Summary
In animals, the sense of smell is often used as a powerful way to attract potential mates, to find food and to explore the environment. Different animals evolved different systems to detect volatile odorants, tuned to the specific needs of each species. Vertebrates and nematodes have been used extensively as models to study the mechanisms of olfaction: the molecular players are olfactory receptors (ORs) expressed in olfactory sensory neurons (OSNs) where they bind to volatile chemicals, acting as the first relay of olfactory processing. These receptors belong to the G protein-coupled receptor (GPCR) superfamily; binding to odorants induces the production and amplification of second messengers, which lead to the depolarization of the neuron. The anatomical features of the insect olfactory circuit are similar to those of mammals, and until recently it was thought that this similarity extended to the ORs, which were originally annotated as GPCRs. Surprisingly, recent evidence shows that insect ORs can act like ligand-gated ion channels, either completely or partially bypassing the amplification steps connected to the activation of G proteins. Although the involvement of G proteins in insect olfactory signal transduction is still under question, this new discovery raises fascinating new questions regarding the function of the sense of smell in insects, its evolution and potential benefits compared with its mammalian counterpart.
Keywords: olfactory receptors, ion channel, GPCR
Introduction
The sense of smell allows insects to detect, discriminate and react to a broad range of different chemicals, even with similar molecular structure, found in the environment. Among thousands of compounds, each insect species has fine-tuned its olfactory system to those that are fundamental for its survival. As a consequence, insects show strong odor-evoked behaviors and change of physiological states in response to chemical cues.
For these reasons, insects have been used as models to study olfaction and olfaction-driven behavior for at least 100 years, since volatile signals influencing moths were first described by the French entomologist Fabre (Fabre, 1911).
Anatomy
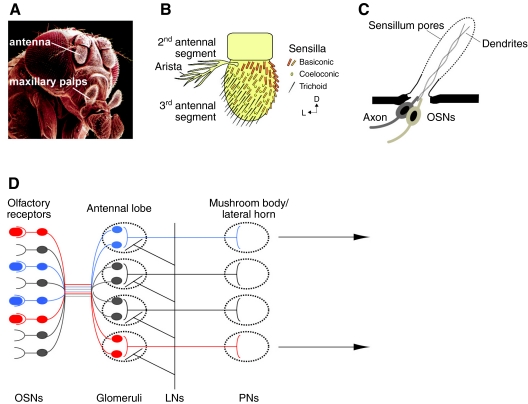
Across the animal kingdom, olfactory systems are remarkably similar. Chemical cues are detected by olfactory sensory neurons (OSNs), which have access to the external environment. In Drosophila, these are located in sensory hairs (sensilla) located on a pair of head appendages, the antennae (∼1200 OSNs each) and the maxillary palps (∼120 OSNs each) (Fig. 1A). The sensilla are categorized into three distinct morphological types: basiconic, coeloconic and trichoid (Shanbhag et al., 1999) (Fig. 1B). Each sensillum is innervated by 2–4 OSNs (Fig. 1C). OSNs are bipolar neurons that extend dendrites into the lumen of the sensillum and project an axon to the antennal lobe (AL), the second relay center of the olfactory system located in the Drosophila brain. In the AL, OSNs expressing the same olfactory receptor (OR) gene make synaptic connections with projection neurons (PN) within globular regions called glomeruli, which are interconnected by inhibitory local interneurons (LNs) (Boeckh and Tolbert, 1993; Galizia and Menzel, 2000). The PNs send their axons to the mushroom body and the lateral horn of the protocerebrum; thus, translating the perception of each odor into a possibly unique temporal and spatial pattern of activity in the brain (Fig. 1D).
Fig. 1.
The olfactory system of Drosophila. (A) Drosophila melanogaster head. The white lines point to the olfactory appendages, the antenna and the maxillary palps. SEM image is courtesy of Jürgen Berger, Max Planck Institute for Developmental Biology, Tübingen, Germany. (B) Drawing of the three different classes of sensilla present on the 3rd antennal segment. The drawing is adapted with permission from Benton et al. (Benton et al., 2006). (C) Anatomy of a sensillum. Each sensillum is innervated by 2–4 olfactory sensory neurons (OSNs), which project an axon to the antennal lobe and dendrites into the sensillum lumen. The sensillum has pores that allow odorants to reach the neurons. (D) Organization of the Drosophila olfactory system. OSNs bind to odorants and send the information to glomeruli, innervated by local interneurons (LNs). The information then travels to higher centers in the brain, the mushroom body and lateral horn, through projection neurons (PNs).
