Abstract
Background
Vagus nerve stimulation (VNS) is a recent intervention for treatment-resistant depression. Electrophysiological recordings in the rat brain showed that VNS increases the firing rate of norepinephrine (NE) neurons after 1 day of stimulation and that of serotonin (5-HT) neurons after 14 days. This study was conducted to further characterize these effects.
Methods
We implanted rats with a VNS electrode and stimulator. We used the selective noradrenergic toxin DSP-4 to lesion NE neurons of the locus coeruleus. We recorded dorsal raphe 5-HT neurons under chloral hydrate anesthesia. We recorded hippocampus CA3 pyramidal neurons using 5-barreled iontophoretic pipettes.
Results
Analysis of a previously published data set revealed that VNS increased not only the spontaneous firing rates of NE neurons, but also the percentage of neurons firing in bursts. The enhancement of the 5-HT neuron firing rate by VNS was abolished by lesioning NE neurons. We found that VNS increased the degree of activation of postsynaptic α1-adrenoceptors on 5-HT neurons, probably through an increased release of endogenous NE. The tonic activation of postsynaptic 5-HT1A receptors in the hippocampus was enhanced after 14 days of VNS, as with other antidepressant treatments.
Limitations
Our study limitations include the fact that we turned off the stimulator during the electrophysiological recordings, which likely decreased the vagal tone to the brain. Also, we obtained the data while the animals were under anesthesia, therefore studies need to be carried out in unanesthetized rats to ascertain whether the anesthetic agent influenced the changes observed between control rats and those treated with VNS.
Conclusion
Vagus nerve stimulation initially increases the firing activity and pattern of NE neurons and subsequently those of 5-HT neurons, presumably as a cascade effect via α1-postsynaptic adrenoceptors. To date, VNS appears to be a unique antidepressant treatment increasing 5-HT transmission and enhancing the firing activity of NE neurons. These effects could contribute to the effectiveness of VNS in treatment-resistant depression.
Introduction
The vagus nerve is usually considered to be a parasympathetic efferent nerve. However, this nerve is in fact composed of about 80% afferent sensory fibres carrying information to the brain.1 Stimulating the afferent fibres has proven to be an effective antiepilepsy treatment,2 and vagus nerve stimulation (VNS) has become the most widely used nonpharmacological treatment for refractory epilepsy. Mood improvement has been observed in epileptic patients treated with VNS, even in those with no change in seizure frequency,3,4 suggesting that VNS might have antidepressant effects. Clinical studies in patients with treatment-resistant depression and without epilepsy reported a 31% response rate and 15% remission rate after 10 weeks of VNS.5 These response and remission rates were sustained at 1 year6 and improved 2 years after surgery.7 In a recent open-label study, VNS has shown antidepressant efficacy in patients with treatment-resistant depression; the level of clinical response increased to 53% and the remission rates reached 33% after 1 year of treatment.8 Recently, it has been shown that VNS produces an antidepressant-like effect in the forced-swim test in rats9 with about the same efficacy as that of the tricyclic antidepressant desipramine and electroconvulsive shock (ECS). Both Canada and Europe approved VNS therapy for use in drug-resistant depression in 2001 and the US Food and Drug Administration approved its use in 2005, but further clinical trials are needed to ascertain its effectiveness in the treatment of major depression.10
The mechanism of action for this therapy is not fully understood. However, it is believed to be mediated by the central projections of the vagus nerve via the nucleus of the tractus solitarius, which in turn innervates multiple brain areas implicated in mood regulation. Functional neuroimaging studies have confirmed that VNS alters the activity of many such cortical and subcortical regions.11 It is well established that the antidepressant efficacy of medications is mediated through monoaminergic neurotransmission, primarily the serotonin (5-HT) and/or norepinephrine (NE) systems.
Serotonin neurons are mainly concentrated in the dorsal raphe nucleus and the median raphe nuclei and project throughout the brain. In turn, 5-HT acts via a variety of sub-types of receptors. The 5-HT1A receptors are located either on the soma of 5-HT neurons, where they subserve an auto-receptor function, or on postsynaptic neurons, with their greatest density in the hippocampus and septal nuclei. The somatodendritic 5-HT1A autoreceptors are important in the regulation of the firing activity of 5-HT neurons. An enhanced activation of these autoreceptors by an increased level of 5-HT decreases the firing activity of 5-HT neurons through a negative feedback action. Thus, acute administration of antidepressants such as selective serotonin reuptake inhibitors (SSRIs), which initially enhance extracellular 5-HT levels, decreases the firing rate of 5-HT neurons. However, following long-term administration of SSRIs, 5-HT1A auto-receptors desensitize and 5-HT neurons in the dorsal raphe nucleus recover their normal firing rate.12
When activated, postsynaptic 5-HT1A receptors also induce a suppression of neuronal firing. These receptors appear to be important in the mediation of antidepressant effects.13,14 In particular, long-term administration of various classes of antidepressants and repeated ECS leads to their enhanced tonic activation by 5-HT.15
Locus coeruleus neurons give rise to 90% of the noradrenergic innervation of the forebrain. This brainstem nucleus receives afferent fibres from the nucleus of the tractus solitarius and sends a monosynaptic projection to the dorsal raphe nucleus. The firing activity of NE neurons is also controlled by autoreceptors of the α2-adrenergic subtype located on their cell bodies.16 The same subtype of α2-adrenergic autoreceptors is located on NE terminals and regulates the amount of NE released per action potential.17,18 Long-term administration of NE reuptake inhibitors desensitizes terminal α2-autoreceptors but not their somatodendritic counter-parts.19,20 Nevertheless, this still results in a net increase of NE levels in the forebrain despite an attenuated firing activity.
At the postsynaptic level, α1-adrenoceptors are present on the soma of 5-HT neurons in the dorsal raphe nucleus. When these receptors are activated by an exogenous agonist like phenylephrine or cirazoline, the firing activity of the neurons on which they are located is increased.21,22 Endogenously released NE activates these α1-adrenoceptors on the cell body of 5-HT neurons in the dorsal raphe nucleus, as revealed by their inhibition produced by the selective α1-adrenoceptor antagonist prazosin.
Recent electrophysiological studies have shown that the basal firing rates of neurons in the dorsal raphe nucleus and locus coeruleus were significantly increased following 2 weeks of VNS, but the enhancement of locus coeruleus neurons firing activity occurred before that of 5-HT neurons in the dorsal raphe nucleus.23 Unexpectedly, a desensitization of 5-HT1A and α2-autoreceptors was not observed following VNS,23 which suggests that other mechanisms might be at play in the enhancement of firing of 5-HT and NE neurons by VNS.
