dr. Bernard Moss (NIAID). Mice were infected i.p. with 2 × 106 PFU of VACV. On day 7 post-infection, the mice were sacrificed and splenic CD8+ T cells were used in mouse IFN-γ ELISPOT assays.
Ex vivo IFN-γ ELISPOT
IFN-γ ELISPOT assays were performed as described [5,9]. In brief, for murine assays, 2 × 105 splenic CD8+ T cells were cultured with 105 human Jurkat cells (expressing the same A2.1/Kb construct as in the Tg mice) pulsed with 10 μg/ml of peptide. For human assays, 2 × 105 PBMC were incubated with 5 μg/ml of peptide. After a 20 h incubation at 37°C, plates were developed, and responses calculated as described [5,9,19]. Criteria for positivity were net spot-forming cells (SFC)/106 cells ≥ 20, stimulation index ≥ 2.0, and p-value ≤ 0.05 using a Student's t test in at least 2 out of 3 experiments.
Abbreviations
A2.1: HLA-A*0201; APC: antigen-presenting cell; CTL: cytotoxic T lymphocyte; DC: dendritic cell; ELISPOT: enzyme-linked immunosorbent spot; HIV-1: human immunodeficiency virus type 1; HLA: human leukocyte antigen; HPLC: high-performance liquid chromatography; IFN-γ: interferon-γ; MHC: major histocompatibility complex; PBMC: peripheral blood mononuclear cells; SFC: spot-forming cells; Tg: transgenic; VACV: vaccinia virus.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
MFK, EA, CO, and VP performed the human and mouse immunoassays. AS conceived the study. BP and HG aided with data analysis. MFK, EA, and AS wrote the manuscript. All authors participated in discussions, and reviewed and approved the final manuscript version.
Supplementary Material
Recognition of additional A2.1-restricted VACV T cell epitopes by human A2-positive VACV vaccinees. The data provided represent the net SFC/106 PBMCs of the human A2-positive VACV vaccinees in response to epitopes originally identified in A2.1 Tg mice.
Acknowledgments
Acknowledgements
This study was supported by NIH Contract HHSN266200400024C. Kyowa Hakko Kirin California Publication No. 1100.
Contributor Information
Maya F Kotturi, Email: maya@liai.org.
Erika Assarsson, Email: erika.assarsson@gmail.com.
Bjoern Peters, Email: bpeters@liai.org.
Howard Grey, Email: hgrey@liai.org.
Carla Oseroff, Email: coseroff@liai.org.
Valerie Pasquetto, Email: vpasquetto@yahoo.com.
Alessandro Sette, Email: alex@liai.org.
References
- Wu SJ, Hayes CG, Dubois DR, Windheuser MG, Kang YH, Watts DM, Sieckmann DG. Evaluation of the severe combined immunodeficient (SCID) mouse as an animal model for dengue viral infection. Am J Trop Med Hyg. 1995;52:468–476. doi: 10.4269/ajtmh.1995.52.468. [DOI] [PubMed] [Google Scholar]
- Man S, Newberg MH, Crotzer VL, Luckey CJ, Williams NS, Chen Y, Huczko EL, Ridge JP, Engelhard VH. Definition of a human T cell epitope from influenza A non-structural protein 1 using HLA-A2.1 transgenic mice. Int Immunol. 1995;7:597–605. doi: 10.1093/intimm/7.4.597. [DOI] [PubMed] [Google Scholar]
- Shirai M, Arichi T, Nishioka M, Nomura T, Ikeda K, Kawanishi K, Engelhard VH, Feinstone SM, Berzofsky JA. CTL responses of HLA-A2.1-transgenic mice specific for hepatitis C viral peptides predict epitopes for CTL of humans carrying HLA-A2.1. J Immunol. 1995;154:2733–2742. [PubMed] [Google Scholar]
- Wentworth PA, Vitiello A, Sidney J, Keogh E, Chesnut RW, Grey H, Sette A. Differences and similarities in the A2.1-restricted cytotoxic T cell repertoire in humans and human leukocyte antigen-transgenic mice. Eur J Immunol. 1996;26:97–101. doi: 10.1002/eji.1830260115. [DOI] [PubMed] [Google Scholar]
- Pasquetto V, Bui HH, Giannino R, Banh C, Mirza F, Sidney J, Oseroff C, Tscharke DC, Irvine K, Bennink JR, Peters B, Southwood S, Cerundolo V, Grey H, Yewdell JW, Sette A. HLA-A*0201, HLA-A*1101, and HLA-B*0702 transgenic mice recognize numerous poxvirus determinants from a wide variety of viral gene products. J Immunol. 2005;175:5504–5515. doi: 10.4049/jimmunol.175.8.5504. [DOI] [PubMed] [Google Scholar]
- Sesma L, Alvarez I, Marcilla M, Paradela A, Lopez de Castro JA. Species-specific differences in proteasomal processing and tapasin-mediated loading influence peptide presentation by HLA-B27 in murine cells. J Biol Chem. 2003;278:46461–46472. doi: 10.1074/jbc.M308816200. [DOI] [PubMed] [Google Scholar]
