Abstract
Histone modification, which affects the rate of transcription without altering DNA sequence, occurs in response to various psychiatric drugs and in several models of psychiatric disease. As increases in histone acetylation have been seen after treatment with antidepressants, we investigated whether directly increasing histone acetylation using a histone deacetylase inhibitor would have antidepressant effects. We administered sodium butyrate (NaB, 100 mg/kg, i.p.) to mice acutely (3 injections over 24 hours) or chronically (twice daily for 21 days) and subjected them to a number of behavioral tests of antidepressant response. This dose of NaB had no effect on overall locomotor activity after either acute or chronic treatment. Acutely treated mice showed an increase in immobility in the forced-swim test (FST) and an increase in latency to consume in the novel environment of the novelty-induced hypophagia (NIH) paradigm, an anxiogenic effect. The effect of NaB on anxiety did not generalize to another test, the elevated zero maze, where it had no effect. Chronic treatment with NaB had no effect on latency to consume in the NIH or immobility in the FST. However, this dose did alter histone acetylation in the hippocampus. While H4 acetylation increased in the hippocampus 30 min following acute NaB, chronic treatment caused a decrease in AcH4. There were no changes in AcH3 following either treatment. While changes in chromatin structure may be involved in the mechanism of action of antidepressant drugs, these data suggest that increasing histone acetylation pharmacologically is not sufficient to produce antidepressant effects.
1. Introduction
Despite its status as the most common psychiatric disorder, depression is poorly understood, both in terms of its pathophysiology and the mechanisms by which antidepressants ameliorate symptoms. The observation of decreased hippocampal volume in depressed humans (Bremner et al., 2000; Sheline et al., 1999), combined with studies showing molecular and cellular changes in the hippocampi of rodents after chronic stress, and their reversal by antidepressants, have focused depression research on this brain region. In particular, antidepressants have been shown to block stress-induced decreases in hippocampal plasticity (Popoli et al., 2002), including decreases in hippocampal neurogenesis (see Dranovsky and Hen, 2006, for a review). Antidepressants have also been shown to upregulate neurotrophic factors such as BDNF, which may contribute to increased plasticity (Castren et al., 2007; Duman and Monteggia, 2006). The mechanism by which antidepressants cause these changes in gene expression and plasticity are still under investigation.
Changes in chromatin structure due to post-translational modifications of histones have been associated with a number of behavioral events, including drug addiction (Kumar et al., 2005; Levine et al., 2005; Pandey et al., 2008; Renthal et al., 2007; Schroeder et al., 2008), memory formation (Alarcon et al., 2004; Lattal et al., 2007; Levenson et al., 2004), seizures (Huang et al., 2002; Tsankova et al., 2004), and stress (Bilang-Bleuel et al., 2005; Chandramohan et al., 2007; Renthal et al., 2007; Tsankova et al., 2006). While a number of post-translational modifications to each of the four core histone proteins (H2A, H2B, H3 and H4) are possible, histone acetylation, which is associated with increased rates of transcription, has received the most attention. Because of the possible role of histone acetylation in a variety of behaviors and disease states, some studies of the effects of histone deacetylase (HDAC) inhibitors in animals have been reported. The HDAC inhibitors trichostatin-A (TSA) and sodium butyrate (NaB) have been shown to increase contextual fear conditioning and extinction (Lattal et al., 2007; Levenson et al., 2004), and both NaB and SAHA, another HDAC inhibitor, have been shown to reduce symptoms in a mouse model of Huntington’s disease (Ferrante et al., 2003; Hockly et al., 2003). NaB was shown to augment the increase in histone acetylation caused by exposure to cocaine (Kumar et al., 2005), as well as to decrease the anxiety-like symptoms associated with alcohol withdrawal (Pandey et al., 2008).
Recently, chromatin modification has also been implicated as an important regulator of the expression of depression-related genes, including BDNF. Electroconvulsive shock, a model of electroconvulsive therapy, the most potent treatment for depression, was shown to increase histone H3 acetylation at the BDNF promoter, which correlated with upregulation of BDNF mRNA (Tsankova et al., 2004). In another study, stress was shown to increase histone H3 di-methylation, a modification associated with repressed transcription, at BDNF promoters (Tsankova et al., 2006). In this same study, antidepressants opposed the effects of stress by increasing H3 acetylation at BDNF promoters, and this effect was associated with a decrease in expression of histone deacetylase 5 (HDAC5), an enzyme that acts to remove acetyl groups from histones. This downregulation in HDAC5 was shown to be necessary for the behavioral effects of chronic antidepressant treatment, but it remains to be shown whether these changes in chromatin are sufficient to produce antidepressant behavioral effects.
To test whether increasing histone acetylation in the hippocampus is sufficient to cause behavioral effects, we studied both acute and chronic treatment with NaB in behavioral models of anxiety and antidepressant response. In the present study, NaB failed to induce antidepressant-like behavioral responses in the forced-swim test (FST) or the novelty-induced hypophagia (NIH) paradigm despite observed alterations in histone acetylation in the hippocampus. Our results demonstrate that increasing histone acetylation in the hippocampus alone is not sufficient to drive antidepressant behavioral changes, suggesting that additional mechanisms must be involved.
2. Materials and Methods
2.1 Animals
All mice used for behavioral and biochemical experiments were F1 hybrid offspring obtained from crosses of 129SvEv and C57Bl/6 mice, a strain which has been shown to respond to antidepressant and anxiolytic compounds in a number of behavioral paradigms in our laboratory (Conti et al., 2002; Gur et al., 2007). Mice (20–40 g, 2–6 months of age, mixed sexes) were group-housed with food and water available ad libitum and maintained on a 12-hour light/dark cycle (lights on at 07:00) in accordance with the University of Pennsylvania Animal Care and Use Committee. All behavioral testing sessions were performed between the hours of 08:00 and 15:00h, and animals were randomly assigned to treatment conditions and tested in counterbalanced order.
