Abstract
Background
Functional brain imaging studies have demonstrated amygdala and insula hyper-reactivity to probes of social threat in participants with generalized social anxiety disorder (gSAD). The amygdala and insula are known to serve broad functions in emotional processing, including integration of affective information. However, few studies have examined brain responses in socially anxious participants during general emotional processing. We examined brain response to emotionally evocative images in patients with gSAD and matched healthy controls.
Methods
Eleven patients with gSAD who were not taking psychotropic medications and did not have psychiatric comorbidities and 11 matched healthy controls underwent functional magnetic resonance imaging while viewing blocks of emotionally salient (positive, negative, neutral) pictures.
Results
Participants with gSAD exhibited enhanced bilateral amygdala and insula reactivity to negative (v. neutral) images compared with healthy controls who did not exhibit enhanced reactivity. Within the gSAD group, the extent of amygdala activation was correlated with social anxiety severity, whereas the extent of insula activation was correlated with trait anxiety.
Limitations
The small sample size may have limited our ability to detect group differences in other relevant brain regions and in behavioural measures.
Conclusion
In addition to prior findings of probes of social information processing, our findings suggest that the amygdala and insula responses are hyper-reactive to general emotional images with negative emotional content and that these brain regions may play divergent roles in their representation of different phenotypes.
Introduction
Generalized social anxiety disorder (gSAD), also known as social phobia, is characterized by an exaggerated fear of negative scrutiny across interpersonal interactions. This exaggerated fear response may be in part due to excessive attention to negative stimuli1,2 and/or deficits in processing positive3,4 or ambiguous5,6 interpersonal stimuli. The neural correlates of this fear response have been extensively investigated by social probes of potential threat (e.g., harsh faces, public speaking, verbal criticism),7–12 and a recent meta-analysis of functional neuroimaging studies13 confirmed that heightened activation of the amygdala and insula is a robust and consistent finding in social phobia. The amygdala and insula are known to serve broad functions in emotional processing, including mind–body integration of affective information,14,15 and their increased sensitivity to negative social stimuli has been specifically implicated as a neural marker of social anxiety severity.8,11,12,16
To date, we are not aware of any studies that have examined brain responses in socially anxious participants during general (nonsocial, e.g., not faces or public speaking) emotional processing. We compared brain response to complex and emotionally evocative images in patients with gSAD and matched healthy controls. We employed stimuli from the International Affective Picture System (IAPS), a standardized set of images known to reliably evoke acute and transient changes in emotional experience and arousal as well as central (e.g., amygdala and insula) indices of emotional reactivity.17 Specifically, we hypothesized that amygdala and insula reactivity in patients with gSAD would be exaggerated compared with healthy controls in response to general emotional probes of negative, but not positive, affect. Although it may be argued that anxious temperament and nonspecific/general anxiety have overlapping characteristics, the Spiel-berger Trait Anxiety Inventory (STAI-trait)18 measures anxiety proneness as a stable temperamental/personality trait (e.g., individual differences in the likelihood that people would experience state anxiety in a stressful situation in their lifetimes), whereas the Hamilton Anxiety Rating Scale (HAM-A)19 and Liebowitz Social Anxiety Scale (LSAS)20 measure state transitory levels of current generalized, non-specific anxiety (e.g., anxiety symptoms over the past week) and social anxiety, and thus represent divergent constructs.21 Interestingly, in individuals with social anxiety or with high anxiety proneness, the extent of their amygdala and/or insula reactivity is positively associated with their levels of trait anxiety and social anxiety but not with their levels of nonspecific anxiety.8,11,22 Thus, we further hypothesized that the magnitude of amygdala and/or insula reactivity would be associated with the level of symptom severity and/or anxious temperament, but not with nonspecific/general anxiety.
