Abstract
Background
There has been an increasing interest in the role of oxidative stress in the pathophysiology of bipolar disorder. To explore this further, we evaluated the activity of glutathione peroxidase (GPx), glutathione reductase (GR) and glutathione S-transferase (GST), as well as 3-nitrotyrosine levels and carbonyl content in patients in the early (within 3 years of illness onset) and late (a minimum of 10 years of illness) stages of bipolar disorder.
Methods
We matched 30 patients in the early stage and 30 patients in the late stage of bipolar disorder, diagnosed according to DSM-IV criteria, with 60 healthy controls (30 matched for each group of patients). We measured symptomatic status using the Hamilton Rating Scale for Depression and the Young Mania Rating Scale.
Results
We found a significant increase in 3-nitrotyrosine levels among patients in the early (p < 0.010) and late (p < 0.010) stages of bipolar disorder. The activity of GR and GST was increased only among patients in the late stage of illness. Glutathione peroxidase activity and carbonyl content did not differ among the groups.
Limitations
Limitations of our study include its cross-sectional design, which did not allow us to examine direct causative mechanisms or the effects of progression of illness, and the potential environmental bias introduced by comparing patient groups recruited from different regions of the world.
Conclusion
Our data indicate a possible tyrosine nitration-induced damage in patients with bipolar disorder that is present from the early stage of illness. Our data also indicate that patients in the late stage of illness demonstrate enhanced activity of GR and GST, which could suggest the involvement of a compensatory system in bipolar disorder.
Introduction
Bipolar disorder is a chronic psychiatric illness characterized by recurrent episodes of mania, hypomania, mixed states and depression. It is a highly disabling condition that is estimated to affect 1%–3% of the population worldwide.1–4 It has been increasingly recognized that individuals with bipolar disorder are at higher risk for chronic general medical conditions such as cardiovascular disease, obesity and diabetes mel-litus,3,5 which are directly associated with the increased morbidity and mortality observed in this disorder. In addition, there is an emerging body of evidence suggesting that chronic and severe medical conditions are associated with oxidative stress.6
Sies7 has defined oxidative stress as “a disturbance in the pro-oxidant–antioxidant balance in favour of the former, leading to potential damage.” Oxidative stress can result from diminished levels of antioxidants or increased production of reactive species from oxygen or nitric oxide. Under physiologic conditions, mitochondria are the major sources of reactive oxygen species, which are quenched by the antioxidant defence system. However, in situations where there is an imbalance between pro-oxidant and antioxidant states, reactive oxygen species may accumulate and oxidative damage may occur. The antioxidant system is composed of 3 key enzymes: superoxide dismutase, which converts the superoxide radical into hydrogen peroxide; and catalase and glutathione peroxidase (Gpx), both of which detoxify the hydrogen peroxide.8 Another important free radical is nitric oxide, which is part of a collective often referred to as “reactive nitrogen species.” Nitric oxide reacts rapidly with the superoxide radical to form peroxynitrite, which is a highly reactive species.8 The central nervous system is extremely vulnerable to peroxidative damage since it is rich in oxidizable substrates, has a high oxygen tension (it metabolizes 20% of total body oxygen) and a relatively low antioxidant capacity.8,9 Reduced glutathione plays a key role as an essential cellular antioxidant in the defence of brain cells against oxidative damage.10 It is a tripeptide, composed of glutamate, cysteine and glycine, and it is used as an electron donor for GPx action, leading to the production of oxidized glutathione. This latter process is assisted by glutathione reductase (GR) to recycle oxidized glutathione to glutathione. In addition, glutathione is also involved in the conjugation of foreign molecules, and these reactions are catalyzed by glutathione S-transferases (GST).9 An important role of GST in the brain is the detoxification of quinones, formed during the oxidation of dopamine (DA) and other catecholamines.6,11–14
If reactive oxygen species or reactive nitrogen species are not effectively eliminated, they can cause oxidative damage to DNA, lipids (cell and organelle membranes) and proteins (receptors and enzymes).8 The oxidative modification of proteins is critical in many biological processes; however, this damage may also lead to the deleterious functional inactivation of proteins and enzymes.15 Reactive oxygen species can cause oxidation of the side chains of lysine, proline, arginine and threonine residues by binding ferrous ion (Fe+2) and copper state (Cu+2), and further attack by hydrogen peroxide or the superoxide radical may lead to the formation of protein carbonyl groups.15,16 Peroxynitrite, a reactive nitrogen species, can cause the nitration of tyrosine residues generating 3-nitrotyrosine.15 In addition, nitration of the tyrosine residues of GST can result in increased enzyme activity.17
The exact neurochemical mechanisms underlying the pathophysiology of bipolar disorder are not completely understood. Recently, the number of studies investigating biochemical aspects of the disorder has increased. Notably, recent studies have demonstrated alterations in diverse oxidative stress parameters in the course of bipolar disorder. For example, studies conducted with peripheral blood cells have demonstrated that the disorder is associated with alterations in antioxidant enzymes,18–21 increased lipid peroxidation,19,20 increased levels of nitric oxide22–24 and increased DNA fragmentation.25–27 Supporting the importance of the glutathione system in reducing the oxidative stress in bipolar disorder, a recent double-blind trial showed that N-acetylcysteine, a precursor of glutathione, improved depressive symptoms in patients with bipolar disorder.28
There is some evidence that patients in the early stages of bipolar disorder have a much better clinical outcome than those with multiple episodes.29,30 For instance, patients with a longer duration of illness or those who have had more than 3 previous episodes are less likely to respond to treatment, particularly to lithium.31,32 Based on these observations, a medical staging model has been recently proposed for bipolar disorder.33
As several studies have demonstrated elevated levels of nitric oxide in patients with bipolar disorder,22,23,34,35 and given the importance of the glutathione system for antioxidant capacity in the brain, our aim in this study was to assess glutathione system (GPx, GR and GST) and protein damage by reactive oxygen species and reactive nitrogen species (carbonylation and 3-nitrotyrosine) among patients in the early (0–3 years of illness) and late (10–20 years of illness) stages of bipolar disorder to determine whether there is a cumulative effect of oxidative stress in bipolar disorder.
Methods
Participants
We conducted a double case–control study of patients aged 18 years or older in the early (within the first 3 years of illness) and late (minimum of 10 years of illness) stages of bipolar disorder, with each group of patients separately matched to a group of healthy controls from the same region. We recruited patients in the early stage of illness and matched controls from the Mood Disorders Centre at the University of British Columbia, Vancouver, BC. We recruited patients in the late stage and matched controls from the Bipolar Disorders Program, Federal University of Rio Grande do Sul, Porto Alegre, Brazil.