2.2 Drugs
All drugs were dissolved in 0.9% saline immediately before use and injected intraperitoneally using a volume of 10 mL/kg. For acute studies, sodium butyrate (NaB) (Sigma, St. Louis, MO) or 0.9% saline was injected on the morning and afternoon of the day preceding testing, as well as on the morning of testing, for a total of three doses before exposure to the behavioral test. For chronic studies, NaB or 0.9% saline was injected twice daily (09:00 and 17:00h) for 21 days before exposure to the behavioral test. As a positive control in the NIH, a third group received desipramine (DMI) (Sigma) (12.5 mg/kg) for chronic studies or chlordiazepoxide (CDP) (10 mg/kg) (Sigma) in the acute study. In the FST, DMI was also used as a positive control with the three injections preceding the test at doses of 10 mg/kg, 10 mg/kg, and 20 mg/kg as described previously (Conti et al., 2002).
2.2 Behavioral studies
For all behavioral studies, mice were given their last injection and brought into the testing room one hour preceding the start of testing.
2.21 Forced swim test
Mice were placed in 15cm of water (22–24°C) in plastic cylinders (23cm tall × 14cm diameter) for 6 min. Mice were video-recorded and time spent immobile vs. swimming and climbing was scored by the Viewpoint Tracking System (Viewpoint, Champagne au Mont d’Or, France).
2.22 Novelty-induced hypophagia
Mice were housed in groups of two for one week before the start of the training period and for the duration of the experiment. During training and home cage testing, mice had daily exposure to a highly palatable food (peanut butter chips) (Nestle, Glendale, CA) in a clear plastic dish in their home cage. Plastic dividers were placed inside the home cage to separate the mice, beginning one hour before training and testing periods. Food was placed in the cage for 15 min and latency to consume the chips was measured. By the 12th day of training, baseline latencies had been established with less than 20% variability amongst mice. For acute experiments, mice received 12 days of training, followed by testing in home cage (Home1), novel environment, and home cage (Home2) on the 3 days following training. For chronic experiments, mice received 12 days of training, followed by 21 days of injections, and then testing in home, novel, home on days 22–24.
Testing in the novel environment consisted of placing mice in an empty standard cage, lacking bedding, which was placed in a white box with bright illumination (2150 lux) and with an added scent (Pine Sol) applied to the cage. Latency to consume in the novel environment was recorded with a 15-minute maximum exposure. Novel testing in both acute and chronic experiments occurred 1 hour after separators were placed in the home cage and 1 hour after mice received their last injection, parallel to the timing of home cage testing.
2.23 Elevated zero maze
The zero maze (Stoelting, Wood Dale, IL) consisted of two open areas (wall height, 0.5”) and two closed areas (wall height, 12”), and was elevated 24” from the ground. Lighting in the maze was 15 lux. At the start of testing, mice were placed into one of the closed areas and allowed to explore the maze for 300s. The Viewpoint Tracking System (Viewpoint) was used to video-record and track the amount of time spent in the open areas, the number of entries into the open areas, and the distance traveled in each area.
2.24 Locomotor activity
Locomotor activity was measured by beam-breaks in a photobeam frame (Med Associates, St. Albans, VT, USA). During the test, mice were placed individually into a clean home cage resting within the photobeam frame, and data were recorded by Med Associates Software. Ambulations, crossings, and rearings were measured in 5-minute bins for 30 (acute study) or 60 minutes (chronic study).
2.3 Histone acetylation western blots
Mice were sacrificed by cervical dislocation 30 minutes following the last injection (both acute and chronic studies). Brains were removed; whole hippocampus was hand-dissected and flash-frozen in liquid nitrogen. Tissues were homogenized in 200 ml of ice-cold extraction buffer containing 250 mM sucrose, 50 mM Tris, pH 7.5, 25 mM KCl, 0.5 mM PMSF, 0.9 mM NaB, as well as protease inhibitors (“complete” protease inhibitor cocktail, Roche, Basel, Switzerland) and phosphatase inhibitors (phosphatase inhibitor cocktail 1, Sigma). The nuclear fraction (pellet) was separated by centrifugation at 7,700xg for 1 min (4°C), and re-suspended in 1 mL 0.4 N H2SO4 and incubated for 30 min (4°C ). Samples were centrifuged at 14,000xg for 30 min (4°C). 250 μL trichloroacetic acid (with 4 mg/mL deoxycholate) was added to the supernatant, and incubated for 30 min (4°C) to precipitate protein. Samples were then spun at 14,000xg for 30 min (4°C) to pellet protein. Pellets were washed for 5 min with 1 mL acidified acetone (0.1% HCl), then for 5 min with acetone. Between washes, protein was collected by centrifuging 5 min at 14,000xg (4°C), and aspirating supernatant. After the last wash, the pellet was re-suspended in 200 μL 10 mM Tris, pH 8.0, and incubated for 15 minutes at room temperature. Protein concentrations were determined using a Bradford assay, with bovine serum albumin as the standard. Equivalent amounts of protein (10 μg) for each sample were resolved with SDS-PAGE using 4–15% gradient Tris-HCl gels. After electrophoresis, proteins were transferred to nitrocellulose membranes for 2 hours at 50V. Membranes were incubated in blocking buffer (LI-COR, Lincoln, Nebraska, USA) 1 hr at room temperature to block non-specific binding. The blots were reacted with primary antibodies (anti-AcH3, 06–599, Millipore, USA; anti-AcH4, 06–598, Millipore; anti-H3, mAbcam 10799, AbCAM, Cambridge, MA, USA) at a concentration of 1:1000 in blocking buffer (LI-COR) overnight at 4°C. After washing (3×15 min in PBS-Tween20), the blots were incubated in secondary antibody (goat anti-mouse IRDye 680 and goat anti-rabbit IRDye 800, LI-COR) in blocking buffer for 1 hr at RT in dark boxes. Membranes were then washed (3×15 min in PBS-Tween20) and dried overnight (also in the dark). Immunolabeling was detected and quantified at two wavelengths simultaneously using the Odyssey infrared imaging system scanner and software (LI-COR). This system allows for accurate quantification of multiple bands on the same membrane at the same time, using different antibodies raised in different species (which appear at different wavelengths), thus allowing quantification of AcH3 and H3 on the same blot. The AcH3/H3 bands were detected at 17 KDa and the AcH4 band was detected at10 kDa. Ratios of AcH3 or AcH4 to total H3 fluorescence were calculated for each sample and analyzed across conditions.
