Abstract
The Selective Estrogen Receptor Modulators (SERMs) are synthetic pharmaceuticals whose relative agonist/antagonist activities are not equivalent in all cells. Their discovery has raised the possibility that endogenous small molecules may exist with similar properties that could play important physiological roles. In support of this hypothesis is the recent demonstration that the oxysterol 27-hydroxycholesterol (27HC) interacts with and modulates the transcriptional activity of both estrogen receptor (ER) subtypes and that the relative agonist/antagonist activity of 27HC is influenced by both cell and promoter context. Although there is limited information available on the role of 27HC in classical estrogen-responsive tissues, that which is available in animal models of cardiovascular disease and cellular models of breast cancer support a role for this ligand in ER signaling. These results provide an interesting potential link between cholesterol and cholesterol metabolism to ER function, the physiological and pathological significance of which remains to be determined.
Introduction
The pathologies associated with long-term estrogen deprivation, such as upon cessation of ovarian function at menopause or following surgical oophorectomy, highlight the important roles of estrogens in reproduction, maintenance of the skeleton, and the central nervous and cardiovascular systems [1]. The increased incidence of climacteric symptoms, the onset of vasomotor instability, and the increased risk of cardiovascular disease and osteoporosis observed in menopausal women has been attributed directly to decreased ovarian estradiol production [2]. Until recently, these pathologies have been treated using estrogen therapy (ET) with or without a concomitant progestin (Hormone Therapy; HT). It is now possible to achieve some of the benefits of ET/HT using Selective Estrogen Receptor Modulators (SERMs), molecules whose relative agonist/antagonist activities permit the treatment of conditions associated with estrogen deprivation but avoid various liabilities associated with standard interventions. The mechanisms underlying the protective actions of estrogens and SERMs in different tissues are slowly being unraveled, but a key observation made thus far is that different ligands acting through the same receptor have different biological activities in different cells. This realization has reinvigorated interest in ER as a therapeutic target and fueled the search for endogenous ligands that exhibit SERM-like activities.
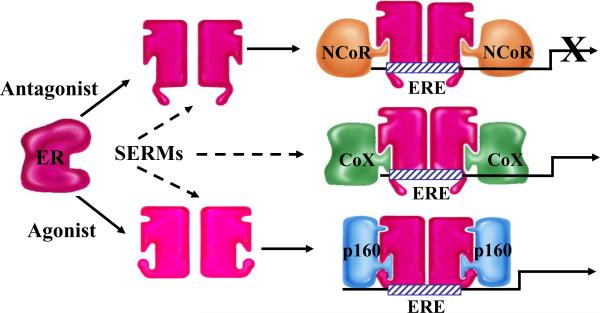
Most, if not all, of the biological actions of estrogens are mediated by the intracellular estrogen receptor (ER) expressed within target cells. A member of the nuclear receptor (NR) superfamily of ligand-inducible transcription factors, ER is expressed as two genetic isoforms, ERα and ERβ, which exhibit distinct and overlapping expression patterns and functions, but share a common mechanism of action [3-5]. In general, ERα is more widely expressed than ERβ, but in some tissues such as the ovary, the lung, and the prostate, ERβ is the predominant isoform [6]. In the absence of hormone, ER exists in an inactive state associated with a large inhibitory heat-shock protein complex. Upon agonist binding, ER undergoes a conformational change that results in displacement of the associated heat-shock proteins and spontaneous homo- or hetero-dimerization between ERα and ERβ. Dimeric ER then interacts with the regulatory regions of target genes either directly through specific estrogen response elements (EREs) or indirectly through a physical interaction with other transcription factors (Sp1, NFκB, AP1) already associated with the promoter [7]. Regardless, DNA-associated ER serves as the nucleation center for complexes comprising NR coactivators and components of the general transcription machinery. Antagonist binding to ER (or in some cases apo-ER) facilitates the recruitment of corepressors (i.e. NCoR and SMRT), which modify chromatin structure to repress target gene transcription. SERMs, like tamoxifen and raloxifene, can be distinguished from classical antagonists, and from each other, by their effect on receptor conformation and the impact this has on differential engagement of coactivators and corepressors (Box 1). It is now well-established that the overall conformation of ER is influenced by the nature of the bound ligand and that this translates into different pharmacological activities secondary to differential cofactor (coactivator or corepressor) recruitment [8].
Box 1. Cofactor Proteins and SERM Action.