The molecular players: ORs
The discovery of the first ORs was elusive for many years due to the nature of the receptors themselves. The presence of a large number of ORs, their sequence divergence and the low expression level made them difficult to detect until, in 1991, the first mammalian ORs were cloned from the rat olfactory epithelium (Buck and Axel, 1991). The newly found proteins showed characteristics that were consistent with their classification as ORs: they are expressed specifically in the olfactory epithelium, they are members of the superfamily of G protein-coupled receptors (GPCRs) with seven membrane-spanning domains as hypothesized by previous studies (Jones and Reed, 1989) and their sequences are related. The protein's physiological function was indeed confirmed a few years later (Zhao et al., 1998), and genes with similar properties were soon described in other organisms (Freitag et al., 1995; Nef et al., 1996; Selbie et al., 1992; Sengupta et al., 1996).
Insect ORs were first identified in Drosophila melanogaster by three independent groups in 1999 (Clyne et al., 1999; Gao and Chess, 1999; Vosshall et al., 1999). Genomic data mining and an accurate analysis of low abundance genes expressed in the olfactory organ revealed a novel protein family with characteristics similar to those described in mammals. Like vertebrate ORs, they had seven predicted transmembrane domains but were surprisingly much more divergent in sequence from ORs described in other organisms, assigning them to a different evolutionary path. Moreover, even among themselves, OR sequences show wide divergence with only ∼20% of similarity on average.
Most Drosophila OSNs co-express two different types of ORs: OR83b, a broadly expressed receptor, and one of the 61 ligand-specific ORs. OR83b is highly conserved among insect species whereas the ligand-specific receptors are highly divergent. Electrophysiological and behavioral experiments in OR83b knock-out fruit flies revealed that OR83b is essential for the correct function of other ORs (Larsson et al., 2004). Benton and colleagues later demonstrated that not only is OR83b a chaperone that transports the ligand-binding ORs from the cell body to the dendrite where ORs can detect odorants but also that is a functional part of the receptor-complex (Benton et al., 2006). However, it still remains to be elucidated whether OR83b is involved at all in the binding to the odorants.
Functional characterization of insect ORs
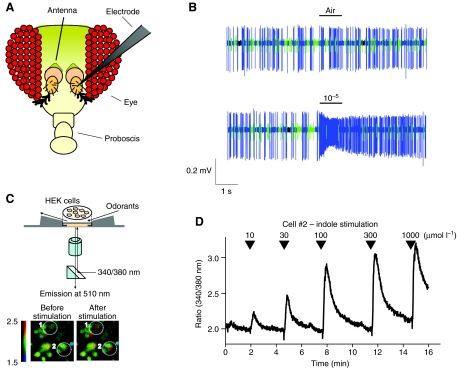
Odorants that pass through pores on the sensillum bind to ORs expressed on the dendrite of OSNs and induce an action potential, which can be monitored using the single sensillum recording (SSR) technique (Bestmann et al., 1996; Stensmyr et al., 2003; Wojtasek et al., 1998); a recording electrode is placed in the desired sensillum and captures voltage changes due to the firing of the OSNs (Fig. 2A). Because the sensillum contains more than one OSN, the resulting trace represents the summed activity of all the neurons housed within the sensillum (Fig. 2B). In some sensilla, it is possible to distinguish the different OSNs because of the different amplitudes of their spikes. Electrophysiological recordings of antennal basiconic sensilla have revealed that OSNs are classified into distinct functional classes, each with a unique odorant response spectrum (de Bruyne et al., 2001). A fundamental step forward was achieved when John Carlson's group established a mutant fly strain with a deletion in the locus of the receptor OR22a/b, thereby abolishing odor-evoked responses in the OSN where the receptor is expressed without eliminating the OSN itself, the so-called `empty neuron' (Hallem et al., 2004a). With this system, thanks to a combination of the SSR technique and the GAL4-UAS system (Hallem et al., 2004a), it is possible to express virtually any OR and study its properties in vivo and use it as a medium-throughput tool for OR de-orphanization, i.e. a simple way to assign ligands to each OR (Hallem et al., 2004b; Kurtovic et al., 2007). Based on this analysis, it was shown that not only is the OR responsible for the odorant response spectrum in OSNs but also for its spontaneous activity and response dynamics (Hallem et al., 2004a). Electrophysiological studies in vivo have been complemented by studies in cell culture: a limited number of insect ORs are in fact functionally expressed in human embryonic kidney 293 (HEK293) cells (Fig. 2C,D), HeLa cells and Xenopus laevis oocytes (Nakagawa et al., 2005; Neuhaus et al., 2004; Sato et al., 2008; Wetzel et al., 2001; Wicher et al., 2008). The functional characterization of insect ORs in heterologous expression systems has provided several new insights into the molecular mechanism of insect ORs, including functional interaction between OR subunits (Neuhaus et al., 2004), novel signaling properties of insect ORs (Sato et al., 2008; Smart et al., 2008; Wicher et al., 2008) and the role of OR83b (Nakagawa et al., 2005; Neuhaus et al., 2004).
Fig. 2.