2.4 Statistics
For the elevated zero maze, Student’s t-test was used to assess statistical significance. For the AcH westerns and FST, ANOVA and Bonferroni post hoc tests were used to assess significant differences between groups. For the NIH and locomotor activity, repeated measures ANOVA was performed, with either time bin (locomotor activity) or day (home1, novel, home2 for the NIH) as the repeated-measures factor. Bonferroni and Newman-Keuls multiple comparison post hoc tests were used to determine significant differences between drug-treated and saline-treated groups at specific time points.
3. Results
3.1 Acute treatment with NaB causes an increase in immobility in the FST, while chronic treatment has no effect
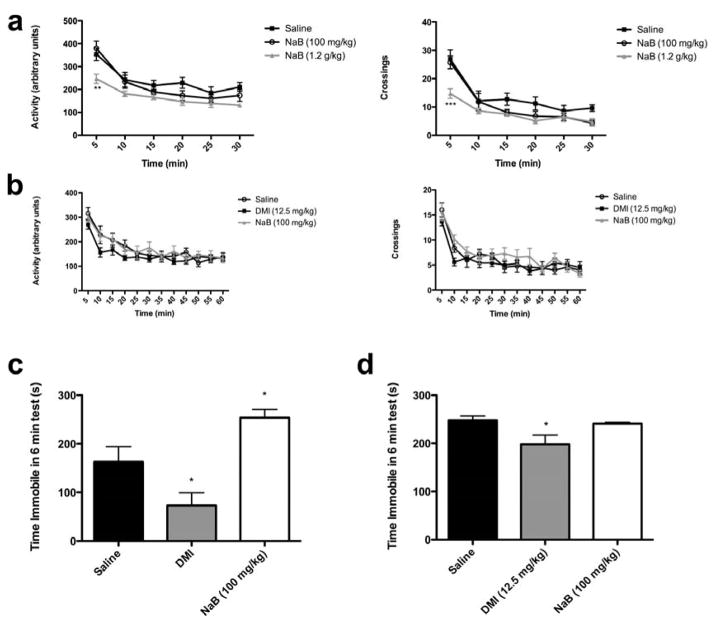
To determine an appropriate dose to evaluate antidepressant effects of NaB, we tested two doses of NaB, 100 mg/kg and 1.2 g/kg (the latter was previously shown to have effects on memory, depression- and reward-related behaviors, see Lattal et al., 2007; Levenson et al., 2004; Schroeder et al., 2006; Schroeder et al., 2008), for their effects on spontaneous locomotor activity (data presented in 5-minute bins). Acute treatment (3 injections) with 1.2 g/kg of sodium butyrate caused a significant decrease in both ambulations and crossings (Ambulations: main effect of treatment, F(2, 105) = 4.690, p = 0.02, RMANOVA; 1.2 g/kg NaB vs. saline, p < 0.01 for first 5 min, Bonferroni post hoc test. Crossings: main effect of treatment, F(2, 105) = 4.144, p = 0.03, RMANOVA; 1.2 g/kg NaB vs. saline, p < 0.001 for first 5 min, Bonferroni post hoc test) (Figure 1a). In contrast, the lower dose of NaB (100 mg/kg) did not cause any significant changes in either measure of locomotor activity (100 mg/kg NaB vs. saline, p > 0.05 for all time points, Bonferroni post hoc test).
Figure 1.
Acute, but not chronic, treatment with NaB increases immobility in the FST at doses that do not affect locomotor activity. Mice were given 3 injections of NaB over 24 hours (a) or treated twice daily for 21 days with NaB or DMI (b), and their locomotor activity was measured 1 hour after the last injection. Ambulations (left) and crossings (right) were measured in 5-minute bins for a total of 30 minutes in acutely treated mice (a). The higher dose of NaB (1.2 g/kg) caused significant hypolocomotion in the first 5 minutes, while the lower dose (100 mg/kg) had no effect (n=8). Chronic treatment with NaB (100 mg/kg) or DMI (12.5 mg/kg) had no significant effect on either ambulations (left) or crossings (right) over 60 minutes (b) (n = 30). A separate cohort of mice was given acute treatment with NaB or DMI, and immobility during a 6 minute FST was measured (c). There was a significant effect of treatment on immobility, with DMI significantly reducing immobility and NaB (100 mg/kg) significantly increasing immobility (n=5–6). After chronic treatment with twice-daily NaB (100 mg/kg) or DMI (12.5 mg/kg), DMI significantly reduced immobility in the FST, whereas NaB had no effect (d). Error bars indicate SEM. *p< 0.05 vs. saline; ** p< 0.01 vs. saline; ***p<0.001 vs. saline.
