Abstract
The kinesin motor protein Kif1b has previously been implicated in the axonal transport of mitochondria and synaptic vesicles1,2. More recently kif1b has been linked with susceptibility to Multiple Sclerosis (MS) 3. Here we show that Kif1b is required for the localization of myelin basic protein mRNA to processes of myelinating oligodendrocytes in zebrafish. We observe the ectopic appearance of myelin-like membrane in kif1b mutants, coincident with the ectopic localization of myelin proteins in kif1b mutant oligodendrocyte cell bodies. These observations suggest the hypothesis that oligodendrocytes localize certain mRNA molecules, namely those encoding small basic proteins such as mbp, to prevent aberrant effects of these proteins elsewhere in the cell. We also find that Kif1b is required for outgrowth of some of the longest axons in the peripheral and central nervous systems. Our data demonstrate new functions of kif1b in vivo and provide insights into its possible roles in Multiple Sclerosis.
The myelin sheath is essential for the normal function of the vertebrate nervous system4. Disruption of myelin underlies the symptoms of many human diseases, including multiple sclerosis (MS). In fish and mammals, a few specific messenger RNA molecules, including myelin basic protein (mbp) mRNA, are localized to processes of myelinating oligodendrocytes (Supplementary Fig. 1)5,6. Previous studies carried out in cell culture have implicated microtubules and associated motors in the localization of mbp mRNAs to myelinating oligodendrocyte processes7,8,9. Nonetheless, the identity of specific factors required for the localization of endogenous mbp mRNAs in vivo has remained unclear. In addition, the function of this evolutionarily conserved mRNA localization mechanism is not known.
In a zebrafish genetic screen, we previously identified a mutation (named st43) that disrupts localization of mbp mRNA in the central nervous system (CNS) and outgrowth of the posterior lateral line nerve (PLLn) in the peripheral nervous system (PNS)10. Using positional cloning, we identified the gene disrupted by the st43 mutation. High-resolution mapping showed that st43 is tightly linked to kif1b, which encodes a kinesin motor. Sequence analysis showed that the st43 mutation disrupts zebrafish kif1b (Fig. 1a), converting a conserved threonine to a proline, which is predicted to break a highly conserved alpha helix in the putative microtubule interaction site of the kinesin motor11,12. kif1b has two prominent isoforms, kif1bα and kif1bβ, which are expressed widely in the developing nervous system (Fig. 1b,c, and data not shown). Zebrafish Kif1bα is 78% identical to human Kif1bα and zebrafish Kif1bβ is 87% identical to human Kif1bβ. These Kif1b isoforms share a common region, which includes the motor domain disrupted by st43, and are distinguished by distinct tail domains that are thought to confer cargo specificity2,13. To confirm the specific requirement for kif1b during mbp mRNA localization and peripheral nerve outgrowth, we designed an antisense morpholino oligonucleotide (MO) to block translation of all kif1b isoforms, and MOs that targeted splice junctions specific to kif1bα and kif1bβ. Injection of either the common-region MO or the kif1bβ specific MO disrupted mbp mRNA localization in the CNS and peripheral nerve outgrowth in the PNS (Fig. 1f,g, Fig. 2a–d and data not shown), whereas MOs targeting kif1bα did not (Fig. 2c). kif1bst43 mutant larvae do not have overt morphological defects at 4 days post fertilization (dpf), but fail to inflate their swim bladders (Fig. 1d,e) and die between 12–14 dpf.
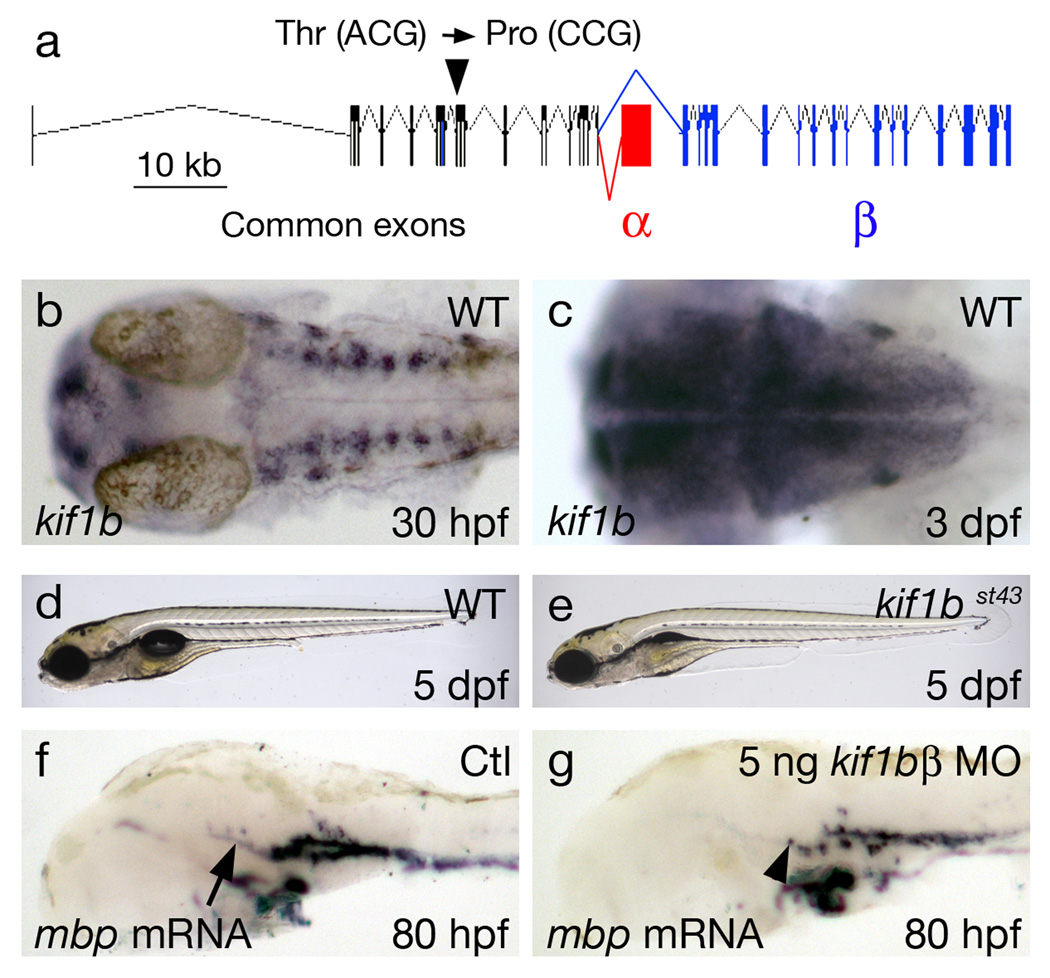
Figure 1. kif1b is essential for myelin basic protein mRNA localization in the CNS.
