Abstract
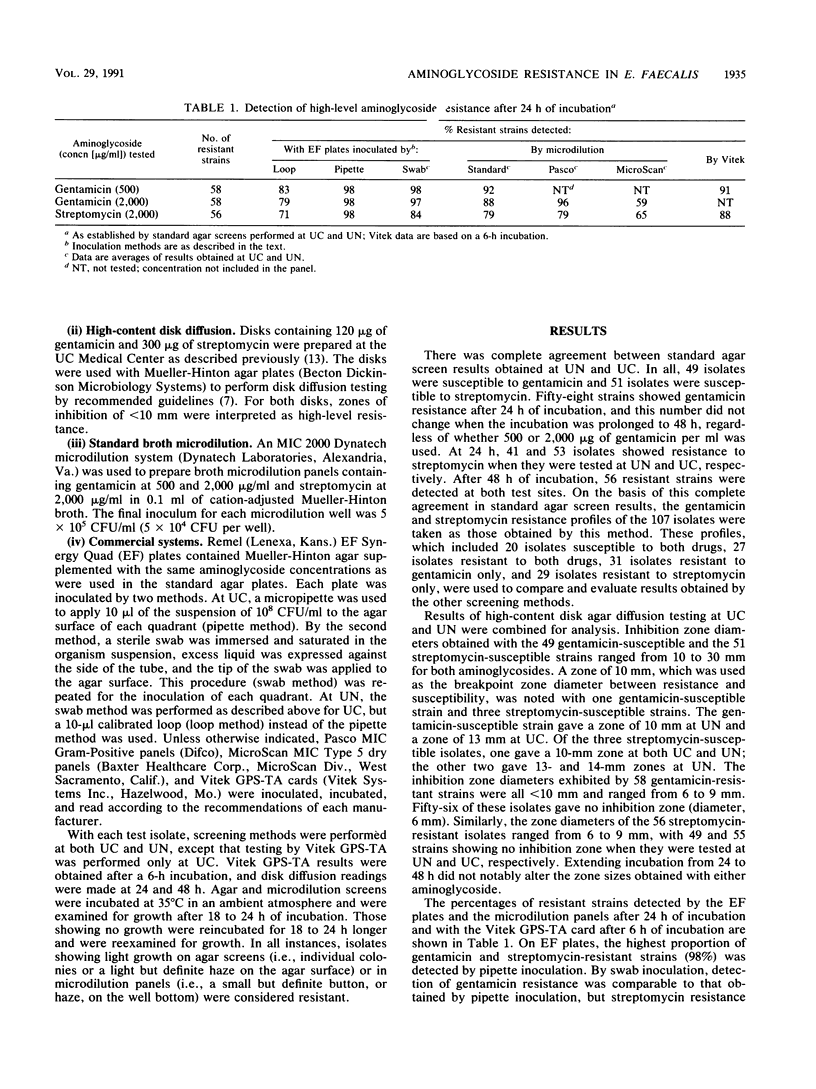
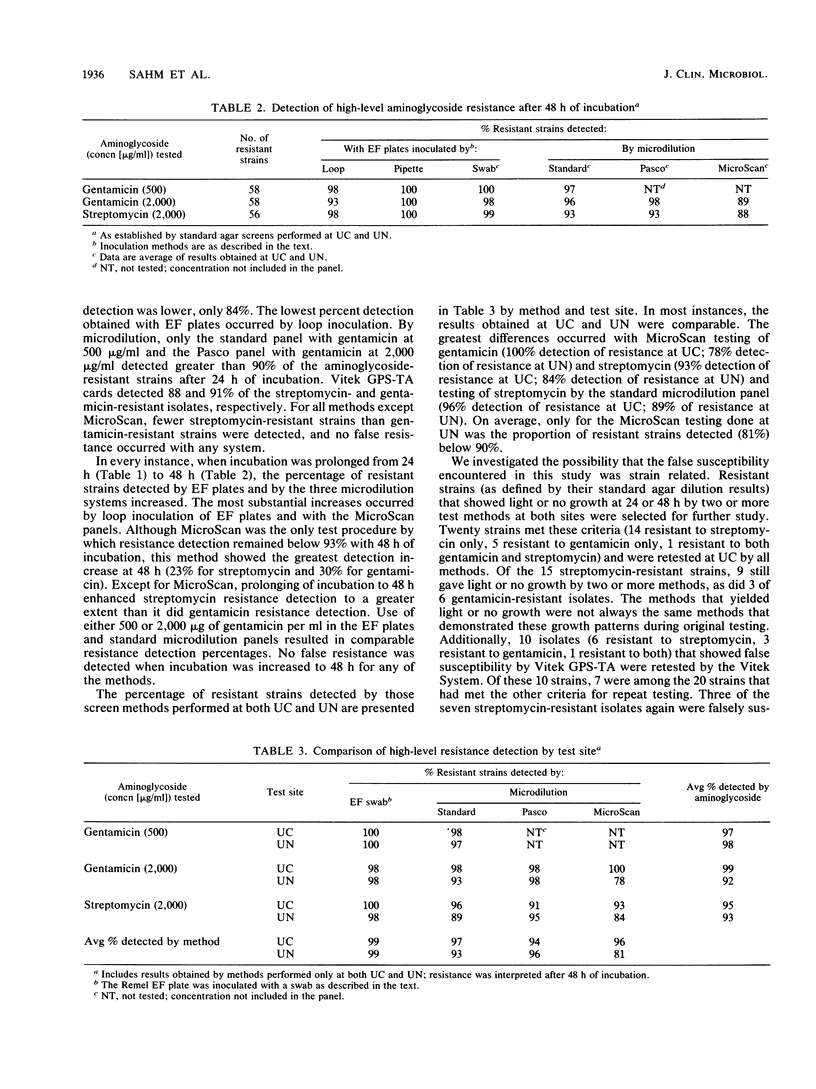
The ability of seven methods to detect high-level gentamicin (58 strains) and streptomycin resistance (56 strains) among 107 Enterococcus faecalis isolates was investigated at the University of Chicago Medical Center and the University of Nebraska Medical Center. Methods included a standard agar screen plate, high-content disk diffusion, Remel (Lenexa, Kans.) EF Synergy Quad plates, standard microdilution panels prepared in house, Pasco MIC Gram-Positive panels (Difco Laboratories, Detroit, Mich.), MicroScan MIC Type 5 dry panels (Baxter Healthcare Corp., MicroScan Div., West Sacramento, Calif.), and Vitek GPS-TA cards (Vitek Systems Inc., Hazelwood, Mo.). Results indicating false resistance were not obtained by any method, and there was 100% agreement between the results of the disk diffusion and standard agar screen methods. Prolonging incubation from 24 to 48 h increased resistance detection for both agar and microdilution screens. EF Synergy Quad plates inoculated with micropipettes detected 100% of the streptomycin- and gentamicin-resistant isolates. Resistance detection for streptomycin and gentamicin, respectively, was 93 and 96% by standard microdilution, 93 and 98% by Pasco panels, 88 and 89% by MicroScan panels, and 88 and 91% by Vitek GPS-TA cards. False susceptibility occurred more frequently with streptomycin-resistant isolates than it did with gentamicin-resistant strains and appeared to be strain related in some instances. The use of an increased inoculum size enhanced resistance detection with these strains, but it complicated interpretation of results and led to the selection of streptomycin-resistant mutants. Until results of further studies delineate optimum test conditions, a delay in the final interpretation of agar and microdilution screen results until 48 h for isolates showing no or light growth at 24 h may help to minimize the occurrence of false susceptibility reporting.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eliopoulos G. M., Farber B. F., Murray B. E., Wennersten C., Moellering R. C., Jr Ribosomal resistance of clinical enterococcal to streptomycin isolates. Antimicrob Agents Chemother. 