Changes in general activity levels can compromise interpretation of results in the FST; therefore, we examined the effect of the lower dose of NaB in this paradigm. Acute treatment (3 injections over 24 hr) with 100 mg/kg NaB caused a significant increase in immobility in the FST (main effect of drug, F(2, 14) = 12.10, p = 0.0009, ANOVA; significant difference between NaB and saline, p < 0.05, Newman-Keuls multiple comparisons post hoc test)(Figure 1c). In the same study, the tricyclic antidepressant desipramine (DMI) caused the expected decrease in immobility (DMI vs. saline, p < 0.05, Newman-Keuls multiple comparisons post hoc test), an antidepressant effect. After chronic treatment (100 mg/kg, twice daily for 21 days), mice injected with NaB showed no difference in immobility from saline-injected mice one hour after the last injection (Figure 1d)(Main effect of treatment, F(2, 24) = 4.201, p = 0.0273, ANOVA; no significant difference between NaB and saline, p > 0.05, Bonferroni’s multiple comparisons post hoc test). The effect of DMI, however, was maintained after chronic treatment, as DMI-injected mice showed a decrease in immobility compared to saline-treated mice (significant difference between DMI and saline, p < 0.05, Bonferroni’s multiple comparisons post hoc test). Locomotor activity was also measured after chronic treatment with both NaB and DMI (Figure 1b). Chronic treatment with NaB did not alter locomotor activity one hour after the last injection (a time point parallel to when other behavioral tests were carried out) (Ambulations: No main effect of treatment, F(2, 209) = 1.204, p = 0.3218; significant time by treatment interaction, F(22,209) = 1.789, p = 0.0196, RMANOVA; NaB vs. saline, p > 0.05 for all time points, Bonferroni post hoc test. Crossings: No main effect of treatment, F(2, 209) = 1.122, p = 0.3462, trend toward significant time by treatment interaction, F(2, 209) = 1.559, p = 0.0586, RMANOVA; NaB vs. saline, p > 0.05 for all time points, Bonferroni post hoc test). Chronic treatment with DMI also did not cause any significant change in locomotor activity one hour after the last injection (Activity: DMI vs. saline, p > 0.05 for all time points, Bonferroni post hoc test. Crossings: DMI vs. saline, p > 0.05 for all time points, Bonferroni post hoc test).
3.2 NaB causes divergent effects in the NIH paradigm depending on the length of treatment
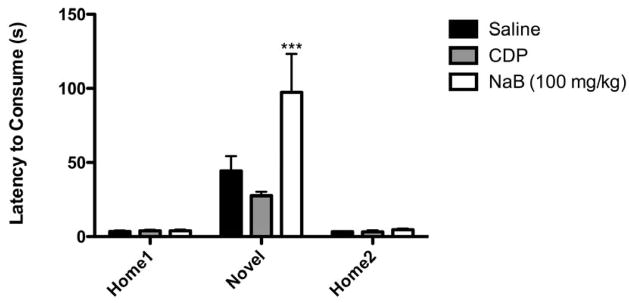
The FST is responsive to acute treatment with antidepressants; therefore, we sought to test the efficacy of NaB in the novelty-induced hypophagia (NIH) paradigm, which is both a test of anxiety and a model of chronic antidepressant response. In this paradigm, latency to approach a familiar food in a novel environment is reduced acutely by anxiolytic compounds, and by chronic treatment with antidepressants (Dulawa et al., 2004; Gur et al., 2007; Merali et al., 2003). We first sought to evaluate the acute effect of NaB in this paradigm, and did so using a dosing regimen identical to that used in the FST; mice received 3 injections of NaB in the 24 hours before testing in the novel environment, with the last injection given one hour before testing. We observed the expected increase in latency to consume in the novel environment as compared to the home cage in all groups (main effect of day, F (2, 52) = 30.81, p < 0.0001, RMANOVA). However, NaB caused a further increase in latency to consume, which was specific to the novel environment (significant day x drug interaction, F (4, 52) = 4.934, p < 0.01; NaB vs. saline on Novel Day, p < 0.001, Bonferroni post hoc test; NaB vs. saline on Home1 and Home2, p > 0.05, Bonferroni post hoc tests)(Figure 2). As a control, we also examined the effects of chlordiazapoxide (CDP), a benzodiazepine that has been shown to decrease latency to consume in the novel environment (Merali et al., 2003). As reported previously in this strain (Gur et al., 2007), CDP reduced latencies; however in this study, the difference did not quite reach significance (CDP vs. saline, p > 0.05, Bonferroni post hoc test).
Figure 2.
Acute treatment with NaB increased latency to consume peanut butter chips in the novel environment of the NIH paradigm. Mean latencies to consume in home and novel environment are shown. Mice were given 3 injections of NaB (AM and PM on Home Day 1 and AM on Novel day, 1 hr before test) or 1 injection of CDP (1 hr before test) before exposure to novel environment. There was an increase in latency to consume in the novel environment relative to the home cage (p<0.0001) and an increase in latency in NaB-treated animals as compared to saline-treated animals (*p<0.001) (n=9–10). Error bars indicate SEM.
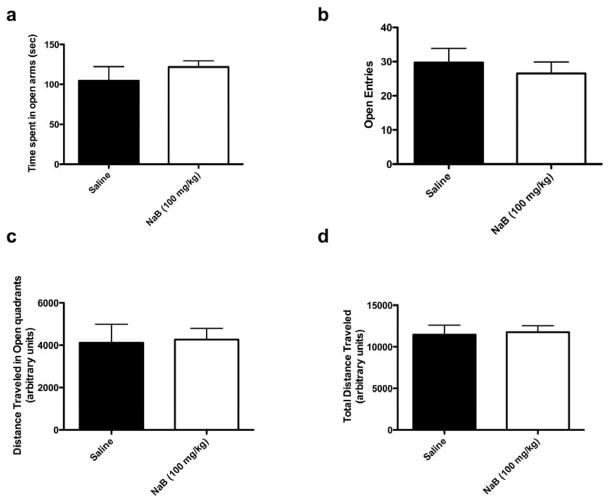
To further investigate this anxiogenic effect of acute treatment with NaB, we examined the effects of NaB in another test of anxiety, the elevated zero maze (EZM). In this paradigm, acute treatment with NaB did not have any significant effect on time spent in the open arms (NaB vs. saline, p = 0.4182, unpaired t-test), entries into the open arms (NaB vs. saline, p = 0.570, unpaired t-test), or distance traveled in the open arms (NaB vs. saline, p = 0.884, unpaired t-test) (Figure 3). In addition, NaB had no effect on total distance traveled during exposure to the EZM (NaB vs. saline, p = 0.8339, unpaired t-test), further confirming that this dose does not affect overall activity levels.
Figure 3.
Acute treatment with NaB had no effect on anxiety behavior in the elevated zero maze. Mice were given 3 injections of NaB over 24 hours and tested in the EZM 1hr following the last injection. NaB had no effect on time spent in the open quadrants (a), entries into the open quadrants (b), distance traveled in the open quadrants (c), or total distance traveled in the maze (d) (n = 6–7). Error bars indicate SEM.