The aim of the present study was to further elucidate the role of 5-HT neurons in the mechanism of action of VNS using electrophysiological paradigms in anaesthetized rats. First, we analyzed the firing pattern of NE and 5-HT neurons after VNS to determine whether burst activity alterations could enhance neurotransmitter release. Second, we investigated the role of NE neurons in altering the firing of 5-HT neurons following VNS by determining whether the lesion of NE neurons would prevent the effect of VNS on 5-HT neurons. Third, we examined the degree of activation of α1-adrenoceptors on 5-HT neurons as a possible mechanism by which VNS could enhance the firing rate of these neurons. Finally, we assessed the tonic activation of postsynaptic 5-HT1A receptors in the hippocampus after sustained VNS to ascertain that a net enhancement of 5-HT transmission could be obtained by this intervention.
Methods
Animals
We performed the experiments on male Sprague–Dawley rats (Charles River, St. Constant, Que.) weighing a minimum of 275 g at the time of the implantation of the VNS device and housed under standard laboratory conditions (12:12 light–dark cycle with free access to food and water). We maintained body temperature at 37°C during the surgery and electrophysiological experiments. We handled all animals according to the guidelines of the Canadian Council on Animal Care, and the McGill University Animal Care Committee approved our experiments.
Surgery
Using sterile techniques, we performed the surgery while animals were under equithesine (1 mL intraperitoneally/300-g rat [4.26% chloral hydrate and 0.96% sodium pentobarbital]). We administered supplemental doses of equithesine intraperitoneally, 0.1 mL at a time, to maintain constant anesthesia and to prevent any nociceptive reaction to a tail pinch. We made a horizontal incision in the ventral aspect of the neck. We carefully separated the skin and muscles and we isolated the left vagus nerve, which lies lateral to the carotid artery. We wrapped bipolar leads around the left carotid artery and vagus nerve, allowing close contact between the vagus nerve and the electrodes. We sutured the leads to the underlying muscle. We tunnelled the leads subcutaneously toward an incision made in the back and connected them to the stimulator. We then placed the stimulator in a dorsal pocket under the back skin wiped with iodine, and we administered antibiotics and fluid replacements to ease recovery. Sham animals (control group) underwent the same surgical procedure with leads and a dummy 102-pulse stimulator in place. We checked the lead impedance to ensure a tight connection between the nerve and the coil, using the device diagnostic setting on the NeuroCybernetic Prosthesis hand-held computer and programming wand (Cyberonics Inc.). After a 2-day recovery, we turned on the stimulator for 2 weeks in treated rats and programmed it with output similar to those used in humans (30 s on and 5 min off, continuous cycle, frequency of 20 Hz, pulse width of 500 μs and intensity of 0.25 mA).5
Noradrenergic neuronal lesion
We injected animals with a single dose of a selective noradrenergic toxin DSP-4 (N[2-chloroethyl]-N-ethyl-2-bromo-benzylamine; Sigma) intraperitoneally at 50 mg/kg in a volume of 2 mL/kg 5 days before the stimulator implant; DSP-4 has been shown to produce a robust decrease (90%) of NE levels in the hippocampus, and its action is restricted to locus coeruleus axons.24,25
Electrophysiological experiments
We anesthetized the rats with 400 mg/kg of chloral hydrate administered intraperitoneally, and we mounted them on a stereotaxic apparatus. Prior to the recording, we inserted a catheter in a lateral tail vein for intravenous administration of drugs. We performed the experiments with the VNS device in place but inactivated for the duration of the experiment (a 2- to 3-hour period) to avoid electrical interference.
Recordings of 5-HT neurons in the dorsal raphe nucleus
We performed in vivo electrophysiological extracellular unitary recordings using single glass micropipettes. We filled the electrodes with a 2-M NaCl solution with an impedance range of 2–4 MΩ. We drilled a burr hole on the midline 1 mm anterior to lambda, and we encountered 5-HT neurons in the dorsal raphe nucleus over a distance of 1 mm starting immediately below the ventral border of the Sylvius aqueduct. We identified 5-HT neurons in the dorsal raphe nucleus using the criteria of Aghajanian and Vandermaelen:26 slow flow (0.5–2.5 Hz), regular firing rate and long duration (0.8–1.2 ms) positive action potentials. To determine the average firing rate for each group, we made at least 5 electrode descents per rat at 100 μm distance from the first descent. We recorded each neuron for at least 1 minute. We calculated neuronal firing rates by adding each discharge per 10-second histogram (as obtained by the Spike2 program [CED, Cambridge, UK] during recording) and dividing by the length of time recorded in seconds. We added together all neuronal firing rates for one group and divided the sum by the number of neurons recorded per group. We used 5–6 rats per group.
We assessed the responsiveness of neurons in the dorsal raphe nucleus to the intravenous administration of the α1-adrenoceptor antagonist prazosin after the injection. We expressed the change in firing rate due to the antagonist for each neuron as a percentage of basal firing rate. We analyzed 1 neuron per rat. We used 2–9 rats per dose of prazosin.
Analysis of the firing pattern of NE and 5-HT neurons
We analyzed the firing pattern of NE neurons by interspike interval burst analysis, using 5–9 rats per group, according to the criteria set by Grace and Bunney.27 We defined the onset of a burst as the occurrence of 2 spikes with an interspike interval shorter than 0.08 s. We defined the termination of burst as an interspike interval of 0.16 s or longer.
We analyzed the firing pattern of 5-HT neurons by inter-spike interval burst analysis, using 5–7 rats per group, according to the criteria set by Hajos and colleagues.28 We defined the onset of a burst as the occurrence of 2 spikes with an interspike interval shorter than 0.01 s. We defined the termination of burst as an interspike interval of 0.012 s or longer.
Recordings from CA3 dorsal hippocampus pyramidal neurons
We performed recording and microiontophoresis using 5-barelled glass micropipettes. We filled the central barrel with a 2-M NaCl solution and used it for extracellular unitary recordings. The side barrels contained 5-HT creatinine sulfate (Sigma; 5 mM in 200 mM NaCl, pH4), quisqualate (Sigma; 1.5 mM in 400 mM NaCl, pH8) and 2 M NaCl used for automatic current balancing. We lowered the microelectrodes at 4.2 mm lateral and 4.2 mm anterior to lambda into the CA3 region of the dorsal hippocampus. We identified the pyramidal neurons by their large amplitude (0.5–1.2 mV), long duration (0.8–1.2 ms) and such simple spikes alternating with complex spike discharges.29 Because most hippocampus pyramidal neurons are not spontaneously active under chloral hydrate anesthesia, we used a leak or a small ejection current of quisqualate (+1 to −2 nA) to activate them within their physiologic range.30 Two minutes before the intravenous administration of WAY 100635, we decreased the firing activity of the quisqualate-activated CA3 pyramidal neurons tested (by a lower ejection current of quisqualate) to less than 5 Hz to allow the detection of possible changes in firing activity after the 5-HT1A receptor antagonist WAY 100635 administration in treated and control rats. We used 6 rats per group.
