Abstract
Fruit flies exhibit robust attraction to food odors, which usually excite multiple glomeruli. To understand how the representation of such odors leads to behavior, we used genetic tools to dissect the contribution of each activated glomerulus. Apple cider vinegar triggers robust innate attraction at a relatively low concentration, which activates six glomeruli. By silencing individual glomeruli, we found that the absence of activity in two glomeruli, DM1 and VA2, markedly reduced attraction. Conversely, when each of these two glomeruli was selectively activated, flies exhibited as robust an attraction to vinegar as wild type flies. Notably, a higher concentration of vinegar excites an additional glomerulus and is less attractive to flies. Here we show that the activation of the additional glomerulus is necessary and sufficient to mediate the behavioral switch. Together, these results indicate that individual glomeruli, rather than the entire pattern of active glomeruli, mediate innate behavioral output.
The olfactory systems of phylogenetically diverse species exhibit numerous common features 1, 2, many of which are found in Drosophila; (1) each olfactory receptor neuron (ORN) expresses one or a few receptor genes which determine its odorant response profile 3–6, (2) all ORNs expressing the same receptor genes project to the same glomerulus 5–8, and (3) most output neurons send dendrites to a single glomerulus 9–11. Thus, each glomerulus can be considered a functional unit. A single odorant typically activates multiple receptor types 12, 13, and therefore elicits a distinct spatial pattern of activated glomeruli in the antennal lobe 14–16. However, the mechanism by which these patterns are actually used to drive behavioral responses remains to be determined. It is possible that the whole pattern is necessary to elicit behavioral output. Alternatively, parts of the pattern, or even individual glomeruli, could be important for olfactory behaviors. This information from the antennal lobe can then be read out by higher brain centers, which probably integrate information from multiple sensory modalities to generate motor responses.
In contrast to the patterns of several glomeruli activated by most odorants, recent studies have identified two odorants that activate single glomeruli – CO2 and the male-specific pheromone, cVA – which trigger innate avoidance and female courtship receptivity, respectively 17–21. By manipulating activity in the cognate receptor neurons, the activation of these single ORN channels was shown to be necessary and sufficient to produce the behavior, suggesting that these receptors are hardwired to specific behavioral outputs 17, 18, 22. These examples could be special cases because these odorants activate only one glomerulus, whereas most odorants excite multiple glomeruli. Furthermore, food odors contain many individual odorants 23, thus activating multiple glomeruli. Here we set out to study innate attraction to cider vinegar, a complex and highly attractive food odor, and to determine the role of individual glomeruli within the odor evoked pattern.
Behavioral assay
Fruit flies are highly attracted to vinegar, as it is associated with their favorite food source – rotting fruit 24. In order to observe this innate attraction behavior in individual flies, we used a four-field olfactometer design, which was recently applied to Drosophila 21. By recording the outcome of multiple decisions in each fly, we were able to obtain a robust and reliable score even when using a relatively small number of flies. We measured attraction by observing single flies walking in a four-field arena, in which each quadrant received a separate air stream. When vinegar was added to one of the air streams, the fly spent most of its time in the corresponding quadrant. We recorded the location of the fly at one-second intervals, and calculated a performance index by measuring the time spent in the odor quadrant. A fly that remained in the odor quadrant for the length of the assay would receive a score of 100%, whereas a fly that distributed its time equally among the four quadrants would score 0%, and a fly that spent no time in the odor quadrant would receive a score of −100%.
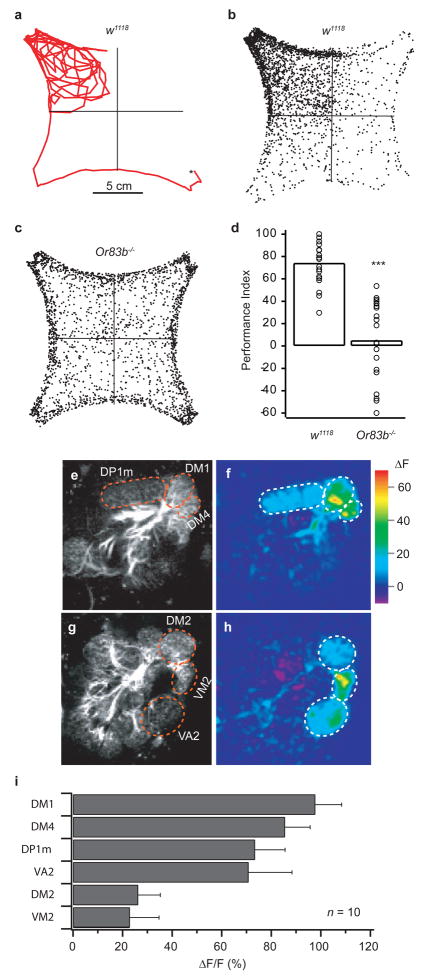
Using a concentration of 3 ppm (isobutylene equivalents) vinegar, we saw an average performance index of 75% (Fig. 1b), which is consistent with previous results 21. In order to verify that the behavior is mediated by the olfactory system, we measured attraction in flies whose antennae had been amputated, and found that they were indifferent to vinegar (PI = −6.7%, n=20). In addition, we tested flies with a targeted mutation of Or83b. Or83b is expressed in 80% of all ORNs 8, and acts in concert with other olfactory receptors (ORs) to generate responses to odorants 25–27. We found that attraction was virtually abolished in Or83b mutant flies (Fig. 1b and c), with the distribution of control w1118 flies almost entirely separated from the Or83b mutant animals (Fig. 1d, Supplementary Fig. S1). In the absence of odors, control and mutant flies are distributed equally in all four quadrants (Supplementary Fig. S2), and Or83b mutant flies showed no impairment in CO2 avoidance (PI = −87 ± 9%, n=12), suggesting that their locomotion capability is normal. Thus, attraction in this assay requires ORNs, and the Or83b mutation provides a useful tool to link ORN activity with behavioral output.
