SUMMARY
Studies have shown that familial risk contributes to etiology of lymphomas. Using large population registries from Sweden, we evaluated risk of lymphoma subtypes among first-degree relatives of 2668 follicular lymphoma (FL) patients, 2517 diffuse large B-cell lymphoma (DLBCL) patients, and 6963 Hodgkin lymphoma (HL) patients compared to first-degree relatives of controls. Relatives were at the highest risk for developing the same lymphoma subtype as the case. DLBCL was 10-fold increased among relatives of DLBCL patients, FL was 4-fold increased among relatives of FL patients and HL was 4-fold increased among relatives of HL patients. These results imply that germline susceptibility genes are specific to lymphoma subtype.
Keywords: non-Hodgkin lymphoma, DLBCL, FL, Hodgkin lymphoma, familial risk
INTRODUCTION
The risk of lymphoid neoplasms among blood relatives of patients with lymphoid neoplasms cases is known to be elevated above that in the general population (Alexander, et al 2007). Case-control studies of non-Hodgkin lymphomas (NHL) patients have reported relative risks of 1.5-3.0 for family history of hematologic malignancy depending on how broadly family history was defined. (Alexander, et al 2007) Some case-control studies have assessed patterns of familial aggregation by specific NHL subtypes but sample sizes have typically been insufficient for reliable risk estimates.
Using large population-based databases from Sweden and Denmark, we previously demonstrated that first-degree relatives of a patient with Hodgkin lymphoma (HL), chronic lymphocytic leukemia (CLL), or lymphoplasmacytic lymphoma (LPL)/Waldenstrom macroglobulinemia (WM) are at particularly high risk for developing the same malignancy as the index case compared to first-degree relatives of matched controls. Similarly, relatives of HL patients were at 3-fold elevated risk for developing HL and higher when index case had a young adult onset, and were also at increased risk for any NHL (Goldin, et al 2004a), relatives of CLL patients were at 7.5-fold increased risk of CLL (Goldin, et al 2004b), and relatives of all NHL cases had a 1.8 increased risk for any NHL (Goldin, et al 2005a). Relatives of multiple myeloma (MM) patients were at 1.7-fold increased risk for MM but they were not at increased risk for other lymphomas (Landgren, et al 2006). In a recent study of familial aggregation patterns for LPL/WM patients, we found relatives of LPL/WM patients to be at 20-fold increased risk for developing LPL/WM and were also at increased risk for CLL and other NHL subtypes (Kristinsson, et al 2008).
Classification of lymphomas using current WHO standards is based on morphology, immunophenotype, genetic features, and clinical characteristics (Jaffe, et al 2008). These subtypes are associated with different incidence rate patterns and etiologic risk factors (Morton, et al 2007). In order to obtain a more complete picture of familial patterns of the more common NHL subtypes as defined by WHO classification, and to better define the familial overlap between NHL and HL, we conducted a new familial aggregation study of NHL and HL using Swedish registries. Our current study quantifies the risk of specific lymphomas among relatives of patients with DLBCL, FL and HL compared to relatives of matched controls.
MATERIAL AND METHODS
Our study design has been described in more detail elsewhere (Kristinsson, et al 2008). For this study, we identified all cases of NHL not including CLL (ICD7 codes 200 and 202) and HL diagnosed 1958-2004 who had linkable relatives and selected 4 matched controls per case. Our case population partially overlaps that in our previous studies but the base sample sizes are substantially increased. (Goldin, et al 2005a, Goldin, et al 2004a) First-degree relatives of cases and controls were obtained from links to the multigenerational registry. All individuals were linked to the Swedish cancer registry as well as our previously created registry of WM/LPL cases to obtain all cancer outcomes 1958-2004. From NHL cases diagnosed 1993 and later, we used available ICD10 and SNOMED codes to classify NHL according to WHO definitions. The Swedish registry codes are based on the Kiel and Rappaport classifications and it is not possible to define all NHL subtypes defined by current WHO. However, WHO provides synonymous definitions across classifications and we used those translations whenever possible (Jaffe and World Health Organization. 2001). As shown in Table 1, we evaluated 8974 relatives of 2517 DLBCL cases, 10188 relatives of 2668 FL cases, and 24053 first-degree relatives of 6963 HL compared to relatives of matched controls. Among relatives, in addition to testing DLBCL and FL as outcomes, we grouped together all B-cell NHL, all T-cell NHL, all indolent B-cell NHL (including CLL, FL, hairy cell leukemia, LPL/WM and mantle cell lymphoma), and HL.