a. Genomic structure of zebrafish kif1b. Exons common to kif1bα and kif1bβ are black, those specific to kif1bα are red and to kif1bβ blue. The st43 mutation changes a threonine codon (ACG) to a proline codon (CCG) in an exon common to both isoforms (arrowhead).
b and c. Dorsal views of kif1b mRNA expression at 30 hours post fertilization (hpf) (b) and 3 dpf (c). Expression is strongest in differentiated neurons at 30 hpf and is widely expressed in the nervous system at 3 dpf.
d and e. Lateral views of live zebrafish larvae at 5 dpf shows that kif1bst43 mutants (e) are morphologically similar to wildtype (d) despite not inflating their swim bladder.
f and g. Lateral views of dissected zebrafish heads at 80 hpf showing that mbp mRNA expression is localized primarily to cell bodies (arrowhead, g) in animals injected with a kif1bβ specific morpholino but localized to cell bodies and myelinating processes (arrow, f) in controls.
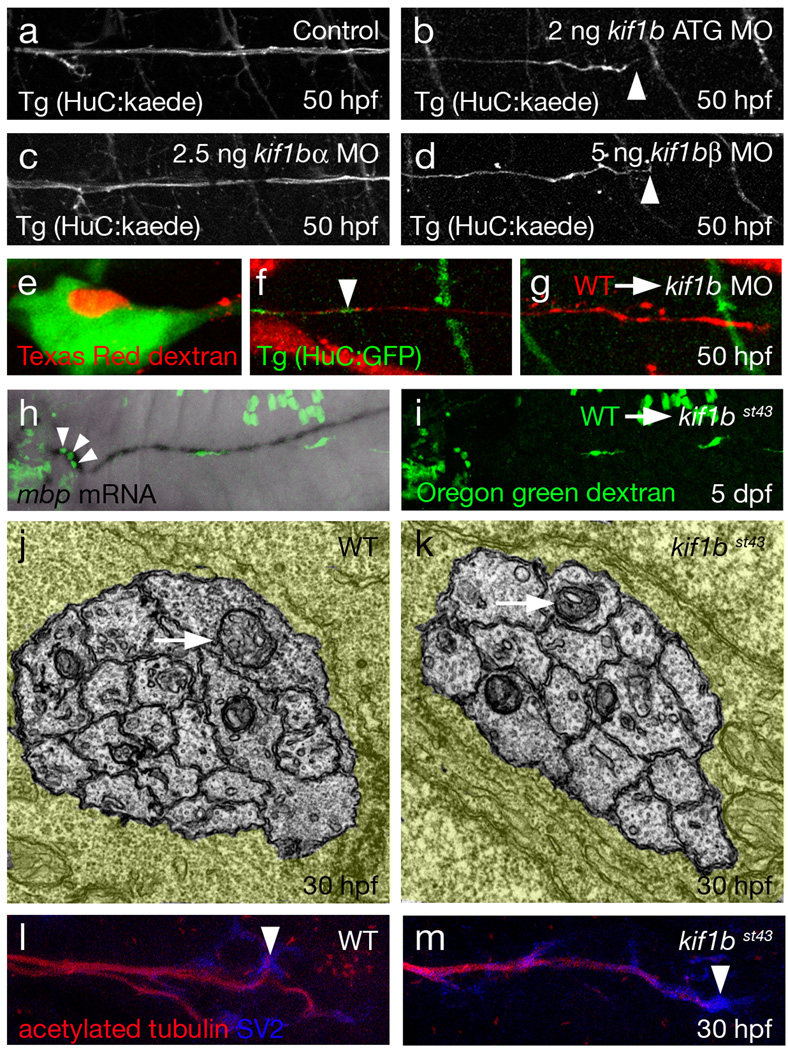
Figure 2. Kif1b is required for normal axonal outgrowth in the PNS.
a–d Lateral views of larvae at 50 hpf expressing Tg(HuC:kaede) show that antisense morpholino oligonucleotide (MO) knockdown of the common start region of kif1b (b) and blocking a splice junction specific to kif1bβ (d) cause truncation of the PLLn (arrowheads). Disruption of kif1bα does not affect PLLn outgrowth (c).