Figure 1. Flies are robustly attracted to apple cider vinegar, which excites six glomeruli.
a, Path of a single fly with 3 ppm vinegar in the upper left quadrant. b, c, Density plot of 20 w1118 and Or83b−/− flies. d, Performance index of w1118 and Or83b−/−flies. *** indicates P < 0.001; T-test. e, g, Pre-stimulation images showing glomerular structure. f, h, Responses to 3 ppm vinegar in flies bearing the GH146-Gal4 and UAS-GCaMP transgenes. i, Quantification of ΔF/F for all six glomeruli over ten flies. Error bars indicate s.e.m.
Visualizing glomerular activity
We next determined which glomeruli are activated by vinegar. We used the genetically encoded calcium sensor G-CaMP to monitor activity in the antennal lobe using two-photon microscopy 15. We imaged flies bearing the GH146-Gal4 and UAS-GCaMP transgenes, which have G-CaMP expression in 83 out of 150 projection neurons 9, 28, 29. Projection neurons (PNs) are the output neurons of the antennal lobe; thus their responses to odorants contain the information that is important for the behavioral response. We also imaged ORNs in flies bearing Or83b-Gal4 and UAS-GCaMP, and found that the PN response pattern is similar to the response of the ORNs (Supplementary Fig. S3), a result that is consistent with previous studies 14, 15. Although excitatory interglomerular connections do exist 30, recent studies have found that ORN input is the main determinant of PN output 31, 32.
From PN and ORN imaging, we found that, at 3 ppm (the concentration used for the behavioral assay) vinegar elicited a response in six glomeruli out of 34 labeled by GH146-Gal4. In the most posterior plane of the antennal lobe, three glomeruli – DM1, DM4, and DP1M – responded quite robustly (Fig. 1f). On a more anterior plane, three more glomeruli – DM2, VA2, and VM2 – also responded to varying degrees (Fig. 1h). Thus, at this behaviorally relevant concentration, vinegar excites six glomeruli. Although vinegar is a complex stimulus with many volatile components, previous studies have shown that several natural stimuli also elicit a surprisingly sparse response in the rodent olfactory bulb 33.
Two glomeruli relevant for attraction
In order to determine what role each activated glomerulus plays in mediating the attraction to vinegar, we silenced each ORN channel in turn, and asked how that affects the attraction behavior. Recently, a nearly complete map of ORN to glomerulus targeting was generated 5, 6, so we were able to match five of the six activated glomeruli with their corresponding ORs (the receptor for DP1m remains unknown). shibirets is a temperature sensitive mutant dynamin, which reversibly prevents neurotransmitter release at the non-permissive temperature (32° C) by blocking endocytosis 34. By generating flies bearing the UAS-shits transgene and selective Or-Gal4 drivers, we should be able to silence five of the six glomeruli. Indeed, silencing individual ORN types resulted in a dramatic reduction in the activity of their cognate PNs, without affecting the non-cognate PN response (Supplementary Fig. S4).
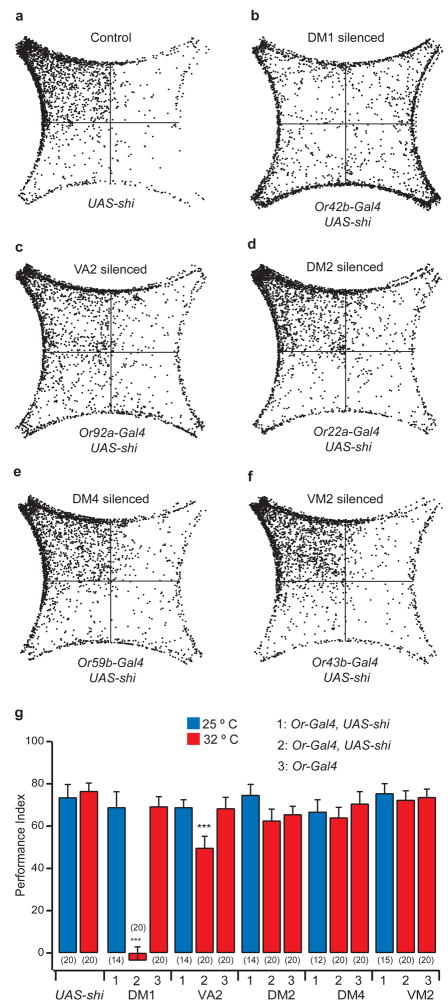
We found that when the Or42b neurons, which innervate the DM1 glomerulus, were silenced, the attraction to vinegar was virtually eliminated (Fig. 2b, g). At the non-permissive temperature, the performance index for these flies was −4%, compared to 69% at the permissive temperature. To independently confirm this result, we have measured attraction behavior in an Or42b mutant 35 and found a similar attraction deficit (PI = −18 ± 14%, n=18). Silencing the Or92a neurons, which innervate the VA2 glomerulus, also had a marked effect on the behavior, with the performance index declining to 50% at 32° C (Fig. 2c, g). Flies with silenced DM4 and VM2 glomeruli showed normal attraction, as did all the genetic background controls (Fig. 2 and Supplementary Fig. S5). The deficits we observed when DM1 or VA2 were silenced suggest that these receptor neuron channels are required for the innate attraction behavior, and could function as labeled lines for attraction. However, a model in which DM1 and VA2 are necessary for attraction in conjunction with other ORNs would also be consistent with these data.