Participants enrolled in both programs received open-label maintenance treatment for bipolar disorder from clinicians with expertise in the management of mood disorders and familiar with the most recent clinical guidelines.36,37 We confirmed the diagnosis of bipolar disorder using the Structured Clinical Interview for DSM-IV Axis I Disorders.38 Patients did not have significant comorbid medical conditions and they were not taking medications other than those prescribed for their psychiatric conditions. Within 1 week of drawing blood, we assessed psychiatric status using clinical rating scales: the Young Mania Rating Scale39 and the Hamilton Rating Scale for Depression, 21-item version (HAM-D).40 We assessed function using the Global Assessment of Functioning scale.41 We collected clinical variables using a standardized protocol. Duration of illness included the time in years from the first mood episode to the time the patient had blood drawn for oxidative stress markers. Although we recruited patients in the early stage of illness after their first manic episodes, many patients in this group had previous depressive episodes, which was accounted for in the duration of illness variable.
Healthy controls had no personal or family history (in first degree relatives) of major psychiatric disorders, dementia, mental retardation, cancer or tumours. We matched them to patients for age, sex and education. Controls were non-smokers and were not taking medication.
The University of British Columbia Clinical Research Ethics Board and the Clinical Hospital of Porto Alegre Ethics Board (Brazil) approved our study. All patients and controls provided written informed consent before taking part in any study-specific activities.
Procedure
Collection and processing of blood samples
Each patient and control provided 5-mL blood samples collected by venipuncture without anticoagulants. We obtained serum by centrifugation at 3000g for 5 minutes and kept it frozen at −70°C for up to 6 months until we performed the biochemical assays.
Assays
We measured GPx, GR, GST and protein carbonyl activity using the corresponding assay kits by Cayman Chemical. The GPx kit utilizes an indirect measure of GPx activity. Oxidized glutathione is produced via reduction of hydrogen peroxide by GPx, and is recycled to its reduced state by GR and nicotinamide adenine dinucleotide phosphate oxidized (NADP+). The oxidation of nicotinamide adenine dinucleotide phosphate reduced (NADPH) to NADP+ is accompanied by decreased absorbance of light at 340 nM. One unit of GPx is defined as the amount of enzyme that will cause the oxidation of 1.0 nmol of NADPH to NADP+ per minute at 25°C. The GR kit measures the rate of oxidation of NADPH to NADP+, which is accompanied by a decrease in absorbance at 340 nM. One unit of GR is defined as the amount of enzyme that will cause the oxidation of 1.0 nmol of NADPH to NADP+ per minute at 25°C. The GST kit measures total GST activity (cytosolic and microsomal) by measuring the conjugation of 1-chloro-2,4-dinitrobenzene with reduced glutathione. This conjugation is accompanied by an increase in absorbance at 340 nM. One unit of enzyme will conjugate 1.0 nmol of 1-chloro-2,4-dinitrobenzene with reduced glutathione per minute per milligram of protein at 25°C. Finally, the protein carbonyl kit utilizes the dinitrophenylhydrazine reaction to measure the protein carbonyl content in the serum. The amount of protein-hydrozone produced is quantified spectrophotometrically by absorbance at 360 nM. We recorded the results of these assays as units per milligram of protein.
We determined plasma 3-nitrotyrosine content using an enzyme-linked immunosorbent assay with a polyclonal anti-3-nitrotyrosine antibody (Abcam) and nitrated bovine serum albumin, as previously described.42,43 Briefly, we prepared a nitrated protein solution for use as a standard by incubating bovine serum albumin (10 mg/mL in phosphate-buffered saline [pH 7.4]) with 100 μM tetranitromethane in 50 mM KH2PO4 (pH 8) for 30 minutes at 37°C. After adjusting the pH to 10 with 3M NaOH, we measured the amount of 3-nitrotyrosine present in this solution by absorbance at 430 nM and expressed the results as nanomoles of 3-nitrotyrosine per milligram of bovine serum albumin (using a ɛM of 4400 M−1 cm−1). We determined the amount of 3-nitrotyrosine in the proteins of human plasma using competitive enzyme-linked immunosorbent assay, as described by Khan and colleagues,42 with some modifications. We performed the assay in 96-well plates at 37°C coated overnight with 4 μg/mL (100 μL per well) of nitro–bovine serum albumin, which we had blocked with nonfat dry milk for 2 hours at 37°C to prevent nonspecific binding. After blocking and washing with phosphate-buffered saline containing 0.6% polysorbate 20 (also called Tween 20), serial diluted standard (200–0.049 μg/mL) and diluted serum (1:10 in phosphate-buffered saline, pH 7.4) were bound to the 96-well plate and incubated for 30 minutes on an orbital shaker, followed by incubation with anti-3-nitrotyrosine (1:1000; Abcam) for 2 hours at 37°C. We then performed sequential incubations with peroxidase-conjugated anti–rabbit immunoglobulin G (Abcam). After further washing, we initiated colour development by adding substrate; we allowed colour to develop for up to 30 minutes at room temperature then terminated development by adding 4 M sulphuric acid. We determined antibody binding from the absorbance at 450 nM. We estimated the concentrations of nitrated proteins that inhibit anti–nitrotyrosine antibody binding from the standard curve and expressed them as nitro–bovine serum albumin equivalents.
Statistical analysis
Data are expressed as means and standard deviations (SD). We performed our statistical analyses using SPSS version 15.0 (SPSS Inc.). We used the Kolmogorov–Smirnov goodness-of-fit test to confirm that the variables (age, education, GPx, GR, GST, protein carbonyl and 3-nitrotyrosine levels) assessed in this study could be represented by a normal distribution. This test compares the observed cumulative distribution function for variables with a specified theoretical normal distribution. Our data were parametrically distributed. We used descriptive statistics to report sociodemographic and clinical characteristics of the sample. We assessed association between dichotomous variables using the χ2 or Fisher exact test where appropriate. We compared continuous demographic and clinical variables between patients and controls using the Student t test or a χ2 test. We performed our main comparisons among the 4 groups (patients in the early and late stages of illness and matched controls) in a model with age, sex and education as covariates (analysis of covariance), followed by post-hoc Tukey tests where appropriate. We performed a correlation (Pearson coefficient) matrix to examine the relation of biochemical marker levels with age, age of onset, duration of illness, education, Young Mania Rating Scale and HAM-D scores and number of mood episodes. Given that the number of mood episodes showed a significant correlation with duration of illness (r = 0.57, p < 0.001) as expected, we only included duration of illness in further analysis, as it is less subject to recall bias. We used a linear regression model to examine the association between biochemical markers (GPx, GR, GST, protein carbonyl and 3-nitrotyrosine) and length of illness, including age, HAM-D and the Young Mania Rating Scale as covariates. To investigate whether there was any association between biochemical markers and classes of medication, we used the linear regression model controlling for potential confounders (sex and age).