Drugs and materials
We dissolved all drugs, DSP-4 (Sigma), prazosin (Sigma) and WAY 100635 (Sigma) in distilled water. Cyberonics provided the leads, 102-pulse stimulators and dummy stimulators; they were not involved in the research.
Statistical analysis
We expressed electrophysiological data as means (and standard errors of the means [SEM]) of the firing rate, number of bursts, percentage of spikes occurring in bursts, mean spikes per burst and mean burst length. We performed statistical comparisons of treated and control rats using linear mixed model analysis to address the number of neurons nested in each rat because we tested several neurons in each rat (we reported type 3 tests of fixed effects). We compared control rats with those treated with 14 days of VNS following DSP-4 lesion using a linear mixed model, in which we treated rats as a random effect (we reported type 3 tests of fixed effects). We used logistic regression analysis to construct dose–response curves and assess the effective dose for inhibiting neuronal activity by 50% (ED50). We performed statistical comparisons of dose–response curves of the effects of WAY 100635 on the CA3 pyramidal neuron firing rate among treated and control rats using 2-way repeated-measures analysis of variance. We compared the percentage of 5-HT and NE neurons discharging in a burst mode among treated and control rats separately using generalized estimating equations (GEE model) to take into account the effect of repeated-measures taken from the same rats. When running the GEE model, we used an exchangeable working correlation matrix (we reported score statistics for type 3 GEE analysis).
Results
Firing pattern of NE and 5-HT neurons
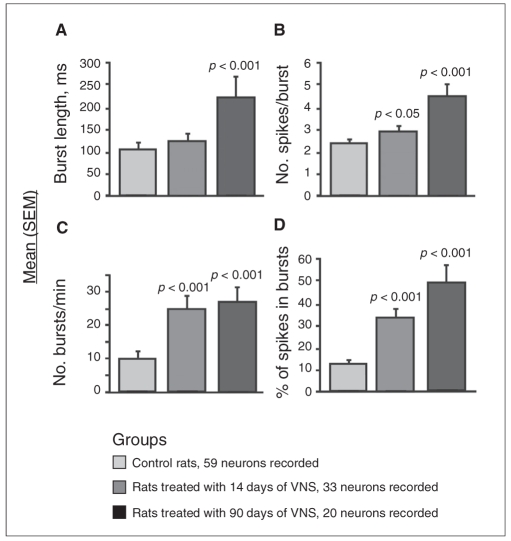
The reanalysis of a published data set23 using the same parameters as those in the present study showed that rats treated with VNS had double the percentage of NE neurons displaying bursts after 14 days of treatment compared with control rats (Table 1; χ21 = 7.6, p = 0.006). Fourteen days of treatment with VNS did not change the mean burst length of locus coeruleus NE neurons, but significantly enhanced the mean spikes per burst by 19% (F1,15 = 4.8, p = 0.040), the number of bursts per minute by 151% (F1,15 = 48.6, p < 0.001) and the percentage of spikes occurring in bursts by 148% (F1,15 = 47.5, p < 0.001; Fig. 1).
Table 1.
Effect of vagus nerve stimulation (VNS) treatment on the neuron firing pattern of norepinephrine (NE) in the locus coeruleus
| NE neurons
|
||
|---|---|---|
| Group | Analyzed, no. | Displaying bursts, no. (%) |
| Control | 59 | 24 (41) |
| VNS 14 d | 33 | 29 (88)* |
| VNS 90 d | 20 | 17 (85)† |
We determined significance using the generalized estimating equations model and comparison with control rats:
p < 0.01
p < 0.05.
Fig. 1.
Histograms representing mean (and standard errors of the mean [SEM]) (A) burst length, (B) spikes per burst, (C) number of bursts per minute and (D) percentage of spikes in bursts of locus coeruleus norepinephrine neurons in control rats and in rats treated with 14 or 90 days of vagus nerve stimulation (VNS). We used 5–9 rats per group. We determined significance using the linear mixed model.
After 90 days of treatment with VNS, the percentage of NE neurons displaying burst activity remained twice as high in treated rats as in control rats (Table 1; χ21= 5.0, p = 0.030). The burst length increased by 110% (F1,12 = 23.1, p < 0.001) and the mean number of spikes per burst increased by 80% (F1,12 = 39.4, p < 0.001). The number of bursts per minute remained stable compared with that in rats treated with 14 days of VNS, and the percentage of spikes occurring in bursts was 365% greater than that in control rats (Fig. 1).
In the case of 5-HT neurons, the mean spikes per burst, burst length, number of bursts per minute and the percentage of spikes in bursts were not significantly altered (data not shown). The percentage of 5-HT neurons discharging in bursts was increased by more than 50% after 90 days of treatment with VNS (Table 2), although this increase did not reach statistical significance.
Table 2.
Effect of vagus nerve stimulation (VNS) on the neuron firing pattern of serotonin (5-HT) in the dorsal raphe nucleus
| 5-HT neurons
|
||
|---|---|---|
| Group | Analyzed, no. | Displaying bursts, no. (%) |
| Control | 56 | 11 (20) |
| VNS 14 d | 70 | 15 (21) |
| VNS 90 d | 65 | 21 (32) |
Effect of VNS on 5-HT neurons in the dorsal raphe nucleus following DSP-4 lesion
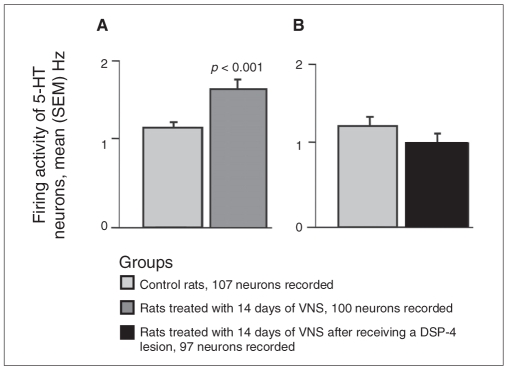
In the current study, 14 days of treatment with VNS significantly increased the basal firing rate of 5-HT neurons in the dorsal raphe nucleus by 44% when compared with control rats (F1,9 = 45.4, p < 0.001; Fig. 2). A selective lesion of locus coeruleus NE neurons following the administration of the selective noradrenergic toxin DSP-4 completely prevented the enhancing action of VNS on 5-HT neuron firing activity in the dorsal raphe nucleus (Fig. 2B).