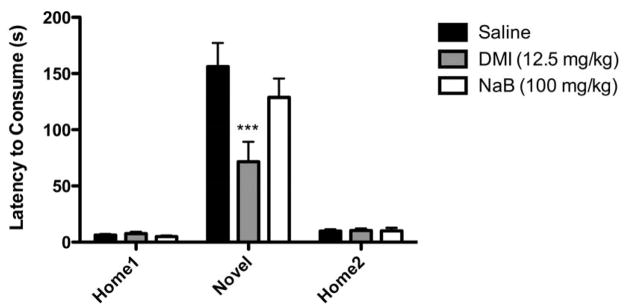
Changes in gene expression brought about by NaB might require longer time periods to take effect; therefore, we examined the effects of chronic treatment (2 injections/day for 21 days) with NaB in the same array of behavioral paradigms. In the NIH paradigm, selective serotonin reuptake inhibitors and tricyclic antidepressants have been shown to reduce latency to consume in the novel environment after similar lengths of treatment, which have led to use of this paradigm as a model of chronic antidepressant response (Dulawa et al., 2004; Gur et al., 2007; Merali et al., 2003). In this study, mice were injected twice daily with a dose of 100 mg/kg NaB, which was well tolerated. We observed no changes in weight during the treatment period (data not shown), as has been previously reported (Schroeder et al., 2006). After chronic treatment with NaB, we again observed an effect of the novel environment to increase latency to consume (main effect of day, F (2, 40) = 96.30, p < 0.0001, RMANOVA) (Figure 4), a main effect of treatment on latency to consume (F (2, 40) = 4.813, p = 0.0197, RMANOVA), and a treatment x day interaction (F (4, 40) = 5.596, p = 0.0011, RMANOVA). However, we saw no effect of chronic treatment with NaB (NaB vs. saline, p > 0.05, Bonferroni post hoc test). DMI, a tricyclic antidepressant, significantly reduced latency to consume in the novel environment (DMI vs. saline, p < 0.001, Bonferroni post hoc test), and the effect was specific to the novel environment (DMI vs. saline on Home1 and Home2, p > 0.05, Bonferroni post hoc test).
Figure 4.
Chronic treatment with NaB had no effect on latency to consume peanut butter chips in the novel environment of the NIH paradigm. Mean latencies to consume in home and novel environment are shown. Mice were treated with NaB or DMI for 22 days before exposure to novel environment. There was an increase in latency to consume in the novel environment relative to the home cage (p<0.001). There was a significant decrease in latencies in the novel environment in DMI-treated mice as compared to saline-treated animals, but no change in NaB-treated mice (*p<0.001) (n=9–10). Error bars indicate SEM.
3.3 NaB causes increases in histone acetylation in the hippocampus after acute, but not chronic treatment
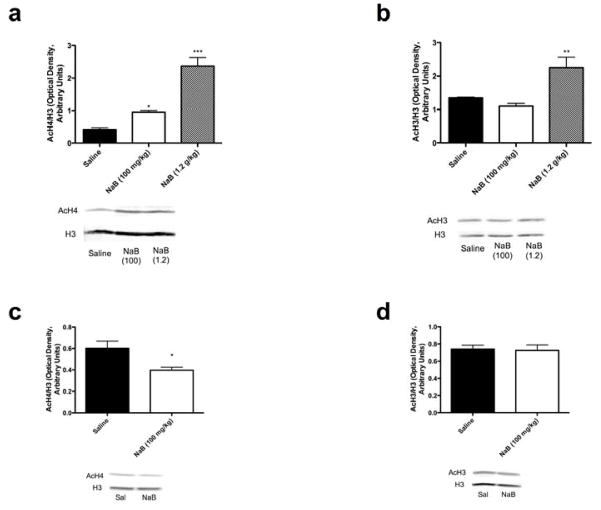
NaB is known to be an inhibitor of HDACs, but its effects in the brain have not been extensively characterized. Thus, we sought to examine the effects of the doses of NaB used in behavioral assays on histone acetylation in the hippocampus, a region known to be important in the activity of antidepressants. Using western blots, we examine the levels of acetylated histones H3 and H4 in the hippocampi of mice 30 min after the last of three injections of NaB (100 mg/kg or 1.2 g/kg). Acute treatment with both doses of NaB increased AcH4 levels in the hippocampus (main effect of treatment F(2, 14) = 35.78, p < 0.0001, ANOVA; 100 mg/kg vs. saline, p < 0.05; 1.2 g/kg vs. saline, p < 0.001, Bonferroni post hoc tests) (Figure 5a). However, only the higher dose significantly increased AcH3 in the hippocampus (main effect of treatment F (2, 15) = 12.93, p = 0.0005, ANOVA; 100 mg/kg vs. saline, p > 0.05; 1.2 g/kg vs. saline, p < 0.01, Bonferroni post hoc tests) (Figure 5b). We also examined histone acetylation after the chronic treatment (twice daily for 21 days) with 100 mg/kg NaB (30 minutes after the final injection). Here, we observed a significant decrease in levels of AcH4 in the hippocampus (p = 0.0166, unpaired t-test) (Figure 5c), and no change in the levels of AcH3 in the hippocampus (p > 0.05, unpaired t-test) (Figure 5d).
Figure 5.
Optical density of AcH staining normalized to total H3 staining is shown. Mice were given 3 injections of NaB over 24 hours and sacrificed 30 min after the last injection (a and b). Acute treatment with NaB dose-dependently increased AcH4 in the hippocampus (a), while only the higher dose of NaB (1.2 g/kg) significantly increased AcH3 in the hippocampus (b) (n = 5–7). A separate cohort of mice was injected with NaB for 21 days and sacrificed 30 minutes after the last injection (c and d). Chronic treatment with NaB decreased AcH4 in the hippocampus (c), while the level of AcH3 in the hippocampus did not change after chronic treatment with NaB (n=7–8). *p<0.05 vs. saline; **p<0.01 vs. saline; ***p<0.001 vs. saline. Error bars indicate SEM.