1984 Mar;25(3):398–399. doi: 10.1128/aac.25.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller S. A., Low D. E., Simor A. E. Evaluation of a commercial microtiter system (MicroScan) using both frozen and freeze-dried panels for detection of high-level aminoglycoside resistance in Enterococcus spp. J Clin Microbiol. 1990 May;28(5):1051–1053. doi: 10.1128/jcm.28.5.1051-1053.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann S. A., Moellering R. C., Jr The enterococcus: "putting the bug in our ears". Ann Intern Med. 1987 May;106(5):757–761. doi: 10.7326/0003-4819-106-5-757. [DOI] [PubMed] [Google Scholar]
- Nachamkin I., Axelrod P., Talbot G. H., Fischer S. H., Wennersten C. B., Moellering R. C., Jr, MacGregor R. R. Multiply high-level-aminoglycoside-resistant enterococci isolated from patients in a university hospital. J Clin Microbiol. 1988 Jul;26(7):1287–1291. doi: 10.1128/jcm.26.7.1287-1291.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ounissi H., Derlot E., Carlier C., Courvalin P. Gene homogeneity for aminoglycoside-modifying enzymes in gram-positive cocci. Antimicrob Agents Chemother. 1990 Nov;34(11):2164–2168. doi: 10.1128/aac.34.11.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller M. A., Niles A. C., Murray P. R. Evaluation of the Kirby-Bauer disc diffusion test as a screening test for high-level aminoglycoside resistance in enterococci. Am J Clin Pathol. 1984 Oct;82(4):458–460. doi: 10.1093/ajcp/82.4.458. [DOI] [PubMed] [Google Scholar]
- Rosenthal S. L., Freundlich L. F. An aminoglycoside disk sensitivity test for use with enterococci. J Antimicrob Chemother. 1982 Nov;10(5):459–462. doi: 10.1093/jac/10.5.459. [DOI] [PubMed] [Google Scholar]
- Sahm D. F., Torres C. Effects of medium and inoculum variations on screening for high-level aminoglycoside resistance in Enterococcus faecalis. J Clin Microbiol. 1988 Feb;26(2):250–256. doi: 10.1128/jcm.26.2.250-256.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahm D. F., Torres C. High-content aminoglycoside disks for determining aminoglycoside-penicillin synergy against Enterococcus faecalis. J Clin Microbiol. 1988 Feb;26(2):257–260. doi: 10.1128/jcm.26.2.257-260.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel C. A., Huycke M. Endocarditis due to streptomycin-susceptible Enterococcus faecalis with high-level gentamicin resistance. Arch Intern Med. 1989 Aug;149(8):1873–1875. [PubMed] [Google Scholar]