Fig. 2.
Histograms representing mean (and standard errors of the mean [SEM]) spontaneous firing rates of serotonin (5-HT) neurons in the dorsal raphe nucleus. We used 5–6 rats per group. (A) Average firing rates for controls and rats treated with 14 days of vagus nerve stimulation (VNS). We determined significance using the linear mixed model. (B) Average firing rates for control rats and rats treated with 14 days of VNS following DSP-4 lesion. There was no significant difference between the 2 groups.
Effect of VNS on the degree of activation of α1–adrenoceptors on 5-HT neurons
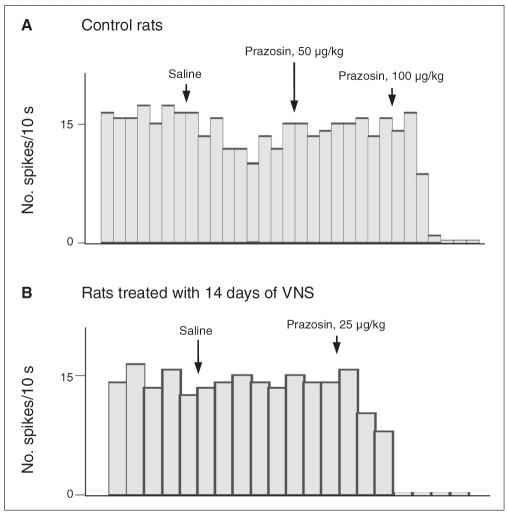
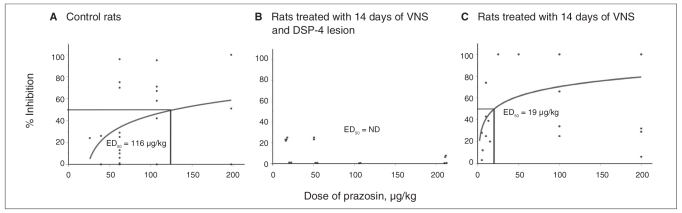
We constructed dose–response curves for the α1-adrenoceptor antagonist prazosin to assess the degree of activation of these receptors. We administered various doses of prazosin intravenously (5–200 μg/kg). The ED50 value for control rats was 116 μg/kg (Fig. 3 and Fig. 4). A selective lesion of locus coeruleus NE neurons following DSP-4 administration nearly abolished the inhibitory effect of prazosin (Fig. 4), therefore indicating the noradrenergic nature of this effect. The ED50 for prazosin in the rats treated with 14 days of VNS was 19 μg/kg (Fig. 3 and Fig. 4), thus indicating an enhanced NE activation of 5-HT neurons.
Fig. 3.
Examples of the response of serotonin (5-HT) neurons in the dorsal raphe nucleus to the α1-adrenoceptor antagonist prazosin. Histograms of one 5-HT neuron in the dorsal raphe nucleus in (A) control rats and (B) rats treated with 14 days of vagus nerve stimulation (VNS).
Fig. 4.
Response of serotonin (5-HT) neurons in the dorsal raphe nucleus to the α1-adrenoceptor antagonist prazosin. Dose–response curves for prazosin in (A) control rats, (B) rats treated with 14 days of vagus nerve stimulation (VNS) following DSP-4 lesion and (C) rats treated with 14 days of VNS. We analyzed 1 neuron per rat. We used 2–9 rats per dose of prazosin. ED50 = effective dose for inhibiting neuronal activity by 50%; ND = not determined.
Effect of VNS on 5-HT1A postsynaptic receptors
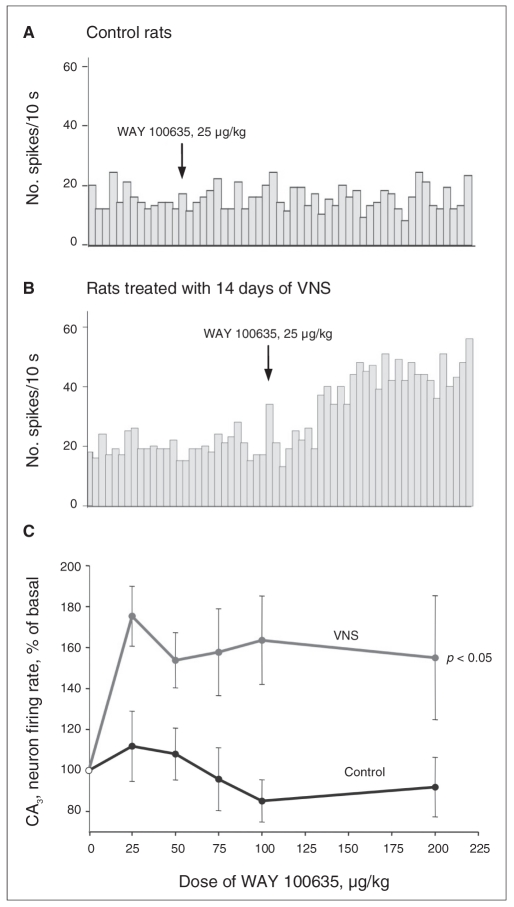
The intravenous administration of the selective 5-HT1A receptor antagonist WAY 100635 (25–200 μg/kg) did not modify the firing activity of dorsal hippocampus CA3 pyramidal neurons in control rats, as previously reported.15 In contrast, in the rats treated with 2 weeks of VNS, the administration of WAY 100635 significantly increased the firing activity of CA3 pyramidal neurons (F1,69 = 9.3, p = 0.012), therefore indicating a greater tonic activation of the inhibitory postsynaptic 5-HT1A receptor (Fig. 5).
Fig. 5.
Integrated firing rate histograms of 2 dorsal hippocampus CA3 pyramidal neurons showing their responsiveness to the intravenous administration of WAY 100635 (25–200 μg/kg) in (A) a control rat and in (B) a rat treated with 14 days of vagus nerve stimulation (VNS). We activated these neurons with quisqualate. (C) Dose–response curves of the effects of WAY 100635 on the CA3 pyramidal neuron firing rate in control rats and rats treated with 14 days of VNS. We used 6 rats per group. We determined significance using 2-way repeated-measures analysis of variance.
