Abstract
Background
Several studies have investigated volumetric brain changes in patients with posttraumatic stress disorder (PTSD) and borderline personality disorder (BPD). Both groups exhibit volume reductions of the hippocampus and amygdala. Our aim was to investigate the influence of comorbid PTSD on hippocampus and amygdala volumes in patients with BPD.
Methods
We compared 2 groups of unmedicated female patients with BPD (10 with and 15 without comorbid PTSD) and 25 healthy female controls. We used T1- and T2-weighted magnetic resonance images for manual tracing and 3-dimensional reconstruction of the hippocampus and amygdala.
Results
Hippocampus volumes of patients with BPD and PTSD were smaller than those of healthy controls. However, there was no significant difference between patients with BPD but without PTSD and controls. Impulsiveness was positively correlated with hippocampus volumes in patients with BPD.
Limitations
Our study did not allow for disentangling the effects of PTSD and traumatization. Another limitation was the relatively small sample size.
Conclusion
Our findings highlight the importance of classifying subgroups of patients with BPD. Comorbid PTSD may be related to volumetric alterations in brain regions that are of central importance to our understanding of borderline psychopathology.
Introduction
Borderline personality disorder (BPD) is a frequent psychiatric disorder, with affective dysregulation and impulsivity as its most prominent features. It is associated with high levels of traumatic stress (e.g., childhood sexual abuse).1 Hippocampus and, to some degree, amygdala atrophy have been found consistently in patients with BPD.2 Studies revealed hippocampus volume reductions between 13% and 20% as well as amygdala volume reductions between 8% and 24%.3–7 The most recent volumetric studies by Zetzsche and colleagues8,9 again revealed reduced hippocampus volume but found an enlargement of the amygdala in patients with BPD and comorbid depression. Similarly, a study by New and collegues10 did not find amygdala volume reductions in patients with BPD.
Patients with BPD often have a variety of comorbidities; the most prominent among them is posttraumatic stress disorder (PTSD). Most epidemiological studies reveal comorbid PTSD in 60%–70% of the investigated patients with BPD.11,12 Hippocampus volume reduction is a major neurobiological finding in PTSD research published in the last 10–15 years. Reduction in hippocampus volume, as assessed by magnetic resonance–based volumetry, has been found in patients with combat-related13–15 and abuse-related PTSD.16–18 A recent meta-analysis19 confirmed the influence of PTSD on hippocampus volume, but also revealed an influence of traumatization, even in the absence of PTSD. In this meta-analysis, a correlation between PTSD and amygdala volume, at least for the left amygdala, could be confirmed. There is an ongoing debate whether these volume reductions are caused by elevated activity of stress-associated neurobiological systems such as the hypothalamic–pituitary–adrenal axis or whether they are genetically determined.14
Taken together, hippocampus and most probably amygdala volumes are reduced in patients with BPD as well as in those with PTSD. Both disorders share etiological factors (e.g., traumatic stress), symptomatology (e.g., states of dissociation, hyperarousal) and biological factors (e.g., aberrant patterns of activation in prefrontal and limbic regions).2 We recently demonstrated that amygdala deactivation in response to painful heat stimuli is present in patients with BPD and comorbid PTSD, but not in those without PTSD.20 Given these important overlapping neurobiological findings and the high co-occurrence of PTSD in patients with BPD, investigation of such patients with and without comorbid PTSD was the major focus of the present study. Using BRAINS2 software (www.psychiatry.uiowa.edu/mhcrc/IPLpages/BRAINS.htm) with T1-, T2.- and tissue-weighted images, a single rater (K.B.) with high intrarater reliability performed manual volumetry of the entire hippocampus and amygdala adjusted for whole-brain volume on a total of 50 human brains.
Methods
Participants
We recruited women with BPD and matched healthy controls over a period of about 18 months at the Central Institute of Mental Health in Mannheim, Germany. In patients, we assessed DSM-IV criteria for Axis-I disorders using the Structured Clinical Interview for DSM-IV Axis-I Disorders (SCID-I21) and for Axis-II disorders using the International Personality Disorder Examination (IPDE22). Qualified diagnosticians (A.K., G.V.) interviewed and screened controls for their mental status using SCID-I and IPDE, and we asked only those who did not meet diagnostic criteria for any Axis-I or Axis-II disorders to participate.