Methods
Participants
We recruited participants with gSAD based on DSM-IV criteria, as confirmed with the Structured Clinical Interview for DSM-IV (SCID-IV)23 with additional probes from the clinician-administered LSAS,20 from the community by advertisement. In addition to the LSAS, participants with gSAD also completed questionnaires to measure trait anxiety and clinical symptomatology measures, including the Speilberger State-Trait Anxiety Inventory (STAI),18 Beck Depression Inventory (BDI)24 and the Hamilton Rating Scales for Depression (HAM-D)25 and Anxiety (HAM-A).19 Severity of social anxiety, trait anxiety and general/nonspecific anxiety were represented by scores on LSAS, STAI-trait and HAM-A, respectively. We recruited matched healthy controls free of prior or current medical, neurologic and psychiatric illness, as confirmed by SCID-IV and medical evaluation by a physician (K.L.P.). Trained clinicians and a board-certified psychiatrist (K.L.P.) performed clincal assessments using SCID-based DSM-IV criteria and clinical judgment based on direct patient interviews and review of all symptom ratings scores. All participants provided written informed consent, and the local university institutional review board approved our study.
Experimental task
We presented 60 IAPS images showing any of 3 affect conditions (i.e., negative, positive, neutral) (Box 1) in 20-second blocks (5 images/block, 2 blocks of each valence/run), alternating with 20-second baseline blocks of blank, greyscale images (6 blocks/run), over 2 functional runs of 4 minutes each. We did not repeat images, and we counterbalanced block order. We selected the negative (i.e., unpleasant/aversive) and positive (i.e., pleasant) images based on normative ratings of low and high valence (pleasantness), respectively, with similar levels of subjective arousal.
Box 1. International Affective Picture System (IAPS) images* by category and number.
| Category, no.
| ||
|---|---|---|
| Neutral | Positive | Negative |
| 1121 | 1440 | 1300 |
| 1313 | 1463 | 2053 |
| 1390 | 1710 | 2205 |
| 1670 | 1920 | 2692 |
| 1945 | 1999 | 2700 |
| 2214 | 2058 | 2710 |
| 2280 | 2070 | 2730 |
| 2372 | 2160 | 2800 |
| 2381 | 2165 | 2900 |
| 2383 | 2340 | 3030 |
| 2440 | 2550 | 3060 |
| 2480 | 2660 | 3150 |
| 2485 | 4220 | 3180 |
| 2487 | 4250 | 3220 |
| 2514 | 4572 | 3266 |
| 2570 | 4599 | 3300 |
| 2575 | 4608 | 6020 |
| 2580 | 4660 | 6230 |
| 2749 | 5450 | 6312 |
| 2840 | 5470 | 6313 |
| 2850 | 5480 | 6560 |
| 2870 | 5831 | 6570 |
| 2880 | 5982 | 7380 |
| 4605 | 7282 | 8230 |
| 5395 | 7330 | 9000 |
| 5531 | 7352 | 9006 |
| 5535 | 7502 | 9040 |
| 5740 | 8034 | 9050 |
| 6150 | 8080 | 9220 |
| 7002 | 8120 | 9290 |
| 7010 | 8161 | 9300 |
| 7130 | 8185 | 9410 |
| 7185 | 8300 | 9440 |
| 7217 | 8370 | 9470 |
| 7233 | 8380 | 9560 |
| 7500 | 8496 | 9561 |
| 7595 | 8500 | 9570 |
| 9070 | 8501 | 9611 |
| 9210 | 8531 | 9800 |
| 9700 | 8540 | 9910 |
The mean (and standard deviation [SD]) ratings for valence were 5.0975 (0.464459) for neutral, 7.4415 (0.504658) for positive and 2.4025 (0.544241) for negative images and the mean (and SD) ratings for arousal were 3.3985 (0.72151) for neutral, 5.4475 (0.820193) for positive and 5.77225 (0.881207) for negative images.
Before scanning, we introduced participants to the task and performed a short practice set containing distinct IAPS images that we did not later present in the scanner. During functional magnetic resonance imaging (fMRI) scanning, we instructed participants to identify the general valence of each image (i.e., negative, positive or neutral) by button-press as soon as they were able to judge the image. After scanning, participants rated each image on a 9-point scale for valence and arousal (1 = most unpleasant/least arousing; 9 = most pleasant/most arousing).