| Variable | DLBCL patients |
Controls | FL patients | Controls | HL patients | Controls |
|---|---|---|---|---|---|---|
| Total number, n (%) | 2517 (100) | 9932 (100) | 2668 (100) | 10468 (100) | 6963 (100) | 28371 (100) |
| Males, n (%) | 1374 (55) | 5421 (55) | 1306 (49) | 5097 (49) | 4122 (59) | 16862 (59) |
| Females, n (%) | 1143 (45) | 4511 (45) | 1362 (51) | 5371 (51) | 2841 (41) | 11509 (41) |
| Age at diagnosis (yrs), mean (s.d.) | 65.5 (16.2) | 63.5 (13.4) | 43.5 (20.8) | |||
| Number of patients by age of dx (%) | ||||||
| <=40 | 184 (7) | 114 (4) | 3361 (48) | |||
| 41-50 | 185 (7) | 265 910) | 794 (11) | |||
| 51-60 | 402 (16) | 601 (22) | 859 (12) | |||
| 61-70 | 503 (20) | 676 (25) | 950 (14) | |||
| > 70 | 1243 (49) | 1012 (938) | 996 (14) | |||
| Numbers of first-degree relatives (%) | ||||||
| Total | 8974 (100) | 35310 (100) | 10188 (100) | 40384 (100) | 24053 (100) | 108180 (100) |
| Parents | 1978 (22) | 7901 (22) | 2442 (24) | 9846 (24) | 7058 (28) | 30354 (29) |
| Siblings | 1718 (19) | 6301 (18) | 2036 (20) | 8210 (20) | 5384 (23) | 24495 (22) |
| Offspring | 5278 (59) | 21108 (60) | 5710 (56) | 22328 (55) | 11611 (49) | 53331 (48) |
The analytic method has been described previously(Goldin, et al 2005a). The age or age at onset of disease in a relative of a proband is modeled by a marginal proportional hazards model. Familial aggregation for each condition is evaluated by testing the hazard ratio (denoted as relative risk) of being a relative of a case compared with being a relative of a control. The model was fitted using the PHREG procedure in SAS v9.1. The robust sandwich covariance matrix accounts for familial dependencies in our sample.
RESULTS
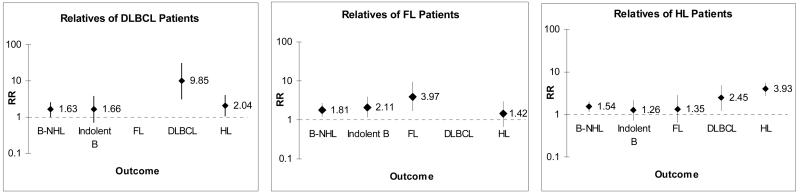
Table 1 describes the patient and control samples and the numbers and types of relatives evaluated. There were more male DLBCL patients but FL patients showed a slight female predominance. As expected from the age distribution of the cases, the majority of the relatives were offspring. Figure 1 shows the RRs for specific lymphomas among first-degree relatives by case group compared to their matched controls. The RRs for T-cell NHL were not increased among relatives of any case group (not shown). The relatives of DLBCL cases were at ∼10-fold increased RR for developing DLBCL (RR=9.8, 95%CI:3.1-31) but were not at increased risk for developing FL or other indolent subtypes. In fact there were no cases of FL among relatives of DLBCL cases compared to 9 cases among relatives of matched controls. We found increased occurrence of DLBCL in parents, siblings, and offspring although numbers were small (not shown). Relatives of DLBCL were at significantly increased risk for developing HL (RR=2.0, 95%CI:1.05-4.0).
Figure 1.
Relative risks (95% CI) for specific lymphoma subtypes among first degree relatives of DLBCL, FL and HL patients. Dotted line is shown at RR=1.
Similarly, the relatives of FL cases were at high risk for FL (RR=4.0, 95%CI:1.6-9.5) but not at increased risk for DLBCL. Relatives were at increased risk for developing other indolent subtypes (RR=2.1, 95%CI:1.1-3.9) including CLL in particular (RR=1.8, 95%CI:1.0-3.3). Parents and offspring of FL cases had increased risk for FL although siblings did not (not shown). Relatives of HL cases were at very high risk for developing HL, at increased risk for developing DLBCL, but not at increased risk for other indolent lymphomas.