4. Discussion
The importance of chromatin remodeling in psychiatric diseases has received much attention recently. In this study we show that, despite causing changes in the level of histone acetylation in the hippocampus, the histone deacetylase (HDAC) inhibitor sodium butyrate (NaB) failed to cause any change in mouse models of chronic antidepressant response. Acute treatment with NaB did induce an increase in immobility in the FST as well as an anxiogenic effect in the novelty-induced hypophagia (NIH) paradigm, with no effects on general locomotor activity. Acute NaB had no effect on anxiety behavior in the elevated zero maze (EZM). While chromatin remodeling has been reported following chronic antidepressant treatment (Tsankova et al., 2006), these data suggest that changes in chromatin structure alone may not be sufficient to induce antidepressant behavioral effects.
We showed an increase in immobility in the FST after acute treatment with NaB. Recently, Schroeder et. al. (2006) showed a similar effect of acute treatment with NaB in the tail suspension test (TST), an analogous behavioral paradigm, although in a different mouse strain (C57BL/6, whereas we use an F1 hybrid of C57BL/6 and 129SvEv mice) and at a much higher dose (1.2 g/kg) than used here (100 mg/kg). At this higher dose (1.2 g/kg), we saw significant hypolocomotion, which could be responsible for the increased immobility seen in Schroeder et. al. (2006), but not for that seen in the present study, as the lower dose of NaB (100 mg/kg) had no such effects on locomotor activity.
We also report here that chronic treatment with 100 mg/kg NaB (twice daily for 21 days) failed to cause any changes in behavior in either the FST or NIH paradigms. Schroeder et. al. (2006) did see an antidepressant effect in the TST after chronic treatment. There are a number of methodological differences between the present study and that done by Schroeder and colleagues, including the dose of NaB (100 mg/kg twice daily here, 1.2 g/kg in the Schroeder et. al. study), the behavioral paradigm (FST vs. TST), the mouse strain (C57BL/6 vs. F1 hybrids of C57BL/6 and 129SvEv), and the length of treatment (21 vs. 28 days). Additionally, the effect observed by Schroeder et. al. (2006) was seen only when the TST was administered on the last day of a 4-day battery of testing and not when administered to behaviorally naïve mice. As the authors suggest, the previous days’ tests may have been a source of stress, and the NaB may have acted to reduce the effects of this stress, rather than having antidepressant effects on its own. This activity of NaB to counteract the effects of stress fits well with results obtained by Tsankova et. al. (2006) using the chronic social defeat (CSD) paradigm. In this study, the tricyclic antidepressant imipramine (IMI) was seen to increase H3 acetylation at BDNF promoters, but only in mice previously exposed to CSD (Tsankova et al., 2006). These stressed mice showed increases in histone methylation, a modification associated with repression of gene expression. The activity of IMI to increase AcH3 countered the decreases in BDNF expression associated with stress-induced increases in di-methylated H3 (Tsankova et al., 2006). In another study, chronic fluoxetine (21 d) was shown to increase AcH3 at the BDNF promoter, but only in mice with increased tri-methylated H3 and decreased AcH3 at the BDNF promoter due to perinatal exposure to methylmercury (Onishchenko et al., 2008). It is therefore possible that the effects of NaB require prior stress experience (or some additional manipulation that causes a repressive chromatin state), and because there was no prior stressful experience in our FST study, NaB did not exert an antidepressant effect. In addition, the FST and TST have been traditionally used to screen novel antidepressants, but these paradigms have mainly validated compounds that act to increase synaptic monoamine levels. Thus, they may not be appropriate to test the efficacy of any antidepressant acting by a novel pathway, e.g. direct changes in gene expression (Cryan et al., 2002).
Due to the potential limitations of the FST, we next examined the effects of NaB in another behavioral paradigm: the NIH. We report here that acute treatment with NaB caused an increase in latency in the novel environment, an anxiogenic effect (Soubrie et al., 1975). This response was not observed in the elevated zero maze (EZM), another test of anxiety behavior. While these results seem to contradict each other, it is important to note that the NIH may measure different aspects of anxiety than the EZM, and also contains appetitive or hedonic components (e.g. consumption of peanut butter chips) not present in the EZM. Increases in histone acetylation (H3 and H4) have been correlated with the anxiolytic effects of alcohol, and decreases in histone acetylation (H3 and H4) in the amygdala were associated with the anxiogenic effects of alcohol withdrawal (Pandey et al., 2008). These decreases in AcH3 and AcH4 and increases in anxiety during alcohol withdrawal were reversed by the HDAC inhibitor TSA, suggesting that increases in histone acetylation are causally related to decreases in anxiety (Pandey et al., 2008). Our study shows an inverted relationship between increased histone acetylation and anxiety as compared to the Pandey et. al. (2008) study. This discrepancy may be explained by the species used (rats were used in the Pandey et. al. study) and/or the presence or absence of alcohol (our mice were alcohol naïve), which may change the valence of the effect of increasing histone acetylation on anxiety.
We also examined the effects of chronic treatment with NaB in the NIH. The NIH has been validated as a test of chronic antidepressant response by a number of laboratories, including our own, as changes in latencies in the novel environment occur after chronic, but not acute, treatment with current antidepressants (Dulawa et al., 2004; Gur et al., 2007; Merali et al., 2003). Chronic treatment with NaB (21 days) did not cause any change in latencies in the NIH. This is in contrast to the increased latencies seen after acute treatment, and also in contrast to the antidepressant effect of chronic treatment with sodium butyrate in the TST seen by Schroeder et. al (2006). In addition to the differences between our study design and the Schroeder et. al. (2006) study discussed above, an additional aspect of the NIH paradigm that could have been influenced by NaB is the motivation of the mice to consume a highly palatable food: peanut butter chips. Based on the control of the home cage tests, the NaB dosing paradigm used in both the acute and the chronic studies did not appear to have any effect on motivation to consume the chips, as home cage latencies were unaffected by NaB. Furthermore, TSA was not seen to affect self-administration of sucrose in a fixed-ratio schedule or breaking points in a progressive-ratio schedule over a 7-day period, or consumption of sucrose in a two-bottle choice preference test after 4 days of treatment (Romieu et al., 2008). Therefore, it is unlikely that the effects of NaB on latency to consume in the NIH are based on changes in motivation for highly palatable food.