Discussion
Our results confirmed the enhancing action of VNS on the firing rate of 5-HT neurons and revealed that VNS markedly enhanced the burst firing activity of NE neurons. In addition, we found that the stimulatory effect of VNS on 5-HT neuronal firing was mediated by a noradrenergic mechanism. Finally, the VNS-induced changes in the activity of 5-HT neurons produced a net increase in 5-HT transmission in the hippocampus.
Prior experiments showed that sustained VNS enhanced the spontaneous firing rate of NE and 5-HT neurons in a time-dependent manner.23 Further reanalysis of this data set showed that the firing pattern of these 2 types of neurons was also altered, albeit not to the same extent. After 14 days of VNS, the percentage of NE neurons displaying bursts and the number of bursts per minute were more than doubled, which lead to a similar enhancement of the percentage of spikes occurring in bursts (Fig. 1). The latter parameter is likely the more reliable indicator that a subset of neurons has significantly shifted its overall firing pattern from single spiking activity to burst discharging. Presumably, this shift would be the most reliable indicator of robust enhancement of NE release. Indeed, it was shown that this mode of firing led to greater NE release than single pulses in the rat frontal cortex.31 After 90 days of VNS, the percentage of neurons displaying bursts remained stable compared with 14 days of VNS, but an increase in spikes per burst and burst length were then detectable, presumably contributing to the further increase in the mean firing rate of NE neurons. Finally, it is important to note that the percentage of spikes occurring in bursts was about 4 times greater among rats having 90 days of VNS when compared with control rats (Fig. 1).
None of the previously mentioned firing pattern parameters of 5-HT neurons was affected after 14 and 90 days of VNS. However, after 90 days of VNS, the percentage of 5-HT neurons discharging in bursts increased to 32% from a value of 20% in controls, although this increase did not reach statistical significance. Taken together, these reanalyses indicate that, despite the observation that the mean firing rate of both NE and 5-HT neurons is doubled by 90 days of VNS,23 NE neurons undergo the most important shift of firing activity. Consequently, based on such parameters, it is expected that VNS would lead to a greater increase in NE than 5-HT release in postsynaptic structures. This hypothesis is currently being tested using microdialysis experiments. It is noteworthy that sustained administration of the NE and dopamine releaser bupropion for 14 days significantly increases the percentage of both NE and 5-HT neurons discharging in bursts, but that only the mean firing rate of 5-HT neurons is significantly increased.32,33 Therefore, whereas VNS and bupropion have some commonality in their action on NE and 5-HT neurons, VNS doubles the NE firing rate and triples the percentage of spikes occurring in bursts after 14 days of treatment (Fig. 1). Thus, VNS seems to exert a more important action on NE neurons than bupropion.
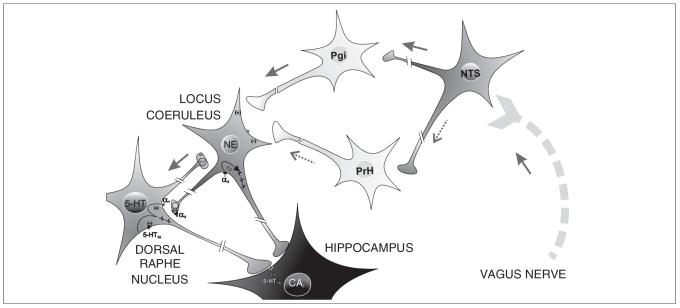
With VNS, the increase of NE neuronal firing activity appeared sooner and was greater than that observed in 5-HT neurons.23 Such an increase in the firing rate of NE neurons likely explains the prompt increase in extracellular NE in postsynaptic areas.34,35 This suggests that the effect of VNS on 5-HT neurons is indirect and might be mediated through an activation of the NE system. To test this hypothesis, we examined the effect of a specific lesion of locus coeruleus neurons on the capacity of VNS to alter the firing of 5-HT neurons using the selective toxin DSP-4. This lesion, which leaves unaltered the firing rate of 5-HT neurons,36 completely prevented the effect of VNS on 5-HT neuronal firing activity in the dorsal raphe nucleus. This confirms the crucial role of NE neurons on the effect of VNS on 5-HT neurons. This observation is therefore consistent with the fact that the vagus nerve sends afferents to the nucleus of the tractus solitarius, which in turn sends direct inputs to the locus coeruleus, but not to the dorsal raphe nucleus.37,38 In fact, the nucleus of the tractus solitarius projects to the locus coeruleus through di-synaptic pathways: a system localized in the nucleus paragigantocellularis containing excitatory amino acids39 and a γ-aminobutyric acid (GABA)ergic inhibitory system localized in the nucleus prepositus hypoglossi, acting primarily on the GABAA receptor subtypes on locus coeruleus neurons.40 Our results suggest that VNS facilitates the excitatory pathway to the locus coeruleus neurons more than the inhibitory one. The locus coeruleus would then modify the firing rate of 5-HT neurons through its monosynaptic input to the dorsal raphe nucleus. Vagus nerve stimulation is therefore believed to act first on the locus coeruleus, and then indirectly on 5-HT neurons in the dorsal raphe nucleus (Fig. 6). This hypothesis is also consistent with the fact that VNS produced an acute activation of the locus coeruleus, which is revealed by an increase of the short-term neuron activation biomarker c-fos, but a more delayed activation of the dorsal raphe nucleus shown by a significant increase of ΔFosB after chronic treatment.41 It remains to be determined whether the firing activity of median raphe 5-HT neurons, the second most important source of 5-HT innervation of the forebrain, could also be affected by VNS.
Fig. 6.
The vagus nerve sends afferents to the nucleus tractus solitarius (NTS), which in turn projects to the locus coeruleus through disynaptic pathways: one via the nucleus paragigantocellularis (Pgi) containing excitatory amino acids and a γ-aminobutyric acid (GABA)ergic inhibitory system via the nucleus prepositus hypoglossi (PrH), acting primarily at the GABAA receptor subtypes on neurons in the locus coeruleus. Vagus nerve stimulation (VNS) would facilitate the excitatory pathway on neurons in the locus coeruleus more than the inhibitory one. The locus coeruleus would then modify the firing rate of serotonin (5-HT) neurons through its monosynaptic input to the dorsal raphe nucleus. Therefore, VNS is believed to act first on the locus coeruleus, and then indirectly on 5-HT neurons in the dorsal raphe nucleus. It leads to a marked enhancement of norepinephrine (NE) and 5-HT transmission.