Results
We enrolled a total of 60 patients with bipolar disorder and 60 healthy controls. Demographic variables are presented in Table 1. We detected no significant differences in age, sex and education among patients in the early stage of bipolar disorder (n = 30) and matched controls (n = 30) and patients in the late stage of bipolar disorder (n = 30) and matched controls (n = 30). Clinical characteristics of patients in the early and late stages of bipolar disorder are shown in Table 2. As expected, there was a significant difference in duration of illness (t58 = 10.9, p < 0.001) and in the number of previous mood episodes between the patients in the early and late stages of illness (Table 2). Patients in the early stage were significantly younger at the onset of illness than those in the late stage (t58 = −4.4, p < 0.001), which could be in part explained by a recall bias because we recruited the late-stage group in a cross-sectional manner and collected the age at onset of illness retrospectively. As expected, patients in the late stage of bipolar disorder had higher Young Mania Rating Scale and HAM-D scores than patients in the early stage. The mean (and SD) Young Mania Rating Scale score was 3.6 (4.1) in the late-stage group versus 1.5 (2.8) in the early-stage group (t58 = 2.2, p = 0.026). The mean (and SD) HAM-D score was 9.2 (6.0) in the late-stage group versus 3.8 (7.1) in the early-stage group (t58 = 3.1, p = 0.002). The Global Assessment of Functioning scores were similar between the 2 groups of patients with bipolar disorder. The medications prescribed for each group of patients with bipolar disorder are listed in Table 2. All patients were prescribed psychotropic medications (mood stabilizers, antipsychotics and/or antidepressants).
Table 1.
Socio-demographic variables among 60 patients in the early and late stages of bipolar disorder and 60 matched controls
| Early stage; mean (SD)
|
Late stage; mean (SD)
|
|||||
|---|---|---|---|---|---|---|
| Variable | Patients (n = 30) | Controls (n = 30) | p value | Patients (n = 30) | Controls (n = 30) | p value |
| Male sex, % | 43.4 (13.0) | 33.3 (8.0) | 0.60* | 30.0 (9.0) | 36.7 (11.0) | 0.78* |
| Age, yr | 22.4 (3.9) | 22.1 (3.6) | 0.73† | 41.1 (8.4) | 43.2 (6.4) | 0.38† |
| Education, yr | 13.5 (2.0) | 12.9 (2.8) | 0.33† | 9.3 (3.8) | 10.6 (1.6) | 0.09† |
SD = standard deviation.
χ2 test.
Student t test.
Table 2.
Clinical features of 60 patients in the early (1–2 years of illness) and late (10 years of illness) stages of bipolar disorder
| Group; mean (SD)
|
|||
|---|---|---|---|
| Variable | Early (n = 30) | Late (n = 30) | p value |
| Age at onset of illness, yr | 20.2 (4.2) | 27.2 (7.4) | < 0.001* |
| Duration of illness, yr | 2.1 (2.9) | 13.9 (5.1) | < 0.001* |
| No. previous episodes | |||
| Depression | 1.1 (1.5) | 6.2 (8.1) | 0.007* |
| Hypomania | 0.8 (2.1) | 0.05 (0.2) | 0.046* |
| Mania | 1.0 (0.0) | 7.1 (9.1) | 0.004* |
| YMRS score | 1.5 (2.8) | 3.6 (4.1) | 0.026* |
| HAM-D score | 3.8 (7.1) | 9.2 (6.0) | 0.002* |
| GAF score | 63.3 (12.5) | 61.4 (17.5) | 0.64* |
| Medication, % | |||
| Mood stabilizer | 83.3 (25.0) | 86.7 (26.0) | 1.00† |
| Antipsychotic | 76.7 (23.0) | 60.0 (18.0) | 0.27† |
| Antidepressant | 10.0 (3.0) | 20.0 (6.0) | 0.47† |
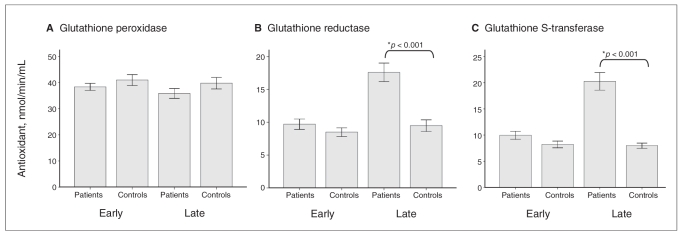
Our first aim in this study was to assess the involvement of glutathione enzymes in patients in the early and late stages of bipolar disorder. Figure 1 shows a significant increase in GST activity in patients in the late stage of illness (F3,116 = 33.96, p < 0.001), but not in those in the early stage. Similarly, GR activity was increased in only the late-stage group (F3,116 = 18.72, p < 0.001). We observed no changes in GPx activity in either group of patients (F3,116 = 1.35, p = 0.26). The findings were similar when we analyzed these variables using age, sex and education as covariates.
Fig. 1.
(A) Glutathione peroxidase, (B) glutathione reductase and (C) glutathione S-transferase activity in serum samples from patients in the early (0–3 years of illness) and late (10–20 years of illness) stages of bipolar disorder. Results are expressed as means and standard deviations. *We compared groups using 1-way analysis of variance, followed by post-hoc analysis using the Tukey test (p < 0.001).
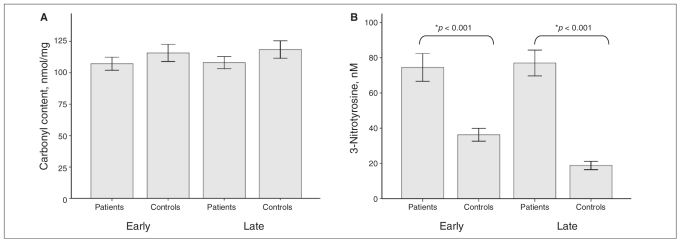
Figure 2 shows that protein carbonyl levels did not differ significantly among either group of patients and their group of matched controls (F3,116 = 2.66, p = 0.06). However, the content of 3-nitrotyrosine was increased in patients in both the early stage (F3,116 = 24.47, p < 0.001) and the late stage of illness (p < 0.001) compared with controls. These results did not change when we included age, sex and education in the analysis.
Fig. 2.
Levels of (A) protein oxidation (carbonyl levels) and (B) tyrosine nitration–induced damage (3-nitrotyrosine) in serum samples from patients in the early (0–3 years of illness) and late (10–20 years of illness) stages of bipolar disorder. Results are expressed as means and standard deviations. *We compared groups using 1-way analysis of variance, followed by post-hoc analysis using the Tukey test (p < 0.001).