The NaB treatment regimens used in these studies were accompanied by changes in the acetylation state of H3 and H4 in the hippocampus. 30 minutes after acute treatment with NaB we observed a significant increase in AcH4 in the hippocampus but no change in AcH3. At the same time point following chronic treatment with NaB, we observed a decrease in AcH4 and no change in AcH3. This transition from an increase in AcH4 acutely to a decrease after chronic treatment may explain the shift in behavioral response to NaB between acute and chronic time points.
The literature contains conflicting evidence regarding the effects of NaB on histone acetylation levels in the brain, and it is important to keep in mind not only dosing regimen and area of the brain, but also whether acetylation is measured globally (e.g. western blots or immunhistochemistry) or at specific promoters (e.g. chromatin immunoprecipitation) (ChIP). In one study, ChIP analysis showed an effect of acute treatment with NaB (200 mg/kg) on AcH4 in the striatum, with AcH3 affected only by chronic treatment with NaB (Kumar et al., 2005). At higher doses, however, increases in both AcH3 and AcH4 in the hippocampus and frontal cortex were measured by western blot 30 minutes after a single injection of NaB (Schroeder et al., 2006). Here, we show an increase in AcH4 after acute treatment with NaB, which shifts to a decrease after chronic treatment. This change in response may be due to a desensitization after chronic treatment with this drug or some compensatory response of neurons to prolonged increases in AcH4. Additionally, it is important to remember that changes at specific promoters may be more complex than changes in global levels of acetylation, as measured in the present study. Recently it has been suggested that regulation of gene expression by histone acetylation is more complex than previously imagined, and deacetylation in some cases may lead to activation of genes (Nusinzon and Horvath, 2005). Indeed, previous work has shown that NaB alters levels of histone acetylation at specific promoters in a time-, dose-, and brain region-specific manner (Schroeder et al., 2008), and thus there may still be increased histone acetylation at some promoters despite the global decrease in AcH4 we observed.
Though limited in number, studies have demonstrated changes in histone acetylation in the brain after chronic treatment with antidepressants. Increases in AcH3 were seen in the hippocampus after chronic treatment with two distinct antidepressants, fluoxetine (an SSRI) and imipramine (a tricyclic), but only after manipulations (stress, methylmercury exposure) that lowered AcH3 and caused increases in repressive post-translational modifications to histones (Onishchenko et al., 2008; Tsankova et al., 2006). In another study, however, chronic treatment with fluoxetine was actually seen to decrease levels of AcH3 in the dentate gyrus of the hippocampus, as well as the frontal cortex and caudate/putamen (Cassel et al., 2006). Because of these discrepancies, it will be of increasing utility to examine histone acetylation at specific promoters, as the global acetylation state may not reflect chromatin structure at genes of interest. In addition, it is important to examine brain regions related to the behaviors in question, as there are clearly region-specific changes brought about by these drugs.
Based on recent evidence, changes in chromatin structure at specific promoters in the hippocampus are likely to play a role in the effects of classic antidepressants. We demonstrate here, however, that global changes in histone acetylation may not be sufficient to produce behavioral effects. It is possible that additional events are required for the full effects of antidepressants to be realized, such as parallel activation of transcription factors. Chromatin remodeling may act as a facilitating event, allowing specific transcription factors to more easily activate their target genes. Alternately, changes in histone acetylation may be more specific and occur only in concert with transcription factor binding, as in the case of CREB and CREB-binding protein, which has intrinsic histone-acetyl transferase activity. Thus, treatment with HDAC inhibitors may be most effective in combination with classic antidepressants, either to increase their efficacy or reduce the lag time before symptoms are ameliorated. Additionally, although BDNF has been implicated as one promoter at which changes in AcH may be involved in the action of antidepressants, it will be important to identify more genes at which these changes are taking place. It is also important to investigate how these changes in chromatin structure interact with the activity of transcription factors (such as CREB) known to be downstream of the activity of antidepressants. Overall, while chromatin modification likely plays some role in depression, the results of this study suggest that direct modulation of histone acetylation levels alone is not sufficient to induce antidepressant behavioral effects.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Alarcon JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, Barco A. Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron. 2004;42:947–959. doi: 10.1016/j.neuron.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Bilang-Bleuel A, Ulbricht S, Chandramohan Y, De Carli S, Droste SK, Reul JM. Psychological stress increases histone H3 phosphorylation in adult dentate gyrus granule neurons: involvement in a glucocorticoid receptor-dependent behavioural response. Eur J Neurosci. 2005;22:1691–1700. doi: 10.1111/j.1460-9568.2005.04358.x. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157:115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- Cassel S, Carouge D, Gensburger C, Anglard P, Burgun C, Dietrich JB, Aunis D, Zwiller J. Fluoxetine and cocaine induce the epigenetic factors MeCP2 and MBD1 in adult rat brain. Mol Pharmacol. 2006;70:487–492. doi: 10.1124/mol.106.022301. [DOI] [PubMed] [Google Scholar]