Figure 2. Silencing DM1 or VA2 reduces attraction to 3 ppm vinegar.
a–f, Density plots composed of 20 flies each. g, Performance indices for flies bearing the OrX-Gal4 and UAS-shits transgenes at permissive and nonpermissive temperatures. ANOVA followed by Tukey’s test was performed on PI values from flies of the experimental group at the permissive and nonpermissive temperatures, and the corresponding genetic background controls at the nonpermissive temperature. ***, P < 0.001; and **, P < 0.01. Error bars indicate s.e.m.
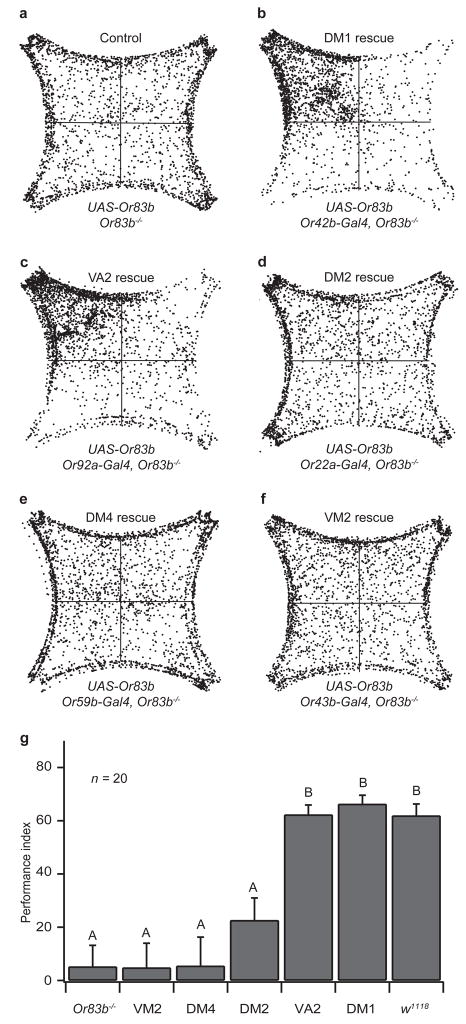
We next asked whether individual receptor neuron channels could elicit attraction when activated alone. Since Or83b mutant flies lack a vital component of the olfactory signaling pathway and are blind to vinegar, we reasoned that by restoring Or83b expression in specific ORNs, we could force vinegar to selectively activate a single Or83b-expressing glomerulus. Thus, we can determine what type of behavioral output each glomerulus would produce. We used Or-Gal4 lines to drive expression of a UAS-Or83b transgene in Or83b mutant flies. Calcium imaging experiments confirmed that the rescue flies exhibit normal olfactory responses in the corresponding ORNs (Supplementary Fig. S6). Remarkably, when the receptor neurons for either DM1 or VA2 were rescued, attraction was restored to normal levels (Fig. 3b, c, g). These results indicate that it is activity in DM1 or VA2, and not the pattern of the six glomeruli, which is read out by higher brain centers to signal the attractiveness of the odor. The finding that VA2 activity is sufficient for attraction may appear inconsistent with the fact that DM1 silenced flies show no attraction to vinegar. However, VA2 may be more robustly activated in the rescue flies, because in the silencing experiments, activation of several remaining ORN channels could result in inhibition of VA2. Indeed, a recent study has shown that adding receptor channel inputs increases lateral inhibition, leading to a reduction in the PN response 36.
Figure 3. Restoring Or83b in DM1 and VA2 ORNs restores attraction to control levels.
a–f, Density plots of 20 flies responding to 3 ppm vinegar. g, Performance indices of flies in which Or83b is selectively restored in individual ORN types. Comparisons between groups were made using ANOVA followed by Tukey’s test. Significant differences (P < 0.05) are denoted by letters. Error bars indicate s.e.m.
Concentration dependent behavioral switch
As odor concentration is increased, odors that are attractive at low concentrations often become less attractive or even repulsive 37. Increasing the odorant concentration often recruits additional receptor neurons, and thus it has been proposed that the change in behavior is mediated by the addition of these glomeruli to the ensemble of activated glomeruli 38, but this hypothesis has not been tested directly. It is also possible that increased activation of the glomeruli that were active at the low concentration could mediate the change in behavioral output 39. Alternatively, the new glomeruli could independently induce aversion.