e–g Three images of a PLLn chimera. Wildtype cells were transplanted into a Tg(HuC:GFP)-expressing kif1b ATG morphant. Wildtype axons (red) grow over a longer distance than kif1b morphant axons (green). Arrowhead in f (somite level 9) points to the tip of the longest kif1b morphant axon in the chimeric PLLn. g shows a wildtype axon at somite 24. Morphant axons never grew beyond the level of somite 10 (n=52 chimeras).
h and i. Wildtype neurons (arrowheads) transplanted into a kif1bst43 mutant are sufficient to restore mbp expressing Schwann cells along the length of the PLLn.
j and k. TEM images of transverse sections cut through the PLLn show mitochondria (arrows) present in kif1bst43 mutant axons (k) and wildtype (j) at 30 hpf.
l and m. Lateral views of embryos at 30 hpf stained with acetylated tubulin (red) and SV2 show that SV2 is present in growth cones at the leading tips of growing axons of the PLLn in both wildtype (1) and kif1bst43 mutant (m) embryos.
In addition to the axonal outgrowth defect we also noted a reduction in the number of neurons in the kif1bst43 mutant PNS. The posterior lateral line ganglion (PLLg) contained an average of 27 neurons (s.d.+/− 2) in wildtype siblings at 28 hpf (n=15) compared to 20 (s.d.+/− 5) in kif1bst43 mutants (n=12), and 38 (s.d.+/− 1) in wildtype (n=13) compared to 25 (s.d.+/− 6) in kif1bst43 mutants (n=6) at 36 hpf. To determine whether kif1b functioned in neurons or Schwann cells to mediate normal peripheral nerve development we performed chimeric analyses, whereby wildtype cells were transplanted into either kif1b morphant or kif1bst43 mutant hosts. Analyses of chimeras showed that wildtype axons could grow significantly further than those with disrupted kif1b function in individual nerves. Whereas kif1b morphant axons never grew past somite ten, wildtype axons could grow to the tip of the tail in the same nerves (Fig. 2e–g, n=4 chimeras). Wildtype neurons were also sufficient to restore the migration and differentiation of kif1bst43 mutant Schwann cells (Fig. 2g,h, n=7 chimeras). These data demonstrate that kif1b is required autonomously in neurons to mediate normal PLLn development.
Analyses of the pan-neuronal marker Tg(HuC:kaede)14 revealed disruption in the outgrowth of some long axons in the kif1bst43 mutant spinal cord (Fig. 3a,b). Transplantation studies showed that kif1b functions autonomously in neurons to ensure normal outgrowth of these axons. Whereas kif1b morphant axons did not grow past somite sixteen, wildtype axons could grow to the posterior tip of the spinal cord in the same animals (Fig. 3c,d, n=7 chimeras). Previous studies have implicated Kif1b in the transport of mitochondria and synaptic vesicles1,2. Our marker and ultrastructural analyses, however, failed to reveal an obvious defect in the distribution of these cargoes in kif1bst43 mutant axons prior to or during disruption of (Fig. 2j–m, Fig. 3d,e, Fig. 5a,b and Fig. 6a–d). Furthermore, kif1bst43 mutants did not have overt disruption in axonal microtubules (data not shown), in contrast to mutants for kbp15, which encodes a putative binding partner of Kif1b. It is also possible, therefore, that the axonal and neuronal defects observed in kif1bst43 mutants might relate to unknown Kif1b cargo, or functions independent of its motor activity, precedent for which has recently been demonstrated16,17,18.
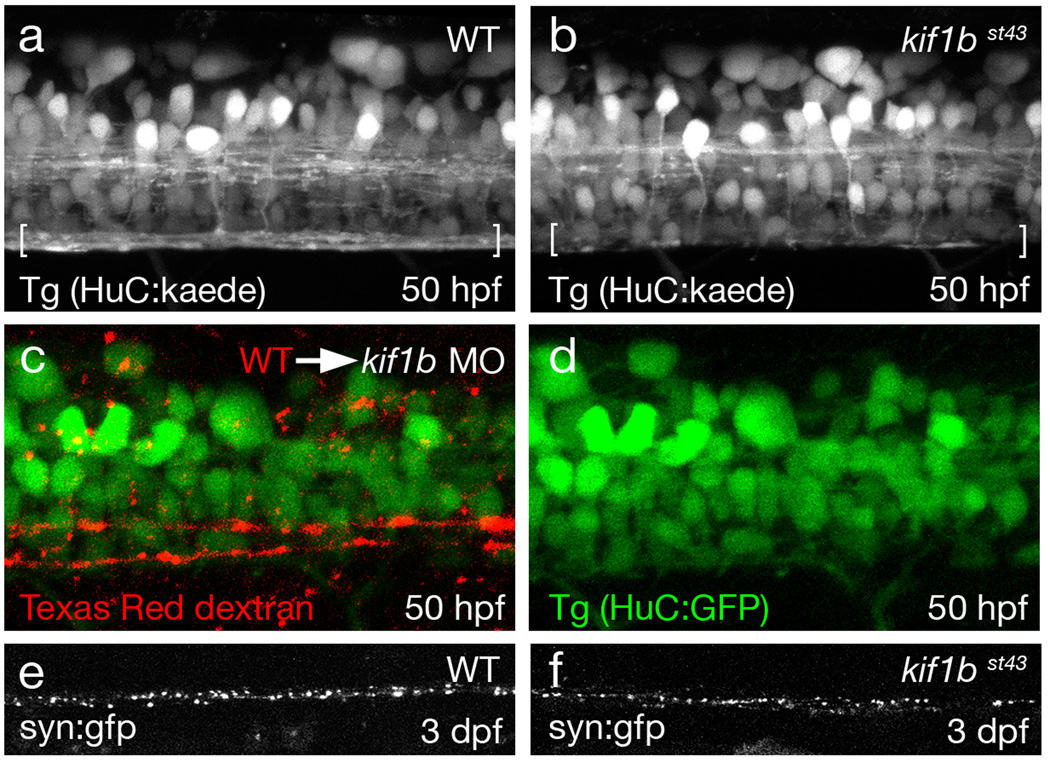
Figure 3. Kif1b is required for normal axonal outgrowth in the CNS.