We examined the correlation between glutathione antioxidant enzymes (GPx, GR and GST) and protein oxidative damage (protein carbonyl and 3-nitrotyrosine) with length of illness. There was a positive correlation between duration of illness and both GR (r = 0.492, p < 0.001) and GST (r = 0.333, p = 0.010) levels (Fig. 3). There was no correlation between duration of illness and the other parameters examined. Given that GR and GST also showed a significant correlation with age and with mania symptom scores (Young Mania Rating Scale), we investigated the association between GR and GST serum levels and duration of illness in a linear regression model controlling for age and the Young Mania Rating Scale scores. The standardized regression coefficients (β) indicated that duration of illness remained a significant correlate with GR levels (β = 0.196, p = 0.030, R2 = 0.242) and GST levels (β = 0.128, p = 0.041, R2 = 0.111) (Fig. 3).
Fig. 3.
Correlation between glutathione reductase (GR) or glutathione S-transferase (GST) levels with duration of illness in all patients with bipolar disorder (n = 60). The duration of illness positively correlates with GR levels (R2 = 0.242) and GST (R2 = 0.111).
Finally, we examined whether there was any association between biochemical markers and classes of medication. We used the linear regression model controlling for potential confounders (sex and age). Results showed a positive association between activity of GST and the use of any antidepressants (β = 0.25, t = 2.06, p = 0.040) or mood stabilizers (β = 0.29, t = 2.01, p = 0.050) (Table 3).
Table 3.
Association between biochemical markers and classes of medication
| GPx; nmol/min/mL
|
GR; nmol/min/mL
|
GST; nmol/min/mL
|
Carbonyl content; nmol/mg
|
3-Nytrotyrosine levels, nM
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted analysis* |
Adjusted analysis* |
Adjusted analysis* |
Adjusted analysis* |
Adjusted analysis* |
||||||||||||||||
| Class of medication | Mean (SD) | β | t | p value | Mean (SD) | β | t | p value | Mean (SD) | β | t | p value | Mean (SD) | β | t | p value | Mean (SD) | β | t | p value |
| Antipsychotic | ||||||||||||||||||||
| No | 33.3 (8.2) | 0.26 | 1.86 | 0.07 | 13.6 (8.9) | 0.03 | 0.21 | 0.83 | 16.8 (9.1) | −0.06 | −0.46 | 0.65 | 110.8 (16.9) | −0.16 | 1.08 | 0.28 | 66.0 (46.5) | 0.21 | 1.50 | 0.14 |
| Yes | 38.8 (8.8) | 13.7 (6.7) | 14.3 (8.6) | 103.7 (27.1) | 80.3 (38.6) | |||||||||||||||
| Antidepressant | ||||||||||||||||||||
| No | 36.0 (8.0) | 0.25 | 1.90 | 0.06 | 13.5 (7.4) | −0.06 | −0.49 | 0.62 | 12.8 (6.6) | 0.25 | 2.06 | 0.041 | 105.5 (24.3) | 0.03 | 0.21 | 0.83 | 77.1 (42.0) | −0.08 | −0.58 | 0.56 |
| Yes | 43.0 (11.0) | 14.4 (7.7) | 15.5 (9.0) | 108.8 (26.7) | 68.3 (39.0) | |||||||||||||||
| Mood stabilizer | ||||||||||||||||||||
| No | 36.2 (10.7) | −0.03 | 0.20 | 0.84 | 12.1 (7.7) | 0.23 | 1.55 | 0.13 | 14.6 (6.6) | 0.29 | 2.01 | 0.055 | 103.3 (16.4) | 0.13 | 0.80 | 0.43 | 65.6 (31.0) | −0.26 | −1.69 | 0.10 |
| Yes | 41.9 (8.5) | 13.9 (7.4) | 15.2 (9.1) | 106.4 (25.7) | 77.6 (43.0) | |||||||||||||||
| Lithium | ||||||||||||||||||||
| No | 37.3 (9.0) | 0.02 | 0.16 | 0.87 | 12.1 (7.4) | −0.06 | −0.44 | 0.66 | 13.7 (7.4) | 0.19 | 1.44 | 0.15 | 102.2 (18.5) | 0.01 | 0.06 | 0.96 | 60.6 (33.8) | 0.38 | 2.63 | 0.010 |
| Yes | 36.9 (9.0) | 15.1 (7.2) | 16.4 (9.8) | 109.5 (28.8) | 90.0 (43.3) | |||||||||||||||
GPx = glutathione peroxidase; GR = glutathione reductase; GST = glutathione S-transferase; SD = standard deviation.
Multiple linear regression adjusted for sex. β represents the standardized regression coefficient.
Discussion
To our knowledge, ours is the first study to examine the activity of glutathione antioxidant enzymes and the levels of protein oxidation and tyrosine nitration in patients in different stages of bipolar disorder. Our results indicate that patients in the late stage of illness show increased activity of GR and GST and increased levels of 3-nitrotyrosine compared with healthy controls, whereas patients in the early stage demonstrate only increased 3-nitrotyrosine levels. Protein oxidation, verified by carbonyl levels, and GPx activity did not differ among patients in the early- or late-stage groups and matched controls. These results indicate that nitration of tyrosine residues occur early in the course of bipolar disorder.
There is evidence that 3-nitrotyrosine can cause inflammation.44 Our previous studies have shown that patients in the early and late stages of bipolar disorder have elevated levels of proinflammatory cytokines interleukin-6 and tumour necrosis factor-α, whereas the anti-inflammatory cytokine interleukin-10 was increased only in patients in the early stage of the disorder.45 This suggests that anti-inflammatory defence mechanisms are no longer operational in late-stage bipolar disorder. With increased duration of illness, it is likely that the cumulative effect of ongoing oxidative stress results in elevation of antioxidant enzymes (GR and GST) as a compensatory mechanism to reduce further oxidative damage and illness progression among patients with bipolar disorder. In this sense, if the compensatory/antioxidants mechanisms do not react in time, these patients’ conditions may progressively worsen. This suggests an important role for compensatory antioxidant mechanisms in illness progression in patients with bipolar disorder. However, it is likely that such compensatory mechanisms are only partially effective, as previous studies have shown that patients with bipolar disorder exhibit increased levels of oxidative damage to proteins, lipids and DNA18–27 as well as reduced brain-derived neurotrophic factor (BDNF) levels.45
Our findings are in line with recent models that highlight the importance of early intervention in bipolar disorder.46,47 The delay in the initiation of suitable treatment strategies early in the course of bipolar disorder can exert substantial functional damage,46,47 as indicated by reduced BDNF levels and increased oxidative damage in patients in the late stage of bipolar disorder. Indeed, currently available treatments for bipolar disorder, such as lithium and divalproex, have proven antioxidant effects48 and thus have the potential to minimize oxidative damage and prevent further deterioration in the course of bipolar disorder.