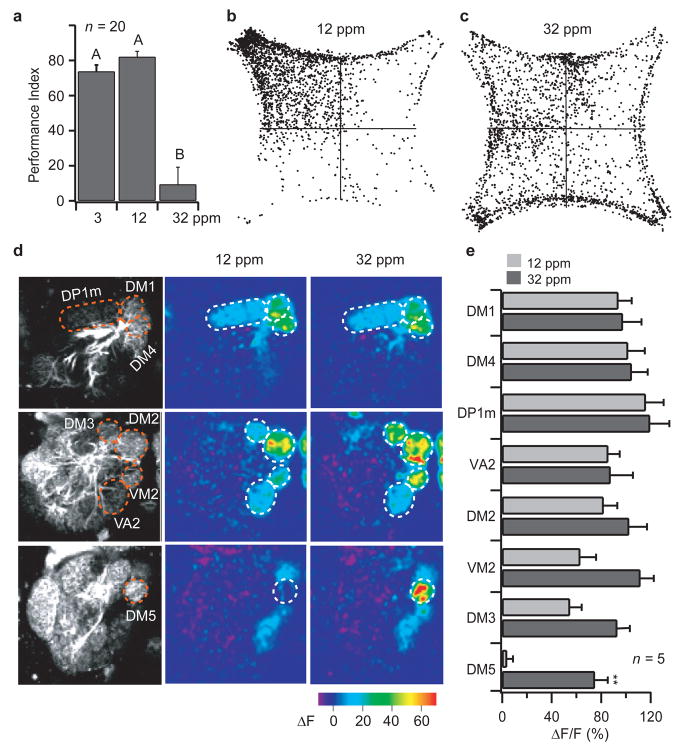
We first measured the olfactory behavior over a range of vinegar concentrations. As we increased the concentration, we observed a slight increase in attractiveness at 12 ppm (Fig. 4a) but then a striking decrease in attractiveness at 32 ppm, with the performance index dropping to 9% (Fig. 4a). We wondered whether the change could be due to recruitment of additional glomeruli, and used calcium imaging to determine the difference in the pattern of glomeruli activated in response to 12 and 32 ppm vinegar. We observed that the DM5 glomerulus, which showed no response at 12 ppm, was strongly activated by 32 ppm (Fig. 4d bottom row, and e). All other glomeruli that were activated by 12 ppm (DM1, DM4, DP1m, DM2, DM3, VM2, and VA2) showed small to moderate increases in response to 32 ppm vinegar.
Figure 4. Vinegar becomes less attractive and activates an additional glomerulus at high concentrations.
a, Performance indices of w1118 flies at various concentrations of vinegar. PI values were compared using ANOVA followed by Tukey’s test. Significant differences (P < 0.05) are denoted by letters. b,c, Density plots of w1118 behavior in response to 12 ppm and 32 ppm vinegar. d, Responses to 12 ppm and 32 ppm vinegar in flies bearing the GH146-Gal4 and UAS-GCaMP transgenes. e, Average ΔF/F. ** indicates P < 0.01; T-test. Error bars indicate s.e.m.
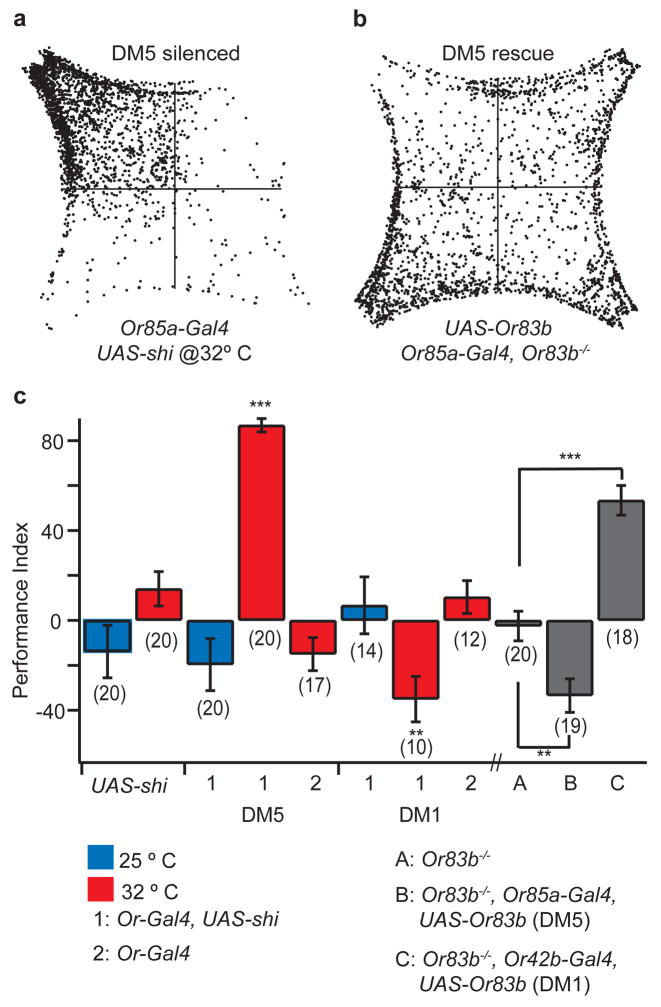
We next asked whether DM5 could be responsible for the decrease in attraction to vinegar observed at 32 ppm. Therefore, we silenced the DM5 glomerulus by expressing shibirets in its cognate ORNs, which express Or85a. At the nonpermissive temperature, we found that the performance index for 32 ppm vinegar increased to 87% (Fig. 5a, c). In contrast, silencing DM1 resulted in repulsion towards 32 ppm vinegar (Fig. 5c). Thus, the activation of DM5 is responsible for the decrease in attractiveness towards 32 ppm vinegar.