- Braud VM, McMichael AJ, Cerundolo V. Differential processing of influenza nucleoprotein in human and mouse cells. Eur J Immunol. 1998;28:625–635. doi: 10.1002/(SICI)1521-4141(199802)28:02<625::AID-IMMU625>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Burgevin A, Saveanu L, Kim Y, Barilleau E, Kotturi M, Sette A, van Endert P, Peters B. A detailed analysis of the murine TAP transporter substrate specificity. PLoS ONE. 2008;3:e2402. doi: 10.1371/journal.pone.0002402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oseroff C, Kos F, Bui HH, Peters B, Pasquetto V, Glenn J, Palmore T, Sidney J, Tscharke DC, Bennink JR, Southwood S, Grey HM, Yewdell JW, Sette A. HLA class I-restricted responses to vaccinia recognize a broad array of proteins mainly involved in virulence and viral gene regulation. Proc Natl Acad Sci USA. 2005;102:13980–13985. doi: 10.1073/pnas.0506768102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Denny TN. HLA-A2-restricted human CD8(+) cytotoxic T lymphocyte responses to a novel epitope in vaccinia virus that is conserved among orthopox viruses. J Infect Dis. 2006;194:168–175. doi: 10.1086/505224. [DOI] [PubMed] [Google Scholar]
- Terajima M, Cruz J, Leporati AM, Demkowicz WE, Jr, Kennedy JS, Ennis FA. Identification of vaccinia CD8+ T-cell epitopes conserved among vaccinia and variola viruses restricted by common MHC class I molecules, HLA-A2 or HLA-B7. Hum Immunol. 2006;67:512–520. doi: 10.1016/j.humimm.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Tang J, Murtadha M, Schnell M, Eisenlohr LC, Hooper J, Flomenberg P. Human T-cell responses to vaccinia virus envelope proteins. J Virol. 2006;80:10010–10020. doi: 10.1128/JVI.00601-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assarsson E, Sidney J, Oseroff C, Pasquetto V, Bui HH, Frahm N, Brander C, Peters B, Grey H, Sette A. A quantitative analysis of the variables affecting the repertoire of T cell specificities recognized after vaccinia virus infection. J Immunol. 2007;178:7890–7901. doi: 10.4049/jimmunol.178.12.7890. [DOI] [PubMed] [Google Scholar]
- Smith CL, Mirza F, Pasquetto V, Tscharke DC, Palmowski MJ, Dunbar PR, Sette A, Harris AL, Cerundolo V. Immunodominance of poxviral-specific CTL in a human trial of recombinant-modified vaccinia Ankara. J Immunol. 2005;175:8431–8437. doi: 10.4049/jimmunol.175.12.8431. [DOI] [PubMed] [Google Scholar]
- Snyder JT, Belyakov IM, Dzutsev A, Lemonnier F, Berzofsky JA. Protection against lethal vaccinia virus challenge in HLA-A2 transgenic mice by immunization with a single CD8+ T-cell peptide epitope of vaccinia and variola viruses. J Virol. 2004;78:7052–7060. doi: 10.1128/JVI.78.13.7052-7060.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler I, Staib C, Kastenmuller W, Stevanovic S, Schmidt B, Lemonnier FA, Rammensee HG, Busch DH, Bernhard H, Erfle V, Sutter G. Identification of vaccinia virus epitope-specific HLA-A*0201-restricted T cells and comparative analysis of smallpox vaccines. Proc Natl Acad Sci USA. 2003;100:217–222. doi: 10.1073/pnas.262668999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terajima M, Ennis FA. Using HLA-transgenic mice to identify immunodominant human CD8+ T cell epitopes – does (genome) size matter? Immunol Lett. 2006;105:97–98. doi: 10.1016/j.imlet.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Terajima M, Cruz J, Raines G, Kilpatrick ED, Kennedy JS, Rothman AL, Ennis FA. Quantitation of CD8+ T cell responses to newly identified HLA-A*0201-restricted T cell epitopes conserved among vaccinia and variola (smallpox) viruses. J Exp Med. 2003;197:927–932. doi: 10.1084/jem.20022222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascolo S, Bervas N, Ure JM, Smith AG, Lemonnier FA, Perarnau B. HLA-A2.1-restricted education and cytolytic activity of CD8(+) T lymphocytes from beta2 microglobulin (beta2m) HLA-A2.1 monochain transgenic H-2Db beta2m double knockout mice. J Exp Med. 1997;185:2043–2051. doi: 10.1084/jem.185.12.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbet S, Nielsen HV, Vinner L, Lauemoller S, Therrien D, Tang S, Kronborg G, Mathiesen L, Chaplin P, Brunak S, Buus S, Fomsgaard A. Optimization and immune recognition of multiple novel conserved HLA-A2, human immunodeficiency virus type 1-specific CTL epitopes. J Gen Virol. 2003;84:2409–2421. doi: 10.1099/vir.0.19152-0. [DOI] [PubMed] [Google Scholar]
- Belz GT, Smith CM, Eichner D, Shortman K, Karupiah G, Carbone FR, Heath WR. Cutting edge: conventional CD8 alpha+ dendritic cells are generally involved in priming CTL immunity to viruses. J Immunol. 2004;172:1996–2000. doi: 10.4049/jimmunol.172.4.1996. [DOI] [PubMed] [Google Scholar]