The main inclusion criteria for patients and controls were that they were free of any psychotropic medication for at least 2 weeks before scanning, right-handed and in a sufficiently stable condition to undergo magnetic resonance imaging (MRI). Exclusion criteria were prior head injury, previous meningoencephalitis, previous seizures or other relevant cerebral pathologies, a lifetime diagnosis of schizophrenia, schizoaffective disorder, bipolar affective disorder, acute suicidal tendencies, acute (< 12 mo) substance abuse problems, pregnancy, metal implants anywhere in the body and the inability to consent to participating in the study.
We compared patient and control groups with regards to body weight, age at examination and years of education.
After complete explanation of the study, we obtained written informed consent from all participants. The ethics committee of the University of Heidelberg, Germany, approved our study.
Psychometric assessment
During the diagnostic screening, we assessed and later used both the Barratt Impulsiveness Scale, 10th version (BIS-1023) and the Borderline Symptom List (BSL24) to calculate possible statistical correlations. We assessed severity of traumatization with the Childhood Trauma Questionnaire.25
Magnetic resonance scanning
We performed the magnetic resonance scanning on a 1.5 T Siemens Magnetom Vision scanner.
We acquired 180 high-resolution sagitally oriented slices in T1 mode (magnetization-prepared rapid acquisition with gradient echo [MPRAGE] sequence) per participant with the following specifications: pixel size 0.86 × 0.86 mm, repetition time (TR) 11.6 ms, echo time (TE) 4.9 ms, flip angle 8°, no. slap 1, slap thickness 180 mm, effective thickness 1 mm, 180 partitions, field of view (FOV) 220 mm. We acquired 104 interleaved sagitally oriented slices per participant in T2 mode with the following specifications: pixel size: 0.87 × 0.86 mm, TR 7280 ms, TE 54 ms, flip angle 180°, no. slap 52, slap thickness 2 mm, distribution factor 1, matrix 252 × 256, FOV 220 mm.
Preprocessing
We used the MRIcro free software package26 (www.sph.sc.edu/comd/rorden/mricro.html) to preprocess the raw image data (T1 and T2 images), converting the Siemens format into the Analyze format. To assess total brain volume, we used BrainSuite2 (http://brainsuite.usc.edu/), a freeware semiautomatic brain-segmenting program for neuroimaging and volumetric purposes offering skull-stripping, tissue classification, surface generation and several other options. Using the implemented Brain Surface Extractor (BSE) tool27–30 on the T1 images, we segmented the brain into different tissue types. We used the software to calculate and display the volumes of all the different tissue types, which we then summed to determine the total volume of the corresponding participant’s brain. We included only grey matter (GM), white matter (WM), grey matter/white matter partial volume (GM/WM) and grey matter/cerebrospinal fluid partial volume (GM/CSF) in the total brain volume calculation; we left out cerebrospinal fluid (CSF) and cerebrospinal fluid/other partial volume (CSF/other).
Manual volumetry
A single rater blind to the diagnostic group (K.B.) performed manual tracing for volumetric assessment using the freeware BRAINS2 software package (www.psychiatry.uiowa.edu/mhcrc/IPLpages/BRAINS.htm). Following the standard image workup guide, we resampled, realigned and accurately fitted both T1 and T2 images onto each other. We then ran an automatic segmentation routine that resulted in the creation of a tissue-segmented image in which we used the data supplied by the input T1 and T2 sets to calculate an enhanced image in terms of tissue recognizability and contrast. We later used this image in conjunction with the T1 and T2 image sets to represent a software-derived suggestion of what type of tissue was likely to be present at any given spatial point.
We measured both traced regions, the hippocampus and amygdala, in their entirety. We performed manual tracing for both sides separately from the posterior to anterior position in the coronal plane, and we then checked the sagittal view. We used a protocol for anatomic definition and demarcation of the hippocampus as a guideline for the tracing operation.31 We defined the most posterior part of the hippocampus as the first slice, which showed the fornix and portions of grey matter that were connected without interruption to the hippocampus tail in more anterior slices. Throughout the tracing process, we defined the medial hippocampus border as the mesial edge of the temporal lobe. If necessary, we facilitated the demarcation of the hippocampus from the parahippocampal gyrus using a diagonal line drawn on the medial edge of the temporal lobe just below the subiculum to separate them. We included the grey matter above and lateral to that imaginary line in the target volume; the rest was neither traced nor counted. The lateral hippocampus boundary comprised both the temporal horn of the lateral ventricle and, if present, the white matter directly adjacent to the hippocampal grey matter. We defined the superior border by the alveus, which we included in the measurement, whereas we excluded white matter, cerebrospinal fluid or any kind of fluid-filled space between the hippocampus and the alveus. We considered the white matter of the parahippocampal gyrus below the hippocampal structure to constitute the inferior boundary, and we included the subiculum in the measurement.