MRI acquisition and analysis
We performed all scanning with blood oxygen level–dependent (BOLD) whole-brain fMRI on a 3.0 T GE Signa System (General Electric) using a standard radiofrequency coil and associated software (LX 8.3, Neuro-optimized gradients; General Electric). We acquired whole-brain functional scans using a T2-weighted reverse spiral gradient-recall echo sequence (echo time [TE] 25 ms, repetition time [TR] 2000 ms, 64 × 64 matrix, flip angle 77°, field of view [FOV] 24 cm, 3.75 mm2 inplane voxels, 30 contiguous 5-mm axial slices per volume) optimized to minimize susceptibility artifacts in the regions of interest (ROIs).26 We also acquired a high-resolution T1-weighted scan (3-dimensional magnetization prepared rapid gradient echo, or 3D-MPRAGE; TR 25 ms; min TE; FOV 24 cm; slice thickness 1.5 mm) for anatomic localization.
Functional MRI data preprocessing
Data from all participants met the criteria for quality with minimal motion correction (< 3 mm displacement in any one direction); we included these data in our analyses. We discarded the first 4 volumes from each run to allow for T1 equilibration effects. We preprocessed and analyzed the data using statistical parametric mapping (SPM5; Wellcome Department of Cognitive Neurology). We spatially realigned the scans to the first scan in each run to correct for head motion, warped (nonlinear) to an echo-planar image (EPI) template in Montreal Neurologic Institute (MNI) space, resampled them to 2-mm3 voxels and smoothed them with an 8-mm3 kernel to maximize signal and minimize residual differences in neuro-anatomy. We combined the general linear model applied to the time series with the canonical hemodynamic response function and with a 128-second high-pass filter.27
Statistical analysis
We performed conventional data processing analyses based on the general linear model using SPM5 software (Wellcome Department of Cognitive Neurology). We generated linear contrasts of interest (negative v. neutral, positive v. neutral) for each participant, and entered them into a second-level random effects model and 2-sample Student t tests to examine between-group differences in brain activation. Within ROIs defined a priori (i.e., amygdala and insula), we set the significance threshold at puncorrected < 0.005, with an extent of greater than 10 contiguous voxels to balance between type I and type II errors.28 Outside the ROIs defined a priori, we set the threshold at p < 0.05, corrected for multiple comparisons using a false discovery rate method.29 Following analyses, we identified the location of activation foci using MARINA software (www.bion.de/index.php?title=MARINA)30 based on the atlas by Tzourio-Mazoyer and colleagues.31 Additionally, we extracted parameter estimates (i.e., β weights, arbitrary units), an index of activation signal change, from 10-mm diameter spherical volumes around the peak voxels within bilateral insula and the amygdala using MarsBaR (http://marsbar.sourceforge.net), and we used 2-tailed Student t tests for between-group comparisons and calculated Pearson correlation coefficients with symptom/trait measures (i.e., LSAS, STAI, HAM-A); we set significance at p < 0.05, 2-tailed.
Results
Participants
We enrolled 22 right-handed participants (11 with gSAD and 11 controls) in our study. The groups did not differ significantly in age (mean 27.45, standard deviation [SD] 8.96, range 19–49 yr in the gSAD group v. mean 30.55, SD 7.69, range 21–49 yr in the control group; p = 0.40) or sex (8 men and 3 women in the gSAD group v. 6 men and 5 women in the control group; p = 0.38). We were able to collect data from the STAI, BDI, HAM-D and HAM-A questionnaires for all participants with gSAD and 7 of the 11 controls. Mean (SD) self-report scores on LSAS, STAI, HAM-A, HAM-D and BDI and between-group comparisons are reported in Table 1. Of note, none of these 3 scores correlated with one another within the gSAD or control groups (Pearson correlations, all p > 0.05).
Table 1.
Self-report measures and between-group comparisons* for 11 participants with generalized social anxiety disorder and 11 healthy controls
| Group; mean (SD)
|
||||
|---|---|---|---|---|
| Measure | gS AD | Control | t16 value | Cohen d |
| LSAS | 75.91 (14.87) | 13.71 (14.26) | 8.79† | 4.27 |
| STAI-state | 41.73 (11.21) | 30.14 (7.49) | 2.40‡ | 1.22 |
| STAI-trait | 46.09 (10.44) | 30.42 (4.72) | 3.71§ | 1.94 |
| BDI | 9.45 (5.72) | 2.29 (3.30) | 3.00§ | 1.53 |
| HAM-D | 4.45 (5.94) | 0.86 (1.57) | 1.55 | 0.83 |
| HAM-A | 4.00 (3.10) | 0.71 (1.11) | 2.67‡ | 1.41 |
BDI = Beck Depression Inventory24; gSAD = generalized social anxiety disorder; HAM-A = Hamilton Rating Scale for Anxiety19; HAM-D = Hamilton Rating Scale for Depression25; LSAS = Liebowitz Social Anxiety Scale20; SD = standard deviation; STAI = State-Trait Anxiety Inventory.18
We performed group comparisons using the Student t test for independent groups.