Upon binding ligand, the estrogen receptor (ER) undergoes a conformational change that permits spontaneous homo- or hetero-dimerization and facilitates its subsequent interaction with estrogen response elements (EREs) located within target genes or with other transcription factors and transcription factor complexes. The functional consequences of different ligand-induced conformational changes were revealed with the discovery of receptor coactivators and corepressors.
Coactivators are proteins that interact either directly or indirectly with activated nuclear receptors (NRs) and facilitate their transcriptional activation. Coactivators often posses enzymatic activity, such as acetyltransferase, methyltransferase, or ubiquitin ligase activity. These proteins also may function as a bridge between an NR and the general transcription machinery. Examples include the p160 family members SRC-1, SRC-2 (GRIP1), and SRC-3 (ACTR, AIB1).
Corepressors also interact either directly or indirectly with NR family members. In contrast to coactivators, corepressors can bind to NRs in the absence or presence of a ligand, depending on the character of that particular ligand, and maintain the receptor in a quiescent state. Corepressors are often part of large protein complexes that contain histone deacetylase activity, and thus repress gene transcription. Examples include NCoR and SMRT.
In general, Selective Estrogen Receptor Modulators (SERMs) permit the receptor to adopt a structure that is intermediate between the inactive and fully activated states. Thus, the relative agonist/antagonist activity of SERMs is a reflection of the ability of the ER-SERM complex to engage coactivators or corepressors. It is also possible that some SERMs allow ER to interact in an ectopic manner with coregulatory proteins (CoX) that would not interact with ER under normal physiological circumstances.
In addition to regulating transcription, there is accumulating evidence that cytoplasmic ER engages in cross-talk with growth-factor signaling pathways through interactions with the adaptor protein Shc, the c-Src protein kinase complex, the regulatory subunit of PI3K (p85), and caveolins [9]. How, and if, these interactions observed in vitro impact the physiological actions of estrogens or the pharmacological actions of SERMs remains to be determined [9].
An endogenous molecule with SERM characteristics
The apparent plasticity in ER structure and its impact on transcriptional activity has raised the question as to whether there exist endogenous molecules with SERM-like activities. Recently, it was determined that the oxysterol 27-hydroxycholesterol (27HC) can interact with and modulate the activity of ER both in vitro and in vivo [10, 11]. Furthermore, the overall structure of the ER-27HC complex is distinct from apo-ER or estradiol-bound ER, a property shared by SERMs [10]. Although the degree to which 27HC occupies ER at the levels present in vivo remains to be determined, it seems likely that the discovery of this “new” ligand will emerge as a significant milestone in our understanding of ER biology.
Hydroxylation of cholesterol by CYP27A1 leads to the formation of 27HC [12]. Subsequently, 27HC can undergo 7α-hydroxylation by CYP7B1, or at any point it can exit the cell and travel through the plasma in a complex with cholesterol in the form of high-density (HDL) or low-density (LDL) lipoprotein. Interestingly, as monocytes differentiate into macrophages, CYP27A1 expression increases, leading to an enhanced capacity to metabolize cholesterol into 27-hydroxylated products such as 27HC and making them key producers of this oxysterol [13, 14]. This ability to convert cholesterol to 27HC is important for proper cholesterol efflux from peripheral tissues, as highlighted by the observation that patients with CYP27A1 gene mutations accumulate cholesterol in peripheral macrophages [15]. However, the role of 27HC itself in regulating cholesterol homeostasis is controversial [16, 17].
In addition to their role in cholesterol homeostasis and bile acid production, certain oxysterols have also been shown to function as ligands for NR family members such as the liver X receptor (LXRα and β). Thus, it was not totally unexpected that the oxysterol 27HC can modulate the activity of ER, another NR family member, in both in vitro and in vivo models of estradiol action [10, 11]. In the cardiovascular system in mice, it was shown that 27HC antagonized the protective effects of estrogen [11]. However, in cellular models of ER-positive breast cancer, 27HC recapitulated many genomic actions of estradiol, albeit with lower potency [10]. Together, these data suggest the possibility that the physiological function of 27HC will differ in a cell- and tissue-dependent manner, and thus would be appropriately considered a potential endogenous SERM [10, 11]. Given that this is a new area of research, we will discuss the existing data and speculate as to the potential significance of 27HC as an ER modulator.