An increase in the firing rate of 5-HT neurons is usually associated with an increase in endogenous 5-HT release42,43 that activates the somatodendritic autoreceptors. However, with VNS, no change in 5-HT1A autoreceptor sensitivity was observed, suggesting that the increased firing was obtained through an alternative mechanism.23 The basal firing activity of 5-HT neurons in the dorsal raphe nucleus is stimulated by NE input44 via α1-adrenergic receptors. It has been established that these receptors are tonically activated, as demonstrated by a reduction of the firing activity of 5-HT neurons following the acute administration of an α1-adrenoceptor antagonist.16,44 Thus, we compared the effect of acute administration of the α1-adrenergic antagonist prazosin in control rats and in rats that had 14 days of VNS. The effect of prazosin on the firing activity of 5-HT neurons was much greater in treated rats than in controls, suggesting that the tonic activation of these dorsal raphe nucleus α1-adrenoceptors was increased through an enhanced release of endogenous NE.
The hippocampus is a structure that appears central in the mediation of the therapeutic effect of various classes of anti-depressant treatments.14 More specifically, the degree of activation of postsynaptic 5-HT1A receptors in this brain region is enhanced after long-term administration of a variety of antidepressant strategies, including electroconvulsive shock.13,15 The CA3 region of the dorsal hippocampus receives its serotonergic input from raphe nuclei. In freely moving rats, the intravenous administration of the 5-HT1A antagonist WAY 100635 increases the firing rate of hippocampal pyramidal neurons, whereas in anesthetized rats this antagonist is without effect, which indicates that there is no tonic activation of these receptors, presumably resulting from the lower firing rate of 5-HT neurons under the latter condition.45 However, after long-term treatment with different anti-depressant strategies, including drug combinations, the administration of the 5-HT1A receptor antagonist WAY 100635 markedly increases the firing activity of CA3 pyramidal neurons.15,46,47 These results indicate that these treatments increase the tonic activation of such receptors. In the present study, the acute administration of WAY 100635 markedly increased the firing activity of CA3 hippocampal pyramidal neurons in rats that had 14 days of VNS, suggesting that VNS, like classical antidepressant treatments, enhances the tonic activation of forebrain postsynaptic 5-HT1A receptors.
Limitations
Our study has some limitations. First, the stimulator was turned off during the electrophysiological recordings, which likely decreased the vagal tone to the brain. Nevertheless, we still observed firing rates higher than those recorded in control rats, thus indicating that sustained changes had been triggered owing only to prolonged stimulation. Therefore, this enhanced activity of the neurons represents their firing rate when the stimulator is off during the 5 minutes of each duty cycle. Second, we obtained the data while the animals were under anesthesia, and it is known that in anesthetized animals the firing activity of presumed 5-HT neurons corresponds to that occurring during slow-wave sleep (about 1 Hz).48 Recordings would therefore need to be carried out in unanesthesized rats to ascertain that the difference between the control rats and those treated with VNS observed herein is not influenced by the anesthetic agent. Third, we did not verify the extent of the NE depletion by DSP-4 in these experiments. In addition, we did not deem it necessary to protect 5-HT neurons using an SSRI when injecting DSP-4 because results from our laboratory have shown that the firing of 5-HT neurons as well as the content in the dorsal raphe nucleus remains unchanged by DSP-4.36,49
Conclusion
In conclusion, this study showed that the effects of VNS on the 5-HT system are mediated through its robust effect on increasing the rate and altering the pattern of firing of NE neurons. In keeping with all the other forms of antidepressant treatments, VNS increased the tonic activation of postsynaptic 5-HT1A receptors. Since VNS appears to be efficient in treatment-resistant depression,5 its effects should not be expected to be the same as the other treatments. Indeed, despite some similarities of VNS and various antidepressant drugs (used alone or in combination) on the 5-HT and NE systems, only VNS has been shown thus far to double the firing rate of NE neurons in a sustained manner and quadruple their number of action potentials occurring in the bursting mode. These features of VNS can therefore begin to provide an explanation for its effectiveness in treatment-resistant depression. Investigation of the effects of VNS on other chemospecific neuronal systems should provide additional insight into its mechanism of action, and ultimately its therapeutic benefits in depression.
Acknowledgements
This work was supported by the Canadian Institutes of Health Research (University-Industry Program-Operating Grant UOP-71713) and by Cyberonics Inc., Houston, Texas. Dr. Blier received the Canadian Research Chair and is a paid consultant for Cyberonics. Cyberonics Inc. provided the leads, 102-pulse simulators and dummy simulators; they had no involvement in the research. We would like to thank Mrs. Lise Martin and Dr. Guillaume Lucas for great assistance.
Footnotes
Competing interests: None declared for Ms. Manta and Dr. Dong. Dr. Blier serves as president of Medical Multimedia Inc. and is a contract employee of Bristol-Myers Squibb, Forest Laboratories, Janssen Pharmaceuticals and Steelbeach Productions. He has received grant funding from AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Forest Laboratories, Janssen Pharmaceuticals, Lundbeck, Mitsubishi Pharma, Organon Pharmaceuticals and Wyeth-Ayerst. He acts as a consultant to Biovail, Bristol-Myers Squibb, Eli Lilly, Forest Laboratories, Janssen Pharmaceuticals, Lundbeck, Novartis, Organon Pharmaceuticals, Pfizer, Sanofi-Aventis, Sepracor, Takeda and Wyeth-Ayerst and served on the speakers bureaus of Cyberonics, Eli Lilly, Forest Laboratories, Janssen Pharmaceuticals, Lundbeck, Organon Pharmaceuticals and Wyeth-Ayerst. Dr. Debonnel was a paid consultant for Cyberonics.
Contributors: Dr. Debonnel designed the study. Ms. Manta and Dr. Dong acquired the data, which Ms. Manta and Drs. Debonnel and Blier analyzed. Ms. Manta wrote the article, which Drs. Dong and Blier reviewed. Ms. Manta and Drs. Dong and Blier approved the final version for publication.