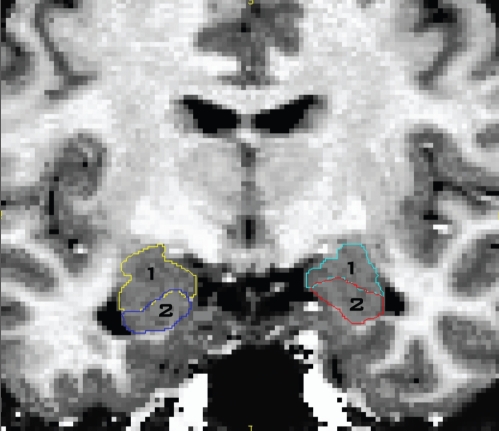
We followed the hippocampus anteriorly until its grey matter dove under that of the amygdala, constituting the separation of both structures with the often serrated-looking alveus. We checked the coronal tracing of every successive slice in the corresponding sagittal view, tracing until both views suggested the hippocampal head to have ended. Figure 1 depicts the manual tracing of the hippocampus–amygdala transition zone.
Fig. 1.
Manual tracing of the hippocampal–amygdalar transition zone showing (1) the amygdala and (2) the hippocampus.
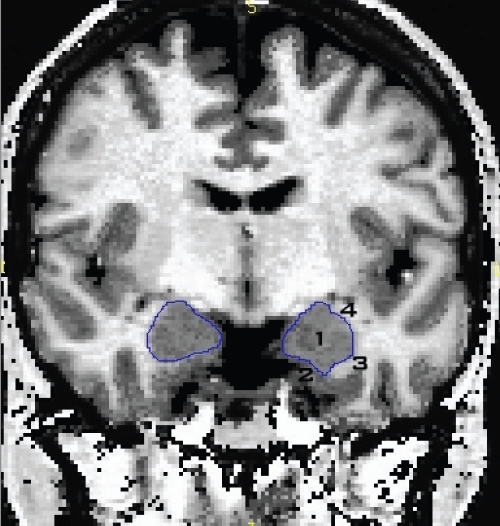
While tracing anteriorly, the amygdala could be seen above the diminishing hippocampal head, thus we traced it whenever it could be recognized on both the coronal and sagittal planes. This marked the posterior boundary of the corpus amygdaloideum. Figure 2 depicts the boundaries of the amygdala. We defined the inferior–medial amygdala border by a layer of white matter separating the amygdala from the entorhinal cortex. We defined the superior–lateral boundary by a layer of white matter, which also demarcated the amygdala from the neighbouring basal ganglia. In the anterior amygdalar region, if no clear demarcation from the surrounding white matter could be established, we defined the inferior border by an artificial line drawn diagonally through the most dorsal point of the entorhinal cortex. However, this was only necessary in a few cases. Finally, we defined the anterior amygdala boundary as the point at which no ovoid shape of grey matter could be segregated from the entorhinal cortex.
Fig. 2.
Boundaries of the amygdala showing the inferior–medial border defined by a layer of white matter (3) separating the amygdala (1) from the entorhinal cortex (2). The superior–lateral boundary was also defined by a layer of white matter (4), which also demarcated the amygdala from the neighbouring basal ganglia.
We established intrarater reliability on 25 consecutive brains of both patients and controls. We traced each of these 25 image sets twice for the mentioned volumes. The resulting intraclass correlation coefficients were 0.969 for the left hippocampus, 0.957 for the right hippocampus, 0.923 for the left amygdala and 0.939 for the right amygdala. These intraclass correlation coefficients indicate a high reliability of measurement.
Statistical analysis
We based our comparisons of hippocampus and amygdala volumes in the 3 groups (BPD with PTSD v. BPD without PTSD v. healthy controls) primarily on multivariate analysis of covariance (MANCOVA) models (i.e., multivariate analysis of variance models that included major variables as covariates). In these analyses, we included the hemisphere (left v. right) as a within-subject factor, we entered the group as a between-subject factor and we included the whole brain volume and age as covariates. We ran 2 separate MANCOVA models for the raw hippocampus and the raw amygdala volumes, respectively. In case of a significant overall group difference, we then computed pairwise contrasts.
We based additional analyses testing for the potential relation between hippocampus and amygdala volumes on the one hand and traumatization in general, physical abuse, comorbid eating disorder, agoraphobia, social phobia, substance abuse and depression on the other hand on the same type of MANCOVA models. As sample sizes in the analyses comparing patients with BPD and depression versus BPD without depression versus healthy controls were imbalanced, we corroborated the analysis on the potential impact of depression with additional analyses including depression as a covariate.