Functional analysis of the olfactory receptor (OR)-evoked responses in vivo and in vitro. (A,B) Single sensillum recording (SSR) technique. (A) Drawing of a Drosophila head. During SSR, the recording electrode is positioned in the sensilla located on the 3rd segment of the antenna. (B) Responses of Drosophila melanogaster antennal basiconic sensilla to methyl acetate using SSR. The A cell (blue spikes) responds to increasing concentrations of the odorant, while the B cell (green spikes) is unaffected. (C,D) Ca2+-imaging technique. (C) Schematic of Ca2+-imaging assay of human embryonic kidney 293 (HEK293) cells. The cells, #1 and #2, are loaded with a Ca2+-sensitive dye and the light emission of the dye is monitored through a microscope while the cells are stimulated with odorants. The cells marked with broken circles show an increase of Ca2+ concentration after odorant stimulation. (D) A representative trace showing the dose-dependent response to indole of cell #2 (panel C) expressing the mosquito Anopheles gambiae receptors GPROR10+GPROR7. Arrowheads indicate the increasing concentration of odorant delivered (μmol l–1).
Signal transduction cascades in olfactory systems
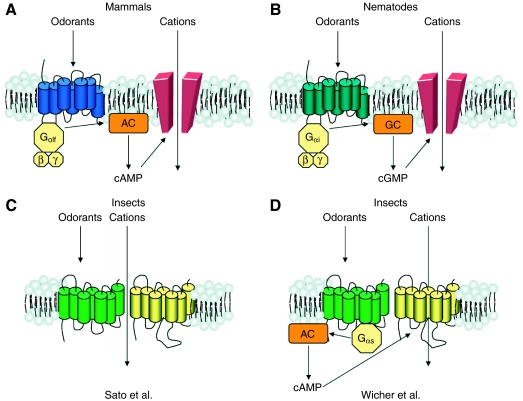
In mammalian and nematode OSNs the binding of odorants to ORs induces the activation of the G protein signaling cascade. Once activated, Gs proteins in mammals, also called Golf, and Gi proteins in nematodes, increase the level of cyclic nucleotides (cAMP and cGMP, respectively) that directly bind and activate cyclic nucleotide-gated (CNG) channels, expressed on the membrane of OSNs. The opening of CNG channels lets cations enter into the neurons, producing an action potential that travels down the axon to the brain (Fig. 3A,B).
Fig. 3.
Models of signal transduction mechanisms in olfactory sensory neurons (OSNs). (A,B) Signal transduction of non-insect olfactory receptors (ORs). (A) Mammalian ORs are G protein-coupled receptors (GPCRs) coupled to a stimulatory G protein, Golf. After binding to an odorant, the G protein activates adenylate cyclase (AC), which increases the intracellular concentration of cAMP. This leads to the opening of a cyclic nucleotide-gated (CNG) channel and the depolarization of the neuron. (B) In nematodes, stimulation of ORs leads to the activation of guanylate cyclase (GC) and an increase in levels of cGMP. This leads to the opening of a CNG channel and the depolarization of the OSN. (C,D) Divergent views on signal transduction of insect ORs. (C) In Sato et al., evidence supports a model in which the OR83b/ORX complex forms an ion channel that is directly opened by the binding of the odorants and is permeable to cations (Sato et al., 2008). (D) By contrast, in Wicher et al.'s model the ligand-binding subunit (in green) is a GPCR that leads to the increase in cAMP through a stimulatory G protein (Wicher et al., 2008). This opens the CNG-like channel OR83b (in yellow).
Before the identification of insect OR genes, there were several hints that pointed toward the involvement of GPCR-mediated second messenger pathways based on biochemical and electrophysiological evidence and the identification of the components of the cAMP and inositol 1,4,5-triphosphate (IP3) signaling pathways in the Drosophila olfactory system. Stimulation with odorants or pheromones on isolated OSNs increases second messenger production like IP3, and in vivo recordings from antennal neurons showed action potentials are generated when IP3 is directly applied to the cells (Stengl, 1993; Talluri et al., 1995). In addition, the reduction of expression of the Drosophila Gαq gene, dgq, and other genes involved in phospholipid signaling induces a decrease of OSNs' odor-evoked responses but not their complete abolishment (Kain et al., 2008; Kalidas and Smith, 2002). These observations lead to the assumption that insect odorant responses were mediated by Gq-coupled GPCRs. However, other groups reported that altering the expression of the genes rut and dnc, affecting the cAMP transduction cascade, showed abnormal electrophysiological and behavioral responses to odorants, suggesting that Gαs is also involved in the transduction mechanism (Gomez-Diaz et al., 2004; Martin et al., 2001). Although the responses are abnormal, it is important to notice that no anosmic phenotypes have been found so far, as expected if G proteins were essential for the transduction mechanisms of odors in OSNs. Are G proteins necessary or sufficient for the correct functioning of the insect olfactory system? General neuronal sickness or the alteration of G protein-mediated signaling pathways downstream or independent of the olfactory receptors could be sufficient to explain the abnormal odor-evoked responses reported in these studies. Altogether, these observations suggest that multiple or alternative signaling cascades are present in the insect olfactory system.