a–b. Lateral views of Tg(HuC:kaede) expressing larvae at 50 hpf showing a reduction in the number of axons in the ventral spinal cord (brackets) in kif1bst43 mutants (b) compared to wildtype (a).
c–d. Images of the posterior spinal cord of a chimera where wildtype cells were transplanted into a Tg(HuC:GFP) expressing kif1b ATG morphant. Wildtype axons (red) grow further than kif1b morphant axons (green), which cannot be seen in the ventral spinal cord at this posterior level (somite 27–28).
e–f. Lateral views of embryos injected with the transgene synaptophysin:gfp show similar distribution of this synaptic vesicle protein in kif1bst43 mutant (f) and wildtype (e) axons.
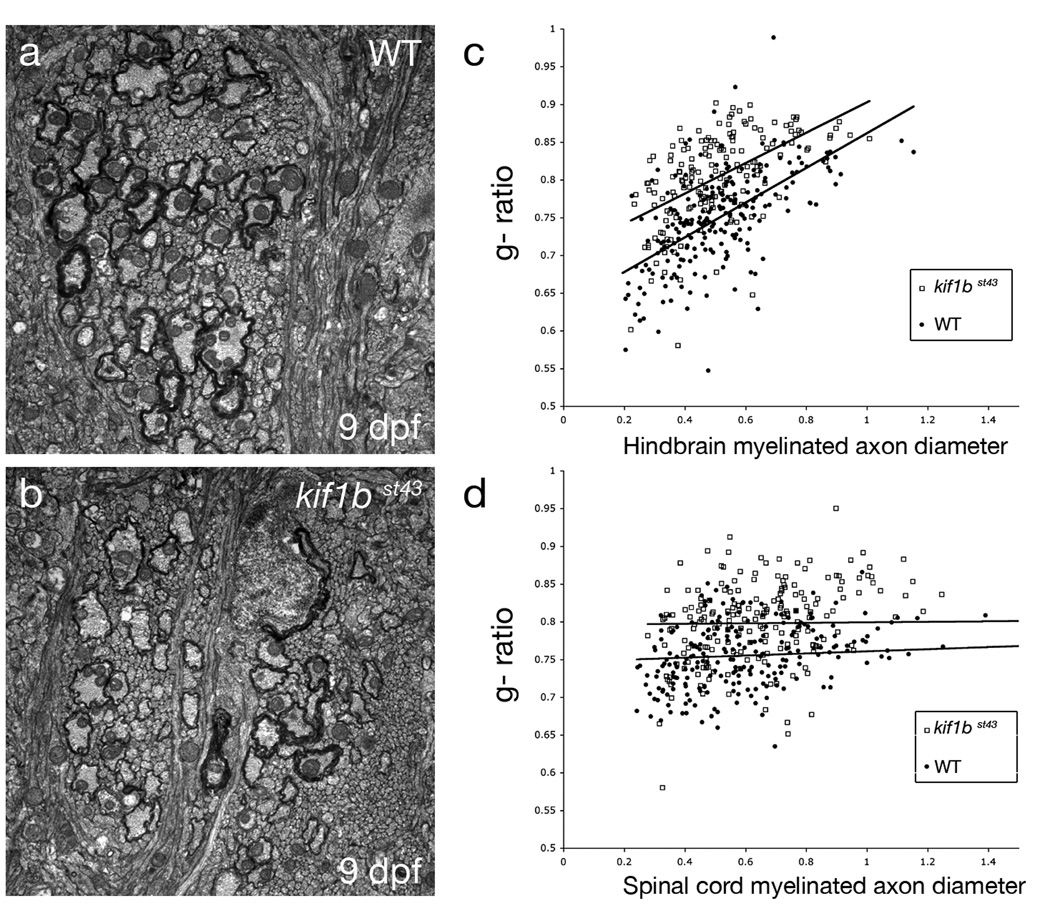
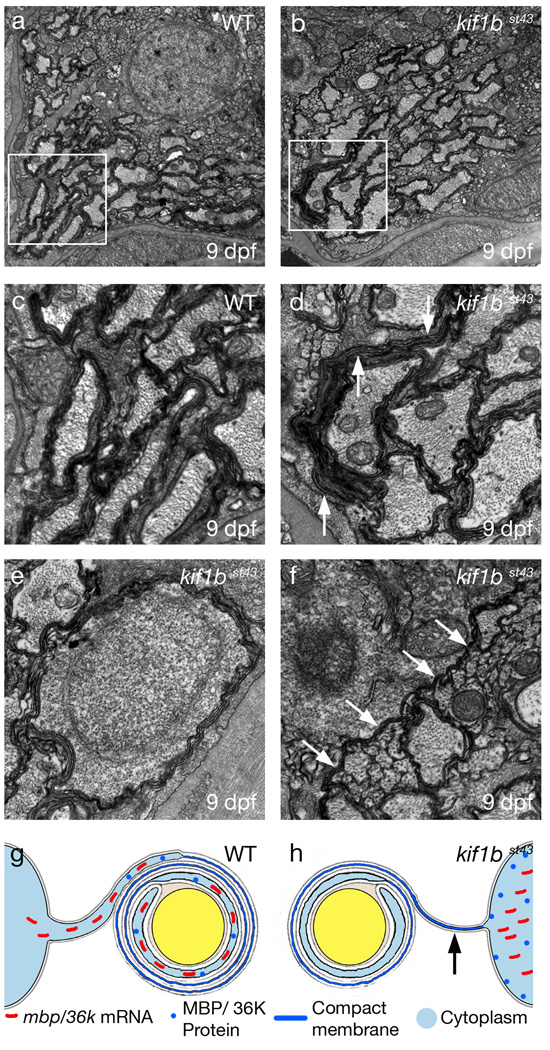
Figure 5. Abnormalities in myelinated axons in kif1bst43 mutants.