We tested the hypothesis of normally distributed data with the Kolmogorov–Smirnov test. In case of normal distribution, we used Student t tests to test for group differences. If the assumption of normality was violated, we performed Mann–Whitney U tests. We calculated Bravais–Pearson correlations. We performed the statistical analyses using the SPSS version 15.0 software package (SPSS Inc.). For all tests, we considered p < 0.05 to be statistically significant.
Results
Participants
We enrolled 25 women with BPD and 25 healthy controls in our study. The sociodemographic and clinical characteristics of patients and controls are provided in Table 1. We found no significant differences in body weight, age at examination and years of education between the groups. Whole-brain volumes were also not significantly different among the 3 groups. Most (n = 15) patients had been exposed to traumatic events. Seven of these 15 patients reported sexual abuse by a familiar person during childhood (ages 4–12 yr). Three patients experienced physical abuse in childhood. Four patients were exposed to rape between the ages of 15 and 17 years. One patient experienced an assault and a life-threatening accident in adulthood.
Table 1.
Socio-demographic and psychometric variables for women with borderline personality disorder with or without comorbid posttraumatic stress disorder and matched controls
| Group; mean (SD)
|
||||||
|---|---|---|---|---|---|---|
| Variable | BPD (n = 25) | BPD with PTSD (n = 10) | BPD without PTSD (n = 15) | Controls (n = 25) | ANOVA* | p value |
| Body weight, kg | 69.58 (18.78) | 70.70 (68.83) | 68.83 (20.77) | 61.88 (12.75) | F2,47 = 1.450 | 0.24 |
| Age [range] yr | 29.00 (6.70) [19–41] | 28.50 (6.06) [21–41] | 29.33 (7.29) [19–40] | 32.84 (9.14) [20–45] | F2,47 = 1.439 | 0.25 |
| Years of education | 11.32 (1.84) | 11.50 (2.01) | 11.20 (1.78) | 12.24 (1.39) | F2,47 = 2.050 | 0.14 |
| BIS-10, total score | 85.61 (11.01) | 81.50 (14.96) | 87.80 (7.99) | — | F1,21 = 1.767 | 0.20 |
| BSL, total score | 162.91 (72.49) | 165.88 (59.22) | 161.33 (80.60) | — | F1,21 = 0.023 | 0.89 |
| CTQ, total score | 69.95 (15.92) | 75.50 (14.50) | 67.73 (16.39) | — | F1,19 = 1.471 | 0.24 |
ANOVA = analysis of variance; BIS-10 = Barratt Impulsiveness Scale, 10th version;23 BSL = Borderline Symptom List;24 CTQ = Childhood Trauma Questionnaire;25 BPD = borderline personality disorder; PTSD = posttraumatic stress disorder; SD = standard deviation.
BPD with PTSD versus BPD without PTSD versus controls or BPD with PTSD versus BPD without PTSD for BIS-10, BSL and CTQ, respectively.
Lifetime psychiatric comorbidities of the patients were as follows: 10 patients had PTSD, 11 agoraphobia (with and without panic disorder), 12 eating disorders (bulimia and anorexia nervosa, binge-eating disorder), 12 substance abuse (stimulants, alcohol or drugs), 9 social phobia, 20 major depression, 2 generalized anxiety disorder and 4 obsessive–compulsive disorder. One patient did not report having comorbidities.
We were able to obtain BIS-10 scores for 24 and BSL scores for 22 of the 25 women with BPD.
Hippocampus volumes
The hippocampal volumes were statistically different among the 3 diagnostic groups (F2,45 = 3.512, p = 0.038). As shown in Table 2, this results from smaller volumes in patients with BPD and comorbid PTSD. We detected no significant main effect for the hemisphere (F1,45 = 1.267, p = 0.27) or for the interaction of group and hemisphere (F2,45 = 1.508, p = 0.23). Post-hoc pairwise comparisons revealed a significant difference between patients with PTSD and controls (F1,31 = 6.114, p = 0.019) and a trend for a difference between patients with and without PTSD (F1,21 = 4.169, p = 0.05). Patients without PTSD did not differ from controls (F1,36 = 0.180, p = 0.67). When splitting the group of patients into those with (n = 15) and without (n = 10) exposure to traumatic events in general (i.e., when using traumatization instead of PTSD diagnostic status for subgrouping the patients with BPD), we also found significant group differences (F2,45 = 3.468, p = 0.040). Splitting the BPD group into those with (n = 10) and without (n = 15) sexual or physical abuse revealed no significant differences in hippocampus volumes (F2,45 = 1.897, p = 0.16).