New insights
Ion channel hypothesis
Structural analysis in silico, in vitro and in vivo surprisingly showed that insect ORs have an inverted topology compared with conventional GPCRs, presenting a cytoplasmic N-terminus and an extracellular C-terminus (Benton et al., 2006; Krogh et al., 2001; Lundin et al., 2007). Furthermore, electrophysiological analysis of moth receptors substantiated the idea of an atypical mechanism of olfactory signal transduction: cell culture expression of moth receptors is enough to confer ligand-dependent responses without the further addition of exogenous G proteins, and the electrophysiological properties of these responses are distinct from currents elicited by GPCRs, as observed for mammalian ORs activation (Katada et al., 2003; Nakagawa et al., 2005).
Further electrophysiological analysis recently carried out provided strong evidence for the idea that insect ORs are, in fact, ligand-gated non-specific cation channels (Sato et al., 2008) (Fig. 3C). Simultaneous measurements of whole-cell currents and Ca2+ influx in HeLa cells expressing insect ORs show that the onset of the response is ∼10-fold faster than what is usually required by GPCRs. Furthermore, general pharmacological inhibition of G proteins does not impair ORs-evoked responses, as would be expected if they were GPCRs. Further experiments with single-channel recordings revealed that the response of insect ORs was not dependent on the cellular cytoplasmic components, including second messengers such as cAMP and cGMP. Finally, different subunit compositions of the OR complex are able to shift the ion selectivity of the measured current. This is an important finding because the ion selectivity is a direct property of ion channels. This makes it unlikely that ORs are associated with a separate ion channel and suggests that ORs themselves are necessary and sufficient to produce an odor-induced response (Sato et al., 2008).
Ion channel–GPCR hypothesis
An alternative hypothesis lies between the provocative ion-channel and classical GPCR theories (Wicher et al., 2008) (Fig. 3D). By electrophysiological recordings of insect ORs expressed in HEK293 cells, Wicher and colleagues show that activation of the Drosophila receptor OR22a is able to induce the opening of a cAMP-dependent CNG channel, suggesting the involvement of Gs proteins directly following OR22a activation. Moreover, the co-receptor OR83b alone can generate currents after an increase of intracellular cAMP/cGMP, similar to the currents recorded after ligand application. Finally, a mutation in OR83b can directly modulate the ion permeability of the OR complex, showing that this protein probably participates in the formation of the channel complex without the involvement of other ion channels (Wicher et al., 2008).
Taken together, the results from independent research groups show an unexpected mechanism of signal transduction in insect OSNs. Both groups focus on the new idea that, unlike the case in vertebrates, insect ORs can function as ligand-gated ion channels activated by odorants. However, there are still unanswered questions that need clarification. To what extent are G proteins and cyclic nucleotides involved in insect OSN signal transduction? The partially conflicting results could be explained by the time scale at which the two groups analyzed the OR activation in cells: while the first group looks at the early onset of OR activation (∼1 s), the second group analyzes the characteristics of longer-lasting dynamics after the fast response (∼1.5 min). This behavior might be due to a double mechanism of ORs activation, where at first the G protein-independent channel component of the complex is activated but it is followed by a G protein-dependent response. The role played by cyclic nucleotides could then be different according to which mechanism is being considered, although there is no clear evidence of a cyclic nucleotide binding domain in the OR family (M.P., unpublished data).
In addition, the OR complexes in the two studies contained different ligand-specific subunits. It would be interesting to determine whether the OR studied by Wicher et al. (Wicher et al., 2008) have the same properties in other heterologous systems and in vivo and, vice versa, whether the long-lasting dynamics of the ORs used by Sato et al. (Sato et al., 2008) are similar to what observed for OR22a.
Finally, the possible dual nature of ORs as both functional GPCRs and CNG channels could raise interesting questions as to how substantially different functions developed within the same protein family.