p < 0.001.
p < 0.01.
p < 0.05.
All participants were free of psychoactive medications at the time of scanning and urine toxicology screens at the time of scanning were negative for all participants. None had current or recent (previous 6 mo) depressive episodes, substance abuse or a history of autism/pervasive developmental disorders, mental retardation or significant medical or neurologic illness, as verified by the SCID-IV and a medical history screening. Two patients with gSAD had concurrent generalized anxiety disorder; however, it did not precede the onset of social anxiety symptoms, nor was it more clinically salient than gSAD.
Behavioural results
Behavioural results are summarized in Table 2. We observed no significant group-by-condition interactions across valence categories; participants with gSAD did not differ from controls on ratings of valence or arousal for negative, positive or neutral picture conditions. Across participants, there was a significant main effect of condition for both valence and arousal ratings. Follow-up Student t tests revealed that, relative to neutral pictures, negative pictures were rated more unpleasant, and positive pictures were rated more pleasant on valence; negative and positive pictures were rated to be equally arousing but more arousing than neutral pictures (data not shown). There were no significant group differences in accuracy or reaction time in the in-scanner task.
Table 2.
Subjective ratings and group-by-condition analysis of variance for 11 participants with generalized social anxiety disorder and 11 healthy controls
| Group; mean (SD)*
|
Group
|
Condition
|
Interaction
|
||
|---|---|---|---|---|---|
| Rating | gSAD | Control | F1,20 value | F2,40 value | F2,40 value |
| Valence | |||||
| Negative | 2.45 (0.35) | 2.1 (0.64) | 2.98 | 323.15† | 0.74 |
| Positive | 6.78 (0.82) | 6.84 (0.53) | |||
| Neutral | 5.25 (0.42) | 4.99 (0.39) | |||
| Arousal | |||||
| Negative | 6.34 (1.12) | 6.35 (1.58) | 0.13 | 44.30† | 0.30 |
| Positive | 5.46 (1.08) | 5.49 (1.32) | |||
| Neutral | 3.30 (1.30) | 2.85 (1.56) | |||
| In-scan accuracy, % | |||||
| Negative | 82.3 (20.4) | 88.6 (14.0) | 0.58 | 3.94‡ | 1.16 |
| Positive | 78.2 (22.6) | 73.2 (17.5) | |||
| Neutral | 66.4 (16.6) | 76.4 (16.7) | |||
| In-scan reaction time, ms | |||||
| Negative | 1605.97 (351.60) | 1519.10 (290.56) | 0.99 | 4.42‡ | 0.97 |
| Positive | 1689.97 (289.06) | 1482.00 (255.91) | |||
| Neutral | 1749.57 (374.07) | 1694.94 (323.63) | |||
gSAD = generalized social anxiety disorder; SD = standard deviation. *Unless indicated otherwise.
p < 0.001.
p < 0.05.