Table 2.
Group statistics (BPD with PTSD v. BPD without PTSD v. controls) for hippocampus and amygdala volumes of women with borderline personality disorder with or without posttraumatic stress disorder and matched controls
| Group; mean (SD) brain volume, mm3 |
|||||
|---|---|---|---|---|---|
| Brain region | BPD with PTSD (n = 10) | BPD witho ut PTSD (n = 15) | Controls (n = 25) | MANCOVA | p value |
| Hippocampus | F2,45 = 3.512 | 0.038 | |||
| Left | 2 870 (470) | 3 160 (304) | 3 084 (393) | ||
| Right | 3 012 (488) | 3 219 (292) | 3 224 (410) | ||
| Amygdala | F2,45 = 0.246 | 0.78 | |||
| Left | 1 960 (261) | 1 910 (180) | 1 903 (284) | ||
| Right | 2 073 (287) | 1 980 (193) | 1 934 (435) | ||
| Whole brain | 1 282 756 (103 436) | 1 281 285 (83 547) | 1 252 628 (122 740) | ||
BPD = borderline personality disorder; MANCOVA = multivariate analysis of covariance; PTSD = posttraumatic stress disorder; SD = standard deviation.
We repeated the analyses with alternative splitting of the BPD group and found no significant group differences when patients were separated into those with and without eating disorders (F2,45 = 1.300, p = 0.28), agoraphobia (F2,44 = 2.034, p = 0.14), social phobia (F2,45 = 2.171, p = 0.13), substance abuse (F2,45 = 1.829, p = 0.17) and major depression (F2,45 = 1.436, p = 0.25). To account for imbalance in sample sizes, we repeated our MANCOVA with depression as an additional covariate. Again, depression did not emerge as a significant predictor of hippocampus volume (F1,44 = 2.388, p = 0.13).
Amygdala volumes
We did not find amygdala volumes to be statistically different among the 3 groups (F2,45 = 0.246, p = 0.78; Table 2). We detected no significant main effect for the hemisphere (F1,45 = 0.677, p = 0.42) or for the interaction of group and hemisphere (F2,45 = 0.434, p = 0.65). Separation of the patient group by neither traumatization in general (F2,45 = 0.060, p = 0.94) nor by abuse (F2,45 = 0.046, p = 0.96) led to significant group differences.
Again, there were no significant group effects when splitting the group of patients with BPD into those with and without eating disorders (F2,45 = 0.450, p = 0.64), agoraphobia (F2,44 = 0.051, p = 0.95), social phobia (F2,45 = 0.057, p = 0.94), substance abuse (F2,45 = 0.056, p = 0.95) and depression (F2,45 = 0.126, p = 0.88). When we repeated the MANCOVA with depression as an additional covariate, depression did not emerge as a significant predictor of amygdala volume (F1,44 = 0.026, p = 0.87).
Correlations between volumes and psychometrics
Correlations between hippocampus and amygdala volumes are depicted in Table 3. Because of small group sizes, we calculated additional correlations for all patients. A significantly positive correlation between hippocampus size and impulsiveness, as assessed with the BIS-10, could be established for all patients.
Table 3.
Correlation coefficients between Barratt Impulsiveness Scale, Borderline Symptom List, Childhood Trauma Questionnaire and Dissociation Scale and target volumes with raw data adjusted for whole brain volume and age for all subgroups of women with borderline personality disorder
| BPD
|
BPD with PTSD
|
BPD witho ut PTSD
|
|||||
|---|---|---|---|---|---|---|---|
| Assessment tool; no. patients | Brain region | coefficient | p value | coefficient | p value | coefficient | p value |
| Barratt Impulsiveness Scale23 | Amygdala | 0.207 | 0.37 | 0.280 | 0.59 | 0.415 | 0.16 |
| n = 22 | Hippocampus | 0.595 | 0.004* | 0.729 | 0.10 | 0.415 | 0.16 |
| without PTSD = 12 | |||||||
| with PTSD = 10 | |||||||
| Borderline Symptom List24 | Amygdala | 0.309 | 0.17 | 0.267 | 0.61 | 0.604 | 0.029 |
| n = 22 | Hippocampus | 0.258 | 0.26 | 0.644 | 0.17 | 0.276 | 0.36 |
| without PTSD = 12 | |||||||
| with PTSD = 10 | |||||||
| Childhood Trauma Questionnaire25 | Amygdala | 0.285 | 0.24 | 0.981 | 0.019 | 0.431 | 0.14 |
| n = 17 | Hippocampus | 0.175 | 0.48 | 0.611 | 0.39 | 0.570 | 0.042 |
| without PTSD = 10 | |||||||
| with PTSD = 7 | |||||||
BPD = borderline personality disorder; PTSD = posttraumatic stress disorder.