If oxidative stress is present in bipolar disorder, what might be responsible for this? There is evidence that increased DA levels are associated with the symptoms of mania and that a reduction in DA transmission through reduction in DA synthesis or blockade of DA D2 receptors may be associated with antimanic effects.49,50 Interestingly, increased DA levels are an important source of oxidative stress in the brain owing to oxidative metabolism of DA.51,52 Dopamine may be metabolized through monoamine oxidase with production of hydrogen peroxide and dihydroxyphenylacetic acid53,54 or through nonenzymatic hydroxylation in the presence of ferrous ion and hydrogen peroxide leading to the formation of 6-hydroxydopamine (6-OHDA).11,55 6-Hydroxydopamine is toxic to the nervous system, and the mechanisms involved in this toxicity include endoplasmic reticulum stress, activation of glycogen synthase kinase-3-β by phosphorylation at tyrosine 216 and inhibition of the protein kinase AKT by phosphorylation at Ser473.14 In-vivo electrochemical measurements have shown that within a few minutes after injection into a rat brain, about 20% of 6-OHDA is converted to p-quinone,14,15 which can also be conjugated with glutathione by GST.6,12 This reaction is similarly thought to combat degenerative processes in the dopaminergic system in the human brain.56 Moreover, Tirmenstein and colleagues,57 using human neuroblastoma cells, showed that after 4 hours of treatment, 6-OHDA substantially depleted cellular and glutathione concentrations, whereas after 24 hours it induced a concentration-dependent increase in glutathione and total glutathione concentrations, suggesting that 6-OHDA induces oxidative stress in SH-SY5Y cells resulting in an adaptive increase in cellular glutathione concentrations. Our results demonstrate that only patients in the late stage of bipolar disorder have increased levels of GR and GST (Fig. 1). In addition, GR and GST levels show positive correlations with duration of illness (Fig. 3). The increase in antioxidant enzymes in patients in the late stage of bipolar disorder may be a consequence of cumulative effect of oxidative stress with progression of bipolar disorder.
Neuroimaging studies have shown increased levels of glutamate+glutamine and lactate levels in discrete subregions of the prefrontal cortex in adult patients with bipolar disorder.58 The N-methyl-D-aspartate receptors are ionotropic glutamate receptors that, when stimulated by glutamate, allow the passage of calcium into the cell, promoting activation of calcium/calmodulin-dependent protein kinase type IV, which may activate nitric oxide synthases (NOS1 and NOS3), thereby leading to increased nitric oxide production. Supporting this scenario, increased nitric oxide levels have been demonstrated in patients with bipolar disorder.22,23,59 In addition, increased intracellular calcium levels are a consistent finding in studies of bipolar disorder.60
Nitric oxide can react with superoxide, leading to the formation of peroxynitrite, which has the ability to nitrosylate free tyrosine and tyrosine residues in proteins, forming 3-nitrotyrosine.61 Our results indicate that patients in the early and late stages of bipolar disorder have increased levels of 3-nitrotyrosine (Fig. 2). The modification of critical cellular proteins by peroxynitrite-induced tyrosine nitration has been proposed as an early event in the process of DA neuronal damage.62 In addition, Ji and Bennet17 showed that microsomal GST is activated on exposure to peroxynitrite by nitration of tyrosine residues. The activation of GST by peroxynitrite may play an important role in limiting the extent of oxidative tissue injury when other cellular antioxidant enzymes such as superoxide dismutase63 and xantine oxidase64 are compromised under pathophysiological conditions of excessive peroxynitrite formation. Given that 3-nitrotyrosine is commonly measured as a biomarker of reactive nitrogen species generation,65 our results suggest that the nitration process may play an important role in the pathophysiology of bipolar disorder.
Recent findings have suggested that mitochondrial dysfunction may be associated with the pathophysiology of bipolar disorder.66 Studies conducted in postmortem brain tissue have demonstrated that mitochondrial genes are downregulated in the hippocampi67 and dorsolateral prefrontal cortices of patients with bipolar disorder,68,69 whereas superoxide dismutase and catalase expression is decreased in the hippocampi of patients with bipolar disorder.70 Interestingly, 3-nitrotyrosine71 and 6-OHDA17 may promote inhibition of the complex I of the mitochondrial electron transport chain. In addition, Ferger and colleagues72 suggest that 6-OHDA can increase protein nitration levels. In our view, reactive nitrogen species may be a link between DA-related oxidative stress and mitochondrial dysfunction. Future studies will need to investigate the levels of 6-OHDA and its relation to increased reactive nitrogen species in patients with bipolar disorder.
Limitations
Some limitations should be considered when interpreting the results of our study. First, we used a cross-sectional design and therefore could only examine associations between the glutathione enzymes activity and protein damage, but not direct causative mechanisms or the effects of progression of illness. Second, the cohort included patients from different regions of the world; however, we reduced the potential confounding effect by matching patients with controls in the same region by sex, age and education. Nevertheless, we cannot exclude the nonspecific environmental bias on the direct comparisons between patient groups. Third, the patients included in our study were taking psychotropic medication, thus our results could reflect effects of chronic medication use. We found a positive association between GST activity with the use of antidepressants or mood stabilizers. Previous studies involving primary cultured neuronal cells have shown that lithium and valproate (first-line mood stabilizers) can increase the mRNA and protein levels of the GST M1 isoenzyme73 and that valproate inhibits FeCl3–induced lipid peroxidation and protein oxidation.48 In addition, Frey and colleagues74 have shown that lithium and valproate exert protective effects against amphetamine-induced oxidative stress in vivo. Long-term use of antidepressants (e.g., desipramine, imipramine, maprotiline, mirtazapine) increased the mRNA levels of glutathione-S-transferase and glutathione reductase in human monocytic U-937 cells,75 and atypical antipsychotics may counteract some of the progressive deteriorative effects by enhancing synaptic plasticity and cellular resilience.76
Conclusion
In conclusion, our study suggests that the cumulative effect of ongoing oxidative stress results in the elevation of antioxidant enzymes (GR and GST) as a compensatory mechanism to reduce further oxidative damage in patients with bipolar disorder. These findings indicate an important role for oxidative stress in illness progression in bipolar disorder. Further prospective studies with larger samples are warranted to confirm these findings.
Footnotes
Competing interests: None declared for Drs. Andreazza, Walz and Gonçalves. Dr. Kapczinski has received speaker fees, educational grants and travel assistance from Eli Lilly. Dr. Kauer-Sant’Anna has been an investigator in clinical trials sponsored by Servier, Canadian Institutes of Health Research and Stanley Medical Research Institute. She is also a NARSAD Young Investigator and has received speaker fees or unrestricted research grants from APA/AstraZeneca, Lilly, CNPq and CAPES. Dr. Yatham has received speaker fees, educational grants and travel assistance from AstraZeneca and Eli Lilly. Dr. Bond has received speaker fees from AstraZeneca and CANMAT and sits on the advisory board of AstraZeneca. Dr. Young has received speaker fees from AstraZeneca and Eli Lilly.