It is clear that 27HC is a low affinity ER ligand, raising the issue as to its physiological relevance. In estrogen-depleted states (males, menopausal women, or breast cancer patients on aromatase inhibitors), it is likely that 27HC would have an opportunity to impact ER function. 27HC circulates in humans at a concentration of 75 - 730 nM, with approximately 90% existing in an esterified form [18, 19]. 27HC levels are higher in males than females and are positively correlated with those of cholesterol [18, 19]. These findings are similar in mice [11, 20]. In vitro competitive ligand-binding assays were used to calculate Ki values for 27HC for ERα (Ki = 1.32μM) and ERβ (Ki = 0.42μM) [11]. Given that the KM of 27HC for its catabolic enzyme CYP7B1 is 24μM, one can deduce that concentrations nearing this value are attainable in vivo, either in circulation or in target cells. As this value is greater than the concentration required to saturate ER, it appears that physiological levels of 27HC will be sufficient to impact ER signaling. It is important to remember that 27HC may not function as a classical endocrine agent, but rather may be an important intracrine or paracrine modulator and may be present at higher concentrations in relevant tissues.
The ER-dependent actions of 27-hydroxycholesterol in the cardiovascular system
27HC is in a unique position to directly impact ER function in the cardiovascular system, as it is the most prevalent circulating oxysterol, is particularly abundant within macrophages resident in atherosclerotic plaques, and is produced in endothelial cells to regulate internal cholesterol stores. The levels of 27HC are approximately 2 orders of magnitude higher in atheromas than in normal aorta, and this concentration rises as a function of lesion size [18]. It has already been established that estrogen, acting through ER, is important for nitric oxide (NO) synthesis in both vascular endothelium and smooth muscle, secondary to its ability to upregulate inducible nitric oxide synthase (iNOS) and endothelial NOS (eNOS) [21, 22]. The generation of NO in the cardiovascular system is diseasepreventative, causing smooth muscle relaxation, promoting wound healing, and reducing thrombosis [23]. Importantly, many cardiovascular pathologies, including hypercholesteremia, are associated with reduced NO synthesis [23]. The observation that 27HC modulates the ability of estradiol to regulate iNOS and eNOS and impacts the repair of artificially damaged vasculature provided strong biological support for a role of this oxysterol in ER signaling [11]. Specifically, wild-type mice treated with exogenous 27HC or fed a cholesterol-supplemented diet had significantly decreased iNOS and eNOS expression in the aorta [11]. This decrease in iNOS and eNOS expression was dependent on expression of ERα and ERβ, as genetic ablation of either receptor blocked the action of 27HC, but not on LXR, the classic oxysterol receptor. Furthermore, genetic disruption of cyp7b1, which results in a modest elevation in plasma of total (6 - 8 fold) and free 27HC levels (from 10% to 50%), leads to impaired estradiol-mediated re-endothelialization of the carotid artery [11].
The implications of 27HC-mediated inhibition of ER signaling in the cardiovascular system are of particular importance in the context of the controversy surrounding the role of ET/HT in the prevention of cardiovascular disease. Estradiol is thought to only protect against the development of new atherosclerotic lesions, and these lesions are often already present at the onset of menopause, even if at a sub-clinical level [24]. As mentioned earlier, the 27HC levels in atherosclerotic lesions are dramatically elevated, creating a local microenvironment in which in vitro data suggest 27HC could effectively compete with post-menopausal estradiol levels. A tantalizing hypothesis emerges, namely that accumulation of 27HC in advanced atherosclerotic lesions antagonizes estradiol, leading to a lack of cardio-protective benefit. Future studies are warranted, given the implication this sort of data would have on the use of ET/HT as a cardio-preventative agent.
The ER-dependent actions of 27-hydroxycholesterol in the breast
27HC is a partial ER agonist in established cellular models of breast cancer, although in vivo confirmation of the significance of this activity is required [10]. 27HC recruits ERα to target gene promoters and controls transcription of ER target genes in a manner similar to estradiol [10]. Of more physiological importance is the demonstrated ability of 27HC to increase the proliferative capacity of ERα-positive breast cancer cells. Despite a great deal of success in treating breast cancer with antiestrogens and aromatase inhibitors, resistance does occur, and recurrent endocrine-resistant breast cancers often still express ER, suggesting a continued dependence on estrogen signaling [25]. Moreover, obesity is known to increase the risk for breast cancer by up-regulating aromatase activity, thus increasing local estradiol production. The coincidence of obesity with hypercholesteremia suggests a second mechanism by which obesity may impact breast cancer through increased circulation of 27HC. Further, cholesterol-reducing statins may indirectly impact breast cancer progression by effecting a global decrease in 27HC production, and in doing so may modulate the effectiveness of estrogen ablative therapies.