Remained significant after Bonferroni correction.
Discussion
We found that patients with BPD and comorbid PTSD had significantly smaller hippocampi than healthy controls. Impulsiveness, as assessed by the BIS-10, was positively correlated with hippocampus volumes. Our measurements and tools (including BRAINS2 software) enabled 3-dimensional reconstruction, resampling, processing and coregistration of T1- and T2-weighted images and tissue-segmented images. The strength of our study lies in the application of both manual and semiautomatic volumetric protocols to minimize error.
Earlier studies, including one by our group, reported smaller hippocampus and, partly, amygdala volumes in patients with BPD.3–9 However, several factors have to be considered in the interpretation of volumetric findings in this context. Three studies3,7,9 so far have investigated the association of hippocampus volume with comorbid PTSD and found no difference between patients with BPD with and without PTSD. In contrast, we found a significant difference between these groups, at least for the left hippocampus. In accordance with our findings, Brambilla and colleagues6 found significant differences in hippocampus volume when patients with BPD who had histories of childhood abuse were compared with healthy controls; this significance disappeared when healthy controls were compared with patients with BPD who did not experience childhood abuse. The fact that we did not confirm earlier findings of amygdala volume reductions may be related to most of our patients having comorbid major depressive disorder, which in the study by Zetzsche and colleagues8 was associated with larger amygdala volumes. To account for the possibility that comorbid major depression might have masked differences in amygdala volume, we tested whether a diagnosis of major depression might account for the amygdala (and hippocampus) volumes by including this variable in the major analyses. Major depression did not emerge as a significant predictor of amygdala and hippocampus volumes. The inconsistent results may also be due to different exclusion criteria; unlike in the present study, several earlier studies5,7–9 did not exclude patients taking psychotropic medications. We further tested whether other comorbid disorders had a similar influence on hippocampus volumes as PTSD; we did not find significant influence of eating disorders, agoraphobia, social phobia, substance abuse or major depression.
Taken together, the interaction of BPD and PTSD in the context of volumetric changes in the limbic system is unclear. It may be that both disorders independently lead to reductions of hippocampus and amygdala volumes, that the presence of both disorders has a supra-additive effect on the volumes, or that the volumetric changes are primarily driven by traumatization or PTSD. Only larger studies will help to disentangle these possibilities. Neuroimaging findings point to a significant influence of comorbid conditions on findings in patients with BPD.8,20 In a functional MRI study, amygdalar deactivation in response to painful stimulation was only found in patients with BPD and comorbid PTSD, but not in those without PTSD.20
Limitations
The pathological processes underlying BPD are not completely understood, but volumetric alterations in the hippocampus and amygdala are of central importance for our understanding of trauma-related and borderline psychopathology.2 Current theories of brain volumetric changes focus on the influence of stress-induced disturbance of the hypothalamic–pituitary–adrenal axis32 and genetic predispositions for volumetric changes.14,33 The design of our study did not allow us to disentangle genetic and environmental influences on brain morphology. Similarly, our study did not allow for disentangling between the effect of a diagnosis of PTSD and traumatization on hippocampus and amygdala volumes. On the one hand, we found significant group differences when patients were separated into those with and without traumatization in general but not when patients were separated based on sexual or physical abuse. On the other hand, we found no significant correlation between Childhood Trauma Questionnaire scores and hippocampus volumes for all patients. The influence of traumatic exposure on hippocampus volumes independent of PTSD diagnosis has been systematically reviewed in a recent meta-analysis.19 For the BPD group, a significant positive correlation between hippocampus volumes and impulsiveness was reported. The meaning of this correlation is currently unclear, but it may be of interest in light of the earlier findings by Zetzsche and colleagues9 of an association between hippocampus volume and aggression but not impulsiveness. Another limitation of our study was its relatively small sample size. With the current sample size, correlation analyses have to be interpreted with great caution.