References
- 1.Foley JO, Dubois F. Quantitative studies of the vagus nerve in the cat. I: the ratio of sensory and motor studies. J Comp Neurol. 1937;67:49–67. [Google Scholar]
- 2.Buoni S, Mariottini A, Pieri S, et al. Vagus nerve stimulation for drug-resistant epilepsy in children and young adults. Brain Dev. 2004;26:158–63. doi: 10.1016/S0387-7604(03)00120-7. [DOI] [PubMed] [Google Scholar]
- 3.Elger G, Hoppe C, Falkai P, et al. Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Res. 2000;42:203–10. doi: 10.1016/s0920-1211(00)00181-9. [DOI] [PubMed] [Google Scholar]
- 4.Schachter SC. Vagus nerve stimulation: mood and cognitive effects. Epilepsy Behav. 2004;5 (Suppl 1):S56–9. doi: 10.1016/j.yebeh.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Sackeim HA, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology. 2001;25:713–28. doi: 10.1016/S0893-133X(01)00271-8. [DOI] [PubMed] [Google Scholar]
- 6.Marangell LB, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for major depressive episodes: one year outcomes. Biol Psychiatry. 2002;51:280–7. doi: 10.1016/s0006-3223(01)01343-9. [DOI] [PubMed] [Google Scholar]
- 7.Nahas Z, Marangell LB, Husain MM, et al. Two-year outcome of vagus nerve stimulation (VNS) for treatment of major depressive episodes. J Clin Psychiatry. 2005;66:1097–104. doi: 10.4088/jcp.v66n0902. [DOI] [PubMed] [Google Scholar]
- 8.Schlaepfer TE, Frick C, Zobel A, et al. Vagus nerve stimulation for depression: efficacy and safety in a European study. Psychol Med. 2008;38:651–61. doi: 10.1017/S0033291707001924. [DOI] [PubMed] [Google Scholar]
- 9.Krahl SE, Senanayake SS, Pekary AE, et al. Vagus nerve stimulation (VNS) is effective in a rat model of antidepressant action. J Psychiatr Res. 2004;38:237–40. doi: 10.1016/j.jpsychires.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Daban C, Martinez-Aran A, Cruz N, et al. Safety and efficacy of vagus nerve stimulation in treatment-resistant depression. A systematic review. J Affect Disord. 2008;110:1–15. doi: 10.1016/j.jad.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Chae JH, Nahas Z, Lomarev M, et al. A review of functional neuro-imaging studies of vagus nerve stimulation (VNS) J Psychiatr Res. 2003;37:443–55. doi: 10.1016/s0022-3956(03)00074-8. [DOI] [PubMed] [Google Scholar]
- 12.Pineyro G, Blier P. Autoregulation of serotonin neurons: role in antidepressant drug action. Pharmacol Rev. 1999;51:533–91. [PubMed] [Google Scholar]
- 13.Blier P, de Montigny C. Current advances and trends in the treatment of depression. Trends Pharmacol Sci. 1994;15:220–6. doi: 10.1016/0165-6147(94)90315-8. [DOI] [PubMed] [Google Scholar]
- 14.Santarelli L, Saxe M, Gross C, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–9. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 15.Haddjeri N, Blier P, de Montigny C. Long-term antidepressant treatments result in a tonic activation of forebrain 5-HT1A receptors. J Neurosci. 1998;18:10150–6. doi: 10.1523/JNEUROSCI.18-23-10150.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Svensson TH, Bunney BS, Aghajanian GK. Inhibition of both noradrenergic and serotonergic neurons in brain by the alpha-adrenergic agonist clonidine. Brain Res. 1975;92:291–306. doi: 10.1016/0006-8993(75)90276-0. [DOI] [PubMed] [Google Scholar]
- 17.Curet O, de Montigny C. Electrophysiological characterization of adrenoceptors in the rat dorsal hippocampus. III. Evidence for the physiological role of terminal alpha 2-adrenergic autoreceptors. Brain Res. 1989;499:18–26. doi: 10.1016/0006-8993(89)91131-1. [DOI] [PubMed] [Google Scholar]
- 18.Schoffelmeer AN, Mulder AH. 3H-noradrenaline release from rat neocortical slices in the absence of extracellular Ca2+ and its presynaptic alpha 2-adrenergic modulation. A study on the possible role of cyclic AMP. Naunyn Schmiedebergs Arch Pharmacol. 1983;323:188–92. doi: 10.1007/BF00497661. [DOI] [PubMed] [Google Scholar]
- 19.Crews FT, Smith CB. Presynaptic alpha-receptor subsensitivity after long-term antidepressant treatment. Science. 1978;202:322–4. doi: 10.1126/science.211589. [DOI] [PubMed] [Google Scholar]
- 20.McMillen BA, Warnack W, German DC, et al. Effects of chronic desipramine treatment on rat brain noradrenergic responses to alpha-adrenergic drugs. Eur J Pharmacol. 1980;61:239–46. doi: 10.1016/0014-2999(80)90126-0. [DOI] [PubMed] [Google Scholar]
- 21.Vandermaelen CP, Aghajanian GK. Electrophysiological and pharmacological characterization of serotonergic dorsal raphe neurons recorded extracellularly and intracellularly in rat brain slices. Brain Res. 1983;289:109–19. doi: 10.1016/0006-8993(83)90011-2. [DOI] [PubMed] [Google Scholar]
- 22.Millan MJ, Rivet JM, Gobert A, et al. 5-HT1A receptors and the tail-flick response. VI. Intrinsic alpha 1A-adrenoceptor antagonist properties can mask the actions of 5-HT1A receptor agonists in the spontaneous tail-flick paradigm. J Pharmacol Exp Ther. 1994;269:121–31. [PubMed] [Google Scholar]
- 23.Dorr AE, Debonnel G. Effect of vagus nerve stimulation on serotonergic and noradrenergic transmission. J Pharmacol Exp Ther. 2006;318:890–8. doi: 10.1124/jpet.106.104166. [DOI] [PubMed] [Google Scholar]
- 24.Grzanna R, Berger U, Fritschy JM, et al. Acute action of DSP-4 on central norepinephrine axons: biochemical and immunohistochemical evidence for differential effects. J Histochem Cytochem. 1989;37:1435–42. doi: 10.1177/37.9.2768812. [DOI] [PubMed] [Google Scholar]
- 25.Logue MP, Growdon JH, Coviella IL, et al. Differential effects of DSP-4 administration on regional brain norepinephrine turnover in rats. Life Sci. 1985;37:403–9. doi: 10.1016/0024-3205(85)90401-1. [DOI] [PubMed] [Google Scholar]