Another intriguing aspect of cancer etiology is the relative contribution of infiltrating macrophages in disease progression. In lung cancer, an emerging ER target tissue, macrophage density positively correlates with micro-vessel density and negatively correlates with patient survival [26]. Additionally, co-culture of lung cancer cell lines and macrophages increases the expression of genes involved in matrix degradation and motility and ultimately increases invasive behavior [26]. Similarly, increased expression of colony stimulating factor 1 (CSF-1), a monocyte/macrophage chemoattractant, is observed in invasive breast cancers, potentially leading to increased tumor infiltration by immune cells, primarily T cells and macrophages [27]. In a study of potential mechanisms for endocrine-resistance in breast cancer, it was shown that macrophages produce cytokines that can de-repress ER-dependent target gene inhibition [28]. Not surprisingly, then, tumor-infiltrating macrophages are an independent risk factor for reduced survival from breast cancer [29]. An unexplored role of these tumor-infiltrating macrophages is whether they provide a local source of 27HC that can be utilized by ER-positive breast cancer cells. As shown in vitro, 27HC increases breast cancer cell proliferation, and thereby may provide another link between macrophage infiltration (and subsequent 27HC production) and the failure of anti-estrogen therapy [10, 28].
Other potential roles of 27-hydroxycholesterol in ER biology
ER signaling is an important regulator of the development and maintenance of reproductive function in both sexes. Thus, it would be predicted that changes in 27HC would effect some, if not all, aspects of reproductive function. Interestingly, no reproductive abnormalities were observed in mouse models displaying different levels of 27HC [11]. Subtle defects may exist or may only be manifest under restricted circumstances; possibilities that remain to be explored. The lack of an observable reproductive phenotype may reflect the SERM nature of 27HC or may relate to the lack of production of this oxysterol by or near cells in the reproductive system where estrogens manifest their activity.
Although macrophages resident in the cardiovascular system produce 27HC and affect estrogen signaling [11], it remains to be shown whether this extends to peripheral macrophages in other estrogen-responsive systems. Estrogen plays a key role in maintaining proper bone micro-architecture by blocking osteoclast differentiation and promoting osteoblast function, thereby increasing bone formation during adolescence and sustaining proper bone function in adulthood. The menopausal decline in estrogen perturbs the balance between osteoclast and osteoblast activities, leading to osteoporosis. Osteoclasts are macrophage-derived mononuclear cells, and thus would be predicted to produce significant amounts of 27HC. The low affinity of 27HC for ER makes it unlikely that 27HC impacts ER activity in pre-menopausal women [11], but in post-menopausal women or in the pathophysiological state of hypercholesteremia, 27HC may significantly impact ER signaling in ways that influence bone structure. Notably, the SERM tamoxifen increases bone mineral density (BMD) in post-menopausal women [30], whereas in the pre-menopausal state it antagonizes ER leading to decreased BMD [31]. Similar context-dependent agonist/antagonist activities would be expected in studies of 27HC action in the bone, and in vivo studies are thus warranted to define these activities.
Since autoimmune diseases are in general more prevalent in women, it has been suggested that the immune system is under partial regulation by the female hormone estradiol [32, 33]. As macrophages are primary producers of 27HC, these cells could be a key target of 27HC-regulated ER signaling in this system. For example, the synovial macrophages implicated in rheumatoid arthritis (RA) express ER, and thus may be a site where 27HC impacts ER function [32]. This may help to explain the sexual dimorphism observed in RA and other pathologies such as multiple sclerosis and systemic lupus erythematosus [32, 34-36]. Interestingly, statins have shown to be effective in several different in vivo models of autoimmune disorders, and have therefore been contemplated as therapeutics [37]. It is interesting to speculate that some of the observed efficacy of statins in this setting may relate to their ability to reduce local or circulating 27HC, a hypothesis that can be tested with available animal models and pharmacological agents.
Final Considerations
While the in vivo studies of the cardiovascular system showed regulation of both genomic and nongenomic ER actions by 27HC, the in vitro studies focused exclusively on nuclear ER actions. Future in vitro studies should define how 27HC impacts the rapid aspects of ER signaling and how this may differ in a tissue-specific manner. Furthermore, it is clear that there is an immediate need for additional in vivo studies to address the specific functions of 27HC in ER-responsive tissues outside the cardiovascular system. In analyzing human populations, polymorphism studies of both CYP27A1 and CYP7B1 would be a great addition to those already published on humans lacking expression of either enzyme [15, 38], and would be crucial to our understanding of how subtle changes in 27HC levels affect biological processes. Finally, using a combination of pharmacological inhibitors of CYP27A1 and CYP7B1, together with established animal models, it should be possible to define which ER-responsive tissues are most sensitive to alterations in 27HC. Even at this early state in our exploration of 27HC biology, the data available provide a compelling case to support the hypothesis that ER is a primary mediator of 27HC action in some target tissues.