a and b. TEM images of transverse sections through myelinated axon tracts in the anterior hindbrain in (a) wildtype and (b) kif1bst43 mutant animals. There are fewer myelinated axons in the in the kif1bst43 mutant and these axons also have a slight reduction in myelin relative to wildtype.
c and d. Scatter plots of myelinated axon g-ratios at different axon diameters in the anterior hindbrain (c) and spinal cord (d). There is an overall increase in g-ratio (reduction in myelin thickness) in kif1bst43 mutant axons (higher trend lines in c and d) compared to wildtype. No myelinated axons have a diameter greater than 1.5µm in either the wildtype or mutant anterior hindbrain at the stage examined. The small number of reticulospinal axons with a diameter greater than 1.5µm in the spinal cord at this stage have been omitted from (d).
Figure 6. Ectopic myelin–like membrane in kif1bst43 mutants.
a and b. TEM images of transverse sections through myelinated axon tracts in the anterior spinal cord. There is a similar distribution of large-diameter axons in wildtype (a) and kif1bst43 mutants (b). Boxes indicate regions of higher magnification in c and d.
c and d. Higher magnification views of areas outlined by white boxes in a and b respectively. Ectopic myelin-like membranes are present in the kif1bst43 mutant (arrows).
e. TEM image of a transverse section through the spinal cord shows a neuronal cell body surrounded by myelin-like membrane in a kif1bst43 mutant.
f. TEM image of a transverse section through the anterior spinal cord showing another ectopic process of myelin-like membrane (arrows).
g–h. Cartoon depicting defects of kif1bst43 mutant oligodendrocytes. Wildtype oligodendrocytes (g) localize mbp and 36k mRNAs to their myelinating processes. mbp and 36k mRNA is localized almost exclusively in the cell body of kif1bst43 mutants (h). kif1bst43 mutants have less myelin surrounding axons than wildtype, and also have ectopic myelin-like membrane along oligodendrocyte processes (arrow). MBP and 36K proteins are expressed ectopically in kif1bst43 mutant oligodendrocyte cell bodies.
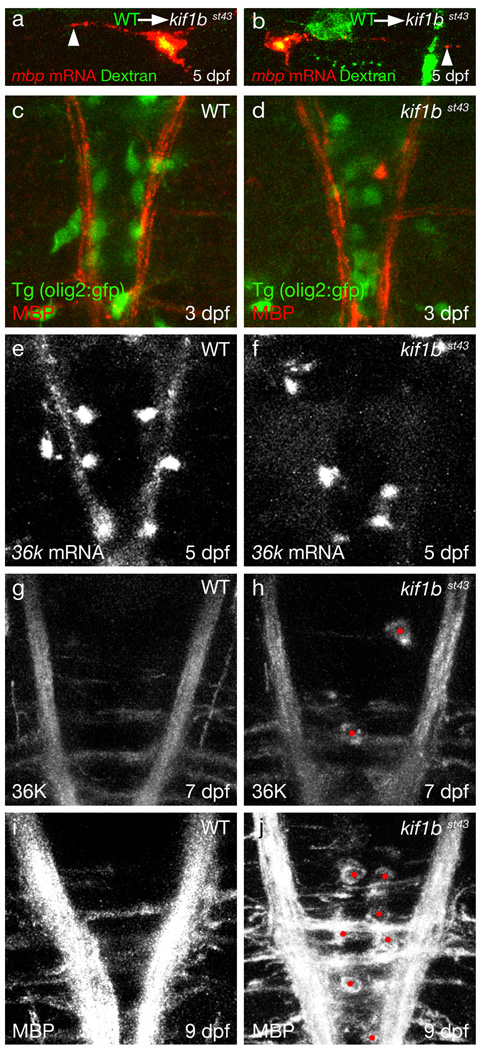
In light of its role in neurons, we investigated whether kif1b functions autonomously in oligodendrocytes or non-autonomously in neurons to mediate normal mbp mRNA localization. Microarray analyses show that kif1b is upregulated in myelinating oligodendrocytes19, but there is no previously defined role for kif1b in these glia. We generated chimeras in which wildtype oligodendrocytes and/or neurons were present in kif1bst43 mutants. In 5 such chimeras we observed mbp mRNA localized in distal oligodendrocyte processes (Fig. 4a,b). In each of these cases, the oligodendrocyte(s) with normally localized mbp mRNA derived from a wildtype animal. We never saw a case where wildtype neurons restored normal mbp mRNA localization to a kif1bst43 mutant oligodendrocyte (n>100 chimeras). These data indicate that kif1b is required autonomously in oligodendrocytes to localize mbp mRNA. To test the possibility that kif1b is required for oligodendrocytes to extend myelinating processes, we analyzed the expression of Tg(olig2:gfp)20, which labels oligodendrocytes, and an antibody directed against MBP protein21. The number and distribution of oligodendrocytes was similar in kif1bst43 mutants and wildtype animals from 3 through 9 dpf, stages when mRNA was always mislocalized in kif1bst43 (Fig. 4c,d, and data not shown). Furthermore, MBP protein was localized in myelinating processes of kif1bst43 mutant oligodendrocytes (Fig. 4c,d,i and j) showing that its translation is not dependent on localization of its mRNA. To investigate whether the localization of other myelin mRNAs requires kif1b function, we analyzed the distribution of 36k, the other mRNA known to be localized to myelinating processes in zebrafish22 (Fig. 4e). As for mbp, 36k mRNA was also restricted to oligodendrocyte cell bodies in kif1bst43 mutants (Fig. 4f). At later stages of development we noticed a striking accumulation of both MBP and 36K protein in cell bodies and proximal processes of kif1bst43 mutant oligodendrocytes, in contrast to wildtype, where these proteins are largely restricted to myelinating processes (Fig. 4g–j). These data indicate that kif1b is specifically required in oligodendrocytes to localize mRNAs encoding a subset of myelin proteins, including MBP and 36K.