Functional MRI results in amygdala and insula
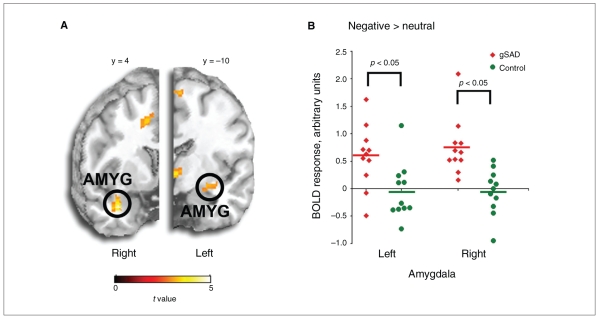
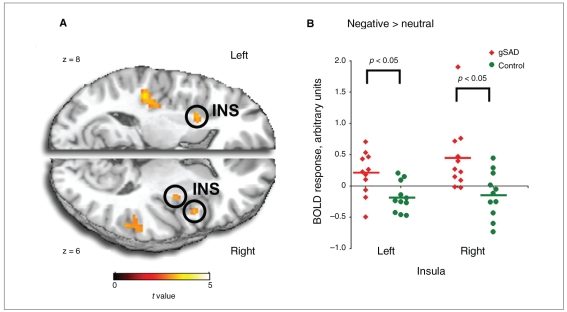
Compared with controls, participants with gSAD exhibited more differential activation to negative (> neutral) images in the right amygdala, extending into the superior temporal gyrus, and the left amygdala, extending into the hippocampus (peak voxel [36,4, − 28], Z = 3.84, 544 mm3 in the right v. peak voxel [−26, −10, −14], Z = 2.86, 128 mm3 in the left; Fig. 1). Compared with controls, participants with gSAD also exhibited more differential activation to negative (> neutral) images in bilateral insula, extending into the putamen (peak voxel [36, −6,4], Z = 2.83, 136 mm3 in the right v. peak voxel [48,8,6], Z = 2.81, 208 mm3 also in the right v. peak voxel [−26,10,6], Z = 2.88, 208 mm3 in the left; Fig. 2). The 2 groups did not differ in amygdala nor insula response to positive (> neutral) images. Of note, we observed no group differences in the amygdala or insula in the neutral > blanks contrast. Activation signal (parameter estimates, mean [SD]) was greater in the amygdala among participants with gSAD than controls (0.75 [0.52] v. −0.06 [0.42], t20 = 4.04, p = 0.001, Cohen d = 1.71 in the right amygdala and 0.59 [0.57] v. −0.05 [0.51], t20 = 2.77, p = 0.012, Cohen d = 1.18 in the left amygdala). Activation signal (parameter estimates, mean [SD]) was also greater in the insula among participants with gSAD than controls (0.45 [0.55] v. −0.13 [0.37], t20 = 2.94, p = 0.008, Cohen d = 1.24 in the right insula and 0.20 [0.35] v. −0.18 [0.24], t20 = 3.00, p = 0.007, Cohen d = 1.27 in the left insula). Among participants with gSAD, the magnitude of BOLD response to negative (> neutral) images in the right amygdala was associated with the intensity of social anxiety symptoms (LSAStotal; r9 = 0.62, p = 0.042) but not with any other symptom/trait measures; response in the left amygdala was not correlated with any symptom measures. There were no significant correlations between insula activation and any symptom measures at the p < 0.05 level. However, the magnitude of both right and left insula activation was correlated with trait anxiety measures at the trend level (r,9 = 0.55, p = 0.08 in the right insula and r9 = 0.55, p = 0.08 in the left insula).
Fig. 1.
Bilateral amygdala hyperactivity in response to negative (> neutral) images in participants with generalized social anxiety disorder (gSAD). (A) Whole-brain, voxel-wise t-map image of group differences depicting greater activation in the bilateral amygdala in patients with gSAD compared with controls. (B) Scatterplot of signal change (negative > neutral images) within the left and right amygdala volumes for each participant with gSAD (red) and control (green). The t-map is superimposed on coronal sections of a canonical brain image (at y-plane coordinates 4 on the right [R], and −10 on the left [L], using the Montreal Neurologic Institute atlas).
Fig. 2.
Bilateral insula hyperactivity in response to negative (> neutral) images in participants with generalized social anxiety disorder (gSAD). (A) Whole-brain, voxel-wise t-map image of group differences depicting greater activation in bilateral insula in participants with gSAD compared with controls. (B) Scatterplot of signal change (negative > neutral images) within the left and right insula volumes for each participant with gSAD (red) and control (green). The t-map is superimposed on axial sections of a canonical brain image (at z-plane coordinates 6 on the right [R], and 8 on the left [L], using the Montreal Neurologic Institute atlas).
Functional MRI results outside a priori ROIs
Outside of the insula and amygdala, there were no ROIs that survived correction for multiple comparisons for gSAD > control or control > gSAD in either negative or positive (v. neutral) pictures.