Acknowledgements
We thank the members of the McDonnell laboratory for comments on this manuscript. This work was supported by the Department of Defense Breast Cancer Program Predoctoral Traineeship Award (W81XWH-06-1-0515 to C.D.D.) and by the National Institutes of Health (5R37-DK048807 to D.P.M.).
References
- 1.Chahal HS, Drake WM. The endocrine system and ageing. J Pathol. 2007;211:173–180. doi: 10.1002/path.2110. [DOI] [PubMed] [Google Scholar]
- 2.Vagenakis AG. Endocrine aspects of menopause. Clin Rheumatol. 1989;8(Suppl 2):48–51. doi: 10.1007/BF02207233. [DOI] [PubMed] [Google Scholar]
- 3.Green S, et al. Human oestrogen receptor cDNA: sequence, expression and homology to v-erbA. Nature. 1986;320:134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- 4.Kuiper GG, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 5.Kuiper GG, et al. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Couse JF, et al. Tissue distribution and quantitative analysis of estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) messenger ribonucleic acid in the wild-type and ERalpha-knockout mouse. Endocrinology. 1997;138:4613–4621. doi: 10.1210/endo.138.11.5496. [DOI] [PubMed] [Google Scholar]
- 7.Bjornstrom L, Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19:833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- 8.Smith CL, Malley BW. Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr Rev. 2004;25:45–71. doi: 10.1210/er.2003-0023. [DOI] [PubMed] [Google Scholar]
- 9.Cheskis BJ, et al. Signaling by estrogens. J Cell Physiol. 2007;213:610–617. doi: 10.1002/jcp.21253. [DOI] [PubMed] [Google Scholar]
- 10.DuSell CD, et al. 27-hydroxycholesterol is an endogenous selective estrogen receptor modulator. Mol Endocrinol. 2008;22:65–77. doi: 10.1210/me.2007-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Umetani M, et al. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nat Med. 2007;13:1185–1192. doi: 10.1038/nm1641. [DOI] [PubMed] [Google Scholar]
- 12.Javitt NB. 25R,26-Hydroxycholesterol revisited: synthesis, metabolism, and biologic roles. J Lipid Res. 2002;43:665–670. [PubMed] [Google Scholar]
- 13.Hansson M, et al. Marked induction of sterol 27-hydroxylase activity and mRNA levels during differentiation of human cultured monocytes into macrophages. Biochim Biophys Acta. 2003;1593:283–289. doi: 10.1016/s0167-4889(02)00398-1. [DOI] [PubMed] [Google Scholar]
- 14.Babiker A, et al. Elimination of cholesterol in macrophages and endothelial cells by the sterol 27-hydroxylase mechanism. Comparison with high density lipoprotein-mediated reverse cholesterol transport. J Biol Chem. 1997;272:26253–26261. doi: 10.1074/jbc.272.42.26253. [DOI] [PubMed] [Google Scholar]
- 15.Bjorkhem I, Leitersdorf E. Sterol 27-hydroxylase deficiency: a rare cause of xanthomas in normocholesterolemic humans. Trends Endocrinol Metab. 2000;11:180–183. doi: 10.1016/s1043-2760(00)00255-1. [DOI] [PubMed] [Google Scholar]
- 16.Bjorkhem I. Do oxysterols control cholesterol homeostasis? J Clin Invest. 2002;110:725–730. doi: 10.1172/JCI16388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meir K, et al. Human sterol 27-hydroxylase (CYP27) overexpressor transgenic mouse model. Evidence against 27-hydroxycholesterol as a critical regulator of cholesterol homeostasis. J Biol Chem. 2002;277:34036–34041. doi: 10.1074/jbc.M201122200. [DOI] [PubMed] [Google Scholar]
- 18.Brown AJ, Jessup W. Oxysterols and atherosclerosis. Atherosclerosis. 1999;142:1–28. doi: 10.1016/s0021-9150(98)00196-8. [DOI] [PubMed] [Google Scholar]
- 19.Dzeletovic S, et al. Determination of cholesterol oxidation products in human plasma by isotope dilution-mass spectrometry. Anal Biochem. 1995;225:73–80. doi: 10.1006/abio.1995.1110. [DOI] [PubMed] [Google Scholar]