Figure 4. Kif1b functions in oligodendrocytes to localize myelin mRNA and protein.
a and b. Confocal images of wildtype oligodendrocytes (yellow) transplanted into a kif1bst43 mutant. Arrowheads indicate mbp mRNA localized in distal processes of the wildtype oligodendrocyte. Such distribution was never seen in mutant oligodendrocytes.
c and d. Dorsal views of the hindbrain at 3 dpf shows that expression of Tg(olig2:gfp) and Myelin Basic Protein (MBP) is similar in wildtype (c) and kif1bst43 mutants (d).
e and f. Dorsal views of the hindbrain of larvae at 5 dpf shows 36k mRNA localized to oligodendrocyte cell bodies and myelinating processes in wildtype (e), but only to oligodendrocyte cell bodies in kif1bst43 mutants (f).
g and h. Dorsal views of the hindbrain at 7 dpf show robust expression of 36K protein in myelinating oligodendrocyte processes in both wildtype (g) and kif1bst43 mutant (h) larvae, and ectopic expression of 36K protein in kif1bst43 mutant cell bodies (red dots).
i and j. Dorsal views of the hindbrain at 9 dpf show robust expression of MBP protein in myelinating oligodendrocyte processes in both wildtype (g) and kif1bst43 mutant (h) larvae, and ectopic expression of MBP protein in kif1bst43 mutant cell bodies (red dots).
To determine if kif1b is required for the formation of myelin in CNS, we analyzed ultrastructure of myelinated axons by TEM. The number of myelinated axons was reduced at 9 dpf in kif1bst43 mutants in both the anterior hindbrain (wildtype average of 57 (s.d.+/−4), n=4, compared to kif1bst43 mutant average of 42 (s.d.+/− 7), n=4) and ventral spinal cord (wildtype average of 59 (s.d.+/− 3), n=4, compared to kif1bst43 mutant average of 50 (s.d.+/−6), n=4). We also noted a small but significant reduction in the amount of myelin surrounding those axons in both the hindbrain and ventral spinal cord (Fig. 5 a–d). Axonal cross sectional area was almost identical between wildtype and kif1bst43 mutants in the hindbrain and spinal cord (Fig. 5 c,d, and data not shown), suggesting that the reduction in myelin did not result from a simple delay in axonal development.
Our ultrastructural analyses also revealed striking ectopic myelin-like membranes in kif1bst43 mutants (Fig. 6a–f) at the same time that we observed robust ectopic expression of myelin proteins in kif1bst43 mutant oligodendrocyte cell bodies. Myelin-like membrane was present in processes that did not ensheath axons, but extended over distances of several microns (Fig. 6b,d,e,f). In almost all of these cases the elongate myelin-like membranes were continuous with “normal” myelin surrounding axons (Fig.6d,f). In addition, we observed neuronal cell bodies surrounded by myelin-like membrane (Fig. 6e). These aberrant membranes were observed in every kif1bst43 mutant examined at 9 dpf (n=8) and were never observed in the wildtype. These observations suggest an unexpected function for kif1b in preventing the ectopic production of myelin-like membrane along primary oligodendrocyte processes (Fig. 6g–h). Thus, in addition to localizing specific mRNAs in order to maintain myelin production, mRNA may be localized to myelinating processes to exclude certain proteins from other regions of the oligodendrocyte, where they may exert deleterious effects. It is interesting in this regard that both MBP and 36K are small, highly basic proteins, as is MOBP, another protein whose mRNA is localized to myelinating processes in mammalian oligodendrocytes23. MBP is sufficient to compact model cell membranes in vitro24, emphasizing the potential need to restrict its localization to the proper region of the cell. Future analyses will identify additional Kif1b cargo(es) and define the minimal set of factors required to generate myelin-like membrane.