Discussion
To our knowledge, ours is the first study to evaluate the neural correlates of general emotional processing in patients with gSAD. The present study provided evidence of greater differential amygdala and insula reactivity in participants with gSAD, relative to matched controls, in response to images with negative, but not positive, emotional content. Moreover, we observed that the magnitude of this reactivity in the amygdala was associated with severity of social anxiety, whereas insula reactivity was associated at a trend level with trait anxiety. These findings add to the growing literature implicating the amygdala and insula as key components to a common neural marker of SAD.
Exaggerated amygdala and insular reactivity to negative emotional stimuli has also been observed in a number of prior functional brain imaging studies of harsh (e.g., emotionally negative) faces,7,8,11,12 unpleasant anticipation before public speaking9,32 and verbal criticism.10 Similar to our results, differences between gSAD and comparison participants in amygdala and insula response to positively valenced stimuli (e.g., happy faces, verbal praise) have not been observed7,8,10,11 in accord with behavioural models linking social anxiety and negative (but not positive) bias in socio-emotional information processing.33 It should be noted that Straube and colleagues3 have observed amygdala hyper-reactivity in participants with SAD to both angry and happy faces. Our findings extend evidence of enhanced amygdala and insula sensitivity to general emotional images with general negative content (e.g., unpleasant/aversive pictures). The amygdala and insula are heavily interconnected34 and are believed to play a concerted role in the regulation of autonomic responses, processing negative affective experiences, and making social judgments (e.g., trustworthiness, approachability) from facial expressions of emotion,14,15,35,36 making them highly plausible neural substrates in the pathophysiology of generalized social phobia.13,14
We found that the magnitude of BOLD response to negative emotional images in the amygdala among participants with gSAD was correlated with their levels of social anxiety severity, but not with anxiety temperament (e.g., trait anxiety) or nonspecific (e.g., nonsocial) anxiety symptoms. This is consistent with a number of prior studies8,11,12,16 and suggests that amygdala reactivity may be a useful biomarker with clinical application; amygdala hyper-reactivity is normalized by and predicts effective treatment of social anxiety.37,38 In addition, insula reactivity was related to levels of trait anxiety at a trend level but not to social anxiety severity. This is consistent with prior findings showing that anxiety-prone (e.g., high-trait anxious) individuals exhibit greater insular reactivity to emotional faces than anxiety-normative controls, and their levels of trait anxiety predict the extent of insula activation.22
Limitations
Our study has limitations worth discussion. Although similar to prior functional neuroimaging studies of gSAD, our sample size was small, which may have limitited our ability to detect group differences in other brain regions or in subjective ratings (e.g., type II error). We were unable to directly test for differences between groups on processes that may have contributed to the observed results (e.g., IQ, habituation to images) because of the study design. This is an important issue that will need to be addressed in future studies. Additionally, our controls did not show amygdala activity in response to negative images relative to neutral images, similar to results of some17,39,40 but not all prior studies.41–43 Several factors may contribute to the inconsistency of these findings. Prior evidence of amygdala response to negative images in controls was generated from contrasts with nonimages41,42 and involved passive viewing of images41 or implicit processing of negative images,42,43 whereas the task in our study involved explicit cognitive appraisal/labelling of the emotional content, which may have diminished amygdala reactivity to unpleasant pictures in healthy controls.44 Moreover, a number of studies, including some conducted in our laboratory, have shown that the amygdala is activated when vieweing neutral pictures17,39 and thereby diminishes the activation difference observable in the negative versus neutral contrast.17,39
Despite limitations, our data suggest that the amygdala and insula may be key brain regions in the common final pathway in the neuropathogenesis of SAD, but that they may play divergent roles in their representation of phenotypic markers. It may be that amygdala reactivity to probes of negative emotions or social threat reflects the disease process of social anxiety rather than a vulnerability marker that increases risk for disease, whereas insula reactivity may be better linked to a temperamental risk, or diathesis, toward the development of social or another anxiety disorder. Therefore, our findings support the inclusion of the amygdala and insula as critical components of a brain-based model of SAD.