- 20.Li-Hawkins J, et al. Disruption of the oxysterol 7alpha-hydroxylase gene in mice. J Biol Chem. 2000;275:16536–16542. doi: 10.1074/jbc.M001811200. [DOI] [PubMed] [Google Scholar]
- 21.Chambliss KL, Shaul PW. Estrogen modulation of endothelial nitric oxide synthase. Endocr Rev. 2002;23:665–686. doi: 10.1210/er.2001-0045. [DOI] [PubMed] [Google Scholar]
- 22.Zhu Y, et al. Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science. 2002;295:505–508. doi: 10.1126/science.1065250. [DOI] [PubMed] [Google Scholar]
- 23.Christopherson KS, Bredt DS. Nitric oxide in excitable tissues: physiological roles and disease. J Clin Invest. 1997;100:2424–2429. doi: 10.1172/JCI119783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grodstein F, et al. Understanding the divergent data on postmenopausal hormone therapy. N Engl J Med. 2003;348:645–650. doi: 10.1056/NEJMsb022365. [DOI] [PubMed] [Google Scholar]
- 25.Chen S, et al. New experimental models for aromatase inhibitor resistance. J Steroid Biochem Mol Biol. 2007;106:8–15. doi: 10.1016/j.jsbmb.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen JJ, et al. Tumor-associated macrophages: the double-edged sword in cancer progression. J Clin Oncol. 2005;23:953–964. doi: 10.1200/JCO.2005.12.172. [DOI] [PubMed] [Google Scholar]
- 27.Tang R, et al. M-CSF (monocyte colony stimulating factor) and M-CSF receptor expression by breast tumour cells: M-CSF mediated recruitment of tumour infiltrating monocytes? J Cell Biochem. 1992;50:350–356. doi: 10.1002/jcb.240500403. [DOI] [PubMed] [Google Scholar]
- 28.Zhu P, et al. Macrophage/cancer cell interactions mediate hormone resistance by a nuclear receptor derepression pathway. Cell. 2006;124:615–629. doi: 10.1016/j.cell.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 29.Steele RJ, et al. A high macrophage content in human breast cancer is not associated with favourable prognostic factors. Br J Surg. 1984;71:456–458. doi: 10.1002/bjs.1800710618. [DOI] [PubMed] [Google Scholar]
- 30.Love RR, et al. Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N Engl J Med. 1992;326:852–856. doi: 10.1056/NEJM199203263261302. [DOI] [PubMed] [Google Scholar]
- 31.Sverrisdottir A, et al. Bone mineral density among premenopausal women with early breast cancer in a randomized trial of adjuvant endocrine therapy. J Clin Oncol. 2004;22:3694–3699. doi: 10.1200/JCO.2004.08.148. [DOI] [PubMed] [Google Scholar]
- 32.Castagnetta L, et al. A role for sex steroids in autoimmune diseases: a working hypothesis and supporting data. Ann N Y Acad Sci. 2002;966:193–203. doi: 10.1111/j.1749-6632.2002.tb04215.x. [DOI] [PubMed] [Google Scholar]
- 33.Jacobson DL, et al. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997;84:223–243. doi: 10.1006/clin.1997.4412. [DOI] [PubMed] [Google Scholar]
- 34.Schwendimann RN, Alekseeva N. Gender issues in multiple sclerosis. Int Rev Neurobiol. 2007;79:377–392. doi: 10.1016/S0074-7742(07)79017-7. [DOI] [PubMed] [Google Scholar]
- 35.Simard JF, Costenbader KH. What can epidemiology tell us about systemic lupus erythematosus? Int J Clin Pract. 2007;61:1170–1180. doi: 10.1111/j.1742-1241.2007.01434.x. [DOI] [PubMed] [Google Scholar]
- 36.Theis KA, et al. Arthritis burden and impact are greater among U.S. women than men: intervention opportunities. J Womens Health (Larchmt) 2007;16:441–453. doi: 10.1089/jwh.2007.371. [DOI] [PubMed] [Google Scholar]
- 37.Weber MS, et al. Spotlight on statins. Int MS J. 2007;14:93–97. [PubMed] [Google Scholar]
- 38.Setchell KD, et al. Identification of a new inborn error in bile acid synthesis: mutation of the oxysterol 7alpha-hydroxylase gene causes severe neonatal liver disease. J Clin Invest. 1998;102:1690–1703. doi: 10.1172/JCI2962. [DOI] [PMC free article] [PubMed] [Google Scholar]