Contributors: Drs. Andreazza, Kapczinski, Gonçalves, Young and Yatham designed the study. Drs. Andreazza, Walz, Bond and Yatham acquired the data, which Drs. Andreazza, Kapczinski, Kauer-Sant’Anna, Walz, Bond, Gonçalves and Yatham analyzed. Drs. Andreazza, Kapczinski, Kauer-Sant’Anna, Walz, Gonçalves and Young wrote the article, which Drs. Kapczinski, Kauer-Sant’Anna, Bond, Gonçalvez Young and Yatham reviewed. All authors approved the final version for publication.
References
- 1.Belmaker RH. Medical progress: bipolar disorder. N Engl J Med. 2004;351:476–86. doi: 10.1056/NEJMra035354. [DOI] [PubMed] [Google Scholar]
- 2.Sajatovic M. Bipolar disorder: disease burden. Am J Manag Care. 2005;11(Suppl):S80–4. [PubMed] [Google Scholar]
- 3.Kupfer DJ. The increasing medical burden in bipolar disorder. JAMA. 2005;293:2528–30. doi: 10.1001/jama.293.20.2528. [DOI] [PubMed] [Google Scholar]
- 4.Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64:543–52. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McIntyre RS, Konarski JZ, Soczynska JK, et al. Medical comorbidity in bipolar disorder: implications for functional outcomes and health service utilization. Psychiatr Serv. 2006;57:1140–4. doi: 10.1176/ps.2006.57.8.1140. [DOI] [PubMed] [Google Scholar]
- 6.Halliwell B. Oxidative stress and neurodegeneration: Where are we now? J Neurochem. 2006;97:1634–58. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 7.Sies H. Oxidative stress: from basic research to clinical application. Am J Med. 1991;91(3C):31S–8S. doi: 10.1016/0002-9343(91)90281-2. [DOI] [PubMed] [Google Scholar]
- 8.Halliwell B. Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging. 2001;18:685–716. doi: 10.2165/00002512-200118090-00004. [DOI] [PubMed] [Google Scholar]
- 9.Takuma K, Baba A, Matsuda T. Astrocyte apoptosis: implications for neuroprotection. Prog Neurobiol. 2004;72:111–27. doi: 10.1016/j.pneurobio.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Dringen R, Hirrlinger J. Glutathione pathways in the brain. Biol Chem. 2003;384:505–16. doi: 10.1515/BC.2003.059. [DOI] [PubMed] [Google Scholar]
- 11.Graham DG. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol Pharmacol. 1978;14:633–43. [PubMed] [Google Scholar]
- 12.Baez S, Segura-Aguilar J, Widersten M, et al. Glutathione transferases catalyse the detoxication of oxidized metabolites (o-quinones) of catecholamines and may serve as an antioxidant system preventing degenerative cellular processes. Biochem J. 1997;324:25–8. doi: 10.1042/bj3240025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stokes AH, Hastings TG, Vrana KE. Cytotoxic and genotoxic potential of dopamine. J Neurosci Res. 1999;55(6):659–65. doi: 10.1002/(SICI)1097-4547(19990315)55:6<659::AID-JNR1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 14.Obata T. Dopamine efflux by MPTP and hydroxyl radical generation. J Neural Transm. 2002;109:1159–80. doi: 10.1007/s00702-001-0683-2. [DOI] [PubMed] [Google Scholar]
- 15.Beal MF. Oxidatively modified proteins in aging and disease. Free Radic Biol Med. 2002;32:797–803. doi: 10.1016/s0891-5849(02)00780-3. [DOI] [PubMed] [Google Scholar]
- 16.Berlett BS, Levine RL, Stadtman ER. Carbon dioxide stimulates peroxynitrite-mediated nitration of tyrosine residues and inhibits oxidation of methionine residues of glutamine synthetase: both modifications mimic effects of adenylylation. Proc Natl Acad Sci U S A. 1998;95:2784–9. doi: 10.1073/pnas.95.6.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji Y, Bennett BM. Activation of microsomal glutathione S-transferase by peroxynitrite. Mol Pharmacol. 2003;63:136–46. doi: 10.1124/mol.63.1.136. [DOI] [PubMed] [Google Scholar]
- 18.Abdalla DS, Monteiro HP, Oliveira JA, et al. Activities of superoxide dismutase and glutathione peroxidase in schizophrenic and manic-depressive patients. Clin Chem. 1986;32:805–7. [PubMed] [Google Scholar]
- 19.Kuloglu M, Ustundag B, Atmaca M, et al. Lipid peroxidation and antioxidant enzyme levels in patients with schizophrenia and bipolar disorder. Cell Biochem Funct. 2002;20:171–5. doi: 10.1002/cbf.940. [DOI] [PubMed] [Google Scholar]
- 20.Andreazza AC, Cassini C, Rosa AR, et al. Serum S100B and antioxidant enzymes in bipolar patients. J Psychiatr Res. 2007;41:523–9. doi: 10.1016/j.jpsychires.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 21.Machado-Vieira R, Andreazza AC, Viale CI, et al. Oxidative stress parameters in unmedicated and treated bipolar subjects during initial manic episode: a possible role for lithium antioxidant effects. Neurosci Lett. 2007;421:33–6. doi: 10.1016/j.neulet.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 22.Savas HA, Herken H, Yürekli M, et al. Possible role of nitric oxide and adrenomedullin in bipolar affective disorder. Neuropsychobiology. 2002;45:57–61. doi: 10.1159/000048677. [DOI] [PubMed] [Google Scholar]
- 23.Savas HA, Gergerlioglu HS, Armutcu F. Elevated serum nitric oxide and superoxide dismutase in euthymic bipolar patients: impact of past episodes. World J Biol Psychiatry. 2006;7:51–5. doi: 10.1080/15622970510029993. [DOI] [PubMed] [Google Scholar]
- 24.Selek S, Savas HA, Gergerlioglu HS, et al. The course of nitric oxide and superoxide dismutase during treatment of bipolar depressive episode. J Affect Disord. 2008;107:89–94. doi: 10.1016/j.jad.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Andreazza AC, Noronha Frey B, Erdtmann B, et al. DNA damage in bipolar disorder. Psychiatry Res. 2007;153:27–32. doi: 10.1016/j.psychres.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 26.Buttner N, Bhattacharyya S, Walsh J, et al. DNA fragmentation is increased in non-GABAergic neurons in bipolar disorder but not in schizophrenia. Schizophr Res. 2007;93:33–41. doi: 10.1016/j.schres.2007.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naydenov AV, MacDonald ML, Ongur D, et al. Differences in lymphocyte electron transport gene expression levels between subjects with bipolar disorder and normal controls in response to glucose deprivation stress. Arch Gen Psychiatry. 2007;64:555–64. doi: 10.1001/archpsyc.64.5.555. [DOI] [PubMed] [Google Scholar]