The generalizibility of our study is restricted. Although we took great care in the acquisition and processing of the images, a possible alteration or difference in volume is not guaranteed to be detected through manual volumetry owing to the limitations in terms of resolution, signal-to-noise ratio and image size. This, however, is a limitation each volumetric study faces, and we tried to mitigate this limitation by simultaneously using high-resolution T1- and T2-weighted images and an automatic segmentation process. Finding an overall volume loss or gain of the examined structure allows no statement about the status of partial volumes within this structure. Also, the real volumetric changes within any given substructure may be masked by a simultaneous change in another substructure. The manual tracing performed by only one rater may have been problematic in terms of a certain bias. Even with high intrarater reliability as in our study, a rater only attests that he traces the same structures consistently without making any statement about the neuroanatomical correctness and accuracy of his tracing process. However, several raters and a high inter-rater reliability only indicate that a neuroanatomic consensus has been established among them, again with no conclusive certainty about the validity of their process concerning the real target volume. With many studies on this subject using different protocols defining the anatomic boundaries of the targeted structures or a slightly modified version of the anatomic guidelines of another group, volumetric results are primarily exclusive to the respective study and comparison between studies has to be done with caution. Reduced hippocampus volumes may be related to cognitive disturbances, which are a prominent feature of PTSD;34 however, this was not explicitly tested in our study.
In sum, our study stresses the importance of PTSD against the backdrop of BPD regarding hippocampus volume reduction. This appears to be an important extension of earlier studies revealing volume reductions in both disorders separately. Further manual or semiautomatic volumetric studies and meta-analyses on this subject seem to be warranted to better understand basic pathophysiological mechanisms in disorders related to traumatic stress.
Acknowledgements
This study was funded by the German Research Foundation (SFB 636).
Footnotes
Competing interests: None declared.
Contributors: Drs. Schmahl and Bohus designed the study. Drs. Schmahl, Berne and Krause acquired and analyzed the data, which Drs. Kleindienst, Valerius, Vermetten and Bohus also analyzed. Drs. Schmahl, Berne, Kleindienst and Bohus wrote the article. Each author reviewed the article and provided final approval for publication.
References
- 1.Lieb K, Zanarini C, Schmahl CG, et al. Borderline personality disorder. Lancet. 2004;364:453–61. doi: 10.1016/S0140-6736(04)16770-6. [DOI] [PubMed] [Google Scholar]
- 2.Schmahl C, Bremner JD. Neuroimaging in borderline personality disorder. J Psychiatr Res. 2006;40:419–27. doi: 10.1016/j.jpsychires.2005.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Driessen M, Herrmann J, Stahl K, et al. Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Arch Gen Psychiatry. 2000;57:1115–22. doi: 10.1001/archpsyc.57.12.1115. [DOI] [PubMed] [Google Scholar]
- 4.Tebartz van Elst L, Hesslinger B, Thiel T, et al. Frontolimbic brain abnormalities in patients with borderline personality disorder. A volumetric MRI study. Biol Psychiatry. 2003;54:163–71. doi: 10.1016/s0006-3223(02)01743-2. [DOI] [PubMed] [Google Scholar]
- 5.Schmahl CG, Vermetten E, Elzinga BM, et al. Magnetic resonance imaging of hippocampal and amygdala volume in women with childhood abuse and borderline personality disorder. Psychiatry Res. 2003;122:193–8. doi: 10.1016/s0925-4927(03)00023-4. [DOI] [PubMed] [Google Scholar]
- 6.Brambilla P, Soloff PH, Sala M, et al. Anatomical MRI study of borderline personality disorder patients. Psychiatry Res. 2004;131:125–33. doi: 10.1016/j.pscychresns.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Irle E, Lange C, Sachsse U. Reduced size and abnormal asymmetry of parietal cortex in women with borderline personality disorder. Biol Psychiatry. 2005;57:173–82. doi: 10.1016/j.biopsych.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Zetzsche T, Frodl T, Preuss UW, et al. Amygdala volume and depressive symptoms in patients with borderline personality disorder. Biol Psychiatry. 2006;60:302–10. doi: 10.1016/j.biopsych.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 9.Zetzsche T, Preuss UW, Frodl T, et al. Hippocampal volume reduction and history of aggressive behavior in patients with borderline personality disorder. Psychiatry Res. 2007;154:157–70. doi: 10.1016/j.pscychresns.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 10.New AS, Hazlett EA, Buchsbaum MS, et al. Amygdala-prefrontal disconnection in borderline personality disorder. Neuropsychopharmacology. 2007;32:1629–40. doi: 10.1038/sj.npp.1301283. [DOI] [PubMed] [Google Scholar]