- Castren E, Voikar V, Rantamaki T. Role of neurotrophic factors in depression. Curr Opin Pharmacol. 2007;7:18–21. doi: 10.1016/j.coph.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Chandramohan Y, Droste SK, Reul JM. Novelty stress induces phospho-acetylation of histone H3 in rat dentate gyrus granule neurons through coincident signalling via the N-methyl-D-aspartate receptor and the glucocorticoid receptor: relevance for c-fos induction. J Neurochem. 2007;101:815–828. doi: 10.1111/j.1471-4159.2006.04396.x. [DOI] [PubMed] [Google Scholar]
- Conti AC, Cryan JF, Dalvi A, Lucki I, Blendy JA. cAMP response element-binding protein is essential for the upregulation of brain-derived neurotrophic factor transcription, but not the behavioral or endocrine responses to antidepressant drugs. J Neurosci. 2002;22:3262–3268. doi: 10.1523/JNEUROSCI.22-08-03262.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- Dranovsky A, Hen R. Hippocampal neurogenesis: regulation by stress and antidepressants. Biol Psychiatry. 2006;59:1136–1143. doi: 10.1016/j.biopsych.2006.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29:1321–1330. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Ferrante RJ, Kubilus JK, Lee J, Ryu H, Beesen A, Zucker B, Smith K, Kowall NW, Ratan RR, Luthi-Carter R, Hersch SM. Histone deacetylase inhibition by sodium butyrate chemotherapy ameliorates the neurodegenerative phenotype in Huntington’s disease mice. J Neurosci. 2003;23:9418–9427. doi: 10.1523/JNEUROSCI.23-28-09418.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur TL, Conti AC, Holden J, Bechtholt AJ, Hill TE, Lucki I, Malberg JE, Blendy JA. cAMP response element-binding protein deficiency allows for increased neurogenesis and a rapid onset of antidepressant response. J Neurosci. 2007;27:7860–7868. doi: 10.1523/JNEUROSCI.2051-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockly E, Richon VM, Woodman B, Smith DL, Zhou X, Rosa E, Sathasivam K, Ghazi-Noori S, Mahal A, Lowden PA, Steffan JS, Marsh JL, Thompson LM, Lewis CM, Marks PA, Bates GP. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, ameliorates motor deficits in a mouse model of Huntington’s disease. Proc Natl Acad Sci U S A. 2003;100:2041–2046. doi: 10.1073/pnas.0437870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Doherty JJ, Dingledine R. Altered histone acetylation at glutamate receptor 2 and brain-derived neurotrophic factor genes is an early event triggered by status epilepticus. J Neurosci. 2002;22:8422–8428. doi: 10.1523/JNEUROSCI.22-19-08422.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, Russo SJ, Laplant Q, Sasaki TS, Whistler KN, Neve RL, Self DW, Nestler EJ. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Lattal KM, Barrett RM, Wood MA. Systemic or intrahippocampal delivery of histone deacetylase inhibitors facilitates fear extinction. Behav Neurosci. 2007;121:1125–1131. doi: 10.1037/0735-7044.121.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson JM, O’Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 2004;279:40545–40559. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- Levine AA, Guan Z, Barco A, Xu S, Kandel ER, Schwartz JH. CREB-binding protein controls response to cocaine by acetylating histones at the fosB promoter in the mouse striatum. Proc Natl Acad Sci U S A. 2005;102:19186–19191. doi: 10.1073/pnas.0509735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merali Z, Levac C, Anisman H. Validation of a simple, ethologically relevant paradigm for assessing anxiety in mice. Biol Psychiatry. 2003;54:552–565. doi: 10.1016/s0006-3223(02)01827-9. [DOI] [PubMed] [Google Scholar]
- Nusinzon I, Horvath CM. Histone deacetylases as transcriptional activators? Role reversal in inducible gene regulation. Sci STKE. 2005;2005:re11. doi: 10.1126/stke.2962005re11. [DOI] [PubMed] [Google Scholar]
- Onishchenko N, Karpova N, Sabri F, Castren E, Ceccatelli S. Long-lasting depression-like behavior and epigenetic changes of BDNF gene expression induced by perinatal exposure to methylmercury. J Neurochem. 2008;106:1378–1387. doi: 10.1111/j.1471-4159.2008.05484.x. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Ugale R, Zhang H, Tang L, Prakash A. Brain chromatin remodeling: a novel mechanism of alcoholism. J Neurosci. 2008;28:3729–3737. doi: 10.1523/JNEUROSCI.5731-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoli M, Gennarelli M, Racagni G. Modulation of synaptic plasticity by stress and antidepressants. Bipolar Disord. 2002;4:166–182. doi: 10.1034/j.1399-5618.2002.01159.x. [DOI] [PubMed] [Google Scholar]
- Renthal W, Maze I, Krishnan V, Covington HE, 3rd, Xiao G, Kumar A, Russo SJ, Graham A, Tsankova N, Kippin TE, Kerstetter KA, Neve RL, Haggarty SJ, McKinsey TA, Bassel-Duby R, Olson EN, Nestler EJ. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron. 2007;56:517–529. doi: 10.1016/j.neuron.2007.09.032. [DOI] [PubMed] [Google Scholar]
- Romieu P, Host L, Gobaille S, Sandner G, Aunis D, Zwiller J. Histone deacetylase inhibitors decrease cocaine but not sucrose self-administration in rats. J Neurosci. 2008;28:9342–9348. doi: 10.1523/JNEUROSCI.0379-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder FA, Lin CL, Crusio WE, Akbarian S. Antidepressant-Like Effects of the Histone Deacetylase Inhibitor, Sodium Butyrate, in the Mouse. Biol Psychiatry. 2006 doi: 10.1016/j.biopsych.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Schroeder FA, Penta KL, Matevossian A, Jones SR, Konradi C, Tapper AR, Akbarian S. Drug-Induced Activation of Dopamine D(1) Receptor Signaling and Inhibition of Class I/II Histone Deacetylase Induce Chromatin Remodeling in Reward Circuitry and Modulate Cocaine-Related Behaviors. Neuropsychopharmacology. 2008 doi: 10.1038/npp.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubrie P, Kulkarni S, Simon P, Boissier JR. [Effects of antianxiety drugs on the food intake in trained and untrained rats and mice (author’s transl)] Psychopharmacologia. 1975;45:203–210. doi: 10.1007/BF00429062. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Kumar A, Nestler EJ. Histone modifications at gene promoter regions in rat hippocampus after acute and chronic electroconvulsive seizures. J Neurosci. 2004;24:5603–5610. doi: 10.1523/JNEUROSCI.0589-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]