Other types of ligand-gated ion channels in sensory perception
Although new in the field of olfaction, ligand-gated ion channels are used in other sensory systems for the perception of the outside world. Notable examples are the mammalian TRPM8 and TRPV1 channels, activated by cold/menthol (Dhaka et al., 2007) and heat/vanilloid (Caterina et al., 1997) compounds, respectively, both involved in nociception. Interestingly, both of these channels are regulated by Ca2+-dependent and -independent pathways and cyclic nucleotides (Bhave et al., 2002; Daniels et al., 2008; De Petrocellis et al., 2007; Vanden Abeele et al., 2006). Other members of the TRP channel family, PKD1L3 and PKD2L1, have been recently implicated in the detection of sour compounds in mammals, while GPCRs are responsible for the detection of umami, sweet and bitter (Chandrashekar et al., 2006; Huang et al., 2006; Ishimaru et al., 2006). In the gustatory system of the fleshfly Boettcherisca peregrina, Murakami and Kijima have also suggested the presence of sugar-activated ion channels but their molecular identity is still unknown (Murakami and Kijima, 2000). Finally, the green alga Chlamydomonas reinhardtii has recruited the ion channel channelrhodopsin to sense photons (Nagel et al., 2002), unlike the GPCR rhodopsin employed by vertebrates. Remarkably, they both make use of retinal as their chromophore. Finally, one of the latest studies on insect olfaction has unraveled a new class of olfactory receptors in Drosophila melanogaster that belong to the ionotropic glutamate receptor family (iGluRs); therefore, adding one more dimension to the role of ion channels in the olfactory system (Benton et al., 2009). This study revealed that iGluR-like receptors (IRs) are expressed in antennal sensory neurons and confer odor-dependent responses to cells. IRs expression patterns are complementary to OR83b-expressing neurons and might explain the remaining olfactory-mediated responses in OR83b-null fruit flies. More importantly, this discovery highlights how multiple receptor families can be recruited to perform similar functions in the same organ but it is yet to be determined if IRs play a special role in fruit fly olfaction.
Open questions
The recent insights on insect olfactory signal transduction mechanisms open the way for new questions to be answered and offer a new way of thinking about old problems. What is the role of the co-receptor and the ligand-binding subunit within the complex? How can different odorants activate the same receptor complex? How can the same receptor complex be activated and inhibited by different odorants?
Structure–function analysis of insect ORs
Despite a weak similarity to known potassium channel pores (Wicher et al., 2008), there is not a clear consensus on where the pore of the channel is located and to what extent different subunits in the OR complex contribute to the pore itself. As a matter of fact, there is little data on the exact stoichiometry of the OR complex. Although we know it must include at least two subunits each of the co-receptor OR83b and the ligand-binding OR (Benton et al., 2006), the composition of the functional complex is still unknown and it might even vary for different OR83b/ORX combinations. Further research on these questions will help us understand how the ORs bind chemicals with different structures and how conformational changes within the proteins play a role in the transmission of the excitatory or inhibitory signal to the OSN.
Insect ORs are likely to undergo post-translational modifications that can modify their behavior, both pre- and post-stimulation. The possible outcomes of such modifications could affect several characteristics of the proteins and the channel activity: expression levels, internalization and turnover, ligand affinity, gating properties, the fraction of time it remains in an open conformation (open probability) and desensitization just to name some. In addition, the exact role of cyclic nucleotides and soluble second messengers needs to be further addressed, and possible differential effects on different OR complexes better explained.
Why do insects use ion channels as ORs?
One of the most interesting questions still remains: why are ion channels the better choice for insect olfaction compared with GPCRs? Bioinformatics analysis of ORs from different animal species suggests that olfactory receptors appeared multiple times during evolution (Dryer, 2000). Most animal species adopted GPCRs to respond to odorants: this involves a signaling cascade with several amplification steps before the neuron fires and the information that a chemical has been encountered is transmitted to higher centers in the brain. By contrast, insects have adopted ion channels that respond directly to environmental chemicals, although there is still an ongoing controversy regarding whether there is or is not G protein amplification. This type of response might lead to a more direct and quantitative correlation between the amount of molecules bound to the receptor and the activity of the neuron and a faster behavioral response by the animal.
Conclusions
Over the past few years, a novel paradigm on the molecular mechanisms underlying insect chemosensation has been revealed despite the common idea that the olfactory system is conserved across the animal kingdom, from its anatomy to the molecular level. Moreover, the discovery that a pair of seven transmembrane receptors functions as ligand-gated ion channels questions the assumption that seven transmembrane proteins belong to the GPCR superfamily. Finally, the discovery that insect ORs belong to a different class of proteins provides a new strategy to design better insect repellents that will specifically affect the insect olfactory system, with little or no effect on humans.
Glossary
-
AL
The antennal lobe is the second relay center of the olfactory system in the insect brain. It receives information from olfactory sensory neurons and sends it to higher brain centers.
-
Anosmia
Anosmia is the inability to perceive odorants.
-
CNG
Cyclic nucleotide-gated channels are a class of ion channels opened by cyclic nucleotides. CNG channels are involved in the olfactory transduction mechanisms in mammals and nematodes.
-
Dendrites
Dendrites are branched projections of neurons. In olfactory neurons, they are responsible for the detection of odorants.
-
GAL4-UAS
GAL4 is a yeast transcription factor that is able to bind to specific upstream activating sequences (UAS) and drive the transcription of the gene downstream the sequence. The GAL4 protein can be expressed under tissue- or cell-specific promoters, specifying the expression of the genes of interest under control of the UAS sequences.
-
GPCR
A G protein-coupled receptor is a seven transmembrane receptor, which activates signaling transduction inside the cells via G proteins after being activated by its cognate ligand.