Figure 5. DM5 mediates the decrease in attraction in response to 32 ppm vinegar.
a, Density plot of twenty flies in which the DM5 ORNs are silenced. b, Density plot of 20 DM5 rescue flies. c, Behavioral responses to 32 ppm vinegar for flies in which DM5 and DM1 are silenced and selectively rescued. For silencing experiments, we performed the same statistical analysis as for Figure 2. DM5 rescue and DM1 rescue flies were compared to Or83b−/− flies by T-test. ***, P < 0.001; and **, P < 0.01.
In light of the above result, it is possible that the activation of DM5 alone mediates aversion, or that activation of DM5 together with other specific glomeruli could mediate aversion. To distinguish between these models, we forced the stimulus to activate only DM5 by expressing Or83b in Or85a ORNs in the Or83b mutant background. We found that these flies were repulsed by 32 ppm vinegar, whereas the Or83b mutant flies showed no preference or aversion to the odorant (Fig. 5b, c). In contrast, when DM1 was selectively activated by expression of Or83b in Or42b ORNs, flies were attracted to 32 ppm vinegar (Fig. 5c and Supplementary Fig. S8). These findings suggest that the higher concentration of vinegar recruits an additional glomerulus that independently mediates aversion. When wild type flies are exposed to 32 ppm vinegar, the activation of an aversive glomerulus may counterbalance the activation of the two attractive glomeruli, resulting in a PI near zero.
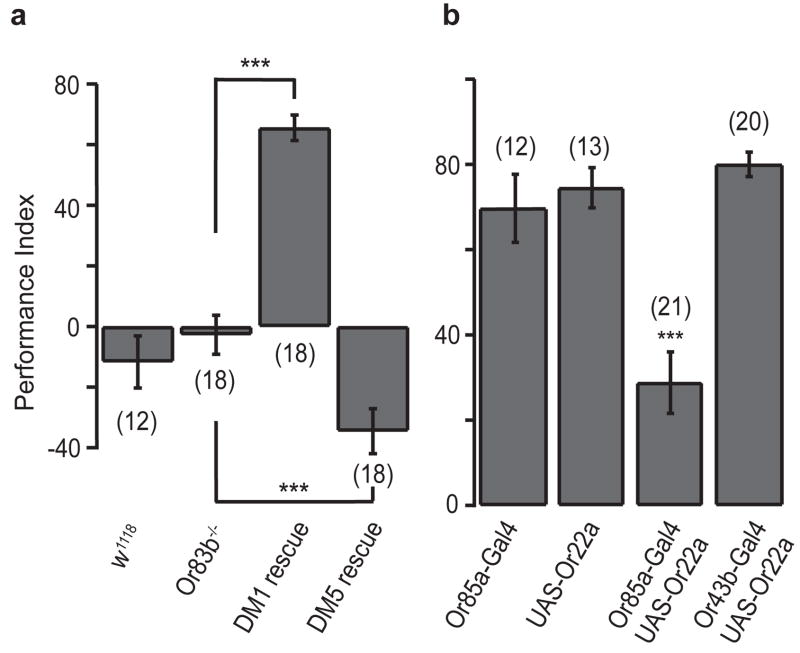
If attraction and aversion are mediated by the activation of specific glomeruli, other odors that activate these glomeruli should give the same behavioral output. For example, an odor that excites DM1 should be attractive to flies in which DM1 ORNs are selectively activated, while an odor that selectively excites DM5 should be repulsive. We have identified an odorant, ethyl butyrate, that excites the DM1, DM2, VM2 and DM5 glomeruli (Supplementary Fig. S7) but has not been detected by gas chromatography in cider vinegar 40. When we selectively restored function in DM1 ORNs, we found that ethyl butyrate triggered attraction behavior, with a PI of 65%. Conversely, when we selectively restored function in the DM5 ORNs, the result was an aversion to ethyl butyrate, with a PI of −34% (Fig. 6a). These results suggest that the activation of DM1 or DM5 by any odor should be sufficient for attraction and aversion, respectively.
Figure 6. DM1 and DM5 mediate attraction and aversion in response to ethyl butyrate.
a, Performance indices in response to 7 ppm ethyl butyrate for DM1 and DM5 rescue flies. ***, P < 0.001; T-test. b, Ectopic expression of Or22a in Or85a ORNs reduced attraction to 12 ppm vinegar. PI values were compared using ANOVA followed by Tukey’s test. *** indicates P < 0.001.
If specific glomeruli are hardwired to generate attraction and aversion behavior, activation of ectopically expressed receptors should give a similar behavioral output. We predict that expression of the Or22a receptor in Or85a ORNs, which project to DM5, should make these neurons sensitive to lower concentrations of vinegar, and bias the behavior towards aversion. Indeed, these flies exhibit a dramatic reduction in PI value in response to 12 ppm vinegar (Fig. 6b), indicating that it is activity in the DM5 ORNs, rather than activation of a particular receptor, that biases the behavior toward aversion.
Discussion
Previous studies have shown that in certain cases, olfactory behaviors are elicited by dedicated receptor channels or labeled lines 17, 18. In this study, we demonstrate that innate attraction to a complex food odor is similarly mediated by a few of the activated glomeruli. However, it is possible that other glomeruli not activated by cider vinegar could also mediate innate attraction to other food odors. A recent study of olfactory behavior in Drosophila larvae also addressed the question of how receptor activation leads to behavioral output, and found that the responses of five ORNs to a panel of odorants can be used to generate a model that accounts for 81% of the variation in olfactory behavior 39. Selective activation of these ORNs should generate robust innate attraction or aversion. In fact, the Or42a ORN, one of the five critical ORNs, has been shown to be sufficient for attraction behavior 41.