Acknowledgements
This research was supported by a Seed Grant from the Brain Research Foundation (K.L.P.) and an NIH grant MH076198 (K.L.P.). We thank Rose McCarron for her assistance in participant recruitment and study coordination.
Footnotes
Competing interests: None declared.
Contributors: Dr. Phan designed the study. Mr. Angstadt acquired the data, which all authors analyzed. Mr. Shah and Drs. Klumpp and Phan wrote the article, which Mrs. Angstadt and Shah and Drs. Nathan and Phan reviewed. All authors gave final approval for publication.
References
- 1.Mogg K, Bradley BP. Selective orienting of attention to masked threat faces in social anxiety. Behav Res Ther. 2002;40:1403–14. doi: 10.1016/s0005-7967(02)00017-7. [DOI] [PubMed] [Google Scholar]
- 2.Mogg K, Philippot P, Bradley BP. Selective attention to angry faces in clinical social phobia. J Abnorm Psychol. 2004;113:160–5. doi: 10.1037/0021-843X.113.1.160. [DOI] [PubMed] [Google Scholar]
- 3.Straube T, Mentzel HJ, Miltner WH. Common and distinct brain activation to threat and safety signals in social phobia. Neuropsychobiology. 2005;52:163–8. doi: 10.1159/000087987. [DOI] [PubMed] [Google Scholar]
- 4.Moser JS, Hajcak G, Huppert JD, et al. Interpretation bias in social anxiety as detected by event-related brain potentials. Emotion. 2008;8:693–700. doi: 10.1037/a0013173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooney RE, Atlas LY, Joormann J, et al. Amygdala activation in the processing of neutral faces in social anxiety disorder: Is neutral really neutral? Psychiatry Res. 2006;148:55–9. doi: 10.1016/j.pscychresns.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Yoon KL, Zinbarg RE. Interpreting neutral faces as threatening is a default mode for socially anxious individuals. J Abnorm Psychol. 2008;117:680–5. doi: 10.1037/0021-843X.117.3.680. [DOI] [PubMed] [Google Scholar]
- 7.Stein MB, Goldin PR, Sareen J, et al. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry. 2002;59:1027–34. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- 8.Phan KL, Fitzgerald DA, Nathan PJ, et al. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biol Psychiatry. 2006;59:424–9. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Tillfors M, Furmark T, Marteinsdottir I, et al. Cerebral blood flow during anticipation of public speaking in social phobia: a PET study. Biol Psychiatry. 2002;52:1113–9. doi: 10.1016/s0006-3223(02)01396-3. [DOI] [PubMed] [Google Scholar]
- 10.Blair K, Geraci M, Devido J, et al. The neural response to self-and-other-referential praise and criticism in generalized social phobia (GSP) Arch Gen Psychiatry. 2008;65:1176–84. doi: 10.1001/archpsyc.65.10.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans KC, Wright CI, Wedig MM, et al. A functional MRI study of amygdala responses to angry schematic faces in social anxiety disorder. Depress Anxiety. 2008;25:496–505. doi: 10.1002/da.20347. [DOI] [PubMed] [Google Scholar]
- 12.Goldin PR, Hakimi S, Manber T, et al. Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Arch Gen Psychiatry. doi: 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60:383–7. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 15.Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Brain Res Rev. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
- 16.Killgore WD, Yurgelun-Todd DA. Social anxiety predicts amygdala activation in adolescents viewing fearful faces. Neuroreport. 2005;16:1671–5. doi: 10.1097/01.wnr.0000180143.99267.bd. [DOI] [PubMed] [Google Scholar]
- 17.Liberzon I, Phan KL, Decker LR, et al. Extended amygdala and emotional salience: a PET activation study of positive and negative affect. Neuropsychopharmacology. 2003;28:726–33. doi: 10.1038/sj.npp.1300113. [DOI] [PubMed] [Google Scholar]
- 18.Spielberger CD, Gorsuch RL, Lushene R. Manual for State-Trait Anxiety Inventory. Palo Alto (CA): Consulting Psychologists Press; 1970. [Google Scholar]
- 19.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 20.Heimberg RG, Horner KJ, Juster HR, et al. Psychometric properties of the Liebowitz Social Anxiety Scale. Psychol Med. 1999;29:199–212. doi: 10.1017/s0033291798007879. [DOI] [PubMed] [Google Scholar]