DISCUSSION
Some case-control studies of familial risk of NHL have tested the effect of family history by subtype of lymphoma. However, family history is usually classified broadly due to sample size restrictions and lack of subtype information among relatives. We have overcome these limitations by using large population-based registries. While most studies quote modest familial risks for NHL (1.5- to 3-fold), we have shown that the risk increases for specific subtypes. Our most prominent finding was that relatives of DLBCL patients were at 10-fold increased risk of developing DLBCL (compared to relatives of controls); however, they were not at increased risk for developing indolent lymphomas (Figure 1. The observed high risks for specific lymphoma subtypes are also consistent with our previously published results for CLL, LPL/WM, and HL.
Our study has some limitations. The ability to classify Kiel and Rappaport categories into WHO is limited and cases have not undergone pathology review. In addition, as molecular and other studies advance, new subtypes have been characterized. For example DLBCL may include 3 subtypes according to gene expression and outcome (Jaffe, et al 2008) and CLL is divided into indolent and aggressive types based on mutation status of IGHV and other factors. In terms of the association of HL and DLBCL, we cannot completely rule out the possibility of misclassification. However, morphological and phenotypic overlap between primary mediastinal large B-cell lymphoma and classical HL has been described. Expression studies found similarity of expression of many genes among these two lymphomas (Rosenwald, et al 2003). The 2008 WHO classification recognizes a provisional category, “B-cell lymphoma unclassifiable, with features intermediate between DLBCL and classical HL”(Jaffe, et al 2008).
One other cohort study also found specific high concordance for DLBCL, FL, and CLL among relatives(Altieri, et al 2005). However, the authors in that study concluded that DLBCL was likely to be autosomal dominant and FL likely to be autosomal recessive. Our results do not support specific Mendelian modes of inheritance. We found similar risks among parents, offspring, and siblings for DLBCL. We did not find an increased risk to siblings of FL cases but numbers were small. Linkage mapping studies in high risk families have not detected mendelian genes with large effects for CLL (Sellick, et al 2007), HL (Goldin, et al 2005b), or WM (McMaster, et al 2006) making it more likely that increased familial risk is due to multiple genes, probably in combination with other factors. A recent whole genome association study of CLL patients compared to controls that found several genes significantly associated with CLL (Di Bernardo, et al 2008).
In summary, our results provide independent support for the WHO classification and support the search for germline genes associated with specific lymphomas. Importantly, clinicians need to keep in mind that the lymphomas are rare in the general population. Although a first-degree blood relative of a lymphoma patient has an increased relative risk for the same lymphoma subtype, the absolute risk is still low.
ACKNOWLEDGEMENTS
This research was supported by the Intramural Research Program of the NIH, NCI and by grants from the Swedish Cancer Society, Stockholm County Council, and the Karolinska Institutet Foundations The authors thank Ms. Shiva Ayobi, The National Board of Health and Welfare, Stockholm, Sweden; Ms. Susanne Dahllöf, Statistics Sweden, and Ms. Emily Steplowski, Information Management Services, Silver Spring, MD, for important efforts in the development of this database.
References
- Alexander DD, Mink PJ, Adami HO, Chang ET, Cole P, Mandel JS, Trichopoulos D. The non-Hodgkin lymphomas: a review of the epidemiologic literature. Int J Cancer. 2007;120(Suppl 12):1–39. doi: 10.1002/ijc.22719. [DOI] [PubMed] [Google Scholar]
- Altieri A, Bermejo JL, Hemminki K. Familial risk for non-Hodgkin lymphoma and other lymphoproliferative malignancies by histopathologic subtype: the Swedish Family-Cancer Database. Blood. 2005;106:668–672. doi: 10.1182/blood-2005-01-0140. [DOI] [PubMed] [Google Scholar]