- Spiegel C. A. Laboratory detection of high-level aminoglycoside-aminocyclitol resistance in Enterococcus spp. J Clin Microbiol. 1988 Nov;26(11):2270–2274. doi: 10.1128/jcm.26.11.2270-2274.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto S., Louie M., Low D. E., Patel M., Simor A. E. Comparison of the new MicroScan Pos MIC Type 6 panel and AMS-Vitek Gram Positive Susceptibility Card (GPS-TA) for detection of high-level aminoglycoside resistance in Enterococcus species. J Clin Microbiol. 1991 Jun;29(6):1258–1259. doi: 10.1128/jcm.29.6.1258-1259.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann D., Spargo J., Wennersten C., Ferraro M. J. Detection of enterococcal high-level aminoglycoside resistance with MicroScan freeze-dried panels containing newly modified medium and Vitek Gram-Positive Susceptibility cards. J Clin Microbiol. 1991 Jun;29(6):1232–1235. doi: 10.1128/jcm.29.6.1232-1235.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtz R., Sahm D., Flaherty J. Gentamicin-resistant, streptomycin-susceptible Enterococcus (Streptococcus) faecalis bacteremia. J Infect Dis. 1991 Jun;163(6):1393–1394. doi: 10.1093/infdis/163.6.1393. [DOI] [PubMed] [Google Scholar]
- Yagupsky P., Petry S., Menegus M. A. Comparison of four methods for testing high-level aminoglycoside resistance in enterococci. Eur J Clin Microbiol Infect Dis. 1990 Feb;9(2):133–135. doi: 10.1007/BF01963639. [DOI] [PubMed] [Google Scholar]
- Zervos M. J., Kauffman C. A., Therasse P. M., Bergman A. G., Mikesell T. S., Schaberg D. R. Nosocomial infection by gentamicin-resistant Streptococcus faecalis. An epidemiologic study. Ann Intern Med. 1987 May;106(5):687–691. doi: 10.7326/0003-4819-106-5-687. [DOI] [PubMed] [Google Scholar]
- Zervos M. J., Patterson J. E., Edberg S., Pierson C., Kauffman C. A., Mikesell T. S., Schaberg D. R. Single-concentration broth microdilution test for detection of high-level aminoglycoside resistance in enterococci. J Clin Microbiol. 1987 Dec;25(12):2443–2444. doi: 10.1128/jcm.25.12.2443-2444.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