- 21.Keedwell P, Snaith RP. What do anxiety scales measure? Acta Psychiatr Scand. 1996;93:177–80. doi: 10.1111/j.1600-0447.1996.tb10627.x. [DOI] [PubMed] [Google Scholar]
- 22.Stein MB, Simmons AN, Feinstein JS, et al. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164:318–27. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- 23.First MB, Spitzer RL, Williams JBW, et al. Structured Clinical Interview for DSM-IV Patient Edition (SCID-P) Washington (DC): American Psychiatric Press; 1995. [Google Scholar]
- 24.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stenger VA, Boada FE, Noll DC. Three-dimensional tailored RF pulses for the reduction of susceptibility artifacts in T(*)(2)-weighted functional MRI. Magn Reson Med. 2000;44:525–31. doi: 10.1002/1522-2594(200010)44:4<525::aid-mrm5>3.0.co;2-l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friston KJ, Holmes AP, Worsley KJ, et al. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- 28.Forman SD, Cohen JD, Fitzgerald M, et al. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 29.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 30.Walter B, Blecker C, Kirsch P, et al. MARINA: an easy to use tool for the creation of masks for region of interest analyses [abstract]. Presented at the 9th International Conference on Functional Mapping of the Human Brain; New York (NY): Available on CD-Rom in NeuroImage; [2003 Jun. 19–22]. 2003. [Google Scholar]
- 31.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 32.Lorberbaum JP, Kose S, Johnson MR, et al. Neural correlates of speech anticipatory anxiety in generalized social phobia. Neuroreport. 2004;15:2701–5. [PubMed] [Google Scholar]
- 33.Bogels SM, Mansell W. Attention processes in the maintenance and treatment of social phobia: hypervigilance, avoidance and self-focused attention. Clin Psychol Rev. 2004;24:827–56. doi: 10.1016/j.cpr.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev. 1996;22:229–44. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- 35.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 36.Winston JS, Strange BA, O’Doherty J, et al. Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nat Neurosci. 2002;5:277–83. doi: 10.1038/nn816. [DOI] [PubMed] [Google Scholar]
- 37.Furmark T, Tillfors M, Marteinsdottir I, et al. Common changes in cerebral blood flow in patients with social phobia treated with citalopram or cognitive-behavioral therapy. Arch Gen Psychiatry. 2002;59:425–33. doi: 10.1001/archpsyc.59.5.425. [DOI] [PubMed] [Google Scholar]
- 38.McClure EB, Adler A, Monk CS, et al. fMRI predictors of treatment outcome in pediatric anxiety disorders. Psychopharmacology (Berl) 2007;191:97–105. doi: 10.1007/s00213-006-0542-9. [DOI] [PubMed] [Google Scholar]
- 39.Phan KL, Fitzgerald DA, Gao K, et al. Real-time fMRI of cortico-limbic brain activity during emotional processing. Neuroreport. 2004;15:527–32. doi: 10.1097/00001756-200403010-00029. [DOI] [PubMed] [Google Scholar]
- 40.Lang PJ, Bradley MM, Fitzsimmons JR, et al. Emotional arousal and activation of the visual cortex: an fMRI analysis. Psychophysiology. 1998;35:199–210. [PubMed] [Google Scholar]
- 41.Britton JC, Taylor SF, Sudheimer KD, et al. Facial expressions and complex IAPS pictures: common and differential networks. Neuroimage. 2006;31:906–19. doi: 10.1016/j.neuroimage.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 42.Hariri AR, Tessitore A, Mattay VS, et al. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 2002;17:317–23. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- 43.Meseguer V, Romero MJ, Barros-Loscertales A, et al. Mapping the apetitive and aversive systems with emotional pictures using a block-design fMRI procedure. Psicothema. 2007;19:483–8. [PubMed] [Google Scholar]
- 44.Taylor SF, Phan KL, Decker LR, et al. Subjective rating of emotionally salient stimuli modulates neural activity. Neuroimage. 2003;18:650–9. doi: 10.1016/s1053-8119(02)00051-4. [DOI] [PubMed] [Google Scholar]