We further show that the decrease in attractiveness in response to a higher concentration of vinegar is due to the activation of an additional glomerulus. It is a common feature of olfactory perception that most odors become less pleasant and eventually repellent as their intensity is increased 37, a phenomenon that has also been observed in Drosophila 23, 42, 43. The recruitment of additional glomeruli has been proposed as a mechanism to mediate this change in behavioral output 38. A recent paper has suggested that different levels of activation in the same ORNs could generate qualitatively different behavioral responses 39. Here, we found that a glomerulus recruited by a high concentration of vinegar, DM5, plays an important role in the behavioral switch. Silencing and selective activation experiments show that DM5 is necessary and sufficient for the behavioral switch.
The present results suggest that certain olfactory receptor neurons in Drosophila are genetically hardwired to generate robust innate olfactory attraction or avoidance behavior, an organizing principle that has been observed in several chemosensory systems 17, 18, 44, 45. In the fly, projection neurons receive input from ORNs and send axons to the mushroom body and lateral horn. Further studies should shed light on the mechanism by which these centers generate the behaviors we observe.
Methods Summary
Behavioral assay
An existing behavioral paradigm was modified to measure the response of single flies to odors 21. The four field olfactometer consisted of a four-pointed star-shaped arena. Air flow was maintained by vacuum suction. such that air entered each quadrant at a rate of 200 mL/min, after passing through a 100 mL bottle. Female flies which had been starved for 50 hours were used. After the addition of an odorant to one quadrant, the fly’s location was measured once per second. The performance index is defined as (2p1/2−1) × 100%, were p is the fraction of time the fly spends in the odor quadrant between 50 and 250 seconds after odor application.
Odor stimuli
Odor concentration was measured using a photoionization detector (Rae Systems, MiniRAE 2000) and an air flow of 200 mL/min through a 100 mL bottle containing the odorant. As the conversion factor to determine the exact concentration of cider vinegar volatiles is unknown, we express the concentration in isobutylene equivalents. The 3 ppm concentration of vinegar corresponds to 40 uL of a 1:2 dilution of apple cider vinegar with water on filter paper. 12 ppm is 80 uL vinegar, 32 ppm is 1 mL vinegar, and 7 ppm ethyl butyrate came from 40 uL of a 1:1000 dilution of ethyl butyrate in mineral oil. The odor source was replenished for each experiment. Odor concentrations stayed constant over the time course of an experiment.
G-CaMP imaging experiments
Calcium imaging was performed as described 15, 46, except that the air flow rate was 200 mL/min. Odorants were administered from 100 mL bottles as described above, and stimuli were given for 2 seconds.
Transgenic Flies
The following fly stocks were used: Or42b-Gal4, Or43b-Gal4, Or92a-Gal4, Or22a-Gal4, and Or92a-Gal4 5; Or59b-Gal4 6; UAS-Or22a, UAS-Or83b, Or83b targeted deletion (Or83b2) 26; UAS-shibirets 34; UAS-GCaMP 15; GH146-Gal4 29; GH146-LexAGAD 47; LexAop-GCaMP-IRES-GCaMP 46.
Methods
Behavioral assay
An existing behavioral paradigm was modified to measure the response of single flies to odors 21. As previously described, the four field olfactometer consisted of a four-pointed star-shaped arena 30 cm across diagonally and 1 cm deep, covered by a glass plate. Air flow was maintained by vacuum suction such that air entered each quadrant at a rate of 200 mL/min, after passing through a 100 mL bottle. Only female flies were used, and at the time of the assay the flies were four days old and had been starved for 50 hours in a vial with a wet kimwipe. After a single fly was introduced into the chamber, its speed was measured for 100 seconds, and only flies with an average speed between 0.5 and 1.0 cm/second were used. At the start of the assay, one of the empty 100 mL bottles was replaced with an odor-containing bottle. The fly’s location was measured once per second using a Logitech quickcam and Labview software (National Instruments). The chamber was illuminated by a panel of LEDs (660 nm). Light reflected from the glass plate was eliminated by polarizing optics. The performance index is defined as (2p1/2−1) × 100%, were p is the fraction of time the fly spends in the odor quadrant during the period between 50 and 250 seconds after odor application. Thus, if the fly is in the odor quadrant for the entire time window, p=1 and the performance index is 100%, whereas if the fly avoids the odor quadrant entirely, p=0 and the performance index will be −100%. Except for the shibirets nonpermissive temperature experiments (which were performed at 32° C), all behavioral experiments were performed at 25° C and 70% humidity. Data were analyzed using Igor Pro (Wavemetrics) and a custom macro. The Jarqe-Bera test was used to verify that the data were normally distributed. Density plots show data collected between 50 and 250 seconds for 20 flies. Each dot indicates one fly spending one second at that location. Odor application was alternated among the four quadrants, and the density plots were created by rotating the positional data so that the odor quadrant becomes the upper left quadrant.