- Yammani RD, Pejawar-Gaddy S, Gurley TC, Weimer ET, Hiltbold EM, Alexander-Miller MA. Regulation of maturation and activating potential in CD8+ versus CD8-dendritic cells following in vivo infection with vaccinia virus. Virology. 2008;378:142–150. doi: 10.1016/j.virol.2008.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Xu Z, Fuhlbrigge RC, Pena-Cruz V, Lieberman J, Kupper TS. Vaccinia virus induces strong immunoregulatory cytokine production in healthy human epidermal keratinocytes: a novel strategy for immune evasion. J Virol. 2005;79:7363–7370. doi: 10.1128/JVI.79.12.7363-7370.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Puig JM, Sanchez L, Roy G, Blasco R. Susceptibility of different leukocyte cell types to Vaccinia virus infection. Virol J. 2004;1:10. doi: 10.1186/1743-422X-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oseroff C, Peters B, Pasquetto V, Moutaftsi M, Sidney J, Panchanathan V, Tscharke DC, Maillere B, Grey H, Sette A. Dissociation between epitope hierarchy and immunoprevalence in CD8 responses to vaccinia virus western reserve. J Immunol. 2008;180:7193–7202. doi: 10.4049/jimmunol.180.11.7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst HC, Tschannen K, Gallimore A, Martinic M, Basler M, Dumrese T, Jones E, Broek MF van den. Immunodominance of an antiviral cytotoxic T cell response is shaped by the kinetics of viral protein expression. J Immunol. 2003;171:5415–5422. doi: 10.4049/jimmunol.171.10.5415. [DOI] [PubMed] [Google Scholar]
- Meyer VS, Kastenmuller W, Gasteiger G, Franz-Wachtel M, Lamkemeyer T, Rammensee HG, Stevanovic S, Sigurdardottir D, Drexler I. Long-term immunity against actual poxviral HLA ligands as identified by differential stable isotope labeling. J Immunol. 2008;181:6371–6383. doi: 10.4049/jimmunol.181.9.6371. [DOI] [PubMed] [Google Scholar]
- Assarsson E, Greenbaum JA, Sundstrom M, Schaffer L, Hammond JA, Pasquetto V, Oseroff C, Hendrickson RC, Lefkowitz EJ, Tscharke DC, Sidney J, Grey HM, Head SR, Peters B, Sette A. Kinetic analysis of a complete poxvirus transcriptome reveals an immediate-early class of genes. Proc Natl Acad Sci USA. 2008;105:2140–2145. doi: 10.1073/pnas.0711573105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotturi MF, Scott I, Wolfe T, Peters B, Sidney J, Cheroutre H, von Herrath MG, Buchmeier MJ, Grey H, Sette A. Naive precursor frequencies and MHC binding rather than the degree of epitope diversity shape CD8+ T cell immunodominance. J Immunol. 2008;181:2124–2133. doi: 10.4049/jimmunol.181.3.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terajima M, Orphin L, Leporati AM, Pazoles P, Cruz J, Rothman AL, Ennis FA. Vaccinia virus-specific CD8(+) T-cell responses target a group of epitopes without a strong immunodominance hierarchy in humans. Hum Immunol. 2008;69:815–825. doi: 10.1016/j.humimm.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidney J, Southwood S, Oseroff C, Guercio MFD, Sette A, Grey H. Measurement of MHC/peptide interactions by gel filtration. In: Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W, editor. Current Protocols in Immunology I. New York: John Wiley & Sons; 1998. pp. 18.13.11–18.13.19. [Google Scholar]
- Kanof ME, Smith PD, Zola H. Isolation of whole mononuclear cells from peripheral blood and cord blood. Curr Protoc Immunol. 2001;Chapter 7:1. doi: 10.1002/0471142735.im0701s19. [DOI] [PubMed] [Google Scholar]
- Alexander J, Oseroff C, Sidney J, Sette A. Derivation of HLA-B*0702 transgenic mice: functional CTL repertoire and recognition of human B*0702-restricted CTL epitopes. Hum Immunol. 2003;64:211–223. doi: 10.1016/S0198-8859(02)00786-3. [DOI] [PubMed] [Google Scholar]
- Alexander J, Oseroff C, Sidney J, Wentworth P, Keogh E, Hermanson G, Chisari FV, Kubo RT, Grey HM, Sette A. Derivation of HLA-A11/Kb transgenic mice: functional CTL repertoire and recognition of human A11-restricted CTL epitopes. J Immunol. 1997;159:4753–4761. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Recognition of additional A2.1-restricted VACV T cell epitopes by human A2-positive VACV vaccinees. The data provided represent the net SFC/106 PBMCs of the human A2-positive VACV vaccinees in response to epitopes originally identified in A2.1 Tg mice.