- 26.Aghajanian GK, Vandermaelen CP. Intracellular identification of central noradrenergic and serotonergic neurons by a new double labeling procedure. J Neurosci. 1982;2:1786–92. doi: 10.1523/JNEUROSCI.02-12-01786.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci. 1984;4:2877–90. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hajos M, Gartside SE, Villa AE, et al. Evidence for a repetitive (burst) firing pattern in a sub-population of 5-hydroxytryptamine neurons in the dorsal and median raphe nuclei of the rat. Neuroscience. 1995;69:189–97. doi: 10.1016/0306-4522(95)00227-a. [DOI] [PubMed] [Google Scholar]
- 29.Kandel ER, Spencer WA. Electrophysiology of hippocampal neurons. II. After-potentials and repetitive firing. J Neurophysiol. 1961;24:243–59. doi: 10.1152/jn.1961.24.3.243. [DOI] [PubMed] [Google Scholar]
- 30.Ranck JB., Jr Studies on single neurons in dorsal hippocampal formation and septum in unrestrained rats. I. Behavioral correlates and firing repertoires. Exp Neurol. 1973;41:461–531. doi: 10.1016/0014-4886(73)90290-2. [DOI] [PubMed] [Google Scholar]
- 31.Florin-Lechner SM, Druhan JP, Aston-Jones G, et al. Enhanced norepinephrine release in prefrontal cortex with burst stimulation of the locus coeruleus. Brain Res. 1996;742:89–97. doi: 10.1016/s0006-8993(96)00967-5. [DOI] [PubMed] [Google Scholar]
- 32.El Mansari M, Ghanbari R, Janssen S, et al. Sustained administration of bupropion alters the neuronal activity of serotonin, norepinephrine but not dopamine neurons in the rat brain. Neuropharmacology. 2008;55:1191–8. doi: 10.1016/j.neuropharm.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 33.Ghanbari R, El Mansari M, Blier P. Electrophysiological effects of the co-administration of escitalopram and bupropion on rat serotonin and norepinephrine neurons. J Psychopharmacol. 2008 Nov. 21; doi: 10.1177/0269881108095714. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 34.Follesa P, Biggio F, Gorini G, et al. Vagus nerve stimulation increases norepinephrine concentration and the gene expression of BDNF and bFGF in the rat brain. Brain Res. 2007;1179:28–34. doi: 10.1016/j.brainres.2007.08.045. [DOI] [PubMed] [Google Scholar]
- 35.Roosevelt RW, Smith DC, Clough RW, et al. Increased extracellular concentrations of norepinephrine in cortex and hippocampus following vagus nerve stimulation in the rat. Brain Res. 2006;1119:124–32. doi: 10.1016/j.brainres.2006.08.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gobbi G, Cassano T, Radja F, et al. Neurokinin 1 receptor antagonism requires norepinephrine to increase serotonin function. Eur Neuropsychopharmacol. 2007;17:328–38. doi: 10.1016/j.euroneuro.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Henry TR. Therapeutic mechanisms of vagus nerve stimulation. Neurology. 2002;59(Suppl 4):S3–14. doi: 10.1212/wnl.59.6_suppl_4.s3. [DOI] [PubMed] [Google Scholar]
- 38.Van Bockstaele EJ, Peoples J, Telegan P. Efferent projections of the nucleus of the solitary tract to perilocus coeruleus dendrites in rat brain: evidence for a monosynaptic pathway. J Comp Neurol. 1999;412:410–28. doi: 10.1002/(sici)1096-9861(19990927)412:3<410::aid-cne3>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 39.Ennis M, Aston-Jones G. Activation of locus coeruleus from nucleus paragigantocellularis: a new excitatory amino acid pathway in brain. J Neurosci. 1988;8:3644–57. doi: 10.1523/JNEUROSCI.08-10-03644.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ennis M, Aston-Jones G. GABA-mediated inhibition of locus coeruleus from the dorsomedial rostral medulla. J Neurosci. 1989;9:2973–81. doi: 10.1523/JNEUROSCI.09-08-02973.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cunningham JT, Mifflin SW, Gould GG, et al. Induction of c-Fos and DeltaFosB immunoreactivity in rat brain by vagal nerve stimulation. Neuropsychopharmacology. 2008;33:1884–95. doi: 10.1038/sj.npp.1301570. [DOI] [PubMed] [Google Scholar]
- 42.Bosker F, Klompmakers A, Westenberg H. Extracellular 5-hydroxytryptamine in median raphe nucleus of the conscious rat is decreased by nanomolar concentrations of 8-hydroxy-2-(di-n-propylamino) tetralin and is sensitive to tetrodotoxin. J Neurochem. 1994;63:2165–71. doi: 10.1046/j.1471-4159.1994.63062165.x. [DOI] [PubMed] [Google Scholar]
- 43.Hery F, Faudon M, Fueri C. Release of serotonin in structures containing serotoninergic nerve cell bodies: dorsalis raphe nucleus and nodose ganglia of the cat. Ann N Y Acad Sci. 1986;473:239–55. doi: 10.1111/j.1749-6632.1986.tb23620.x. [DOI] [PubMed] [Google Scholar]
- 44.Baraban JM, Aghajanian GK. Suppression of firing activity of 5-HT neurons in the dorsal raphe by alpha-adrenoceptor antagonists. Neuropharmacology. 1980;19:355–63. doi: 10.1016/0028-3908(80)90187-2. [DOI] [PubMed] [Google Scholar]
- 45.Kasamo K, Suzuki T, Tada K, et al. Endogenous 5-HT tonically inhibits spontaneous firing activity of dorsal hippocampus CA1 pyramidal neurons through stimulation of 5-HT(1A) receptors in quiet awake rats: in vivo electrophysiological evidence. Neuropsychopharmacology. 2001;24:141–51. doi: 10.1016/S0893-133X(00)00181-0. [DOI] [PubMed] [Google Scholar]
- 46.Besson A, Haddjeri N, Blier P, et al. Effects of the co-administration of mirtazapine and paroxetine on serotonergic neurotransmission in the rat brain. Eur Neuropsychopharmacol. 2000;10:177–88. doi: 10.1016/s0924-977x(00)00069-9. [DOI] [PubMed] [Google Scholar]
- 47.Szabo ST, Blier P. Effects of the selective norepinephrine reuptake inhibitor reboxetine on norepinephrine and serotonin transmission in the rat hippocampus. Neuropsychopharmacology. 2001;25:845–57. doi: 10.1016/S0893-133X(01)00284-6. [DOI] [PubMed] [Google Scholar]
- 48.Trulson ME, Jacobs BL. Raphe unit activity in freely moving cats: correlation with level of behavioral arousal. Brain Res. 1979;163:135–50. doi: 10.1016/0006-8993(79)90157-4. [DOI] [PubMed] [Google Scholar]
- 49.Haddjeri N, Blier P. Neurokinin-1 receptor antagonists modulate brain noradrenaline and serotonin interactions. Eur J Pharmacol. 2008;600:64–70. doi: 10.1016/j.ejphar.2008.10.001. [DOI] [PubMed] [Google Scholar]