In conclusion, our study shows that kif1b is required to localize myelin mRNA to oligodendrocyte processes, to elaborate the correct amount of myelin around axons, and to prevent the ectopic production of myelin-like membrane. We also show that kif1b is required for the normal development of certain axons in the PNS and CNS. How these functions might relate to increasing susceptibility to MS remains unclear. Given increasing evidence that some of the most debilitating symptoms of MS may derive from damage to axons25 it is possible, for example, that kif1b related disruption of axons or neurons could contribute to the disease. In addition, our analysis supports the possibility that a function for kif1b in oligodendrocytes might underlie a role in MS. Disruption of kif1b might also reduce the capacity of oligodendrocytes to remyelinate demyelinated axons characteristic of MS, and thus exacerbate symptoms of the disease. It is also possible that Kif1b may play a causal role in onset of the disease. MBP, for example, is a prominent autoantigen for human T-cells26, and certain MBP epitopes can activate specific autoimmune defects in MS patients27,28. Therefore mislocalization of MBP or other factors following disruption of kif1b may play a role in MS by increasing autoimmune attacks characteristic of the disease directly, or indirectly, by damaging the oligodendrocyte. Future work on the role of kif1b in MS susceptibility should consider its functions in both oligodendrocytes and neurons.
Methods
Positional Cloning of kif1bst43
We previously mapped st43 to linkage group 2310. To pursue the positional cloning of the mutated gene, we scored polymorphic markers in the region of st43 in large mutant mapping crosses. This analysis identified a marker approximately 0.1 cM (1 recombinant among 1092 meioses) from st43. Partial sequencing of candidate genes in the vicinity of this marker indicated that the st43 mutation converts a conserved threonine to a proline in zebrafish kif1b. The st43 mutation was scored by PCR as described (Supplementary Methods and Supplementary Table 1). Full-length kif1bβ was cloned from larval cDNA using Phusion (Finnzyme), (Supplementary Methods and Supplementary Table 1).
Immunohistochemistry
The following antibodies were used: mouse anti-acetylated-tubulin (1:1000, Sigma), rabbit anti-MBP21, mouse anti-SV2 (1:200, DSHB), rabbit anti-36K22, mouse and rabbit anti-GFP (Molecular probes), anti-Oregon green (Molecular probes), AlexaFluor-conjugated secondary antibodies (1:2000, Molecular Probes).
Synaptic vesicle protein labeling
25 pg of plasmid DNA encoding synaptophysin:GFP was injected into embryos at the one cell stage.
Kif1b morpholinos
Antisense MOs (Gene Tools) were designed to target a region surrounding the start codon of kif1b, the kif1bα specific splice junction and a kif1bβ specific splice junction (Supplementary Methods). The efficacy of specific splice junction MOs was assayed by RT-PCR (Supplementary Methods and Supplementray Table 1).
In situ hybridization
PCR products specific to the common domain of kif1b, to kif1bα, and to kif1bβ were cloned into pCRII-TOPO (Invitrogen) and sequenced. Riboprobes were synthesized using SP6 RNA polymerase for antisense and T7 for sense.
Transplants
Transplants were carried out as described 15 with modifications outlined in Supplementary Methods.
Transmission electron microscopy was carried out as described15. We used the g-ratio to assess the extent of myelination of axons (see Supplementary Methods).
Supplementary Material
Acknowledgements
We thank Isaac Middendorf, Tuky Reyes, and Chenelle Hill, for technical support and fish care. We thank Hitoshi Okamoto, Bruce Appel, Gunnar Jeserich, and Martin Meyer sharing reagents. We thank Ben Barres, Peter Brophy, and members of our lab for comments on the manuscript. This work was supported by grants from the National Institutes of Health (NS050223, W.S.T.) and the National Multiple Sclerosis Society (RG 3943-A-2, W.S.T.), a postdoctoral fellowship from the Muscular Dystrophy Association (MDA4061, D.A.L.) and a David Phillips Fellowship from the Biotechnology and Biological Sciences Research Council, U.K. (D.A.L.).
Footnotes
The Genbank Accession number for zebrafish kif1bβ is FJ756939.
Author Information
The authors declare no competing financial interests.