- Di Bernardo MC, Crowther-Swanepoel D, Broderick P, Webb E, Sellick G, Wild R, Sullivan K, Vijayakrishnan J, Wang Y, Pittman AM, Sunter NJ, Hall AG, Dyer MJ, Matutes E, Dearden C, Mainou-Fowler T, Jackson GH, Summerfield G, Harris RJ, Pettitt AR, Hillmen P, Allsup DJ, Bailey JR, Pratt G, Pepper C, Fegan C, Allan JM, Catovsky D, Houlston RS. A genome-wide association study identifies six susceptibility loci for chronic lymphocytic leukemia. Nat Genet. 2008;40:1204–1210. doi: 10.1038/ng.219. [DOI] [PubMed] [Google Scholar]
- Goldin LR, Landgren O, McMaster ML, Gridley G, Hemminki K, Li X, Mellemkjaer L, Olsen JH, Linet MS. Familial aggregation and heterogeneity of non-Hodgkin lymphoma in population-based samples. Cancer Epidemiol Biomarkers Prev. 2005a;14:2402–2406. doi: 10.1158/1055-9965.EPI-05-0346. [DOI] [PubMed] [Google Scholar]
- Goldin LR, McMaster ML, Ter-Minassian M, Saddlemire S, Harmsen B, Lalonde G, Tucker MA. A genome screen of families at high risk for Hodgkin lymphoma: evidence for a susceptibility gene on chromosome 4. J Med Genet. 2005b;42:595–601. doi: 10.1136/jmg.2004.027433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin LR, Pfeiffer RM, Gridley G, Gail MH, Li X, Mellemkjaer L, Olsen JH, Hemminki K, Linet MS. Familial aggregation of Hodgkin lymphoma and related tumors. Cancer. 2004a;100:1902–1908. doi: 10.1002/cncr.20189. [DOI] [PubMed] [Google Scholar]
- Goldin LR, Pfeiffer RM, Li X, Hemminki K. Familial risk of lymphoproliferative tumors in families of patients with chronic lymphocytic leukemia: results from the Swedish Family-Cancer Database. Blood. 2004b;104:1850–1854. doi: 10.1182/blood-2004-01-0341. [DOI] [PubMed] [Google Scholar]
- Jaffe ES, Harris NL, Stein H, Isaacson PG. Classification of lymphoid neoplasms: the microscope as a tool for disease discovery. Blood. 2008;112:4384–4399. doi: 10.1182/blood-2008-07-077982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe ES, World Health Organization . Pathology and genetics of tumours of haematopoietic and lymphoid tissues. IARC Press; Oxford University Press (distributor); Lyon Oxford: 2001. [Google Scholar]
- Kristinsson SY, Bjorkholm M, Goldin LR, McMaster ML, Turesson I, Landgren O. Risk of lymphoproliferative disorders among first-degree relatives of lymphoplasmacytic lymphoma/Waldenstrom macroglobulinemia patients: a population-based study in Sweden. Blood. 2008;112:3052–3056. doi: 10.1182/blood-2008-06-162768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgren O, Linet MS, McMaster ML, Gridley G, Hemminki K, Goldin LR. Familial characteristics of autoimmune and hematologic disorders in 8,406 multiple myeloma patients: a population-based case-control study. Int J Cancer. 2006;118:3095–3098. doi: 10.1002/ijc.21745. [DOI] [PubMed] [Google Scholar]
- McMaster ML, Goldin LR, Bai Y, Ter-Minassian M, Boehringer S, Giambarresi TR, Vasquez LG, Tucker MA. Genomewide linkage screen for Waldenstrom macroglobulinemia susceptibility loci in high-risk families. Am J Hum Genet. 2006;79:695–701. doi: 10.1086/507687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton LM, Turner JJ, Cerhan JR, Linet MS, Treseler PA, Clarke CA, Jack A, Cozen W, Maynadie M, Spinelli JJ, Costantini AS, Rudiger T, Scarpa A, Zheng T, Weisenburger DD. Proposed classification of lymphoid neoplasms for epidemiologic research from the Pathology Working Group of the International Lymphoma Epidemiology Consortium (InterLymph) Blood. 2007;110:695–708. doi: 10.1182/blood-2006-11-051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwald A, Wright G, Leroy K, Yu X, Gaulard P, Gascoyne RD, Chan WC, Zhao T, Haioun C, Greiner TC, Weisenburger DD, Lynch JC, Vose J, Armitage JO, Smeland EB, Kvaloy S, Holte H, Delabie J, Campo E, Montserrat E, Lopez-Guillermo A, Ott G, Muller-Hermelink HK, Connors JM, Braziel R, Grogan TM, Fisher RI, Miller TP, LeBlanc M, Chiorazzi M, Zhao H, Yang L, Powell J, Wilson WH, Jaffe ES, Simon R, Klausner RD, Staudt LM. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med. 2003;198:851–862. doi: 10.1084/jem.20031074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellick GS, Goldin LR, Wild RW, Slager SL, Ressenti L, Strom SS, Dyer MJ, Mauro FR, Marti GE, Fuller S, Lyttelton M, Kipps TJ, Keating MJ, Call TG, Catovsky D, Caporaso N, Houlston RS. A high-density SNP genome-wide linkage search of 206 families identifies susceptibility loci for chronic lymphocytic leukemia. Blood. 2007;110:3326–3333. doi: 10.1182/blood-2007-05-091561. [DOI] [PMC free article] [PubMed] [Google Scholar]