-
G proteins
Guanine nucleotide-binding proteins are involved in second messenger cascades. Mammals and nematodes employ trimeric G proteins in olfactory transduction mechanisms. Gα subunits are divided in different classes, depending on the effector protein they modulate. For example, Gαs activates adenylyl cyclase, Gαi inhibits it and Gαq activates phospholipase C.
-
HEK293
The human embryonic kidney cell line 293 is a heterologous cell line originally obtained from human embryonic kidney. It is often used as an expression system for GPCRs.
-
IP3
Inositol 1,4,5-triphosphate is a secondary messenger molecule used in signaling transduction induced by the activation of phospholipase C.
-
LN
A local interneuron is a multipolar neuron, which modifies the output from the AL to higher brain centers through intra- and interglomerular communication.
-
Nociception
Nociception is the perception of pain.
-
OR
Olfactory receptors are proteins that bind odorants in the olfactory sensory neurons.
-
OSN
An olfactory sensory neuron, which is the primary center of the olfactory system, detects odorants through the ORs expressed on its dendrites and transmits the information to glomeruli.
-
PN
Projection neurons synapse with OSNs in the glomeruli and transmit the olfactory information to the AL and higher brain centers.
-
Sensillum
A sensillum is a sensory hair, which contains neurons surrounded by lymph; within a sensillum, a variable number of neurons can be housed. Olfactory sensilla found on the antenna of Drosophila melanogaster can be divided in three types, based on their shape and size: basiconic, ceoloconic and trichoid.
-
SSR
Single sensillum recording is an extracellular recording of voltage differences generated by the activation of ORs.
The authors thank L. B. Vosshall and R. Benton for insightful criticisms of the manuscript, and L. B. Vosshall for support. We apologize to colleagues for being unable to cite all relevant primary literature due to space constraints. This work was funded in part by a grant to R. Axel and L.B.V. from the Foundation for the National Institutes of Health through the Grand Challenges in Global Health Initiative, by grants to L.B.V. from the National Institutes of Health (RO1 DC008600), and a postdoctoral fellowship from the Japan Society for the Promotion of Science (JSPS) to T.N. Deposited in PMC for release after 12 months.
References
- Benton, R., Sachse, S., Michnick, S. W. and Vosshall, L. B. (2006). Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 4, e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton, R., Vannice, K. S., Gomez-Diaz, C. and Vosshall, L. B. (2009). Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell 136, 149-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestmann, H. J., Roth, K. D., Rehefeld, C., Leinemann, B., Kern, F. and Vostrowsky, O. (1996). Ab initio calculations on (Z)-5-decenyl acetate, a component of the pheromone complex of Agrotis segetum (Lepidoptera: Noctuidae) and electrophysiological studies with chain elongated analogues. Bioorg. Med. Chem. 4, 473-477. [DOI] [PubMed] [Google Scholar]
- Bhave, G., Zhu, W., Wang, H., Brasier, D. J., Oxford, G. S. and Gereau, R. W. t. (2002). cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron 35, 721-731. [DOI] [PubMed] [Google Scholar]
- Boeckh, J. and Tolbert, L. P. (1993). Synaptic organization and development of the antennal lobe in insects. Microsc. Res. Tech. 24, 260-280. [DOI] [PubMed] [Google Scholar]
- Buck, L. and Axel, R. (1991). A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell 65, 175-187. [DOI] [PubMed] [Google Scholar]
- Caterina, M. J., Schumacher, M. A., Tominaga, M., Rosen, T. A., Levine, J. D. and Julius, D. (1997). The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816-824. [DOI] [PubMed] [Google Scholar]
- Chandrashekar, J., Hoon, M. A., Ryba, N. J. and Zuker, C. S. (2006). The receptors and cells for mammalian taste. Nature 444, 288-294. [DOI] [PubMed] [Google Scholar]
- Clyne, P. J., Warr, C. G., Freeman, M. R., Lessing, D., Kim, J. and Carlson, J. R. (1999). A novel family of divergent seven-transmembrane proteins: candidate odorant receptors in Drosophila. Neuron 22, 327-338. [DOI] [PubMed] [Google Scholar]
- Daniels, R. L., Takashima, Y. and McKemy, D. D. (2008). The activity of the neuronal cold sensor TRPM8 is regulated by phospholipase C via the phospholipid phosphoinositol-4,5-bisphosphate. J. Biol. Chem. 284, 1570-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruyne, M., Foster, K. and Carlson, J. R. (2001). Odor coding in the Drosophila antenna. Neuron 30, 537-552. [DOI] [PubMed] [Google Scholar]
- De Petrocellis, L., Starowicz, K., Moriello, A. S., Vivese, M., Orlando, P. and Di Marzo, V. (2007). Regulation of transient receptor potential channels of melastatin type 8 (TRPM8): effect of cAMP, cannabinoid CB(1) receptors and endovanilloids. Exp. Cell Res. 313, 1911-1920. [DOI] [PubMed] [Google Scholar]
- Dhaka, A., Murray, A. N., Mathur, J., Earley, T. J., Petrus, M. J. and Patapoutian, A. (2007). TRPM8 is required for cold sensation in mice. Neuron 54, 371-378. [DOI] [PubMed] [Google Scholar]
- Dryer, L. (2000). Evolution of odorant receptors. BioEssays 22, 803-810. [DOI] [PubMed] [Google Scholar]
- Fabre, J. H. (1911). Social Life In The Insect World. Harmondsworth, UK: Penguin.