Supplementary Material
Acknowledgments
We would like to thank David Anderson, Richard Axel, Charles Zuker, and Marco Gallio for comments on the manuscript. We thank Monica Hilker for help with the design of the four field olfactometer. We thank Cory Root for generating the data presented in Supplementary Figure S4. We thank Leslie Vosshall, Barry Dickson, and Tzumin Lee for providing fly stocks. This work was partially supported by a research grant from the Whitehall Foundation to J.W.W. and a grant from the National Institute of Deafness and other Communication Disorders to J.W.W. (R01DC009597). J.W.W. is a Beckman Young Investigator, a Hellman Faculty scholar, and a Searle scholar.
References
- 1.Hildebrand JG, Shepherd GM. Mechanisms of olfactory discrimination: converging evidence for common principles across phyla. Annu Rev Neurosci. 1997;20:595–631. doi: 10.1146/annurev.neuro.20.1.595. [DOI] [PubMed] [Google Scholar]
- 2.Bargmann CI. Comparative chemosensation from receptors to ecology. Nature. 2006;444:295–301. doi: 10.1038/nature05402. [DOI] [PubMed] [Google Scholar]
- 3.Vosshall LB, Amrein H, Morozov PS, Rzhetsky A, Axel R. A spatial map of olfactory receptor expression in the Drosophila antenna. Cell. 1999;96:725–36. doi: 10.1016/s0092-8674(00)80582-6. [DOI] [PubMed] [Google Scholar]
- 4.Goldman AL, Van der Goes van Naters W, Lessing D, Warr CG, Carlson JR. Coexpression of two functional odor receptors in one neuron. Neuron. 2005;45:661–6. doi: 10.1016/j.neuron.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 5.Fishilevich E, Vosshall LB. Genetic and functional subdivision of the Drosophila antennal lobe. Curr Biol. 2005;15:1548–53. doi: 10.1016/j.cub.2005.07.066. [DOI] [PubMed] [Google Scholar]
- 6.Couto A, Alenius M, Dickson BJ. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr Biol. 2005;15:1535–47. doi: 10.1016/j.cub.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 7.Gao Q, Yuan B, Chess A. Convergent projections of Drosophila olfactory neurons to specific glomeruli in the antennal lobe. Nat Neurosci. 2000;3:780–5. doi: 10.1038/77680. [DOI] [PubMed] [Google Scholar]
- 8.Vosshall LB, Wong AM, Axel R. An olfactory sensory map in the fly brain. Cell. 2000;102:147–59. doi: 10.1016/s0092-8674(00)00021-0. [DOI] [PubMed] [Google Scholar]
- 9.Wong AM, Wang JW, Axel R. Spatial representation of the glomerular map in the Drosophila protocerebrum. Cell. 2002;109:229–41. doi: 10.1016/s0092-8674(02)00707-9. [DOI] [PubMed] [Google Scholar]
- 10.Marin EC, Jefferis GS, Komiyama T, Zhu H, Luo L. Representation of the glomerular olfactory map in the Drosophila brain. Cell. 2002;109:243–55. doi: 10.1016/s0092-8674(02)00700-6. [DOI] [PubMed] [Google Scholar]
- 11.Stocker RF, Lienhard MC, Borst A, Fischbach KF. Neuronal architecture of the antennal lobe in Drosophila melanogaster. Cell Tissue Res. 1990;262:9–34. doi: 10.1007/BF00327741. [DOI] [PubMed] [Google Scholar]
- 12.de Bruyne M, Foster K, Carlson JR. Odor coding in the Drosophila antenna. Neuron. 2001;30:537–52. doi: 10.1016/s0896-6273(01)00289-6. [DOI] [PubMed] [Google Scholar]
- 13.Hallem EA, Ho MG, Carlson JR. The molecular basis of odor coding in the Drosophila antenna. Cell. 2004;117:965–79. doi: 10.1016/j.cell.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Ng M, et al. Transmission of olfactory information between three populations of neurons in the antennal lobe of the fly. Neuron. 2002;36:463–74. doi: 10.1016/s0896-6273(02)00975-3. [DOI] [PubMed] [Google Scholar]
- 15.Wang JW, Wong AM, Flores J, Vosshall LB, Axel R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell. 2003;112:271–82. doi: 10.1016/s0092-8674(03)00004-7. [DOI] [PubMed] [Google Scholar]
- 16.Fiala A, et al. Genetically expressed cameleon in Drosophila melanogaster is used to visualize olfactory information in projection neurons. Curr Biol. 2002;12:1877–84. doi: 10.1016/s0960-9822(02)01239-3. [DOI] [PubMed] [Google Scholar]
- 17.Suh GS, et al. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature. 2004;431:854–9. doi: 10.1038/nature02980. [DOI] [PubMed] [Google Scholar]
- 18.Kurtovic A, Widmer A, Dickson BJ. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature. 2007;446:542–6. doi: 10.1038/nature05672. [DOI] [PubMed] [Google Scholar]
- 19.Clyne P, Grant A, O’Connell R, Carlson JR. Odorant response of individual sensilla on the Drosophila antenna. Invert Neurosci. 1997;3:127–35. doi: 10.1007/BF02480367. [DOI] [PubMed] [Google Scholar]
- 20.Ha TS, Smith DP. A pheromone receptor mediates 11-cis-vaccenyl acetate-induced responses in Drosophila. J Neurosci. 2006;26:8727–33. doi: 10.1523/JNEUROSCI.0876-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faucher C, Forstreuter M, Hilker M, de Bruyne M. Behavioral responses of Drosophila to biogenic levels of carbon dioxide depend on life-stage, sex and olfactory context. J Exp Biol. 2006;209:2739–48. doi: 10.1242/jeb.02297. [DOI] [PubMed] [Google Scholar]
- 22.Suh GS, et al. Light activation of an innate olfactory avoidance response in Drosophila. Curr Biol. 2007;17:905–8. doi: 10.1016/j.cub.2007.04.046. [DOI] [PubMed] [Google Scholar]