References
- 1.Nangaku M, et al. KIF1B, a novel microtubule plus end-directed monomeric motor protein for transport of mitochondria. Cell. 1994;79:1209–1220. doi: 10.1016/0092-8674(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 2.Zhao C, et al. Charcot-Marie-Tooth disease type 2A caused by mutation in a microtubule motor KIF1Bbeta. Cell. 2001;105:587–597. doi: 10.1016/s0092-8674(01)00363-4. [DOI] [PubMed] [Google Scholar]
- 3.Aulchenko YS, et al. Genetic variation in the KIF1B locus influences susceptibility to multiple sclerosis. Nat Genet. 2008;40:1402–1403. doi: 10.1038/ng.251. [DOI] [PubMed] [Google Scholar]
- 4.Sherman DL, Brophy PJ. Mechanisms of axon ensheathment and myelin growth. Nat Rev Neurosci. 2005;6:683–690. doi: 10.1038/nrn1743. [DOI] [PubMed] [Google Scholar]
- 5.Brosamle C, Halpern ME. Characterization of myelination in the developing zebrafish. Glia. 2002;39:47–57. doi: 10.1002/glia.10088. [DOI] [PubMed] [Google Scholar]
- 6.Colman DR, Kreibich G, Frey AB, Sabatini DD. Synthesis and incorporation of myelin polypeptides into CNS myelin. J Cell Biol. 1982;95:598–608. doi: 10.1083/jcb.95.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carson JH, Worboys K, Ainger K, Barbarese E. Translocation of myelin basic protein mRNA in oligodendrocytes requires microtubules and kinesin. Cell Motil Cytoskeleton. 1997;38:318–328. doi: 10.1002/(SICI)1097-0169(1997)38:4<318::AID-CM2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 8.Song J, Carson JH, Barbarese E, Li FY, Duncan ID. RNA transport in oligodendrocytes from the taiep mutant rat. Mol Cell Neurosci. 2003;24:926–938. doi: 10.1016/s1044-7431(03)00254-9. [DOI] [PubMed] [Google Scholar]
- 9.Ainger K, et al. Transport and localization of exogenous myelin basic protein mRNA microinjected into oligodendrocytes. J Cell Biol. 1993;123:431–441. doi: 10.1083/jcb.123.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pogoda HM, et al. A genetic screen identifies genes essential for development of myelinated axons in zebrafish. Dev Biol. 2006;298:118–131. doi: 10.1016/j.ydbio.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 11.Kikkawa M, et al. Switch-based mechanism of kinesin motors. Nature. 2001;411:439–445. doi: 10.1038/35078000. [DOI] [PubMed] [Google Scholar]
- 12.Marchler-Bauer A, et al. CDD: a conserved domain database for interactive domain family analysis. Nucleic Acids Res. 2007;35:D237–D240. doi: 10.1093/nar/gkl951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirokawa N, Takemura R. Molecular motors and mechanisms of directional transport in neurons. Nat Rev Neurosci. 2005;6:201–214. doi: 10.1038/nrn1624. [DOI] [PubMed] [Google Scholar]
- 14.Sato T, Takahoko M, Okamoto H. HuC:Kaede, a useful tool to label neural morphologies in networks in vivo. Genesis. 2006;44:136–142. doi: 10.1002/gene.20196. [DOI] [PubMed] [Google Scholar]
- 15.Lyons DA, Naylor SG, Mercurio S, Dominguez C, Talbot WS. KBP is essential for axonal structure, outgrowth and maintenance in zebrafish, providing insight into the cellular basis of Goldberg-Shprintzen syndrome. Development. 2008;135:599–608. doi: 10.1242/dev.012377. [DOI] [PubMed] [Google Scholar]
- 16.Schlisio S, et al. The kinesin KIF1Bbeta acts downstream from EglN3 to induce apoptosis and is a potential 1p36 tumor suppressor. Genes Dev. 2008;22:884–893. doi: 10.1101/gad.1648608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeh IT, et al. A germline mutation of the KIF1B beta gene on 1p36 in a family with neural and nonneural tumors. Hum Genet. 2008;124:279–285. doi: 10.1007/s00439-008-0553-1. [DOI] [PubMed] [Google Scholar]
- 18.Munirajan AK, et al. KIF1Bbeta functions as a haploinsufficient tumor suppressor gene mapped to chromosome 1p36.2 by inducing apoptotic cell death. J Biol Chem. 2008;283:24426–24434. doi: 10.1074/jbc.M802316200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cahoy JD, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shin J, Park HC, Topczewska JM, Mawdsley DJ, Appel B. Neural cell fate analysis in zebrafish using olig2 BAC transgenics. Methods Cell Sci. 2003;25:7–14. doi: 10.1023/B:MICS.0000006847.09037.3a. [DOI] [PubMed] [Google Scholar]
- 21.Lyons DA, et al. erbb3 and erbb2 are essential for schwann cell migration and myelination in zebrafish. Curr Biol. 2005;15:513–524. doi: 10.1016/j.cub.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 22.Morris JK, et al. The 36K protein of zebrafish CNS myelin is a short-chain dehydrogenase. Glia. 2004;45:378–391. doi: 10.1002/glia.10338. [DOI] [PubMed] [Google Scholar]
- 23.Gould RM, Freund CM, Barbarese E. Myelin-associated oligodendrocytic basic protein mRNAs reside at different subcellular locations. J Neurochem. 1999;73:1913–1924. [PubMed] [Google Scholar]
- 24.Rispoli P, et al. A thermodynamic and structural study of myelin basic protein in lipid membrane models. Biophys J. 2007;93:1999–2010. doi: 10.1529/biophysj.106.103820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hauser SL, Oksenberg JR. The neurobiology of multiple sclerosis: genes, inflammation, and neurodegeneration. Neuron. 2006;52:61–76. doi: 10.1016/j.neuron.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 26.Meinl E, Hohlfeld R. Immunopathogenesis of multiple sclerosis: MBP and beyond. Clin Exp Immunol. 2002;128:395–397. doi: 10.1046/j.1365-2249.2002.01879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hedegaard CJ, et al. T helper cell type 1 (Th1), Th2 and Th17 responses to myelin basic protein and disease activity in multiple sclerosis. Immunology. 2008;125:161–169. doi: 10.1111/j.1365-2567.2008.02837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lambracht-Washington D, et al. Antigen specificity of clonally expanded and receptor edited cerebrospinal fluid B cells from patients with relapsing remitting MS. J Neuroimmunol. 2007;186:164–176. doi: 10.1016/j.jneuroim.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.