- Freitag, J., Krieger, J., Strotmann, J. and Breer, H. (1995). Two classes of olfactory receptors in Xenopus laevis. Neuron 15, 1383-1392. [DOI] [PubMed] [Google Scholar]
- Galizia, C. G. and Menzel, R. (2000). Odour perception in honeybees: coding information in glomerular patterns. Curr. Opin. Neurobiol. 10, 504-510. [DOI] [PubMed] [Google Scholar]
- Gao, Q. and Chess, A. (1999). Identification of candidate Drosophila olfactory receptors from genomic DNA sequence. Genomics 60, 31-39. [DOI] [PubMed] [Google Scholar]
- Gomez-Diaz, C., Martin, F. and Alcorta, E. (2004). The cAMP transduction cascade mediates olfactory reception in Drosophila melanogaster. Behav. Genet. 34, 395-406. [DOI] [PubMed] [Google Scholar]
- Hallem, E. A., Ho, M. G. and Carlson, J. R. (2004a). The molecular basis of odor coding in the Drosophila antenna. Cell 117, 965-979. [DOI] [PubMed] [Google Scholar]
- Hallem, E. A., Nicole Fox, A., Zwiebel, L. J. and Carlson, J. R. (2004b). Olfaction: mosquito receptor for human-sweat odorant. Nature 427, 212-213. [DOI] [PubMed] [Google Scholar]
- Huang, A. L., Chen, X., Hoon, M. A., Chandrashekar, J., Guo, W., Trankner, D., Ryba, N. J. and Zuker, C. S. (2006). The cells and logic for mammalian sour taste detection. Nature 442, 934-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimaru, Y., Inada, H., Kubota, M., Zhuang, H., Tominaga, M. and Matsunami, H. (2006). Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc. Natl. Acad. Sci. USA 103, 12569-12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, D. T. and Reed, R. R. (1989). Golf: an olfactory neuron specific-G protein involved in odorant signal transduction. Science 244, 790-795. [DOI] [PubMed] [Google Scholar]
- Kain, P., Chakraborty, T. S., Sundaram, S., Siddiqi, O., Rodrigues, V. and Hasan, G. (2008). Reduced odor responses from antennal neurons of G(q)alpha, phospholipase Cbeta, and rdgA mutants in Drosophila support a role for a phospholipid intermediate in insect olfactory transduction. J. Neurosci. 28, 4745-4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalidas, S. and Smith, D. P. (2002). Novel genomic cDNA hybrids produce effective RNA interference in adult Drosophila. Neuron 33, 177-184. [DOI] [PubMed] [Google Scholar]
- Katada, S., Nakagawa, T., Kataoka, H. and Touhara, K. (2003). Odorant response assays for a heterologously expressed olfactory receptor. Biochem. Biophys. Res. Commun. 305, 964-969. [DOI] [PubMed] [Google Scholar]
- Krogh, A., Larsson, B., von Heijne, G. and Sonnhammer, E. L. (2001). Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305, 567-580. [DOI] [PubMed] [Google Scholar]
- Kurtovic, A., Widmer, A. and Dickson, B. J. (2007). A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature 446, 542-546. [DOI] [PubMed] [Google Scholar]
- Larsson, M. C., Domingos, A. I., Jones, W. D., Chiappe, M. E., Amrein, H. and Vosshall, L. B. (2004). Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron 43, 703-714. [DOI] [PubMed] [Google Scholar]
- Lundin, C., Kall, L., Kreher, S. A., Kapp, K., Sonnhammer, E. L., Carlson, J. R., Heijne, G. and Nilsson, I. (2007). Membrane topology of the Drosophila OR83b odorant receptor. FEBS Lett. 581, 5601-5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, F., Charro, M. J. and Alcorta, E. (2001). Mutations affecting the cAMP transduction pathway modify olfaction in Drosophila. J. Comp. Physiol. A 187, 359-370. [DOI] [PubMed] [Google Scholar]
- Murakami, M. and Kijima, H. (2000). Transduction ion channels directly gated by sugars on the insect taste cell. J. Gen. Physiol. 115, 455-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel, G., Ollig, D., Fuhrmann, M., Kateriya, S., Musti, A. M., Bamberg, E. and Hegemann, P. (2002). Channelrhodopsin-1: a light-gated proton channel in green algae. Science 296, 2395-2398. [DOI] [PubMed] [Google Scholar]
- Nakagawa, T., Sakurai, T., Nishioka, T. and Touhara, K. (2005). Insect sex-pheromone signals mediated by specific combinations of olfactory receptors. Science 307, 1638-1642. [DOI] [PubMed] [Google Scholar]