- 23.Stensmyr MC, Giordano E, Balloi A, Angioy AM, Hansson BS. Novel natural ligands for Drosophila olfactory receptor neurones. J Exp Biol. 2003;206:715–24. doi: 10.1242/jeb.00143. [DOI] [PubMed] [Google Scholar]
- 24.Zhu J, Park KC, Baker TC. Identification of odors from overripe mango that attract vinegar flies, Drosophila melanogaster. J Chem Ecol. 2003;29:899–909. doi: 10.1023/a:1022931816351. [DOI] [PubMed] [Google Scholar]
- 25.Sato K, et al. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature. 2008;452:1002–U9. doi: 10.1038/nature06850. [DOI] [PubMed] [Google Scholar]
- 26.Larsson MC, et al. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–14. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 27.Wicher D, et al. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature. 2008;452:1007–U10. doi: 10.1038/nature06861. [DOI] [PubMed] [Google Scholar]
- 28.Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci. 2007;30:505–33. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- 29.Stocker RF, Heimbeck G, Gendre N, de Belle JS. Neuroblast ablation in Drosophila P[GAL4] lines reveals origins of olfactory interneurons. J Neurobiol. 1997;32:443–56. doi: 10.1002/(sici)1097-4695(199705)32:5<443::aid-neu1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 30.Shang Y, Claridge-Chang A, Sjulson L, Pypaert M, Miesenbock G. Excitatory local circuits and their implications for olfactory processing in the fly antennal lobe. Cell. 2007;128:601–12. doi: 10.1016/j.cell.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olsen SR, Bhandawat V, Wilson RI. Excitatory interactions between olfactory processing channels in the Drosophila antennal lobe. Neuron. 2007;54:89–103. doi: 10.1016/j.neuron.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Root CM, Semmelhack JL, Wong AM, Flores J, Wang JW. Propagation of olfactory information in Drosophila. Proc Natl Acad Sci U S A. 2007;104:11826–31. doi: 10.1073/pnas.0704523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin DY, Shea SD, Katz LC. Representation of natural stimuli in the rodent main olfactory bulb. Neuron. 2006;50:937–49. doi: 10.1016/j.neuron.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 34.Kitamoto T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J eurobiol. 2001;47:81–92. doi: 10.1002/neu.1018. [DOI] [PubMed] [Google Scholar]
- 35.Bellen HJ, et al. The BDGP gene disruption project: single transposon insertions associated with 40% of Drosophila genes. Genetics. 2004;167:761–81. doi: 10.1534/genetics.104.026427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asahina K, Louis M, Piccinotti S, Vosshall LB. A circuit supporting concentration-invariant odor perception in Drosophila. J Biol. 2009;8:9. doi: 10.1186/jbiol108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laing DG, Panhuber H, Baxter RI. Olfactory Properties of Amines and N-Butanol. Chemical Senses & Flavour. 1978;3:149–166. [Google Scholar]
- 38.Malnic B, Hirono J, Sato T, Buck LB. Combinatorial receptor codes for odors. Cell. 1999;96:713–23. doi: 10.1016/s0092-8674(00)80581-4. [DOI] [PubMed] [Google Scholar]
- 39.Kreher SA, Mathew D, Kim J, Carlson JR. Translation of sensory input into behavioral output via an olfactory system. Neuron. 2008;59:110–24. doi: 10.1016/j.neuron.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aurand LW, Singleto Ja, Bell TA, Etchells JL. Volatile Components in Vapors of Natural and Distilled Vinegars. Journal of Food Science. 1966;31:172. [Google Scholar]
- 41.Fishilevich E, et al. Chemotaxis behavior mediated by single larval olfactory neurons in Drosophila. Curr Biol. 2005;15:2086–96. doi: 10.1016/j.cub.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 42.Heimbeck G, Bugnon V, Gendre N, Keller A, Stocker RF. A central neural circuit for experience-independent olfactory and courtship behavior in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2001;98:15336–15341. doi: 10.1073/pnas.011314898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, et al. Blockade of neurotransmission in Drosophila mushroom bodies impairs odor attraction, but not repulsion. Curr Biol. 2003;13:1900–4. doi: 10.1016/j.cub.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 44.Troemel ER, Kimmel BE, Bargmann CI. Reprogramming chemotaxis responses: sensory neurons define olfactory preferences in C. elegans. Cell. 1997;91:161–9. doi: 10.1016/s0092-8674(00)80399-2. [DOI] [PubMed] [Google Scholar]
- 45.Zhao GQ, et al. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–66. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]
- 46.Root CM, et al. A presynaptic gain control mechanism fine-tunes olfactory behavior. Neuron. 2008;59:311–21. doi: 10.1016/j.neuron.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lai SL, Awasaki T, Ito K, Lee T. Clonal analysis of Drosophila antennal lobe neurons: diverse neuronal architectures in the lateral neuroblast lineage. Development. 2008;135:2883–93. doi: 10.1242/dev.024380. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.