- 28.Berk M, Copolov DL, Dean O, et al. N-acetylcysteine for depressive symptoms in bipolar disorder — a double-blind randomized placebo-controlled trial. Biol Psychiatry. 2008;64:468–75. doi: 10.1016/j.biopsych.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 29.Schuepbach D, Novick D, Haro JM, et al. EMBLEM Advisory Board. Determinants of voluntary vs. involuntary admission in bipolar disorder and the impact of adherence. Pharmacopsychiatry. 2008;41:29–36. doi: 10.1055/s-2007-993213. [DOI] [PubMed] [Google Scholar]
- 30.Tohen M, Waternaux CM, Tsuang MT. Outcome in mania. A 4-year prospective follow-up of 75 patients utilizing survival analysis. Arch Gen Psychiatry. 1990;47:1106–11. doi: 10.1001/archpsyc.1990.01810240026005. [DOI] [PubMed] [Google Scholar]
- 31.Gelenberg AJ, Kane JM, Keller MB, et al. Comparison of standard and low serum levels of lithium for maintenance treatment of bipolar disorder. N Engl J Med. 1989;321:1489–93. doi: 10.1056/NEJM198911303212201. [DOI] [PubMed] [Google Scholar]
- 32.Swann AC, Bowden CL, Calabrese JR, et al. Differential effect of number of previous episodes of affective disorder on response to lithium or divalproex in acute mania. Am J Psychiatry. 1999;156:1264–6. doi: 10.1176/ajp.156.8.1264. [DOI] [PubMed] [Google Scholar]
- 33.Berk M, Conus P, Lucas N, et al. Setting the stage: from prodrome to treatment resistance in bipolar disorder. Bipolar Disord. 2007;9:671–8. doi: 10.1111/j.1399-5618.2007.00484.x. [DOI] [PubMed] [Google Scholar]
- 34.Yanik M, Vural H, Tutkun H, et al. The role of the arginine-nitric oxide pathway in the pathogenesis of bipolar affective disorder. Eur Arch Psychiatry Clin Neurosci. 2004;254:43–7. doi: 10.1007/s00406-004-0453-x. [DOI] [PubMed] [Google Scholar]
- 35.Savas HA, Gergerlioglu HS, Gurel A, et al. Increased xanthine oxidase and malondialdehyde levels in euthymic bipolar patients. Klinik Psikiyatri Dergisi. 2005;8:180–5. [Google Scholar]
- 36.Yatham LN, Kennedy SH, O’Donovan C, et al. Canadian network for mood and anxiety treatments (CANMAT) guidelines for the management of patients with bipolar disorder: consensus and controversies. Bipolar Disord. 2005;7 (Suppl 3):5–69. doi: 10.1111/j.1399-5618.2005.00219.x. [DOI] [PubMed] [Google Scholar]
- 37.Yatham LN, Kennedy SH, O’Donovan C, et al. Guidelines Group. Canadian network for mood and anxiety treatments (CANMAT) guidelines for the management of patients with bipolar disorder: update 2007. Bipolar Disord. 2006;8:721–39. doi: 10.1111/j.1399-5618.2006.00432.x. [DOI] [PubMed] [Google Scholar]
- 38.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York (NY): Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 39.Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity, and sensitivity. Br J Psychiatry. 1978;133:429–35. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 40.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diagnostic and statistical manual of mental disorders. 4th ed. American Psychiatric Association; Washington: The Association; 1994. [Google Scholar]
- 42.Khan J, Brennand DM, Bradley N, et al. 3-Nitrotyrosine in the proteins of human plasma determined by an ELISA method. Biochem J. 1998;332:807–8. doi: 10.1042/bj3320807v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davis IC, Zajac AJ, Nolte KB, et al. Elevated generation of reactive oxygen/nitrogen species in hantavirus cardiopulmonary syndrome. J Virol. 2002;76:8347–59. doi: 10.1128/JVI.76.16.8347-8359.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ckless K, Lampert A, Reiss J, et al. Inhibition of arginase activity enhances inflammation in mice with allergic airway disease, in association with increases in protein S-nitrosylation and tyrosine nitration. J Immunol. 2008;181:4255–64. doi: 10.4049/jimmunol.181.6.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kauer-Sant’Anna M, Kapczinski F, Andreazza AC, et al. Brain-derived neurotrophic factor and inflammatory markers in patients with early- vs. late-stage bipolar disorder. Int J Neuropsychopharmacol. 2008;12:447–58. doi: 10.1017/S1461145708009310. [DOI] [PubMed] [Google Scholar]
- 46.Berk M, Malhi GS, Hallam K, et al. Early intervention in bipolar disorders: clinical, biochemical and neuroimaging imperatives. J Affect Disord. 2009;114:1–13. doi: 10.1016/j.jad.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 47.Schimmelmann BG, Huber CG, Lambert M, et al. Impact of duration of untreated psychosis on pre-treatment, baseline, and outcome characteristics in an epidemiological first-episode psychosis cohort. J Psychiatr Res. 2008;42:982–90. doi: 10.1016/j.jpsychires.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 48.Wang JF, Azzam JE, Young LT. Valproate inhibits oxidative damage to lipid and protein in primary cultured rat cerebrocortical cells. Neuroscience. 2003;116:485–9. doi: 10.1016/s0306-4522(02)00655-3. [DOI] [PubMed] [Google Scholar]
- 49.Yatham LN. Translating knowledge of genetics and pharmacology into improving everyday practice. Bipolar Disord. 2005;7(Suppl 4):13–20. doi: 10.1111/j.1399-5618.2005.00211.x. [DOI] [PubMed] [Google Scholar]
- 50.Berk M, Dodd S, Kauer-Sant’Anna M, et al. Dopamine dysregulation syndrome: implications for a dopamine hypothesis of bipolar disorder. Acta Psychiatr Scand Suppl. 2007;434:41–9. doi: 10.1111/j.1600-0447.2007.01058.x. [DOI] [PubMed] [Google Scholar]
- 51.Rees JN, Florang VR, Anderson DG, et al. Lipid peroxidation products inhibit dopamine catabolism yielding aberrant levels of a reactive intermediate. Chem Res Toxicol. 2007;20:1536–42. doi: 10.1021/tx700248y. [DOI] [PubMed] [Google Scholar]