- 11.Zanarini MC, Frankenburg FR, Dubo ED, et al. The Axis I comorbidity of borderline personality disorder. Am J Psychiatry. 1998;155:1733–9. doi: 10.1176/ajp.155.12.1733. [DOI] [PubMed] [Google Scholar]
- 12.Zimmermann M, Mattia JI. Axis I diagnostic comorbidity and borderline personality disorder. Compr Psychiatry. 1999;40:245–52. doi: 10.1016/s0010-440x(99)90123-2. [DOI] [PubMed] [Google Scholar]
- 13.Bremner JD, Randall P, Scott TM, et al. MRI-based measurement of hippocampal volume in patients with combat-related post-traumatic stress disorder. Am J Psychiatry. 1995;152:973–81. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilbertson MW, Shenton ME, Ciszewski A, et al. Smaller hippo-campal volume predicts pathological vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–7. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gurvits TV, Shenton ME, Hokama H, et al. Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biol Psychiatry. 1996;40:1091–9. doi: 10.1016/S0006-3223(96)00229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bremner JD, Randall P, Vermetten E, et al. Magnetic resonance imaging-based measurement of hippocampal volume in post-traumatic stress disorder related to childhood physical and sexual abuse — a preliminary report. Biol Psychiatry. 1997;41:23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bremner JD, Vythilingam M, Vermetten E, et al. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am J Psychiatry. 2003;160:924–32. doi: 10.1176/appi.ajp.160.5.924. [DOI] [PubMed] [Google Scholar]
- 18.Stein MB, Koverola C, Hanna C, et al. Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med. 1997;27:951–9. doi: 10.1017/s0033291797005242. [DOI] [PubMed] [Google Scholar]
- 19.Karl A, Schaefer M, Malta LS, et al. A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev. 2006;30:1004–31. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Kraus A, Esposito F, Seifritz E, et al. Amygdala deactivation as a neural correlate of pain processing in patients with borderline personality disorder and co-occurrent posttraumatic stress disorder. Biol Psychiatry. 2009;65:819–22. doi: 10.1016/j.biopsych.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 21.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 22.Loranger AW. International Personality Disorder Examination (IPDE): DSM-IV and ICD-10 modules. Odessa (FL): Psychological Assessment Resources; 1999. [Google Scholar]
- 23.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. J Clin Psychol. 1995;51:768–74. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 24.Bohus M, Limberger MF, Frank U, et al. Psychometric properties of the Borderline Symptom List (BSL) Psychopathology. 2007;40:126–32. doi: 10.1159/000098493. [DOI] [PubMed] [Google Scholar]
- 25.Bernstein DP, Fink L. A retrospective self-report manual. San Antonio (TX): The Psychological Corporation; 1998. Childhood Trauma Questionnaire . [Google Scholar]
- 26.Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- 27.Shattuck DW, Leahy RM. Automated graph-based analysis and correction of cortical volume topology. IEEE Trans Med Imaging. 2001;20:1167–77. doi: 10.1109/42.963819. [DOI] [PubMed] [Google Scholar]
- 28.Shattuck DW, Sandor-Leahy SR, Schaper KA, et al. Magnetic resonance image tissue classification using a partial volume model. Neuroimage. 2001;13:856–76. doi: 10.1006/nimg.2000.0730. [DOI] [PubMed] [Google Scholar]
- 29.Shattuck DW, Leahy RM. BrainSuite: an automated cortical surface identification tool. Med Image Anal. 2002;6:129–42. doi: 10.1016/s1361-8415(02)00054-3. [DOI] [PubMed] [Google Scholar]
- 30.Sandor S, Leahy R. Surface based labeling of cortical anatomy using a deformable atlas. IEEE Trans Med Imaging. 1997;16:41–54. doi: 10.1109/42.552054. [DOI] [PubMed] [Google Scholar]
- 31.Honeycutt NA, Smith PD, Aylward A, et al. Mesial temporal lobe measurements on magnetic resonance imaging scans. Psychiatry Res. 1998;83:85–94. doi: 10.1016/s0925-4927(98)00035-3. [DOI] [PubMed] [Google Scholar]
- 32.Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–35. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- 33.Geuze E, Vermetten E, Bremner JD. MR-based in vivo hippocampal volumetrics: 2. Findings in neuropsychiatric disorders. Mol Psychiatry. 2005;10:160–84. doi: 10.1038/sj.mp.4001579. [DOI] [PubMed] [Google Scholar]
- 34.Vasterling JJ, Duke LM, Brailey K, et al. Attention, memory, and learning performances and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology. 2002;16:5–14. doi: 10.1037//0894-4105.16.1.5. [DOI] [PubMed] [Google Scholar]