- Nef, S., Allaman, I., Fiumelli, H., De Castro, E. and Nef, P. (1996). Olfaction in birds: differential embryonic expression of nine putative odorant receptor genes in the avian olfactory system. Mech. Dev. 55, 65-77. [DOI] [PubMed] [Google Scholar]
- Neuhaus, E. M., Gisselmann, G., Zhang, W., Dooley, R., Stoertkuhl, K. and Hatt, H. (2004). Odorant receptor heterodimerization in the olfactory system of Drosophila melanogaster. Nat. Neurosci. 8, 15-17. [DOI] [PubMed] [Google Scholar]
- Sato, K., Pellegrino, M., Nakagawa, T., Nakagawa, T., Vosshall, L. B. and Touhara, K. (2008). Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature 452, 1002-1006. [DOI] [PubMed] [Google Scholar]
- Selbie, L. A., Townsend-Nicholson, A., Iismaa, T. P. and Shine, J. (1992). Novel G protein-coupled receptors: a gene family of putative human olfactory receptor sequences. Brain Res. Mol. Brain Res. 13, 159-163. [DOI] [PubMed] [Google Scholar]
- Sengupta, P., Chou, J. H. and Bargmann, C. I. (1996). odr-10 encodes a seven transmembrane domain olfactory receptor required for responses to the odorant diacetyl. Cell 84, 899-909. [DOI] [PubMed] [Google Scholar]
- Shanbhag, S. R., Muller, B. and Steinbrecht, R. A. (1999). Atlas of olfactory organs of Drosophila melanogaster. 1. Types, external organization, innervation and distribution of olfactory sensilla. Int. J. Insect Morphol. Embryol. 28, 377-397. [Google Scholar]
- Smart, R., Kiely, A., Beale, M., Vargas, E., Carraher, C., Kralicek, A. V., Christie, D. L., Chen, C., Newcomb, R. D. and Warr, C. G. (2008). Drosophila odorant receptors are novel seven transmembrane domain proteins that can signal independently of heterotrimeric G proteins. Insect Biochem. Mol. Biol. 38, 770-780. [DOI] [PubMed] [Google Scholar]
- Stengl, M. (1993). Intracellular-messenger-mediated cation channels in cultured olfactory receptor neurons. J. Exp. Biol. 178, 125-147. [DOI] [PubMed] [Google Scholar]
- Stensmyr, M. C., Giordano, E., Balloi, A., Angioy, A. M. and Hansson, B. S. (2003). Novel natural ligands for Drosophila olfactory receptor neurones. J. Exp. Biol. 206, 715-724. [DOI] [PubMed] [Google Scholar]
- Talluri, S., Bhatt, A. and Smith, D. P. (1995). Identification of a Drosophila G protein alpha subunit (dGq alpha-3) expressed in chemosensory cells and central neurons. Proc. Natl. Acad. Sci. USA 92, 11475-11479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Abeele, F., Zholos, A., Bidaux, G., Shuba, Y., Thebault, S., Beck, B., Flourakis, M., Panchin, Y., Skryma, R. and Prevarskaya, N. (2006). Ca2+-independent phospholipase A2-dependent gating of TRPM8 by lysophospholipids. J. Biol. Chem. 281, 40174-40182. [DOI] [PubMed] [Google Scholar]
- Vosshall, L. B., Amrein, H., Morozov, P. S., Rzhetsky, A. and Axel, R. (1999). A spatial map of olfactory receptor expression in the Drosophila antenna. Cell 96, 725-736. [DOI] [PubMed] [Google Scholar]
- Wetzel, C. H., Behrendt, H. J., Gisselmann, G., Störtkuhl, K. F., Hovemann, B. and Hatt, H. (2001). Functional expression and characterization of a Drosophila odorant receptor in a heterologous cell system. Proc. Natl. Acad. Sci. USA 98, 9377-9380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicher, D., Schafer, R., Bauernfeind, R., Stensmyr, M. C., Heller, R., Heinemann, S. H. and Hansson, B. S. (2008). Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature 452, 1007-1011. [DOI] [PubMed] [Google Scholar]
- Wojtasek, H., Hansson, B. S. and Leal, W. S. (1998). Attracted or repelled? A matter of two neurons, one pheromone binding protein, and a chiral center. Biochem. Biophys. Res. Commun. 250, 217-222. [DOI] [PubMed] [Google Scholar]
- Zhao, H., Ivic, L., Otaki, J. M., Hashimoto, M., Mikoshiba, K. and Firestein, S. (1998). Functional expression of a mammalian odorant receptor. Science 279, 237-242. [DOI] [PubMed] [Google Scholar]