- 52.Chen L, Ding Y, Cagniard B, et al. Unregulated cytosolic dopamine causes neurodegeneration associated with oxidative stress in mice. J Neurosci. 2008;28:425–33. doi: 10.1523/JNEUROSCI.3602-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maker HS, Weiss C, Silides DJ, et al. Coupling of dopamine oxidation (monoamine oxidase activity) to glutathione oxidation via the generation of hydrogen peroxide in rat brain homogenates. J Neurochem. 1981;36:589–93. doi: 10.1111/j.1471-4159.1981.tb01631.x. [DOI] [PubMed] [Google Scholar]
- 54.Berman SB, Hastings TG. Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria: implications for Parkinson’s disease. J Neurochem. 1999;73:1127–37. doi: 10.1046/j.1471-4159.1999.0731127.x. [DOI] [PubMed] [Google Scholar]
- 55.Hastings TG, Lewis DA, Zigmond MJ. Role of oxidation in the neurotoxic effects of intrastriatal dopamine injections. Proc Natl Acad Sci U S A. 1996;93:1956–61. doi: 10.1073/pnas.93.5.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 57.Tirmenstein MA, Hu CX, Scicchitano MS, et al. Effects of 6-hydroxydopamine on mitochondrial function and glutathione status in SH-SY5Y human neuroblastoma cells. Toxicol In Vitro. 2005;19:471–9. doi: 10.1016/j.tiv.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 58.Yildiz-Yesiloglu A, Ankerst DP. Review of 1H magnetic resonance spectroscopy findings in major depressive disorder: a meta-analysis. Psychiatry Res. 2006;147:1–25. doi: 10.1016/j.pscychresns.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 59.Gergerlioglu HS, Savas HA, Bulbul F, et al. Changes in nitric oxide level and superoxide dismutase activity during antimanic treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:697–702. doi: 10.1016/j.pnpbp.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 60.Kato T. Role of mitochondrial DNA in calcium signaling abnormality in bipolar disorder. Cell Calcium. 2008;44:92–102. doi: 10.1016/j.ceca.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 61.Ischiropoulos H. Biological selectivity and functional aspects of protein tyrosine nitration. Biochem Biophys Res Commun. 2003;305:776–83. doi: 10.1016/s0006-291x(03)00814-3. [DOI] [PubMed] [Google Scholar]
- 62.Ara J, Przedborski S, Naini AB, et al. Inactivation of tyrosine hydroxylase by nitration following exposure to peroxynitrite and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Proc Natl Acad Sci U S A. 1998;95:7659–63. doi: 10.1073/pnas.95.13.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.MacMillan-Crow LA, Crow JP, Thompson JA. Peroxynitrite-mediated inactivation of manganese superoxide dismutase involves nitration and oxidation of critical tyrosine residues. Biochemistry. 1998;37:1613–22. doi: 10.1021/bi971894b. [DOI] [PubMed] [Google Scholar]
- 64.Lee C-I, Liu X, Zweier J. Regulation of xanthine oxidase by nitric oxide and peroxynitrite. J Biol Chem. 2000;275:9369–76. doi: 10.1074/jbc.275.13.9369. [DOI] [PubMed] [Google Scholar]
- 65.Crow JP, Ischiropoulos H. Detection and quantitation of nitrotyrosine residues in proteins: in vivo marker of peroxynitrite. Methods Enzymol. 1996;269:185–94. doi: 10.1016/s0076-6879(96)69020-x. [DOI] [PubMed] [Google Scholar]
- 66.Kato T, Kato N. Mitochondrial dysfunction in bipolar disorder. Bipolar Disord. 2000;2:180–90. doi: 10.1034/j.1399-5618.2000.020305.x. [DOI] [PubMed] [Google Scholar]
- 67.Konradi C, Eaton M, MacDonald ML, et al. Molecular evidence for mitochondrial dysfunction in bipolar disorder. Arch Gen Psychiatry. 2004;61:300–8. doi: 10.1001/archpsyc.61.3.300. [DOI] [PubMed] [Google Scholar]
- 68.Iwamoto K, Bundo M, Kato T. Altered expression of mitochondria-related genes in postmortem brains of patients with bipolar disorder or schizophrenia, as revealed by large-scale DNA microarray analysis. Hum Mol Genet. 2005;14:241–53. doi: 10.1093/hmg/ddi022. [DOI] [PubMed] [Google Scholar]
- 69.Sun X, Wang JF, Tseng M, et al. Downregulation in components of the mitochondrial electron transport chain in the postmortem frontal cortex of subjects with bipolar disorder. J Psychiatry Neurosci. 2006;31:189–96. [PMC free article] [PubMed] [Google Scholar]
- 70.Benes FM, Matzilevich D, Burke RE, et al. The expression of proapoptosis genes is increased in bipolar disorder, but not in schizophrenia. Mol Psychiatry. 2006;11:241–51. doi: 10.1038/sj.mp.4001758. [DOI] [PubMed] [Google Scholar]
- 71.Ma TC, Mihm MJ, Bauer JA, et al. Bioenergetic and oxidative effects of free 3-nitrotyrosine in culture: selective vulnerability of dopamin-ergic neurons and increased sensitivity of non-dopaminergic neurons to dopamine oxidation. J Neurochem. 2007;103:131–44. doi: 10.1111/j.1471-4159.2007.04735.x. [DOI] [PubMed] [Google Scholar]
- 72.Ferger B, Themann C, Rose S, et al. 6-hydroxydopamine increases the hydroxylation and nitration of phenylalanine in vivo: implication of peroxynitrite formation. J Neurochem. 2001;78:509–14. doi: 10.1046/j.1471-4159.2001.00429.x. [DOI] [PubMed] [Google Scholar]
- 73.Wang JF, Shao L, Sun X, et al. Glutathione S-transferase is a novel target for mood stabilizing drugs in primary cultured neurons. J Neurochem. 2004;88:1477–84. doi: 10.1046/j.1471-4159.2003.02276.x. [DOI] [PubMed] [Google Scholar]
- 74.Frey BN, Valvassori SS, Réus GZ, et al. Effects of lithium and valproate on amphetamine-induced oxidative stress generation in an animal model of mania. J Psychiatry Neurosci. 2006;31:326–32. [PMC free article] [PubMed] [Google Scholar]
- 75.Schmidt AJ, Heiser P, Hemmeter UM, et al. Effects of antidepressants on mRNA levels of antioxidant enzymes in human monocytic U-937 cells. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1567–73. doi: 10.1016/j.pnpbp.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 76.Lieberman JA, Perkins DO, Jarskog LF. Neuroprotection: a therapeutic strategy to prevent deterioration associated with schizophrenia. CNS Spectr. 2007;12 (3 Suppl 4):1–13. doi: 10.1017/s1092852900025906. [DOI] [PubMed] [Google Scholar]